Abstract
The high altitude regions around the world consist of an interesting group of landscapes with diverse features and microbial diversity. These regions differ individually and possess their own characteristic features such as lakes, glaciers, desserts, volcanoes, and forests. The microbial diversity found in these remote areas is uniquely adapted to the challenges of high altitude such as cold temperature, lack of water and biomass, seasonal variation in climatic conditions, and solar radiations. The unfortunate lack of studies on the microbial communities at high altitudes has made it necessary for the researchers to explore unique microbes surviving in the extreme conditions of higher altitude along with the enzymes they produce to survive. The novel characteristics of the enzymes obtained from these regions are expected to be industrially important which demands the need of their in depth understanding. The mountainous locations are among the highly prone areas to be affected by the global warming which ultimately leads to changes in the structure of microbial community and even extinction of microbial species. In this chapter, we discuss various higher altitude sites, their climatic conditions and the factors affecting the microbial community structure. We also present the seasonal variation in the enzyme activities and their correlations with various factors such as C/N ratio, amount of biomass, fungal/bacterial number ratio, and change in altitude. In addition, the predominant microbial species found at various high altitude niche regions were discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
The studies on microbial diversity in higher altitude regions are of much interest nowadays. As there is a growing effort to document all existing life, it is essential to reach all corners of the globe to find rare species that may have a chance of going extinct due to global warming. High altitude regions are among those extreme geological location that has been ignored in this effort of microbial search and discovery (Connon et al. 2007). High altitude locations are characterized with cold temperature, rapid changes in temperature, rise in total amount of radiation in a year, and decrease in vegetation. The amount of precipitation received may vary from one geographic location to another which in turn impacts the vegetation level and biomass. The correlation between the amount of plant biomass and the number of microorganisms is pretty straightforward, so it stands the reason that the high altitude regions with lesser plants will have lower amount of microorganisms (Jha et al. 1992; Xu et al. 2015).
Mostly pyschrophilic microbes are present in higher altitude regions and their enzymes show activity at low temperature and site-specific stress conditions (Dumorné et al. 2017). Spore forming variants are usually found to be more successful at higher altitude regions due to their ability to survive at extreme temperature, pH, and pressure (Mandic-Mulec and Prosser 2011) and are also important nitrogen recyclers and biodegradation agents. Some species of Pseudomonas and Bacillus have been reported for their role as biocontrol agents and plant growth enhancers in colder regions (Pandey et al. 1999). Bacterial species such as Carnobacterium, Rhodococcus, and Serratia isolated from higher altitude regions have found their applications in bioremediation due to their biodegradation characteristics (Kaira et al. 2015; Leisner et al. 2007), whereas strains that are pathogenic in nature are important for clinical relevance (Wenzler et al. 2015; Brooke 2008). Enzymes produced by the cold adapted microbes isolated from alpine soils have found their role in industries due to their numerous advantages, thus are important targets for the biotechnology industry (Dumorné et al. 2017; Barria et al. 2013; Barroca et al. 2017). High demand of novel psychrophiles and enzymes that shows optimal activity at lower temperature attracts the scientific community to explore extreme locations of high elevation around the world.
Majority of the work has been done on the altitudinal gradients and existence of flora and fauna across low altitude landscapes (Kessler et al. 2011; Mccain 2005; Rahbek 2005). But the most abundant and diverse class of organisms of earth are still not well studied (Torsvik and Øvreås 2002). The current literature lacks the studies that considered elevation and climate at the same time (Siles et al. 2016). Microbial community present in the soil circulates the carbon, nitrogen, sulfur, phosphorus, and numerous metals, thus plays an important role in the foundation of earth’s biosphere. These microbes are also crucial for the decomposition of litter in an efficient manner (Schimel and Bennett 2004; Balser and Firestone 2005; Carney and Matson 2005).
The processes involved in the ecosystem are strongly affected by the microbial diversity of a community because these microbes play an important role in the respective processes. It is well known that fungal and Gram-positive bacterial strains are more susceptible in utilizing complex compounds when compared with the Gram-negative bacterial strains (Waldrop and Firestone 2006). The functioning of an ecosystem is influenced by the variations in community structure and microbial diversity which ultimately affected the overall turnover of soil organic matter (Stroud et al. 2007; Baumann et al. 2013; Wang et al. 2013). Diversity of microbes in the soil plays a significant part in the interactions and functioning (Hines et al. 2006; Stroud et al. 2007), changes in climate (Singh et al. 1989), and biogeography of an ecosystem (O’Malley 2007; Fierer et al. 2009) which demands the thorough study of their spatial patterns and driving factors. Hence, there is a need for improvement in the thorough understanding of microbial biogeography and the environmental factors associated with them.
A fewer studies are reported on the altitudinal patterns of soil bacterial and fungal communities which mainly projected the variation in the structures of microbial communities with the increase in elevation (Fierer et al. 2011). The factors including soil pH, carbon/nitrogen ratio (C/N), and climatic conditions showed positive correlation with the diversity and composition of the microbial community (Fierer et al. 2011; Lucas-Borja et al. 2012; Shen et al. 2013). The seasonal variation leads to change in the temperature and water level on high altitudes which ultimately affects the microbial biomass and the production of enzymes (López-Mondéjar et al. 2015). The climatic conditions also affected the plant biomass which can be seen during the leaf falling in the autumn season (Koranda et al. 2013). Not all mountain ecosystems are similar though. There is huge amount of variations at different sites of the same altitude such as the presence of lakes, forests, completely dry volcanic soil, etc. Some mountains with active or inactive volcanoes are found to have similar chemosynthetic modes of obtaining energy as are found in deep sea vents (Connelly et al. 2012). The study of such sites may even shed some light on the theoretical functioning of microbes on planets that may harbor life due to the possible presence of water, like Mars (McEwen et al. 2011). There is an urgent need to study the mountainous microbial communities as the threat of global warming looms over their existence. The global warming leads to receding glaciers which exposed the underneath soils to different environmental conditions, thereby altering the microbial community structures (Allison et al. 2010).
A study of the colonization of deglaciated soils at high altitudes revealed that the cyanobacteria were the ones to colonize during the initial period which leads to increase in soil stability and then arrival of moss, lichen, and vascular plants takes place (Schmidt et al. 2008). The microbes present in the soil play a major role in carbon flux between soil and atmosphere (Falkowski et al. 2008). The 2007 report of IPCC mentioned that the concentration of CO2 was 380 ppm in the year 2005, which was 80 ppm more than the average of 650,000 years before it. The microbial biomass remains stable or decreases with the increase in global warming but there is no case of increase (Vanhala et al. 2011; Feng and Simpson 2009). The fungal numbers were also found to decrease with increase in global warming (Vanhala et al. 2011). The usual mode of studying high altitude microbes has been by typical culture techniques in which only the microbes that grow in nutrient rich media grow with high growth rates, which unfortunately excludes a large number of microorganisms that live in these areas (Kaeberlein et al. 2002). This is why the recent attempts at isolating microorganisms make use of the genetic sequences and other advanced techniques. The major techniques being used today for high altitude microbial studies are 16S rRNA, MALDI-TOF, diffusion chamber based isolation, and the use of metagenomic tools (Pandey et al. 2019) (Table 11.1). This chapter presents the detailed analysis on the effect of change in altitude and season on the microbial community structure and enzyme activities. We also discussed various sites around the world that were actively studied for their mountainous microbiota and their specific biogeographical features.
11.2 Study Sites and Their Climatic Conditions
Several higher altitude locations were explored for their microbial diversity and microbial activities around the world. The higher altitude regions studied are mainly from India, China, Nepal, Italy, Mexico, borders of Argentina and Chile, Africa, and Spain (Fig. 11.1).
11.2.1 Higher Altitude Locations Studied in India
A study was conducted in Meghalaya state of India at two different sites Byrnihat (100 m) and Shillong (1500 m). These locations are at a distance of 90 km away from each other and are situated between latitude 26″N and longitude 92″45′E at Byrnihat and latitude 25″34′N and longitude 92″47′E at Shillong (Table 11.2). The climatic condition in Byrnihat and Shillong is monsoonic type which varies from tropical to subtropical. December and January are among the coldest months of these places, whereas May to September time period remains moist and warm. The average maximum and minimum temperatures measured were 28 and 17.5 °C at Byrnihat, while at Shillong they were 20 and 10.9 °C, respectively. The average rainfall recorded was 367 mm at Shillong and 212 mm at Byrnihat. Gneisses, granite, and schists were the major type of soil present in these areas which were originated from the hard rock, whereas the texture of the soil is mainly reddish brown sandy loam formed through laterization (Kshattriya et al. 1992). A fungal strain was isolated for the production of laccase enzyme from the soil of cold desert, Mana (30°46′24.8″N; 79°29′33.4″E; located around 3238 m above mean sea level), and nearly 4 km from Badrinath, in Chamoli district of Garhwal Himalaya, India (Dhakar and Pandey 2016). Kumar et al. investigated the microbial diversity and physiochemical properties of the soil from the Himalayan cold desert of Gangotri. The higher altitude soil ecosystem was finalized for the collection of soil samples of Gangotri located at the 30.9947°N and 78.9398°E (Kumar et al. 2019). Whereas Pandey et al. (2019) studied the bioprospectation of cold adapted microbes of Pindari Glacier region located at 33°5′–30°10′N to 79°48′–79°52′E and cold desert at 30°46′24.8″N; 79°29′33.4″. In this study, they covered a wide range of altitudes between 1800 and 3610 m above sea level including the alpine, subalpine, and temperate zones of the Indian Himalayan region. The location is known for heavy rainfall and snowfalls. The mean temperature measured in these regions lies near about 5.5 °C in the month of January and 21.5 °C in August, whereas pH of the soil lies in the range of 4.5–6.5 at the sampling site.
11.2.2 Higher Altitude Locations Studied in China
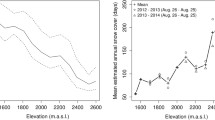
The variation in structure and functioning of microbial diversity of Changbai Mountain in the Northeast China (Jilin Province) located at the 126°55′-129°00′E; 41°23′-42°36′N was investigated. This mountain is among the most promising locations for the study of highly conserved ecosystems on earth to explore novel microbial communities that spread along the border of North Korea and China. In the northeast region of China, Changbai mountain is the highest mountain which possesses a well-defined vertical distribution of forests. The mean annual temperature (MAT) increases from the highest part (2691) of the mountain to the lowest part of the mountain (540), whereas mean annual precipitation (MAP) decreases from 1340 to 750 mm (He et al. 2005). The changes in climatic conditions and topography of the mountain resulted in four vertical zones of vegetation and are categorized as: (a) mixed coniferous and broad-leaved forest (MCB) which is located below 1100 m; (b) dark-coniferous spruce-fir forest (DCF) which is located between 1100 and 1700 m; (c) Ermans birch forest (EB) which is located between 1700–2000 m, and (d) alpine tundra (AT) which is located above 2000 m. The type of soil found in MCB region is Albi-Boric Argosols and in DCF, EB, and AT region is cambisols (Shen et al. 2013), all of which are derived from volcanic ash (Xu et al. 2015). Tan et al. studied the effect of removal of snow from the microbial biomass in a Tibetan alpine forest. The site of study is located in a 120-year-old natural alpine forest of China which is in the Bipenggou Nature Reserve of Lixian County of Sichuan, China. This site is located at 31°15′28.10″N, 102°53′29.34″E and 3580 m a.s.l. in the Eastern Tibetan Plateau (Fig. 11.2). The annual mean precipitation was recorded as 850 mm, while annual mean temperature is 3.0 °C with maximum of 23.1 °C in the month of July and minimum of −18.0 °C in the month of January, respectively. Soil temperature shifted to below 0 °C and during the whole cold snow season remains frozen from late November to mid-April (Wu et al. 2010). The sample soil is categorized as Cambic Umbrisols with a 15 cm deep layer of organic matter (Tan et al. 2014). The enzyme activities and nutrient composition of soil collected from dry-hot valley region of southwestern China was studied along an altitudinal gradient which lies between 680 and 1840 m a.s.l. The study site lies in the lower region of the Jinsha River in eastern Ningnan County of Sichuan province which is located at 26°54′-27°09′N, 102°54′-103°02′E. The major soil type present in this area is classified as an Ustic Ferrisols under the Chinese taxonomic system. The climatic conditions and vegetation vary in this region along the elevational gradient. Elevations >1500 m refer to a subtropical climate having mean annual precipitation of 700–850 mm, a mean annual evapotranspiration of about 1600 mm, and a mean annual temperature of 14.2 °C. Elevations <1500 m belong to the typical dry-hot valley region and possess a mean annual precipitation of 600–700 mm, a mean annual evapotranspiration of about 3500 mm, and a mean annual temperature of 21.8 °C (Xiao et al. 2019). Zhang et al. (2014) reported the Bacterioplankton communities from four different sites of the Luoshijiang Wetland. The Luoshijiang Wetland, a typical freshwater wetland in the Yunnan-Kweichow Plateau, is situated in Dali City of Yunnan Province. The wetland is surrounded by farmlands and occupies an area of 1 km2 with an elevation of approximately 2056 m. This wetland is adjacent to the Erhai Lake, which is also the second largest high altitude lake in Yunnan Province. The annual precipitation and the annual mean air temperature at this site were 1000–1200 mm and 15.7 °C, respectively. The study sites include A as the no vegetation zone located at 25°57′25″N–100°06′06″E, B as the reed-planted zone at 25°57′12″N–100°05′59″E, C as the densely water-lily-planted zone at 25°57′4″N–100°06′00″E, and D as the sparsely water-lily planted zone at 25°56′55″N–100°05′59″E. Tang et al. (2012) investigated the diversity in bacterial communities in the Zoige Wetland soil of the Qinghai-Tibetan Plateau of China.
11.2.3 Higher Altitude Locations Studied in Other Parts of the World
Siles et al. investigated the four types of forests in the South Tyrol region of Italian Alps. The alpine region consists of four diverse vegetation zones that dominate in the reported area including submontane, montane, subalpine, and alpine. The zone submontane is located 8 km south of Bozen/Bolzano at an elevation of 545–570 m a.s.l. and is above the Small Lake Montiggl. The mean annual temperature was recorded to be 11 °C and the annual precipitation was found to be approximately 900 mm at this zone with the mild continental climatic conditions consisting of submediterranean influences (Bonavita et al. 1998; Margesin et al. 2014).The montane zone is situated 300 m north of the village center of Klobenstein on a small hill at an elevation of 1175–1200 m a.s.l. The mean annual air temperature recorded as 7.4 °C with an annual precipitation of 950 mm, whereas the climate is montane-continental. The soil type was classified as dystric cambisol in case of submontane and montane sites. The subalpine site is located below the Rittner Horn and is around 7 km north of Bozen/Bolzano at an elevation of about 1724–1737 m a.s.l. The soil contained a thick layer of raw humus and its type was categorized as Haplic Podzol. The mean annual air temperature measured as 4.0 °C with an annual precipitation of 1000 mm with the subalpine-continental climatic conditions (Bonavita et al. 1998; Margesin et al. 2014). The alpine site is located at a height of 1965–2000 m a.s.l. at the tree line below the mountain Schwarzseespitze. The mean annual temperature of this site is 2.4 °C and an annual precipitation is of 1050 mm with alpine-continental climatic conditions. The subalpine and alpine soil type is Haplic Podzol. The pedogenic substratum consists of rhyolite in all of the four zones (Siles et al. 2016). In another study, the soil samples were collected from different places of Kathmandu Valley (Kirtipur, Balkhu, Dhobighat, Gwarko, and Putalisadak) and the Rautahat District of Nepal to produce the secretory enzyme laccase. The altitude of these sites ranges from 1600 to 2303 m above sea level (Yadav et al. 2019). The microbial diversity was investigated in the mineral soils of the Atacama region. Soil samples were collected in mid-February during the austral summer at elevations ranging from 5500 to 6330 m a.s.l. on Volcán Socompa and Volcán Llullaillaco, located at the border of Argentina and Chile. This work was a part of global survey of biological diversity at high altitudes in the Andes, Rockies, and Himalayan mountain range (Lynch et al. 2012).The seasonal and vertical distribution of picoplankton were reported in the Alchichica lake which is situated at 19°24′N, 97°24′W; 2340 m a.s.l. The lake belongs to a group of six maar lakes, thus considered as a crater lake, located on the eastern region of the Trans-Mexican Volcanic belt of the central Mexican Plateau. It covers surface area of 2.3 km2. It possesses maximum depth of about 62 m, while its mean depth is around 41 m; thus, the lake is one of the deepest lakes of Mexico. The Alchichica lake is oligotrophic with a salinity of 8.5 g/L and alkaline pH between 8.6 and 9.4. The Chl a concentration of the lake was recorded as <5 μg/L (Ramírez-Olvera et al. 2009) and high concentration of sodium–magnesium and chloride–bicarbonate ions was present. The climatic conditions of the location are semi-arid with annual precipitation of <500 mm and a mean temperature of 12.9 °C. The lake is warm-monomictic type, with mixing during the cold dry season and thermal stratification from April to December (Pajares et al. 2017). Margesin et al. studied the microbial community structure and their activities in the soil of alpine and subalpine regions in the Grossglockner mountain area of Austrian central Alps. Soil samples were taken along the southern and northern slopes at subalpine area with an elevation of 1500–1900 m, under packed vegetation, up to the forest line and alpine area with an elevation of 2300–2530 m, under scattered vegetation, above the forest line. All the soil samples were predominantly gneiss (silicate) type (Margesin et al. 2009). The soil samples of Mount Kilimanjaro of Africa were studied for the decomposition of soil organic matter with the increase in altitude via enzymatic catalysis. The temperature sensitivity of the process was also investigated. The site is located adjacent to the Machame route at 3°4′33″S 37°21′12″E. The altitude gradient lies in between the colline zone to middle subalpine zone within a range of 950–3020 m a.s.l. (Blagodatskaya et al. 2016). Lucas-Borja et al. presented the characteristics of content present in the Mediterranean humid soil with the diversity of microbes present in the location. The study was conducted in the Cuenca Mountains of east-central Spain. During the experimentation, six forest areas and two tree diversity levels including monospecific and mixed pine forest were considered at three stages of altitudes. At the lower altitude of up to 960 m a.s.l. a monospecific Spanish black pine forest stand and a mixed forest stand were studied. At medium altitudes of up to 1350 m, a monospecific Spanish black pine forest stand and a mixed forest stand named Scots pine and Spanish black pine were investigated, whereas at the altitude of above 1670 m, a monospecific Spanish black pine forest stand and a mixed forest stand named Scots pine and Spanish black pine were studied (Lucas-Borja et al. 2012).
11.3 Microbial Diversity of High Altitude Regions
The mountainous regions of the world are home to numerous and interesting microbial populations with their own unique adaptations for survival. Various higher altitude locations around the globe showed novel characteristics and diverse range in the microbial communities. In this section, we take a look at the different studies that sought to catalogue the variety of microorganisms in various mountains around the world. The Central Alps of Austria have availability of both Alpine and subalpine regions making a suitable location for high altitude microbial study. A change in microbial community structure is to be expected due to the change in altitude with the total number of microorganisms decreasing in general (Uchida et al. 2000; Lipson 2007), but the proteobacteria showed an increase along with psychrophilic Gram-negative bacteria and fungi (Margesin et al., 2009) (Fig. 11.3). The method of investigation also had a bearing on the strains obtained, like proteobacteria and β-proteobacteria which showed up majorly on the culture-independent methods, while γ-proteobacteria and Firmicutes were shown to be present in high number in the culture dependent studies with bacteroidetes present in lower numbers (Margesin and Miteva 2011). The Gram-positive bacteria were outnumbered by Gram negative as the altitude increased, which may be due to their tolerance to freeze-thaw cycle and changes in pH. The Himalaya and the Arctic region have been shown to have psychrotolerant fungal species like Aspergillus, Cladosporium, Penicillium, and Trichoderma (Dhakar and Pandey 2016). Dhakar et al. isolated a novel strain of psychrotolerant fungus identified as Cladosporium Tenuissimum with the ability to produce laccase (Dhakar and Pandey 2016). Similarly, Yadav et al. isolated several Actinomycetes and Streptomycetes strains from the Kathmandu Valley and observed the production of laccase from a strain identified as Pestalotiopsis spp (Yadav et al. 2019). Himalayas contain both vegetated areas and arid regions. But there are some mountain ranges that remain permanently without water and vegetation, which make them interesting to study for the microorganisms that survive there. One such study involved the higher regions of Atacama region spanning Argentina and Chile. The study took place at approximately 6000 m a.s.l. and in the extremes of daily high and low temperatures as well as intense solar radiation (Lynch et al. 2012). In these mountains, at elevations of 5000 m a.s.l. and above, a Mars like atmosphere prevails devoid of any plant or animal life. There has been a lot of interest in the Atacama region but it has received little attention from a biology point of view (Connon et al. 2007). The 16S and 18S targeted microbial study revealed that at lower levels Pseudonocardia dominates, while the dominance shifts to Ktedonobacter with increase in altitude. Eukaryotic microorganisms were found in only 7% of the total samples, while the Xerotolerant Cryptococcus albidus clade members were present majorly. There was a minor number of archae as well from the phylum Thaumarchaeota, which can utilize trace quantities of ammonia and oxidize it for energy. Autofluorescence at 680 nm was used to discover low levels of algae and cyanobacteria containing chlorophyll in the higher reaches of Himalayan soils.
Similar method used in Atacama indicated the absence of any chlorophyll containing microorganisms which made it necessary to look for other sources of energy the existing microbes may use from the volcanic areas of the peaks. Another factor affecting the survival of microorganisms is the cooling rate which drastically decreases their number at 1.5 °C/h, while at slower cooling rates they were largely unaffected. The cooling rates recorded on Volcán Llullaillaco, the major site of this study, has linear cooling rates faster than 1.5 °C/h in the range of 1.83 °C/h. The only other place having similar cooling rates is the Peruvian Andes and measured during the austral winter in barren, peri-glacial soils and is found to be faster than in some studies on Himalayas, especially Tibet. The sequence data revealed that the major Actinobacteria is very similar to Pseudonocardia asaccharolytica which is known to be capable of oxidizing dimethyl sulfide (DMS). The other dominant lineage is from a branch of Ktedonobacter racemifer which is a facultative “carboxydovore.” The upper atmosphere contains microorganisms that can be dispersed globally by precipitation, which may be the source of these microbes in the Atacama mountains (Lynch et al. 2012).
There are similar mountain ranges to the one in above study that are approximate in environmental and geological conditions like the subnival zones of Colorado Rockies and Central Andes. Although these soils appear barren, and the deep ice pack slows down any soil evolution, unexpected amount of nitrogen and carbon cycling have been observed by some studies. The process of mineralization by microorganisms has been documented by the researchers, who observed that the fungi was majorly involved in organic material mineralization during the melting of snow, while in the summertime, bacteria dominated the process. Although the Andes sites in Peru were higher and drier than the sites in Colorado (82 μg C/g), the Andes (140 μg C/g) was found to be hosting much more life forms as indicated by the amount of microbial biomass. But there was correlation between water content and biomass as well (King et al. 2008)
The features that can be found on some of the mountains around the world are water bodies such as lakes, which can be formed by inactive volcanoes or other kinds of depressions. Lakes at high altitudes may differ in the type of microbial life they have from other areas of the same mountain. It is thus necessary to study and contrast microbial life in high altitude lakes from mountains or other low level lakes. One such study was done by Pajares et al. (2017) that examined seasonal and altitude variations in picoplankton and their ability for ammonium oxidation and denitrification through effects on their gene distribution in the Lake Alchichica (2340 m a.s.l.) of Central Mexican Plateau. Another such crater lake has been studied in the Iztaccihuatl Volcanic complex in Mexico (Calvillo-Medina et al. 2019). It is a Pleistocene stratovolcano complex, with an active volcano, three glaciers, and two crater lakes. The study done by 16S rRNA gene amplicon method revealed the presence of several extremophiles well adjusted to this kind of climatic conditions. Three sites were chosen for sample isolation, namely Monte de Venus crater lake (4950 m a.s.l.), glaciers La Panza (5065 m a.s.l.) and El Pecho (5200 m a.s.l.). In all three samples taken together the Proteobacteria pervaded with a comparative percentage of 31–93% distantly followed by Actinobacteria with 6–15% of the total and Bacteroidetes at 1–17%. Other smaller groups present were Deinococcus-Thermus, Armatimonadetes, Candidatus Saccharibacteria, Parcubacteria, Cyanobacteria, Firmicutes, and Acidobacteria.
Another study in similar vein was done by Zhang et al. (2014) which was concerned with high altitude wetlands and its microbial communities. The site of study was Luoshijiang wetland situated in the Yunnan-Kweichow Plateau, China. The 16S rRNA study revealed the presence of Actinobacteria, Bacteroidetes, Gemmatimonadetes, Proteobacteria, Cyanobacteria, and Verrucomicrobia with only Bacteroidetes, Proteobacteria, and Verrucomicrobia common to all the testing sites. The study of correlation between the various microbes and the chemical properties of water revealed that positive correlations exist between pairs of Actinobacteria and Gemmatimonadetes with nitrile nitrogen and Alphaproteobacteria with dissolved phosphorus. Also present were members of genus Luteolibacter and Haloferula, but only in a few samples and in lower percentages. Of special interest among these is Verrucomicrobia, which is universally distributed and capable of using several carbon compounds (Arnds et al. 2010). Although the dominant nature of Verrucomicrobia in freshwater lakes has been studied, but its correlation with the dissolved phosphorus and nitrogen has been ignored (Arnds et al. 2010; Kolmonen etal. 2011). Another predominant freshwater microorganism is Proteobacteria which is supported by the findings of Sommaruga and Casamayor (2009) showing the dominant presence of beta-proteobacteria in lakes around the Mount Everest as well as the largest Tibetan lake called Lake Namco (Liu et al. 2013). Alpha and gamma proteobacteria take precedence over beta-proteobacteria in more saline lakes situated at high altitudes as shown with the study of 16 high altitude lakes on the Tibetan Plateau (Zhang et al. 2014).
While the Tibetan Plateau is consistently very dry place, the western part of Himalayas is very diverse. A study by Kumar et al. (2019) in the Gangotri Glacier, which is also the second largest glacier in Himalayas after Siachen, has revealed several novel microbial communities. The study was spurred by the fact that the Gangotri Glacier is receding very fast and the soil left uncovered may change its microbial mix and rapid documentation is required before that happens. Two sites were selected for comparison purposes, one at Kandakhal (1532 m a.s.l.) and Gangotri (3415 m a.s.l.) using the metagenomic analysis, several bacterial lineages were revealed among which the ten major phyla in order of decreasing percentages were Proteobacteria (38.49%), Acidobacteria (17.88%), Actinobacteria (14.48%), Bacteroidetes (7.89%), Gemmatimonadetes (7.87%), Chloroflexi (5.94%), Nitrospirae (1.08%), Cyanobacteria (0.93%), Firmicutes (0.9%), TM7 (0.84%), and Elusimicrobia (0.55%). A number of unidentified OTUs were also present suggesting the exciting possibility of finding novel strains. In terms of abundance, the bacterial classes in decreasing order were Alphaproteobacteria (16.88%), Beta-proteobacteria (9.44%), Acidobacteria-6 (7.86%), and Actinobacteria (7.33%). There were both culturable bacteria on Gangotri site as well as a major number which are unculturable by normal methods. The authors of the study used a specially devised diffusion chamber to facilitate growth of some of the unculturable strains. The resulting cultures were found to be of Acidovorax facilis, Arthrobacter pascens, Arthrobacter equi, Dyadobacter endophyticus, Paenarthrobacter nirogunajacolicus, Pseudomonas baetica, Paenarthrobacter siccitolerans, Pseudomonas mandelii, Pantoea gaviniae, and Pseudomonas frederiksbergensis. In addition, there were some psychrophilic strains that were easily cultured using low temperatures, such as Arthrobacter humicola, Pseudomonas helmanticensis, Brevibacillus invocatus, and Pseudomonas mandelii.
Another study in the Himalayas by Pandey et al. sheds more light on the types of microorganisms that survive in harsh high altitude climates. The researchers here did not stick to a single site but studied various altitudes and regions such as temperate, subalpine, alpine, and cold desert areas of Pindari Glacier with altitudes ranging from 1800 to 3610 m a.s.l. The study made use of both 16S rDNA sequencing and MALDI-TOF analysis to study the diversity of microbes. A comparison of the MALDI-TOF, 16S rRNA, and biochemical methods confirmed that the use of MALDI-TOF for bacterial isolate identification is efficient and fast. The use of this technique revealed the major bacterial genera as Bacillus, Rhodococcus, Pseudomonas, Stenotrophomonas, Serratia, Lysinibacillus, Paenarthrobacter, Alcaligenes, Carnobacterium, and Microbacterium with Bacillus being the largest group, largely due to their spore forming ability making it easier for them to survive under harsh conditions of pH, temperature. and pressure. Another dominating genus was Pseudomonas which has already been known to survive easily in colder environments. The rest of the represented species are known majorly for bioremediation (Pandey et al. 2019).
All the above studies have shown us how much variations there can be at different altitudes and differing mountain ranges of the same altitude. Yet, there are some common groups that are consistently found at higher altitudes such as Proteobacteria. Other factors that modify the microbial biodiversity at these high altitudes are moisture content, nutrients available as well as the presence of special features like volcanoes.
11.4 Characteristics of Microbial Enzymes Produced at Higher Altitude Sites
The enzymatic activity of several microbial communities at higher altitudes has been studied in detail by the researchers around the globe. The major focus of their study lies on the variation of activities in different seasons throughout the year. It is suggested from the literature that the type of vegetation and litter affects the production of enzymes. The climate conditions, respiratory activity, and chemical properties of soil directly influence the quantity of enzymes (Sinsabaugh and Linkins 1989; Kshattriya et al. 1992). The activities of enzymes such as cellulase, amylase, and invertase isolated from the extracts of leaf litters of four different forests of different ages of lower and higher altitudes located in Northeast India were studied. It was observed that the enzyme activities were lower in case of litters at higher altitude than the litters of lower altitude, whereas cellulase and amylase showed the variation in their activities with the change in season at both altitudes. Cellulase and amylase activities were found to be increased during the decomposition of litter, whereas invertase activity increased only at the beginning stages of litter decomposition. The numbers of fungi and bacteria significantly correlated with the amylase and cellulase activities, whereas the activity of invertase remained unaffected with the microbial population. Angiosperm litters showed higher enzyme activities than the coniferous litters in the higher altitude regions (Kshattriya et al. 1992). The activities of dehydrogenase, urease, and phosphatase enzymes were determined from the microbial population of four different forest areas of lower and higher altitude regions. The forest stands considered in the study did not have the same regeneration stage and it was noticed that the activity of dehydrogenase enzyme positively correlated with fungal numbers in the four locations. The correlation was significantly positive regarding microbial number and urease activity at the lower altitudes of less degraded forests. But at higher altitudes, only fungal population numbers showed positive relation with urease activity. However, no significant correlation was observed between phosphatase activity and microbial population in case of higher altitude regions, but it was significantly positively correlated to number of fungi and organic C in soil, in the case of lower altitude (Jha et al. 1992). Xu et al. reported the higher activity of N-acetylglucosaminidase, β-glucosidase, leucine aminopeptidase, and acid phosphatase at mixed coniferous with broad-leaved forest than the other studied sites of high altitude regions. The soil nutrients and soil organic matter decomposition displayed positive correlation with the enzyme activities (Xu et al. 2015). A psychrotolerant fungus Cladosporium tenuissimum isolated from the Indian Himalayan region specifically a cold desert has been reported to be producing extracellular laccase enzyme. The enzyme showed optimum activity at temperature and pH of 14 °C and 5.5, respectively (Dhakar and Pandey 2016). Siles et al. (2016) reported a positive correlation of microbial activity with the increase in altitude. The increase in microbial activity ranges from 1.7 times (sulfatase) to 4.8 times (β-glucosidase). During the study, it was observed that enzymes showed highest activity at alpine site except xylanase, whereas Schinner (1982) demonstrated higher xylanase activity and SOM content in alpine soils. PLFA-related analysis displayed a location specific effect of enzyme production. The production of enzymes such as acidic phosphatase, arylsulfatase, protease, and cellobiohydrolase was increased in spring than in autumn, while β-glucosidase and xylanase remain unaffected with the change in season (Siles et al. 2016). The production of laccase enzyme was investigated from a fungal strain Pestalotiopsis spp. CDBT-F-G1 which possesses an approximate molecular mass of 43 kDa with pH and temperature optima of 6 and 60 °C, respectively (Yadav et al. 2019). Pastor et al. (2019) studied the role of various factors that control the enzymes during the processing of organic matter present in the streams of high arctic region. It was observed that phenol oxidase and phosphatase activities were lower in alluvial regions than the solifluction regions. Major drivers controlling the enzyme activities at high arctic are dissolved organic carbon and availability of nitrogen (Pastor et al. 2019).
Xiao et al. 2019 studied the enzymatic activities of cellulase (CEL), polyphenol oxidase (PPO), saccharase (SAC), urease (URE), catalase (CAT), alkaline phosphatase (ALP) in the increasing order of altitude of a dry valley in Southwest China. They reported a direct positive correlation of absolute enzyme activities with altitude but the specific activities were not that straightforward, which first decreased and then increased, except for phosphatase. The enzyme activities increased with an increase in nutrients such as nitrogen, which had an increasing trend with altitude rise and this abundant nitrogen concentration may be responsible for overcoming the excretion and other metabolic limitations of microbes (Sinsabaugh et al. 2002; Xu et al. 2015). But this does not apply to enzymes that degrade biomass and form elemental cycles, which may not be affected by changes in elevation (Nottingham et al. 2015; Chang et al. 2016). They found that the changes in soil SOC does not have to be reflected in the changes in enzyme activity per unit SOC and its different from the activity calculated based on soil mass. The specific activities of immobilized extracellular enzymes were more informative than the absolute activities regarding ecology and enzymatic efficiency (Raiesi and Beheshti 2014; Zhang et al. 2015). For this study site in the dry-hot valley region of China, the increase in altitude correlated more to specific enzymatic activity than absolute enzymatic activity. King et al. (2008) reported the higher phosphatase and peptidase activities in the soil of Peru and higher activities of phosphatase and β-glucosidase in the soil of Colorado. β-Glucosidase isolated from Colorado had positive correlation to C from microbial biomass, while phosphatase showed correlation with dissolved organic C and with microbial biomass C but none of them had significant correlation to water content in the soil. The phosphatase and peptidase enzyme activities in Peru had no correlation with either soil carbon content or the amount of water in soil. The dehydrogenase activity of soil collected from the Grossglockner mountain area (Austrian central Alps) shown to be decreased with the increase in altitude. But higher activity of dehydrogenase was observed in alpine soils than the subalpine soils (Margesin et al. 2009).
11.5 Factors Affecting the Microbial Community and Their Activities at High Altitude
The climatic conditions, chemical properties, and respiratory activity of soil directly influence the quantity of enzymes (Sinsabaugh and Linkins 1989; Kshattriya et al. 1992). Various studies suggested that microbial communities of soil at higher altitude depend on wide range of factors. It is reported from the literature that majority of the enzymes including dehydrogenase (Margesin et al. 2009) showed decrease in their activity along the altitude gradients but in a report on the Mediterranean soil showed lower enzyme activities at lower altitudes. The studies suggested that the factors related to altitude including soil moisture and temperature play major role in levels of enzyme activities (Lucas-Borja et al. 2012). It was observed that the increase in pH values increases the levels of total phospholipid fatty acids (PLFAs) in the soils of beech/beech oak forest located in the Northern Germany (Bååth and Anderson 2003). The high altitude regions generally possess low temperature conditions; therefore, fungal strains are more prominent in these areas which ultimately increased the fungal/bacterial ratio (Margesin et al. 2009), whereas Djukic et al. (2010) reported that variation in the composition of vegetation and decomposition condition affected the structure of microbial community in the higher altitude regions of Austrian limestone alps. The change in climatic conditions with the season tends to change the soil enzyme activities of that particular site. It was observed from the previous studies that increase in elevation leads to harshness in the environmental conditions such as cold climate, change in vegetation, and improper nutrient conditions, which ultimately affected the microbial activities, thus climate shifts also alter enzyme activities (Xu et al. 2015).
In addition, fraction of organic carbon in the soil and microbial biomass carbon was effectively related to the soil enzyme activities (Wang et al. 2013). However, there is still unclear image of the major influencing factors of soil enzyme activities.
Xu et al. (2015) reported that change in altitude tends to change the structural and functional patterns with the microbial community of the soil. Thus, they hypothesized the following three patterns in the microbiological characteristics of the soil:
-
1.
Increase in altitude leads to decrease in soil microbial biomass.
-
2.
Enzyme activities are primarily controlled by the nutrients present in the soil, whereas changes in the microbial composition of the soil are mainly regulated by the C/N ratios and pH of the soil.
-
3.
There should be a positive correlation between the microbial biomass, SOM decomposition, and enzyme activity.
Forests located in the Italian alpines were investigated to observe the effect of change in season from the spring to autumn in high altitude regions on the microbial properties of the soil. Therefore, they studied several factors including soil temperature, microbial activities, community level physiological profiles (CLPP), microbial abundance and community structure, PLFA profiles, and physiochemical properties in each season. The concentration of nutrients in the soil and soil organic matter (SOM) was found to be increased, whereas enzyme production was highly dependent on the site and season. The correlation between site factors and incubation temperature for soil microbial activities confirms the variation in microbial communities. Site-specific effects were noticed for CLPP. It was observed that site-specific, altitudinal and seasonal effects influence the microbial community structure and its functioning. Correlation study suggested that altitude was the major factor for the determination of variation in the biotic and abiotic characteristics of the sites, while seasonal variation does not show any major effect (Siles et al. 2016). Singh et al. presented a report on diversity of microbes at various extreme locations. According to their study, the extremity of the environment increases with the altitude gradient which ultimately resulted in the lower levels of microbial biomass and enzyme activity. It was also hypothesized that the composition of microbial community varies with the change in environmental conditions (Margesin and Miteva 2011). An increase in culturable psychrophilic heterotrophic Gram-negative bacteria and fungi was observed with the increase in altitude in the Austrian Central Alps soils and the dominance of proteobacteria was found among them. The microbial activity of dehydrogenases also decreases with increasing altitude (Margesin et al. 2009). A decrease in microbial species was noticed with the elevational gradients in the cold soils of Himalayan mountains and the abundance of proteobacteria and β-proteobacteria was also reported in a culture-independent study, whereas the culture dependent study displayed the dominance of γ-proteobacteria, Firmicutes, and low number of bacteroidetes (Margesin and Miteva 2011; Singh et al. 2019).
The enzyme activities were lower in case of litters at higher altitude than the litters of lower altitude, whereas cellulase and amylase showed the variation in their activities with the change in season at both altitudes. Cellulase and amylase activities were found to be increased during the decomposition of litter, whereas invertase activity increased only at the beginning of litter decomposition. The number of fungi and bacteria significantly correlates with the activity of cellulase and amylase; however, invertase activity remains unaffected with the microbial population (Kshattriya et al. 1992).
The forest stands considered under study were at different stages of regeneration and it was found that microbial population numbers were lower in highly degraded forests than in less degraded ones. The factors including fungal and bacterial population numbers, enzyme activities, organic C, and soil moisture were considered for the measurement of their correlation coefficient. It was noticed in all the forest stands that fungal population numbers develop a positive correlation with dehydrogenase activity. A notable level of positive correlation was observed between microbial population numbers and urease activity at the lower altitudes of less degraded forests. But at higher altitudes, only fungal population numbers showed positive relation with urease activity. However, no significant correlation was observed between phosphatase activity and the abovementioned factors in case of higher altitude regions, but in lower altitude regions, phosphatase enzyme possesses positive correlation with fungal population numbers and the concentration of soil organic C (Jha et al. 1992). Xu et al. reported a variation in PLFAs profiles with the change in altitude. The ratios of fungal/bacterial (F/B) number and the Gram-positive/Gram-negative (G+/G−) bacteria hiked with the increase in altitude. Various factors including the soil moisture, silt and clay fraction, mean annual temperature (MAT), mean annual precipitation (MAP), phosphorus concentration, and nitrate nitrogen correlate with the changes in the composition of microbial community of the soil. The nutrients present in the soil were significantly correlated with the variation in soil enzyme activities. The rate of decomposition of soil organic matter (SOM) showed positive correlation with the microbial enzyme activities and PLAs profiles (Xu et al. 2015).
11.6 Concluding Remarks and Future Perspectives
The mountainous regions around the world have always been a source of life owning to their role in regulating the water cycle. The large number of glaciers that adorn the upper levels of the highest mountains are the source of constant supply of freshwater to the inhabitants living in all the areas lying below. The microbial communities on these locations are adapted to extreme conditions like cold temperature and different levels of moisture content. But the threat of global warming is the first to reach and affect these areas, eroding glaciers and exposing underlying soils to new environments. These processes cause the loss of existing species of microorganisms and they may be replaced by a different set of microbes. Therefore it is of utmost importance to study the existing microbial community in these areas with an alarming approach but sadly that has not been the case as the impact on microbial life has largely been ignored. There is a distinct possibility of finding new species and their associated enzymes that may be useful to us industrially. Although the remote dry mountains have been studied geologically, the biological studies have had a very slow start. The study of barren high altitude zones like Atacama, Andes, or Colorado Rockies has revealed that the microorganisms surviving in such areas are carbon limited due to the absence of any plant life and dependent on the presence of ice packs whose melting may endanger the particular ecosystem. Although the old system of microbe culturing had limitations in growing various strains that existed in high altitudes, thus recent developments in metagenomics and techniques like 16S rRNA and MALDI-TOF have increased the chances that we can document most of the life forms that exist on the mountains. Soil organic matter (SOM) decomposition is yet another issue that is still very unclear in terms of how it occurs and requires a lot of further research, although an increase in SOM content with altitude and proportionally the number of fungi/bacteria have been observed. Study of microorganisms in these remote and untouched environments will also help us in hypothesizing the kind of atmosphere and life that existed on prehistoric earth.
References
Allison SD, Wallenstein MD, Bradford MA (2010) Soil-carbon response to warming dependent on microbial physiology. Nat Geosci 3:336–340. https://doi.org/10.1038/ngeo846
Arnds J, Knittel K, Buck U, Winkel M, Amann R (2010) Development of a 16S rRNA-targeted probe set for Verrucomicrobia and its application for fluorescence in situ hybridization in a humic lake. Syst Appl Microbiol 33:139–148. https://doi.org/10.1016/j.syapm.2009.12.005
Bååth E, Anderson TH (2003) Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol Biochem 35:955–963. https://doi.org/10.1016/S0038-0717(03)00154-8
Balser TC, Firestone MK (2005) Linking microbial community composition and soil processes in a California annual grassland and mixed-conifer forest. Biogeochemistry 73:395–415. https://doi.org/10.1007/s10533-004-0372-y
Barria C, Malecki M, Arraiano CM (2013) Bacterial adaptation to cold. Microbiol Soc 159:2437–2443. https://doi.org/10.1099/mic.0.052209-0
Barroca M, Santos G, Gerday C, Collins T (2017) Biotechnological aspects of cold-active enzymes. In: Margesin R (ed) Psychrophiles: from biodiversity to biotechnology. Springer, Cham, pp 461–475
Baumann K, Dignac MF, Rumpel C, Bardoux G, Sarr A, Steffens M, Maron PA (2013) Soil microbial diversity affects soil organic matter decomposition in a silty grassland soil. Biogeochemistry 114:201–212. https://doi.org/10.1007/s10533-012-9800-6
Blagodatskaya Е, Blagodatsky S, Khomyakov N, Myachina O, Kuzyakov Y (2016) Temperature sensitivity and enzymatic mechanisms of soil organic matter decomposition along an altitudinal gradient on Mount Kilimanjaro. Sci Rep 6:22240. https://doi.org/10.1038/srep22240
Bonavita P, Chemini C, Minerbi S, Salvadori C, Furlanello C (1998) Biodiversity and stress level in four forests of the Italian Alps. Chemosphere 36:1055–1060. https://doi.org/10.1016/S0045-6535(97)10171-0
Brooke JS (2008) Pathogenic bacteria in sink exit drains. J Hosp Infect 70:198–199. https://doi.org/10.1016/j.jhin.2008.06.017
Calvillo-Medina RP, Reyes-Grajeda JP, Moreno-Andrade VD, Barba-Escoto L, Bautista-de Lucio V, Jones GH, Campos-Guillén J (2019) Bacterial diversity based on a 16S rRNA gene amplicon data set from a high-altitude crater lake and glacial samples of the Iztaccihuatl Volcanic Complex (Mexico). Microbiol Resour Announc 8:e01636–e01618. https://doi.org/10.1128/MRA.01636-18
Carney KM, Matson PA (2005) Plant communities, soil microorganisms, and soil carbon cycling: does altering the world belowground matter to ecosystem functioning? Ecosystems 8:928–940. https://doi.org/10.1007/s10021-005-0047-0
Chang EH, Chen TH, Tian GL, Chiu CY (2016) The effect of altitudinal gradient on soil microbial activity and structure in moso bamboo plantations. Appl Soil Ecol 98:213–220. https://doi.org/10.1016/j.apsoil.2015.10.018
Connelly DP, Copley JT, Murton BJ, Stansfield K, Tyler PA, German CR, Van Dover CL, Amon D, Furlong M, Grindlay N, Hayman N (2012) Hydrothermal vent fields and chemosynthetic biota on the world’s deepest seafloor spreading centre. Nat Commun 3:620. https://doi.org/10.1038/ncomms1636
Connon SA, Lester ED, Shafaat HS, Obenhuber DC, Ponce A (2007) Bacterial diversity in hyperarid Atacama desert soils. J Geophys Res Biogeosci 112:1–9. https://doi.org/10.1029/2006JG000311
Dhakar K, Pandey A (2016) Extracellular laccase from a newly isolated psychrotolerant strain of Cladosporium tenuissimum (NFCCI 2608). Proc Natl Acad Sci India Sect B Biol Sci 86:685–690. https://doi.org/10.1007/s40011-015-0507-z
Djukic I, Zehetner F, Mentler A, Gerzabek MH (2010) Microbial community composition and activity in different alpine vegetation zones. Soil Biol Biochem 42:155–161. https://doi.org/10.1016/j.soilbio.2009.10.006
Dumorné K, Córdova DC, Astorga-Eló M, Renganathan P (2017) Extremozymes: a potential source for industrial applications. J Microbiol Biotechnol 27:649–659. https://doi.org/10.4014/jmb.1611.11006
Falkowski PG, Fenchel T, Delong EF (2008) The microbial engines that drive Earth’s biogeochemical cycles. Science 320:1034–1039. https://doi.org/10.1126/science.1153213
Feng X, Simpson MJ (2009) Temperature and substrate controls on microbial phospholipid fatty acid composition during incubation of grassland soils contrasting in organic matter quality. Soil Biol Biochem 41:804–812. https://doi.org/10.1016/j.soilbio.2009.01.020
Fierer N, Strickland MS, Liptzin D, Bradford MA, Cleveland CC (2009) Global patterns in belowground communities. Ecol Lett 12:1238–1249. https://doi.org/10.1111/j.1461-0248.2009.01360.x
Fierer N, McCain CM, Meir P, Zimmermann M, Rapp JM, Silman MR, Knight R (2011) Microbes do not follow the elevational diversity patterns of plants and animals. Ecology 92:797–804. https://doi.org/10.1890/10-1170.1
He HS, Hao Z, Mladenoff DJ, Shao G, Hu Y, Chang Y (2005) Simulating forest ecosystem response to climate warming incorporating spatial effects in north-eastern China. J Biogeogr 2043–2056. https://doi.org/10.1111/j.1365-2699.2005.01353.x
Hines J, Megonigal JP, Denno RF (2006) Nutrient subsidies to belowground microbes impact aboveground food web interactions. Ecology 87:1542–1555. https://doi.org/10.1890/0012-9658(2006)87[1542:NSTBMI]2.0.CO;2
Jha DK, Sharma GD, Mishra RR (1992) Soil microbial population numbers and enzyme activities in relation to altitude and forest degradation. Soil Biol Biochem 24:761–767. https://doi.org/10.1016/0038-0717(92)90250-2
Kaeberlein T, Lewis K, Epstein SS (2002) Isolating “uncultivable” microorganisms in pure culture in a simulated natural environment. Science 296:1127–1129. https://doi.org/10.1126/science.1070633
Kaira GS, Dhakar K, Pandey A (2015) A psychrotolerant strain of Serratia marcescens (MTCC 4822) produces laccase at wide temperature and pH range. AMB Express 5(1):92. https://doi.org/10.1186/s13568-014-0092-1
Kessler M, Kluge J, Hemp A, Ohlemüller R (2011) A global comparative analysis of elevational species richness patterns of ferns. Glob Ecol Biogeogr 20:868–880. https://doi.org/10.1111/j.1466-8238.2011.00653.x
King AJ, Meyer AF, Schmidt SK (2008) High levels of microbial biomass and activity in unvegetated tropical and temperate alpine soils. Soil Biol Biochem 40:2605–2610. https://doi.org/10.1016/j.soilbio.2008.06.026
Kolmonen E, Rantala-Ylinen A, Rajaniemi-Wacklin P, Lepitö LA, Haukka K, Sivonen K (2011) Bacterioplankton community composition in 67 Finnish lakes differs according to trophic status. Aquat Microb Ecol 62:241–250. https://doi.org/10.3354/ame01461
Koranda M, Kaiser C, Fuchslueger L, Kitzler B, Sessitsch A, Zechmeister-Boltenstern S, Richter A (2013) Seasonal variation in functional properties of microbial communities in beech forest soil. Soil Biol Biochem 60:95–104. https://doi.org/10.1016/j.soilbio.2013.01.025
Kshattriya S, Sharma GD, Mishra RR (1992) Enzyme activities related to litter decomposition in forests of different age and altitude in north East India. Soil Biol Biochem 24:265–270. https://doi.org/10.1016/0038-0717(92)90228-P
Kumar S, Suyal DC, Yadav A, Shouche Y, Goel R (2019) Microbial diversity and soil physiochemical characteristic of higher altitude. PLoS One 14:e0213844. https://doi.org/10.1371/journal.pone.0213844
Leisner JJ, Laursen BG, Prévost H, Drider D, Dalgaard P (2007) Carnobacterium: positive and negative effects in the environment and in foods. FEMS Microbiol Rev 31:592–613. https://doi.org/10.1111/j.1574-6976.2007.00080.x
Lipson DA (2007) Relationships between temperature responses and bacterial community structure along seasonal and altitudinal gradients. FEMS Microbiol Ecol 59:418–427. https://doi.org/. https://doi.org/10.1111/j.1574-6941.2006.00240.x
Liu YQ, Yao TD, Jiao NZ, Liu XB, Kang SC, Luo TW (2013) Seasonal dynamics of the bacterial community in Lake Namco, the largest Tibetan lake. Geomicrobiol J 30:17–28. https://doi.org/10.1080/01490451.2011.638700
López-Mondéjar R, Voříšková J, Větrovský T, Baldrian P (2015) The bacterial community inhabiting temperate deciduous forests is vertically stratified and undergoes seasonal dynamics. Soil Biol Biochem 87:43–50. https://doi.org/10.1016/j.soilbio.2015.04.008
Lucas-Borja ME, Candel Pérez D, López Serrano FR, Andrés M, Bastida F (2012) Altitude-related factors but not Pinus community exert a dominant role over chemical and microbiological properties of a Mediterranean humid soil. Eur J Soil Sci 63:541–549. https://doi.org/10.1111/j.1365-2389.2012.01438.x
Lynch RC, King AJ, Farías ME, Sowell P, Vitry C, Schmidt SK (2012) The potential for microbial life in the highest-elevation (> 6000 m.a.s.l.) mineral soils of the Atacama region. J Geophys Res 117:G02028. https://doi.org/10.1029/2012JG001961
Mandic-Mulec I, Prosser JI (2011) Diversity of endospore-forming bacteria in soil: characterization and driving mechanisms in endospore-forming soil bacteria. In: Logan NA, Vos PD (eds) Soil biology, vol 27. Springer, Berlin, pp 31–59
Margesin R, Miteva V (2011) Diversity and ecology of psychrophilic microorganisms. Res Microbiol 162:346–361. https://doi.org/10.1016/j.resmic.2010.12.004
Margesin R, Jud M, Tscherko D, Schinner F (2009) Microbial communities and activities in alpine and subalpine soils. FEMS Microbiol Ecol 67:208–218. https://doi.org/10.1111/j.1574-6941.2008.00620.x
Margesin R, Minerbi S, Schinner F (2014) Long-term monitoring of soil microbiological activities in two forest sites in South Tyrol in the Italian Alps. Microbes Environ 29:277–285. https://doi.org/10.1264/jsme2.ME14050
McCain CM (2005) Elevational gradients in diversity of small mammals. Ecology 86:366–372. https://doi.org/10.1890/03-3147
McEwen AS, Ojha L, Dundas CM, Mattson SS, Byrne S, Wray JJ, Cull SC, Murchie SL, Thomas N, Gulick VC (2011) Seasonal flows on warm Martian slopes. Science 333:740–743. https://doi.org/10.1126/science.1204816
Nottingham AT, Turner BL, Whitaker J, Ostle NJ, McNamara NP, Bardgett RD, Salinas N, Meir P (2015) Soil microbial nutrient constraints along a tropical forest elevation gradient: a belowground test of a biogeochemical paradigm. Biogeosciences 12:6071–6083. https://doi.org/10.5194/bg-12-6071-2015
O’Malley MA (2007) The nineteenth century roots of ‘everything is everywhere’. Nat Rev Microbiol 5:647–651. https://doi.org/10.1038/nrmicro1711
Pajares S, Merino-Ibarra M, Macek M, Alcocer J (2017) Vertical and seasonal distribution of picoplankton and functional nitrogen genes in a high-altitude warm-monomictic tropical lake. Freshw Biol 62:1180–1193. https://doi.org/10.1111/fwb.12935
Pandey A, Durgapal A, Joshi M, Palni LMS (1999) Influence of Pseudomonas corrugata inoculation on root colonization and growth promotion of two important hill crops. Microbiol Res 154:259–266. https://doi.org/10.1016/S0944-5013(99)80023-8
Pandey A, Jain R, Sharma A, Dhakar K, Kalra GS, Rahi P, Dhyani A, Pandey N, Adhikari P, Shouche YS (2019) 16S rRNA gene sequencing and MALDI-TOF mass spectrometry based comparative assessment and bioprospection of psychrotolerant bacteria isolated from high altitudes under mountain ecosystem. SN Appl Sci 1:278. https://doi.org/10.1007/s42452-019-0273-2
Pastor A, Freixa A, Skovsholt LJ, Wu N, Romaní AM, Riis T (2019) Microbial organic matter utilization in high-arctic streams: key enzymatic controls. Microb Ecol 10:1–6. https://doi.org/10.1007/s00248-019-01330-w
Rahbek C (2005) The role of spatial scale and the perception of large-scale species-richness patterns. Ecol Lett 8:224–239. https://doi.org/10.1111/j.1461-0248.2004.00701.x
Raiesi F, Beheshti A (2014) Soil specific enzyme activity shows more clearly soil responses to paddy rice cultivation than absolute enzyme activity in primary forests of Northwest Iran. Appl Soil Ecol 75:63–70. https://doi.org/10.1016/j.apsoil.2013.10.012
Ramírez-Olvera MA, Alcocer J, Merino-Ibarra M, Lugo A (2009) Nutrient limitation in a tropical saline lake: a microcosm experiment. Hydrobiologia 626:5–13. https://doi.org/10.1007/s10750-009-9733-9
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602. https://doi.org/10.1890/03-8002
Schinner F (1982) Soil microbial activities and litter decomposition related to altitude. Plant Soil 65:87–94. https://doi.org/10.1007/BF02376806
Schmidt SK, Reed SC, Nemergut DR, Stuart Grandy A, Cleveland CC, Weintraub MN, Hill AW, Costello EK, Meyer AF, Neff JC, Martin AM (2008) The earliest stages of ecosystem succession in high-elevation (5000 metres above sea level), recently deglaciated soils. Proc R Soc Lond [Biol] 275:2793–2802. https://doi.org/10.1098/rspb.2008.0808
Shen C, Xiong J, Zhang H, Feng Y, Lin X, Li X, Liang W, Chu H (2013) Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol Biochem 57:204–211. https://doi.org/10.1016/j.soilbio.2012.07.013
Siles JA, Cajthaml T, Minerbi S, Margesin R (2016) Effect of altitude and season on microbial activity, abundance and community structure in alpine forest soils. FEMS Microbiol Ecol 92(3). pii: fiw008. https://doi.org/10.1093/femsec/fiw008
Singh JS, Raghubanshi AS, Singh RS, Srivastava SC (1989) Microbial biomass acts as a source of plant nutrients in dry tropical forest and savanna. Nature 338:499–500. https://doi.org/10.1038/338499a0
Singh P, Jain K, Desai C, Tiwari O, Madamwar D (2019) Microbial community dynamics of extremophiles/extreme environment. In: Microbial diversity in the genomic era. Academic Press, New York, pp 323–332. https://doi.org/10.1016/B978-0-12-814849-5.00018-6
Sinsabaugh RL, Linkins AE (1989) Cellulase mobility in decomposing leaf litter. Soil Biol Biochem 21:205–209
Sinsabaugh RL, Carreiro MM, Repert DA (2002) Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 60:1–24. https://doi.org/10.1023/A:1016541114786
Sommaruga R, Casamayor EO (2009) Bacterial ‘cosmopolitanism’ and importance of local environmental factors for community composition in remote high-altitude lakes. Freshw Biol 54(5):994–1005. https://doi.org/10.1111/j.1365-2427.2008.02146.x
Stroud JL, Paton GI, Semple KT (2007) Microbe-aliphatic hydrocarbon interactions in soil: implications for biodegradation and bioremediation. J Appl Microbiol 102:1239–1253. https://doi.org/10.1111/j.1365-2672.2007.03401.x
Tang J, Ding X, Wang LM, Xu QR, Yang ZR, Zhao J, Sun Q, Feng S, Zhang J (2012) Effects of wetland degradation on bacterial community in the Zoige Wetland of Qinghai-Tibetan Plateau (China). World J Microbiol Biotechnol 28:649–657. https://doi.org/10.1007/s11274-011-0858-4
Tan B, Wu FZ, Yang WQ, He XH (2014) Snow removal alters soil microbial biomass and enzyme activity in a Tibetan alpine forest. Appl Soil Ecol 76:34–41. https://doi.org/10.1016/j.apsoil.2013.11.015
Torsvik V, Øvreås L (2002) Microbial diversity and function in soil: from genes to ecosystems. Curr Opin Microbiol 5:240–245. https://doi.org/10.1016/S1369-5274(02)00324-7
Uchida M, Nakatsubo T, Kasai Y, Nakane K, Horikoshi T (2000) Altitudinal differences in organic matter mass loss and fungal biomass in a subalpine coniferous forest, Mt. Fuji, Japan. Arct Antarct Alp Res 32:262–269. https://doi.org/10.1080/15230430.2000.12003363
Vanhala P, Karhu K, Tuomi M, Björklöf K, Fritze H, Hyvärinen H, Liski J (2011) Transplantation of organic surface horizons of boreal soils into warmer regions alters microbiology but not the temperature sensitivity of decomposition. Glob Chang Biol 17:538–550. https://doi.org/10.1111/j.1365-2486.2009.02154.x
Waldrop MP, Firestone MK (2006) Response of microbial community composition and function to soil climate change. Microb Ecol 52:716–724. https://doi.org/10.1007/s00248-006-9103-3
Wang Q, He T, Wang S, Liu L (2013) Carbon input manipulation affects soil respiration and microbial community composition in a subtropical coniferous forest. Agric For Meteorol 178–179:152–160. https://doi.org/10.1016/j.agrformet.2013.04.021
Wenzler E, Kamboj K, Balada-Llasat JM (2015) Severe sepsis secondary to persistent Lysinibacillus sphaericus, Lysinibacillus fusiformis and Paenibacillus amylolyticus bacteremia. Int J Infect Dis 35:93–95. https://doi.org/10.1016/j.ijid.2015.04.016
Wu F, Yang W, Zhang J, Deng R (2010) Litter decomposition in two subalpine forests during the freeze–thaw season. Acta Oecologica 36:135–140. https://doi.org/10.1016/j.actao.2009.11.002
Xiao L, Li P, Shi P, Liu Y (2019) Soil nutrient stoichiometries and enzymatic activities along an elevational gradient in the dry-hot valley region of southwestern China. Arch Agron Soil Sci 65:322–333. https://doi.org/10.1080/03650340.2018.1502882
Xu Z, Yu G, Zhang X, Ge J, He N, Wang Q, Wang D (2015) The variations in soil microbial communities, enzyme activities and their relationships with soil organic matter decomposition along the northern slope of Changbai Mountain. Appl Soil Ecol 86:19–29. https://doi.org/10.1016/j.apsoil.2014.09.015
Yadav M, Bista G, Maharjan R, Poudyal P, Mainali M, Sreerama L, Joshi J (2019) Secretory laccase from Pestalotiopsis species CDBT-F-G1 fungal strain isolated from high altitude: optimization of its production and characterization. Appl Sci 9:340. https://doi.org/10.3390/app9020340
Zhang J, Zhang X, Liu Y, Xie S, Liu Y (2014) Bacterioplankton communities in a high-altitude freshwater wetland. Ann Microbiol 64:1405–1411. https://doi.org/10.1007/s13213-013-0785-8
Zhang X, Dong W, Dai X, Schaeffer S, Yang F, Radosevich M, Xu L, Liu X, Sun X (2015) Responses of absolute and specific soil enzyme activities to long term additions of organic and mineral fertilizer. Sci Total Environ 536:59–67. https://doi.org/10.1016/j.scitotenv.2015.07.043
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sharma, V., Dahiya, D., Vasanth, D. (2020). Characteristics of Microbial Community and Enzyme Activities in Higher Altitude Regions. In: Goel, R., Soni, R., Suyal, D. (eds) Microbiological Advancements for Higher Altitude Agro-Ecosystems & Sustainability. Rhizosphere Biology. Springer, Singapore. https://doi.org/10.1007/978-981-15-1902-4_11
Download citation
DOI: https://doi.org/10.1007/978-981-15-1902-4_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1901-7
Online ISBN: 978-981-15-1902-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)