Abstract
Recruitment of new individuals is essential for community sustainability and resilience. Spatial variation in coral recruitment was quantified for nine sites in Amitori Bay of Iriomote Island, Okinawa Prefecture, Japan, and compared with adult abundance. In addition, potential source sites for recruits, larval trajectories from source to sink sites, and travel distances of larvae were estimated by numerical modeling using real biological and physical data at the main spawning periods. As a result, acroporid recruitment was dominant in Amitori Bay and was positively correlated with adult abundance. Numerical modeling demonstrated a potential that recruits in Amitori Bay could be originated from nearby areas, and that most of recruits traveled less than 400 m with the maximum travel distance of less than 5.5 km. Therefore, estimated dispersal distance of larvae could be relatively short as the mouth of Amitori Bay is about 2 km across and the length of the bay is 4 km. These suggested that the acroporid community in Amitori Bay could be maintained by recruits from within the bay and from immediately surrounding areas.
This chapter is based on work reported by Nakamura et al. (2017).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
For the last several decades, scleractinian corals have been facing serious biological and physical disturbances, and it is estimated that 50–80% of the world’s scleractinian corals have been destroyed (Gardner et al. 2003; De’ath et al. 2012). Recently, mass bleaching of corals, followed by mass mortality, has been reported in most of the world’s coral reefs due to abnormally high seawater temperatures during the summer of 2016 (GBRMPA 2016; Biodiversity Center of Japan 2016). Because decreased coral abundance reduces diversity and abundance of other coral reef fauna (Jones et al. 2004; Graham et al. 2015), mechanisms to protect, maintain, and recover coral communities after severe disturbances are necessary in order to effectively protect coral reef ecosystems.
Maintenance and recovery of heavily damaged coral communities depend on numbers of newly recruited individuals and their species composition, acquired via planktonic larval dispersal (Connell et al. 1997; Hughes et al. 2000). Trajectories of dispersing larvae have been estimated using current circulation models and/or by analyzing genetic connectivity among different populations (Treml et al. 2008; Cowen et al. 2009). In addition to these analyses, substantial larval supply and recruitment data are essential for understanding the processes.
Larvae of acroporid corals, which are broadcast-spawners, have been thought to disperse over long distances by strong, stable currents such as the Kuroshio Current. In contrast, acroporid larvae have also been estimated from surveys on recruitment and GPS-equipped surface drifters to disperse locally via local coastal currents and to recruit within several tens of kilometers of their origins. Therefore, larval dispersal and recruitment in an inland bay might be affected by currents working at this scale, e.g., estuary circulation patterns. However, studies focusing on larval dispersal patterns in relation to current circulation patterns and subsequent larval supply at local scales, such as an inland bay, are scarce. As a result, knowledge of larval dispersal and subsequent recruitment patterns at this scale is limited. This limitation could be due to difficulties of observation and the paucity of numerical analyses with high accuracy and high resolution.
In this study, using field surveys and a coastal ocean current model with a multi-sigma coordinate system (CCM, see Chap. 3) to overcome difficulties in numerical analyses, we quantified larval supply and estimated source areas, dispersal trajectories, and distances traveled by supplied larvae in Amitori Bay, where mass bleaching occurred in the summer of 2016. This study should lead to a better understanding of spatio-temporal variability in larval supply and subsequent recruitment, and it should permit us to estimate connectivity among populations and to construct substantial protective measures for corals in Amitori Bay and for coral communities in similar inland bays.
2 Materials and Methods
-
(1)
Research Sites
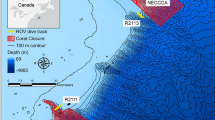
Nine sites were selected along both banks from the entrance to the end of Amitori Bay. These sites, including five sites on the west bank (AmSt.1–5) and four sites on the east bank (SaSt.1–4), encompass significant environmental gradients due to topographical characteristics and river flow (see Sect. 1.1) (Fig. 15.1). Three subsites were also established at each site.
Research sites along Amitori Bay, Iriomote Island, Okinawa Prefecture, Japan (referred from Nakamura et al. 2017). Map data © 2018 Google
-
(2)
Field Surveys
Surveys of larval supply and community structure of corals were conducted at these nine sites. Larval supply was quantified using artificial settlement plates, which consisted of a pair of 10 × 10 cm panels made of fiber-reinforced cement (Fig. 15.2). To precondition the plates, ten plates were deployed at each subsite (30 plates per site) about one month before the predicted major coral spawning periods. These were retrieved about one month later, after the observed spawning on May 19, 2016 in the field. Retrieved plates were bleached with a chlorine solution to eliminate organic matter and then dried for observation under a stereomicroscope. Settled juvenile corals on the plates were identified by skeletal morphology and classified to family (Acroporidae, Pocilloporidae, Poritidae, etc.) (Fig. 15.3), and the numbers of individuals pertaining to each family were counted.
Artificial settlement plate (referred from Nakamura et al. 2017)
Juvenile corals settled on artificial settlement plates. a Acroporidae, b Pocilloporidae, and c Poritidae (referred from Nakamura et al. 2017)
Community structures were quantified using the belt transect method with 1 m × 1 m quadrats. One 10 m × 2 m belt transect was established per subsite (three transects per site). Pictures of five randomly selected 1 m × 1 m quadrats were taken and % cover of Acroporidae, Pocilloporidae, Poritidae, and other families were determined using Image J. Relationships between larval supply and % cover were analyzed with Pearson’s correlation analysis.
-
(3)
Numerical Analyses
Visual observation of coral larval trajectories from source to sink cannot be made because of small larval sizes. Therefore, a coastal ocean current model with a multi-sigma coordinate system (CCM, see Chap. 3) was employed for estimation of sources and trajectories of larvae supplied to Amitori Bay. Initial values for CCM were wind speed data from mesoscale model I (MSM) of the Japan Meteorological Agency, flow data for the Yonada, Udara, and Paita Rivers from our own observations, and those of the Urauchi, Nakara, and Kuira Rivers from Prof Osamu Watanabe from the Iriomote Project of the Research Institute for Humanity and Nature, as well as astronomical data on tides from the NAO model. Particles were considered as coral larvae under reproduced ocean flow field and tracked with a Lagrangian description. Other detailed conditions for analyses were as given below.
-
Date and time for larval releases: Large slicks of coral eggs and embryos were observed along the northwestern coast of Iriomote Island from 9:00 to 11:00 am on May 20, 2016 (Fig. 15.4). Slicks were not observed before this day. Therefore, mass spawning of corals occurred during the night of May 19, 2016, establishing the date of larval release. In addition, release time was set as 22:00–22:25, in accordance with Murakami et al. (2015).
Fig. 15.4 Slicks of coral eggs and embryos observed along the northwestern coastal area of Iriomote Island (referred from Nakamura et al. 2017)
-
Areas of larval release: Using 15-m topographic grids, areas less than 5 m deep were considered as regions of potential larval release.
-
Number of released larvae: From each 225 m2 quadrat of potential larval release, 10 particles, considered as coral larvae, were released from 22:00 to 22:25 (Murakami et al. 2015). In total, 616,180 particles were released.
-
Settlement timing: According to Suzuki et al. (2011), particles treated as coral larvae could settle within 4–15 days after release. Therefore, in this analysis, anytime a particle passed through one of the nine research sites within 15 days, it was considered to have “settled.”
3 Results and Discussion
-
(1)
Larval Supply and Community Structure
Acroporids were the most abundant juveniles established on settlement plates in Amitori Bay, comprising 63% of all juveniles settled on the installed plates. These included 223.7 ± 62.4 acroporids, 49.8 ± 19.6 pocilloporids, 41.0 ± 11.7 poritids, and 37.4 ± 11.4 others (Fig. 15.5). This was not surprising, given that acroporid corals are the major component of coral communities along both banks of Amitori Bay, covering 66.4%.
Average numbers of juveniles settled on settlement plates in Amitori Bay (referred from Nakamura et al. 2017)
The number of settled juveniles decreased toward the apex of the bay on both banks (Fig. 15.6). On the west bank, Am.St2 showed the highest number, 19.7 ± 3.5 per plate, and the lowest number was found at Am.St5, 2.5 ± 0.5. Numbers at sites on the east bank were lower than those on the west bank. The highest number was observed at Sa.St1, 6.2 ± 5.8 and the lowest was at Sa.St4, 0.6 ± 0.2.
Average numbers of juveniles settled on settlement plates at nine sites in Amitori Bay (referred from Nakamura et al. 2017)
Positive correlations were observed between the number of settled juveniles (larval supply) and % cover for acroporid corals on both banks of Amitori Bay. A stronger relationship was found with the east bank (Fig. 15.7; west bank, r = 0.70, and east bank, r = 0.90). These results suggested that sites with higher acroporid cover have higher larval supply and/or that survival rates of settled juveniles are higher at sites with higher acroporid cover, resulting higher coral cover. That is, larval supply or survival rates of settled individuals may decrease from the mouth to the apex of the bay. In Amitori Bay, influenced by flow rates of the Ayanda and Udara Rivers, daily fluctuations in salinity and sediment inflow were higher near to the apex (Murakami et al. 2012), factors that can affect larval settlement and survival. In addition, our results also suggest the potential for self-seeding within Amitori Bay. Previous studies of larval settlement and % cover of acorporid corals have not found any such correlation; therefore, acroporid larvae were thought to have come from other areas (Hughes et al. 1999; Nakamura and Sakai 2010). However, larval settlement and % cover of acorporid corals in Amitori Bay showed strong positive correlations; thus larval supply may originate nearby.
Relationship between the number of settled juveniles and % cover for acroporid corals at sites on both banks of Amitori Bay (referred from Nakamura et al. 2017)
-
(2)
Estimation of Larval Sources
Figure 15.8 shows probable source areas for juveniles settled at site Am.St2. This suggests that juveniles settled at Am.St2 came from near Am.St2 and a relatively high number of juveniles originated from Sakiyama Bay. The other eight sites (Am.St1, 3–5 and Sa.St1–4) showed similar patterns that juveniles could be originated from nearby areas.
Estimated source areas of juveniles settled in Am.St2 (referred from Nakamura et al. 2017)
Dispersal distances of juveniles settled at each study site were calculated (Fig. 15.9). Most juveniles traveled < 1,200 m and the maximum distances were within 4.0 km for the west bank and within 5.5 km for the east bank. Moreover, 26.0–97.7% of juveniles settled at Am.St2–St5, Sa.St3, and Sa.St4 traveled <400 m. Considering that the mouth and depth of the Bay are about 2 km and 4 km, respectively, acroporid larvae arriving in the bay disperse relatively short distances.
Frequency of larvae in relation to travel distances from estimated source to sink areas (referred from Nakamura et al. 2017)
It has been reported that acroporid larvae start to settle three days after mass spawning and that the main settlement occurs within 8 days (Suzuki et al. 2011). In addition, coral larvae can travel over 10 km in three days with coastal currents and several tens km within seven days (Nakamura et al. 2015). However, estimated dispersal distances of larvae in Amitori Bay were relatively short while distances increased toward the entrance of the bay. This may have resulted from larval retention due to tidal fluctuations and local currents resulting from the complex topography of the bay. Therefore, further studies with numerical analyses based on detailed physical field observations are necessary.
4 Conclusions
This study quantified larval supply through settlement on artificial plates in coral communities inhabiting an inner bay and demonstrated a positive correlation with % local coral cover. Moreover, numerical analyses estimated that source areas of larvae are often located within 400 m of the settlement sites. These results suggest the potential of larval supply from nearby areas. That is, coral communities in an inner bay might be maintained by self-seeding. Therefore, to sustain acroporid coral communities in Amitori Bay, protection and maintenance of existing corals, especially sexually mature colonies, is the highest priority. In addition to the further studies mentioned above, it could be useful to conduct a study estimating larval supply from wider areas.
References
Biodiversity Center of Japan (2016) Report on monitoring 1000 coral reef survey in fiscal year 2016. The Ministry of the Environment, Japan
Connell JH, Hughes TP, Wallace CC (1997) A 30-year study of coral abundance, recruitment, and disturbance at several scales in space and time. Ecol Monogr 67:461–488
Cowen RK, Sponaugle S (2009) Larval dispersal and marine population connectivity. Ann Rev Mar Sci 1:443–466
De’ath G, Fabricius KE, Sweatman H, Puotinen M (2012) The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc Natl Acad Sci 109(44):17995–17999
Gardner TA, Côté IM, Gill JA, Grant A, Watkinson AR (2003) Long-term region-wide declines in caribbean corals. Science 301:958–960
GBRMPA (Great Barrier Reef Marine Park Authority) (2016) Interim report: coral bleaching event on the Great Barrier Reef. GBRMPA, Townsville, pp 1–27
Graham NAJ, Jennings S, MacNeil MA, Mouillot D, Wilson SK (2015) Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature 518:94–97
Hughes TP, Baird AH, Dinsdale EA, Moltschanlwskyj NA, Pratchett MS, Tanner JE, Wills BL (1999) Patterns of recruitment and abundance of corals along the Great Barrier Reef. Nature 396:59–63
Hughes TP, Baird AH, Dinsdale EA, Moltschaniwskyj NA, Pratchett MS, Tanner JE, Willis BL (2000) Supply-side ecology works both ways: the link between benthic adults, fecundity, and larval recruits. Ecology 81:2241–2249
Jones GP, McCormick MI, Srinivasan M, Eagle V (2004) Coral decline threatens fish biodiversity in marine reserves. Proc Natl Acad Sci 101(21):8251–8253
Murakami T, Ukai A, Khono H, Mizutani A, Shimokawa S, Nakase K, Noguchi K, Yasuda T (2012) Relationships between distributions of corals and physical environments in Amitori Bay, Iriomote Island, Japan. J Japan Soc Civil Eng B3, 68:I_1133–I_1138
Murakami T, Khono H, Yamamoto Y, Mizutani A, Shimokawa S (2015) Numerical simulation for initial dynamic state of bundle based on field observations of Acroporidae spawning in Amitori Bay, Iriomote Island, Japan. J Japan Soc Civil Eng B2 71(2):I_1225–I_1230 (In Japanese with English abstract)
Nakamura M, Sakai K (2010) Spatiotemporal variability in recruitment around Iriomote Island, Ryukyu Archipelago, Japan: implications for dispersal of spawning corals. Mar Biol 157:801–810
Nakamura M, Kumagai NH, Sakai K, Okaji K, Mitarai S (2015) Spatial variability in recruitment of acroporid corals and predatory starfish along the Onna coast, Okinawa, Japan. Mar Ecol Prog Ser 540:1–12
Nakamura M, Murakami T, Khono H, Noda W, Matsushita Y, Mizutani A (2017) Coral recruitment on a local scale in Amitori Bay, Iriomote Island, estimated by settlement plates and numerical analysis. J Japan Soc Civil Eng B2 72(2):I_1279–I_1284 (In Japanese with English abstract)
Suzuki G, Arakaki S, Hayashibara T (2011) Rapid in situ settlement following spawning by Acropora corals at Ishigaki, southern Japan. Mar Ecol Prog Ser 421:131–138
Treml EA, Halpin PN, Urban DL, Pratson LF (2008) Modeling popualtion connectivity by ocean currents, a graph-theoreric apporach for marine conservation. Landscape Ecol 23:19–36
Acknowledgement
We are very grateful to Wataru Noda and Yusuke Matsushita for helping to conduct this research. This study was supported by JSPS KAKENHI Grant Numbers 16K07527. We also thank OIST’s technical editor, Dr. Steven D. Aird, for helping to polish this manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Nakamura, M., Murakami, T., Kohno, H., Mizutani, A. (2020). Coral Recruitment on a Local Scale in Amitori Bay, Iriomote Island, Estimated by Filed Surveys and Numerical Analyses. In: Shimokawa, S., Murakami, T., Kohno, H. (eds) Geophysical Approach to Marine Coastal Ecology. Springer Oceanography. Springer, Singapore. https://doi.org/10.1007/978-981-15-1129-5_15
Download citation
DOI: https://doi.org/10.1007/978-981-15-1129-5_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1128-8
Online ISBN: 978-981-15-1129-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)