Abstract
A 50-year-old smoker, a known case of chronic obstructive pulmonary disease (COPD) with dilated cardiomyopathy, was admitted to the intensive care unit (ICU) with altered sensorium and acute respiratory distress. His arterial blood gases showed severe hypoxemia and acute respiratory acidosis. He was intubated and put on controlled mechanical ventilation (CMV). He improved in 2 days. Spontaneous breathing trial (SBT) was tried and the patient was extubated after successful SBT. After 5 h, the patient again developed severe respiratory distress and had to be reintubated. He failed several trials of SBT . Tracheostomy was done on the ninth day of ventilation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
A 50-year-old smoker, a known case of chronic obstructive pulmonary disease (COPD) with dilated cardiomyopathy, was admitted to the intensive care unit (ICU) with altered sensorium and acute respiratory distress. His arterial blood gases showed severe hypoxemia and acute respiratory acidosis. He was intubated and put on controlled mechanical ventilation (CMV). He improved in 2 days. Spontaneous breathing trial (SBT) was tried and the patient was extubated after successful SBT. After 5 h, the patient again developed severe respiratory distress and had to be reintubated. He failed several trials of SBT . Tracheostomy was done on the ninth day of ventilation.
Weaning from mechanical ventilation means the transition from total ventilatory support to spontaneous breathing. It is usually a rapid and smooth process in most of the patients however this can become a progressive and prolonged process in 20–25% of the cases. These patients require a systematic approach for successful liberation from the ventilator. It should be started early once the patients fulfils the criteria for weaning trial. This weaning has two components: liberation from the ventilator and extubation. Liberation process itself has multiple components like, assessment for readiness for weaning (once daily in the morning), assessment for predictors of a successful weaning (2–5 min) followed by a proper weaning trial (30–120 min) The sooner the patient is liberated from the ventilator, the lesser are the chances of ventilator-associated pneumonias, ventilator-induced lung injury, decreased ICU length of stay, and overall reduced mortality.
Step 1: Identify Readiness for Weaning
-
1.
Weaning should never be hurried as it can be successful only when the patient is ready both physically and mentally. At the same time, it should also not be delayed as it is associated with complications. During readiness testing, objective clinical criteria are preferred to determine whether a patient is ready to begin weaning. It is important to assess patients daily who are mechanically ventilated for more than 24 h. Any patient on MV should be considered for weaning if he/she fulfils the readiness criteria.
-
2.
Prerequisites “readiness criteria”.
Screen patient daily for readiness to wean either by clinical criteria or automated readiness testing
-
The underlying reason for MV has been stabilized and the patient is improving.
-
The patient is hemodynamically stable on minimal-to-no pressors.
-
Oxygenation is adequate (e.g., PaO2/FiO2 > 150 PEEP < 5–8 cm H2O, FiO2 < 0.5) or SpO2 > 90% on FiO2 < 40%.
-
The patient is able to initiate spontaneous inspiratory efforts.
-
Besides these criteria, the patient should be afebrile (temperature < 38 °C), have stable metabolic status (pH ≥ 7.25), adequate hemoglobin (e.g., Hb > 8–10 g/dL), and adequate mentation (e.g., arousable, Glasgow coma scale >13) and optimum fluid balance.
-
-
3.
Understand the predictors of successful weaning.
-
If the patient fulfils readiness for weaning criteria an assessment for prediction of successful weaning should be performed before starting a proper weaning trial.
-
The predictors of successful weaning have been designed from physiologic parameters to help the decision-making process. Weaning predictors can be classified as measurements of oxygenation and gas exchange, measurements of respiratory system load, measurements of respiratory muscle capacity, and integrative indices.. Numerous weaning predictors have been studied, however, none of the tests alone are particularly powerful, and clinical judgment is of paramount importance.
-
The Rapid Shallow Breathing Index (RSBI) is the most widely studied and popular weaning predictor. Rapid shallow breathing index) is assessed by putting patient on T-piece for 2 min.
-
F (frequency)/Vt (tidal volume in litres) less than 100 is predictor of successful weaning.
-
The threshold of 100 is not binding and can be relaxed by 10–20% in patients with endotracheal tube size less than 7 and in women.
-
RSBI >100 breaths/min/L is better at identifying patients who will fail weaning than a RSBI<100 breaths/min/L is at identifying patients who can be successfully weaned.
-
-
Minute ventilation less than 10 L/min.
-
Respiratory ate (RR) less than 35 breaths/min.
-
Maximum inspiratory pressure more negative than −30 cm H2O.
-
Ultrasound of the diaphragm that measures diaphragmatic excursion and diaphragmatic thickening fraction is a promising predictor to predict weaning outcome.
-
A comprehensive list of weaning predictors can be seen in Table 9.1.
-
Protocols have been used by many ICUs to encourage the early identification of patients that are ready to wean.
-
Automated weaning systems use closed-loop control to interpret clinical and ventilator data and then change ventilator settings and potentially enable earlier weaning that is less labor-intensive and decreases ICU length of stay.
Traditional methods of weaning include spontaneous breathing trials (SBTs), progressive decreases in the level of pressure support during pressure support ventilation (PSV), and progressive decreases in the number of ventilator-assisted breaths during intermittent mandatory ventilation (IMV).
Step 2: Prepare for Weaning
Stop continuous infusion of sedation daily to awaken the patient to do spontaneous awakening trial (SAT) . In patients whose sedation has been titrated for an appropriate sedation goal may not need daily SAT.
-
Communicate with patient, explain the procedure, and calm them.
-
Record baseline parameters and keep flow sheet at the patient’s bedside.
-
Keep a calm peaceful environment and have the nurse or physician remain at the bedside to offer encouragement and support.
-
If patient fails SAT (Table 9.2), restart sedation on half of the previous dose.
-
If patient passes the SAT after stopping sedation, assess the patient for spontaneous breathing trial (SBT) based on the prerequisites criteria mentioned in step 1.
Step 3: Do Spontaneous Breathing Trial (SBT)
An SBT refers to a patient breathing through the endotracheal tube either without any ventilator support (e.g., through a T-piece) or with minimal ventilator support (e.g., a low level of pressure support, automatic tube compensation [ATC], or continuous positive airway pressure (CPAP). Once it has been determined that a patient is ready to be weaned, many prefer weaning via once-daily SBTs, rather than PSV or IMV.
-
Whenever possible, position the patient upright in bed.
-
Thoroughly suction the endotracheal tube and ensure patency.
-
Any of the following modes can be chosen for SBT:
-
A.
T-piece:
-
Patients are disconnected from the ventilator and made to breathe humidified oxygen—air mixture through a T-piece connected to the endotracheal/tracheostomy tube for 30–120 min.
-
Increased respiratory load is offered by the endotracheal tube. Dyspnea and fatigue should be carefully avoided.
-
-
B.
Pressure support:
-
The pressure support level is to be gradually reduced, titrated to RR and patient comfort.
-
A level of 6–8 cm H2O pressure support is considered to overcome the tube resistance.
-
Put the patient on PS of 6–8 cm H2O and PEEP of 4 cm H2O.
-
For patients with a small, high resistance endotracheal tube (size ≤7 mm), we suggest using low level pressure support or ATC, rather than a T-piece.
-
-
A.
Duration
The duration should be 30–120 min—shorter time for the patients on the ventilator for less than 1 week and longer for the patients on prolonged MV. An initial SBT of 30 min duration is generally sufficient to determine whether mechanical ventilation can be discontinued. For patients who fail their initial SBT, or required prolonged mechanical ventilation prior to the initial SBT (e.g., more than 10 days), it is suggest that subsequent trials be 120 min, rather than 30 min.
Step 4: Monitor Closely During SBT Trial
-
Patient comfort, dyspnea, and all vital and respiratory parameters should be closely monitored. SBT should be terminated if it fails (Table 9.3).
-
SBT should be tried at least once in 24 h. More frequent SBTs do not help.
-
At the end of the trial, if it succeeds, the patient is considered for extubation.
-
Patient may be rested for few hours on assisted ventilation after a successful T Piece trial before extubation.
Step 5: Extubate the Patient
After undergoing a successful SBT, a few more criteria should be fulfilled before deciding about extubation:
-
Adequate cough reflex—spontaneously or while suctioning.
-
Adequate cough strength (cough peak expiratory flow > 60 L/min)
-
Patient should be able to protect airways, and they should follow simple commands.
-
Secretions should not be copious. (requirement of suction < every 2–3 h)
-
A cuff leak of less than 110 mL measured during assist-control ventilation may help to identify patients who are at high risk of developing postextubation stridor/obstruction of airway. (prolonged intubation, traumatic intubation, reintubation, large endotracheal tube).If it is less than 110 mL it points out that patient may have significant laryngeal edema and stridor may develop.
-
Presence of a cuff leak (>110 mL or >25% of delivered tidal volume) is suggestive of a patent airway, on the other hand absence of cuff leak is not always associated with a blocked airway.
-
For most patients cuff leak test does not need to be performed, except in anticipated post extubation stridor cases.
-
No radiological or surgical procedure is being planned in the near future.
-
Extubation should not be done at the end of the day.
-
GCS > 8.
Step 6: Monitor for Extubation Failure
After extubation , the patient should be observed closely for signs of extubation failure as mentioned below:
-
RR more than 25/min for 2 hrs
-
Heart rate more than 140 beats/min or sustained increase or decrease of more than 20%
-
Clinical signs of respiratory muscle fatigue or increased work of breathing
-
SaO2 less than 90%; PaO2 less than 80 mmHg, on FiO2 more than 0.50
-
Hypercapnia (PaCO2 > 45 mmHg or > 20% from preextubation), pH < 7.33
Step 7: Try Noninvasive Ventilation (NIV)
-
If the signs of extubation failure are present, the physician should try NIV particularly in conditions where its role is proved; for example, in COPD, postoperative failure after lung resection surgery, or decompensated obstructive sleep apnea.
-
NIV has the advantage of reduced complications and better patient interactions. However, it is important to keep in mind that it should not delay reintubation (if required), and every hour that a patient spends on NIV when intubation is clearly required increases mortality and delays recovery.
-
NIV is used in three clinical settings:
-
As an alternative weaning technique for the patients who failed SBT: Extubate and put on NIV—well-documented role in COPD patients without significant comorbidities and in centers with expertise in NIV use.
-
As a prophylactic measure for the patients with a high risk of reintubation: Studied in postoperative patients. Start NIV electively after successful SBT and extubation.
-
As the treatment of respiratory insufficiency after extubation (postextubation failure): Useful in COPD.
-
In conditions where role of NIV is not proved, the patients should be reintubated.
High Flow nasal oxygen (HFNC) may be used in non COPD patients at risk of extubation failure .
Reintubation carries higher mortality either because of underlying medical condition or because of possible complications such as aspiration or ventilator-associated pneumonia.
Intravenous corticosteroid (Methylprednisolone 20 mg) four hours before extubation, and every four hourly for four doses, or a single dose of 40 mg four hours before extubation may be tried in patients with high risk for post extubation stridor (prolonged ventilation, difficult intubation, reintubation case) specially in the absence of cuff leak.
-
Assess safety of starting feed after extubation, preferably after a swallow assessment, to avoid aspiration
Step 8: Identify Difficult Weaning
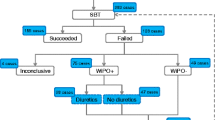
Weaning success is defined as extubation and the absence of ventilator support 48 h following extubation.
-
Weaning failure is defined as one of the following:
-
Failed SBT
-
Reintubation and/or resumption of ventilator support following successful extubation
-
Death within 48 h following extubation
-
-
The term weaning in progress is used for the patients who are extubated, but remain supported by NIV.
-
Difficult weaning—Patients who fail initial weaning and require up to three SBT or as long as 7 days from the first SBT to achieve successful weaning.
-
Prolonged weaning—Patients who fail more than three weaning attempts or require more than 7 days of weaning after the first SBT.
Step 9: Ascertain the Cause of Weaning Difficulty
-
Carry out a detailed examination of the patient, and look for the cause of difficult weaning.
-
Difficult or prolonged weaning is usually due to incomplete resolution of initial indication for ventilation.
-
Make a checklist based on pathophysiologic mechanisms:
-
I.
Inadequate respiratory drive
-
Nutritional deficiencies
-
Excess of sedatives
-
Central nervous system abnormality
-
Sleep deprivation
-
-
II.
Inability of the lungs to carry out gas exchange effectively
-
Unresolving pneumonia
-
Unresolved pulmonary edema/fluid overload
-
Undiagnosed pulmonary embolism
-
The splinting effect of obesity, abdominal distension, or ascites
-
Respiratory muscle fatigue/weakness
-
Nutritional and metabolic deficiencies
-
Critical illness polyneuropathy/myopathy
-
Hypokalemia
-
Hypomagnesemia
-
Hypocalcemia
-
Hypophosphatemia
-
Hypoadrenalism
-
Hypothyroidism
-
Corticosteroids: myopathy, hyperglycemia
-
Chronic renal failure
-
Systemic disease sepsis: impaired diaphragmatic force generation
-
Refractory hypoxemia and hypercapnia
-
Persistently increased work of breathing
-
Ineffective triggering, auto-PEEP
-
Increased resistance due to ventilator tubings or humidification devices
-
Poor cardiac performance
-
Neuromuscular dysfunction/disease
-
Drugs
-
-
III.
Anxiety
It is difficult to distinguish anxiety from ventilatory failure. If in doubt, always presume it to be ventilatory failure.
-
IV.
Psychological dependency in difficult weaning
-
I.
Step 10: Treat All the Reversible Causes Identified
-
Provide good nutrition, but avoid overfeeding.
-
Have good glycemic control (110–140 mg/dL).
-
Correct metabolic factors (especially metabolic alkalosis).
-
Maintain hemoglobin above 7–8 g/dL.
-
Maintain adequate cardiac output and tissue perfusion.
-
Treat arrhythmia.
-
Treat hypothyroidism and steroid deficiency or excess.
-
Control the patient’s underlying illness.
-
Abolish ventilator dyssynchrony with appropriate inspiratory flow and trigger settings.
-
Change of the mode of ventilation may help improve patient–ventilator interactions.
-
Reverse bronchospasm as much as possible and reduce dynamic hyperinflation.
-
Drain out significant pleural effusions and ascites.
-
Treat intraabdominal hypertension.
-
Treat pulmonary edema aggressively.
-
Discontinue the use of steroids, aminoglycosides, colistin, and statins, if possible.
-
Avoid fluid overload in renal failure and cardiac failure—do dialysis if indicated.
-
Make the patient comfortable.
-
Aggressive physiotherapy and mobilization.
-
Reverse oversedation.
-
Treat anxiety: Improve patient communication, use relaxation techniques, and give low-dose benzodiazepines.
-
Diagnose and treat narcotic/benzodiazepine withdrawal.
-
Treat delirium and depression.
-
Ensuring nighttime sleep may be helpful. Zolpidem may be added
Step 11: Plan the Weaning Process in Difficult Weaning
-
1.
Select the mode of ventilation
The mode of ventilation used should provide adequate respiratory support and prevent diaphragmatic atrophy.
-
Pressure support ventilation: It is most commonly used, and has been shown to be better than SIMV for weaning.
-
Continuous positive airway pressure (CPAP): Besides the usual benefits of improved oxygenation and improved left ventricular function, it has beneficial role in selected patients with hypoxemic respiratory failure.
-
Automatic tube compensation: It may be helpful in narrow endotracheal tubes to overcome tube resistance.
-
Proportional assist ventilation: It has been studied with CPAP and shown to improve respiratory mechanics.
-
Adaptive support ventilation: It has been shown to be better than SIMV in postcardiac surgery patients.
-
Control Mechanical Ventilation (CMV): It has logical use in patients showing respiratory fatigue on spontaneous mode. So it is recommended to use this mode in cases of difficult weaning at night to give rest to the muscles.
-
-
2.
Plan tracheostomy.
-
Percutaneous tracheostomy has been shown to have fewer complications than surgical tracheostomy and to be more cost-effective
-
Potential benefits of using tracheostomy in difficult-to-wean patients are as follows:
-
Decreased work of breathing
-
Reduced requirement of sedation and improved patient comfort and cooperation
-
Earlier reinstitution of oral feeding
-
Less chances of accidental extubation
-
-
In spite of the above-mentioned benefits, tracheostomy has not been consistently shown to decrease mortality. It has resulted in a number of dependent survivors. It facilitates easier bedside management of such patients.
-
-
3.
Do aggressive physiotherapy and mobilization
-
Physiotherapy and mobilization are prerequisites for successful weaning. Early institution of physiotherapy in a protocol-driven approach and daily assessment to achieve maximum mobility is now an integral part of ICU management.
-
-
4.
Select proper place for weaning
-
Cost-effective care has been shown to be provided in respiratory intermediate care units and specialized regional weaning centers. It requires team effort and expertise.
-
Step 12: Choose a Weaning Protocol
Protocol-driven weaning has more chances of success, reduced costs, and probably reduced mortality. Two basic weaning protocols are used for a prolonged weaning patient:
-
Progressive reduction of ventilator support
-
Progressively longer periods of SBTs
No significant difference in weaning success and mortality rate, duration of ventilatory assistance, or total hospital length of stay is reported between these two weaning techniques in the difficult-to-wean patients. A combination of both the protocols can also be used.
Step 13: Decide About Home Ventilation or Transfer to a Long Term Care Facility
Indications
-
An inability to be completely weaned from ventilatory support including NIV
-
A progression of disease etiology that requires increasing ventilatory support
Patients should have stable physiology and proper resources, personnel, and motivation.
Suggested Reading
AARC, Respiratory Home Care Focus Group. AARC clinical practice guideline. Long-term invasive mechanical ventilation in the home—2007 revision & update. Respir Care. 2007;52(8):1056–62. This article discusses multiorganizational effort to clear the controversies concerning the best method for conducting weaning, liberation from mechanical ventilation, and extubation.
Ambrosino N, Gabbrielli L. The difficult-to-wean patient. Expert Rev Respir Med. 2010;4(5):685–92. The study describes the epidemiology, causes, and management strategies for difficult-to-wean patients.
Blackwood B, Alderdice F, Burns K, Cardwell C, Lavery G, O’Halloran P. Use of weaning protocols for reducing duration of mechanical ventilation in critically ill adult patients: Cochrane systematic review and meta-analysis. Br Med J. 2011;342:c7237. This review included 11 trials that included 1971 patients. They found that compared with usual care, the mean duration of MV in the weaning protocol group was reduced by 25%, the duration of weaning was reduced by 78%, and the ICU length of stay by 10%.
Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Statement of the sixth international consensus conference on intensive care medicine. Eur Respir J. 2007;29(5):1033–56. Home MV is now increasingly being used to rehabilitate long-term ventilator-dependent patients.
Jubran A, Lawm G, Kelly J, Duffner LA, Gungor G, Collins EG, et al. Depressive disorders during weaning from prolonged mechanical ventilation. Intensive Care Med. 2010;36:828–35. Depressive disorders were diagnosed in 42% of the patients who were weaned from prolonged ventilation. Patients with depressive disorders were more likely to experience weaning failure and death.
MacIntyre NR, Epstein SK, Carson S, Scheinhorn D, Christopher K, Muldoon S. Management of patients requiring prolonged mechanical ventilation: report of a NAMDRC consensus conference. Chest. 2005;128:3937–54. The goal of this conference was not only to review existing practices but also to develop recommendations on a variety of assessment, management, and reimbursement issues.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Chawla, R., Kansal, S., Bali, R.K., Jain, A.C. (2020). Weaning. In: Chawla, R., Todi, S. (eds) ICU Protocols. Springer, Singapore. https://doi.org/10.1007/978-981-15-0898-1_9
Download citation
DOI: https://doi.org/10.1007/978-981-15-0898-1_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-0897-4
Online ISBN: 978-981-15-0898-1
eBook Packages: MedicineMedicine (R0)