Abstract
45-year-old male patient presented to emergency department with abdominal pain and vomiting. On evaluation he was found to have pancreatic tumor and subsequently underwent whipples procedure (pancreato-duodenectomy). He was doing well till fifth postoperative day when he deteriorated and was reexplored and found to have leak at pancreas jejunum junction which was corrected. Arterial line and internal jugular vein central line were inserted intraoperatively On seventh POD from re-exploration he developed fever spikes of 101 °F and was worked up for development of nosocomial infection. His chest X-ray did not show any infiltrates, surgical site was clean. Urinary catheter was changed. Paired blood cultures were sent with one set from periphery.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
45-year-old male patient presented to emergency department with abdominal pain and vomiting. On evaluation he was found to have pancreatic tumor and subsequently underwent whipples procedure (pancreato-duodenectomy). He was doing well till fifth postoperative day when he deteriorated and was reexplored and found to have leak at pancreas jejunum junction which was corrected. Arterial line and internal jugular vein central line were inserted intraoperatively On seventh POD from re-exploration he developed fever spikes of 101 °F and was worked up for development of nosocomial infection . His chest X-ray did not show any infiltrates, surgical site was clean. Urinary catheter was changed. Paired blood cultures were sent with one set from periphery.
Central line (Central Venous Catheter or CVC) is an intravascular access device or catheter that terminates at or close to the heart or in one of the great vessels like internal jugular vein, subclavian vein, superior vena cava, inferior vena cava, brachiocephalic veins, pulmonary artery, external iliac veins, common iliac veins or femoral veins. It can be short term catheter (<14 days) or long-term catheter (>14 days).
CVC infection implies central line colonization with accompanying clinical manifestations suggestive of infection which can be local (phlebitis) or systemic (CRBSI).CRBSI occurrence in the ICU is common.
Central Line Associated Blood Stream Infections (CLABSI) Versus Central Line Related Blood Stream Infection (CRBSI)
-
CLABSI is a surveillance definition used by the Centre for Disease Control, Atlanta, USA (CDC) and is defined as recovery of a pathogen from a blood culture in a patient who had central line at the time of infection or within 48 h before development of infection and is not due to an infection at another site. Simply put CLABSI is a laboratory confirmed bloodstream infection where an eligible BSI organism is identified, and an eligible central lines present on the lab confirmed blood stream infection (LCBI) on the day of event (DOE) or the day before. This epidemiological definition overestimates the true incidence of BSI.
CRBSI: Catheter Related Blood Stream Infection is a diagnostic definition given by IDSA. It includes laboratory confirmed BSI in patient with an intravascular access device and at least
-
1 positive culture obtained from a peripheral vein, clinical manifestations of infection (fever, chills, hypotension, and no apparent cause of BSI except the vascular access) + one of the following:
-
Positive semiquantitative culture (>15 CFU/catheter segment) with the same organism.
-
Positive Quantitative culture (>103 CFU/catheter segment) with the same organism.
-
Simultaneous quantitative blood cultures with a ≥ 5:1 ratio CVC vs. peripheral.
-
Differential period of CVC cultures vs. peripheral blood culture positivity of >2 h. i.e blood culture is positive at least two hours earlier than the culture drawn from the peripheral blood.
Approach to CRBSI
Step 1: Suspect CRBSI and Diagnosis
-
One or more of the following should raise suspicion of CRBSI in a patient with central line.
-
Fever.
-
Hemodynamic instability.
-
Catheter dysfunction.
-
Catheter inserted in emergency situation.
-
Catheter in place for >7 days.
-
Femoral catheters.
-
Difficult catheter insertion.
-
Clinical signs of sepsis occurring abruptly after starting intravenous infusion through the line.
-
Central line infection is generally a diagnosis of exclusion and should be suspected in patients with a central line who do not have an obvious source of bacteremia due to an infection at another site (CAUTI, VAP, SSI).
-
Most common reasons are: A breach of standard aseptic precautions during insertion of CVC, emergency placement of CVC, a breach of standard aseptic precautions to access hub.
-
Identify patient at risk of CRBSI (Table 57.1).
Step 2: Examine CVC Insertion Site
Whenever there is clinical suspicion, the CVC insertion site should be examined for discharge, erythema and other signs of infection.
Step 3: Send Blood Culture
-
Ideally blood culture should be drawn prior to initiation of antibiotics
-
Send minimum two sets of blood cultures drawn at the same time, of which one should be from peripheral site. One culture may be taken from a catheter hub and the other from a peripheral vein.
-
Adequate volume of blood (at least 10 ml) should be drawn for each culture bottle.
-
Note time of blood culture drawn from both sites, to ascertain time difference of blood culture positivity time from both sites, though utility of this method to ascertain central line as the source of bacteremia is doubtful.
-
Blood cultures should not be taken only from the catheter port, as these are frequently colonized with contaminants, thereby increasing the likelihood of a false-positive blood culture result.
-
All aseptic precautions should be taken for withdrawing samples for blood culture.
-
Start appropriate empirical antibiotics after drawing samples.
-
If the central line is removed in patients with suspected CRBSI, tip and subcutaneous part should be sent for culture.
-
There is no role of routine catheter culture at the time of catheter removal in noninfected patient.
-
Growth of staphylococcus aureus, Staphylococcus epidermidis or Candida is suggestive of CRBSI, in the absence of other source of infection.
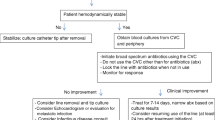
Step 3: Initial Management (Fig. 57.1)
-
Antibiotic therapy is usually not indicated in the absence of clinical signs of infection in following circumstances:
-
Positive catheter tip culture.
-
Positive blood cultures obtained through a catheter with negative cultures through a peripheral vein.
-
Phlebitis in the absence of infection.
-
-
In general, empiric antibiotic therapy must be instituted before culture and susceptibility results are revealed. The therapy should be tailored once microbiology results are available.
-
Initiation of empirical antibiotics requires knowledge of organisms commonly associated with line related infections specifically in your hospital.
-
If there is discharge from insertion site, then send swab for culture.
-
Vancomycin or Teicoplanin is commonly recommended for empirical therapy in medical settings with high prevalence of MRSA. If vancomycin MIC values >2 μ g/mL, daptomycin may be used.
-
Linezolid should not be used for empirical therapy.
-
Empirical coverage for GNB should be based on local antimicrobial susceptibility data and the severity of disease. (fourth generation cephalosporin, carbapenem, or β-lactam/β-lactamase combination, with or without an aminoglycoside may be used).
-
In neutropenics, empirical combination antibiotic coverage for multidrug resistant gram negative bacilli (MDR-GNB), such as Pseudomonas aeruginosa, is recommended, as they are known to be colonized with such bacteria.
-
De-escalation of the antibiotic regimen should be done, once the culture and susceptibility data are available.
-
Empirical therapy for suspected CRBSI involving femoral catheters in critically ill patients should include coverage for GNB and Candida species.
-
Empirical therapy for suspected catheter-related candidemia should be used for septic patients who have any of the following risk factors:
-
Prolonged use of broad-spectrum antibiotics.
-
Femoral catheterization.
-
Total parenteral nutrition.
-
Hematologic malignancy.
-
Colonization due to Candida species at multiple sites.
-
Receipt of bone marrow or solid-organ transplant.
-
-
Echinocandins are the preferred agents. Fluconazole should be used only if no azole exposure in the previous 3 months and the risk of Candida krusei or Candida glabrata infection is very low.
Step 3: Decision on Central Line
-
If a central line is no longer required, then it should be removed.
-
If continuation of central line is indicated then whether to retain same line or insert new CVC line depends on organism isolated in culture, duration of central line, complications.
-
Retaining CVC is not a good option in following situations:
-
Septic shock.
-
Persistent bacteremia inspite of >72 h on appropriate antibiotics.
-
Complications like endocarditis, septic thrombophlebitis, osteomyelitis.
-
When organism isolated is Staphylococcus aureus, pseudomonas and candida.
-
There is no evidence to support routine catheter exchange.
-
Catheter removal is not mandatory for hemodynamically stable patients with unexplained fever in the absence of documented bloodstream infection and without endovascular device/graft.
-
Guide wire exchange should not be performed and preferably a new central line should be inserted.
-
Catheter Salvage
-
Catheter salvage with antibiotic lock and systemic antibiotic treatment are usually not recommended for critically ill patients.
-
Salvage therapy is limited to cases without septic shock, growth of organism other than Pseudomonas, Saphylococci and Candida, high risk of catheter reinsertion and for long term catheter like hemodialysis and Hickman catheters.
Antibiotic Lock Therapy (Table 57.2)
-
Antibiotic lock is used for patients with CRBSI involving long-term catheters with no signs of exit site or tunnel infection for whom catheter salvage is needed.
-
For CRBSI, antibiotic lock therapy should not be used as monotherapy. It should be combined with systemic antimicrobial therapy.
-
Dwell times for an antibiotic lock is 48 h before reinstallation of lock solution. The reinstallation should be done every 12–24 h.
-
Catheter removal is generally recommended for CRBSI due to S. aureus and Candida species instead of treatment with antibiotic lock and catheter retention.
-
When CRBSI is due to CONS, a trial of retaining CVC can be tried with systemic antibiotics for 10–14 days combining it with antibiotic lock solution.
-
Antibiotic lock solution can be used in recurrent CRBSI, for dialysis catheter.
-
Antibiotic lock solution should be renewed every 24 h. and in case of hemodialysis catheter it is renewed after each session of dialysis.
Step 4: Specific Therapy (Fig. 57.2)
Antibiotics should be narrowed down to specific organism based on culture sensitivity, once the blood culture reports are available.
-
If Coagulase negative Staphylococci (CONS) is grown in culture, it should be isolated in >1 set of culture to make sure it is not a contaminant.
-
Duration of antibiotic is dependent on the organism identified.
-
Staphylococcus aureus: 14 days.
-
Coagulase-negative staphylococci: 7 days.
-
Enterococci and Gram-negative bacilli: 10 to 14 days.
-
Candida: 14 days.
-
Gram Negative Nonfermenters—Acinetobacter or Pseudomonas—14 days.
-
Step 5: Rule Out Complications
-
If the organism isolated is Staphylococcus aureus or candida, then echocardiography (preferably transesophageal) is indicated to rule out endocarditis. One should also look for candida endophthalmitis, metastatic abscess at distant site as they require prolong antibiotic therapy.
-
Endocarditis should be ruled out in line related bacteremia irrespective of organism in following—prosthetic valve, implanted defibrillator, pacemaker, persistent bacteremia/fungemia or fever >3 days after initiation of antibiotics.. Ideally it should be done 5–7 days after onset of bacteremia, as the false negativity is high. TEE can be repeated later, whenever there is doubt.
Step 6: Prevention
-
Education, training and adequate staffing for controlling CRBSI.
-
Use aseptic precautions while inserting catheters.
-
Follow hand hygiene.
-
Use clean gloves for peripheral IV catheters and sterile gloves for CVC.
-
Use maximum sterile barrier precautions: cap, mask, sterile gown, sterile gloves, and a sterile full body drape.
-
Avoid femoral lines for CVC access.
-
Use subclavian vein to minimize infection risks as compared to IJV and Femoral veins.
-
Use ultrasonic guidance (USG) for CVC insertion.
-
Use CVC with minimum number of ports necessary to manage the patient.
-
Replace CVC inserted in emergency within 48 h.
-
Prepare clean skin with a > 0.5% chlorhexidine preparation with alcohol before CVC insertion. If there is a contraindication to chlorhexidine, tincture of iodine, an iodophor, or 70% alcohol can be used as alternatives.
-
Do not use topical antibiotic ointment or creams on insertion sites, except for dialysis catheters, because of their potential to promote fungal infections and antimicrobial resistance.
-
Replace dressings used on CVC sites at least every 7 days for transparent dressings and every two days for gauze dressings.
-
When the CRBSI rate is not decreasing even after successful implementation of measures to reduce CRBSI, a chlorhexidine/silver sulfadiazine or minocycline/rifampin impregnated CVC should be used in patients whose catheter is expected to remain in place >5 days.
-
Use a sutureless securement device to reduce the risk of infection for intravascular catheters.
-
Do not administer systemic antimicrobial prophylaxis routinely before insertion or during use of an intravascular catheter to prevent catheter colonization or CLABSI.
-
Use prophylactic antimicrobial lock solution in patients with long term catheters who have a history of multiple CRBSI despite optimal maximal adherence to aseptic technique.
-
Use a 2% chlorhexidine wash for daily skin cleansing to reduce CRBSI.
-
Replace IV sets that are continuously used, including secondary sets and add-on devices, no more frequently than at 96-h intervals, but at least every 7 days in patients not receiving blood, blood products or fat emulsions.
-
Replace tubing used to administer blood, blood products, or fat emulsions (those combined with amino acids and glucose in a 3-in-1 admixture or infused separately) within 24 h of initiating the infusion (IB).
-
Propofol infusion tubing should be changed every 6 or 12 h.
Suggested Reading
Bouzidi H, Emirian A. Differential time to positivity of central and peripheral blood cultures is inaccurate for the diagnosis of Staphylococcus aureus long-term catheter-related sepsis. J Hosp Infect. 2018;99(2):192–9. To ascertain accuracy of Differential time to positivity of cultures of blood drawn simultaneously from central venous catheter and peripheral sites which is widely used to diagnose catheter-related bloodstream infections without removing the catheter. Because of the high number of false-negative cases, the classic cut-off limit of 120 min showed 100% specificity but only 42% sensitivity for the diagnosis of catheter-related bloodstream infection due to S. aureus.
Central Line Associated Blood Stream Infections (CLABSI)–CDC. https://www.cdc.gov/nhsn/pdfs/training/2018/clabsi-508.pdf
Chong HY, Lai NM. Comparative efficacy of antimicrobial central venous catheters in reducing catheter-related bloodstream infections in adults: abridged cochrane systematic review and network meta-analysis. Clin Infect Dis. 2017;64(suppl_2):S131–40. A cochrane systematic review of antibiotic impregnanted central venous catheter.
Guenezan J, Drugeon B, Marjanovic N, et al. Treatment of central line associated blood stream infections. Crit Care. 2018;22:303.
Ling ML, Apisarnthanarak A, Jaggi N, Harrington G. APSIC guide for prevention of central line associated bloodstream infections (CLABSI). Antimicrob Resist Infect Control. 2016;5:16. A guide for the prevention of CLABSI.
O’Grady NP, et al. Healthcare infection control practices advisory committee (HICPAC). Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52:e162–93. Guidelines for the prevention of CRBSI.
Raad I, Chaftari AM. Successful salvage of central venous catheters in patients with catheter-related or central line-associated bloodstream infections by using a catheter lock solution consisting of minocycline, EDTA, and 25% ethanol. Antimicrob Agents Chemother. 2016;60(6):3426–32. An article on use of antibiotic lock solution in cancer patients with central line.
Russell TA, Fritschel E, et al. Minimizing central line-associated bloodstream infections in a high-acuity liver transplant intensive care unit. Am J Infect Control. 2019;47(3):305–12. Multidisciplinary collaboratives are essential to minimize CLABSI and optimize value and quality in a challenging, high-acuity patient population.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Samavedam, S., Reddy, R., Pande, R. (2020). Central Line Related Blood Stream Infections (CRBSI). In: Chawla, R., Todi, S. (eds) ICU Protocols. Springer, Singapore. https://doi.org/10.1007/978-981-15-0898-1_57
Download citation
DOI: https://doi.org/10.1007/978-981-15-0898-1_57
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-0897-4
Online ISBN: 978-981-15-0898-1
eBook Packages: MedicineMedicine (R0)