Abstract
Selective detection of heavy metal ions remains an area of broad and current interest, especially in the areas of biological and environmental chemistry considering their inseparable roles in bodily functions as well as omnipresence in the environment. In this mini-review, specific fluorogenic chemosensors are accommodated in accordance with their ability to selectively detect heavy metal ions in aqueous medium or physiological conditions and involve a variety of platforms including pyrene, benzothiazole, thiophene, coumarin, naphthalimide, and quinoline.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The inseparable roles of heavy metals in the modern world and their ubiquitous nature have rendered their detection as a matter of immediate concern [1]. Heavy metal ions act as two-edged swords, with some of them playing inseparable roles in living systems and at the same time causing serious environmental and biological hazards [2].
A wide variety of analytical and instrumental techniques such as inductively coupled plasma mass spectrometry (ICP-MS), atomic absorption spectroscopy (AAS), and electrochemical biosensing are available for qualitative as well as quantitative detection of heavy metal ions [3]. Of the several prevailing techniques, the use of fluorescence-based analytical techniques deserve special mention owing to their ready usability, low cost of operation, rapid response times, and high sensitivity [4]. Fluorescent probes also hold the critical advantage of being able to be modified for the detection of multiple chemical species as well as consequent detection of multiple analytes depending on the target species. The use of fluorescence-based techniques are preferred due to their simple sample preparation practices and also, at the same time, enabling real-time detection of samples pertaining to biology, environment, and industry. A wide variety of chemosensors employing different mechanistic modules are, therefore, being used for selective detection of heavy metal ions [5]. However, the development of fluorometric sensors for sensing of heavy metal ions poses their own unique challenges as most of the heavy metal ions act as fluorescence quenchers.
An ideal chemosensor must be designed so as to accommodate (a) a receptor (binding moiety), (b) a chromophore/fluorophore (signaling moiety), (c) incorporation of groups that facilitate water solubility, (d) extensive conjugation to enable long-range emissions for all practical purposes (Fig. 4.1).
The specific detection of any particular metal ion requires designing the ligand keeping in the mind the response of the target metal ion to any particular atom or functional group [6]. For instance, comparatively “soft” metal ions such as Hg2+ tend to preferentially bind to “soft” S-donor groups rather than the “hard” O-donor or N-donor groups, but the spectroscopic silent d10 configuration of Hg2+ makes it all the more challenging for fluorogenic detection [7]. On the other hand, oxophilic metal ions such as Pb2+ coordinate better with groups containing O atoms [8]. Synchronizing such basic principles with a variety of signaling processes, the detection of a particular is made possible over others [9]. Another challenge lies in the discriminatory analyte of either Zn2+ or Cd2+ considering the striking similarity in coordination properties. However, owing to the availability of completely occupied their d-orbitals, the development of Photoinduced Electron Transfer (PET) sensors has made the detection more feasible [10].
2 Optical Sensing of Heavy Metals in Aqueous Medium
Herein, we present a summary of some of the reported optical and fluorometric sensors for heavy metal ions in aqueous medium.
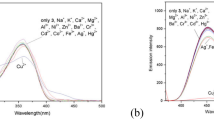
Das and co-workers reported a pyrene-based amphiphilic ligand (L1) that could selectively sense Hg2+ in aqueous medium within a broad range of pH with nanomolar range detectability (Fig. 4.2) [11]. The existence of a 1:1 L-Hg stoichiometric complex was proved by fluorescence spectroscopy, NMR studies, and mass spectrometry. The probe was also used for the detection of Hg2+ inside live human cancer cells (HeLa). The hydrophobic fragment of the ligand was effectively used for the quantitative removal of Hg2+ from aqueous medium into the organic medium. The extraction utility of L1 was also studied by NMR, fluorescence spectroscopy, and atomic absorption spectroscopy with results approaching approximately 99% Hg2+ ion removal.

(adapted from [11] with permission from The Royal Society of Chemistry)
Schematic representation of Hg2+ sensing by the amphiphilic probe L1
Das and co-workers reported a fluorogenic probe (L2) that could selectively detect Pb2+ ions in a “Turn-on” fashion in aqueous medium (Fig. 4.3) [12]. The reversibility of the detection process was repeatable for a minimum of five cycles. The probe was additionally used for the detection of Pb2+ in real water samples. The mechanism of the sensing process was proposed as Chelation-Enhanced Fluorescence (CHEF) mechanism and substantiated by Intramolecular Charge Transfer (ICT) upon binding of L2 to Pb2+ ions. Subsequently, the Pb2+-probe ensemble was further used for the detection of a wide variety of biothiols along with real sample detection in onion and garlic extract.

(adapted from [12] with permission from The Royal Society of Chemistry)
Structure and proposed 2:1 binding mode of L2 with Pb2+ ions
Singh and co-workers have reported a thiophene-based probe (L3) for selective “Turn-on” fluorogenic sensing as well as optical detection of Hg2+ ions in aqueous medium (Fig. 4.4) [13]. A 2:1 stoichiometric complex was proposed for L3-Hg2+ binding, and the mechanism of interaction of the probe with Hg2+ ions was studied through UV–Visible and emission spectroscopy along with 1H NMR and MALDI-TOF mass spectrometry.

(adapted from [13] with permission from The Royal Society of Chemistry)
Structure of the probe L3 and its proposed 2:1 binding mode with Hg2+ ions
Moreover, DFT and cyclic voltammetry studies were undertaken to further approve for the CHEF-based detection mechanism for Hg2+ by the thiophene-based ligand.
Das and co-workers reported a Schiff base (L4) for differential fluorophoric detection of Hg2+ and Ag+ ions: a “Turn-on” response for Hg2+ ions and a “Turn-off” signal for Ag+ ions in aqueous medium (Fig. 4.5) [14]. Hg2+ sensing was proposed to proceed via conventional 2:1 L-Hg2+ complex via the Chelation Enhanced Fluorescence mechanism (CHEF) based on the absorbance, emission, 1H-NMR titration, and mass spectrometry. On the other hand, detection of Ag+ was advocated to be due to the formation of nano-sized aggregates in solution which was confirmed by Dynamic Light Scattering (DLS), Field Emission Scanning Electron Microscope (FESEM), and Field Emission Transmission Electron Microscope (FETEM) studies.

(adapted from [14] with permission from Elsevier)
Pictorial representation of differential sensing of Ag+ and Hg2+ ions by L4
A fluorogenic probe (L5) based on a benzothiazole–quinoline platform for selective “Turn-on” detection of Cd2+ ions in aqueous medium at physiological pH was reported by Das and co-workers (Fig. 4.6) [15]. Emission wavelengths over 600 nm were achieved which allowed for possible practical applications of the probe. A 2:1 coordination complex was proposed from absorbance, fluorescence, and mass spectrometric experiments, and the sensing was proposed via CHEF in conjunction with PET mechanism. In a similar manner, the probe was used for the detection of Zn2+ ions at 612 nm and the L5-Cd2+ ensemble was used for consequent detection of inorganic phosphates in the same medium. In addition, the practical utility of the fluorescence detection of metal ions was enhanced by detection studies in real water samples.

(adapted from [14] with permission from The Royal Society of Chemistry)
Structure and proposed 2:1 binding mode of Cd2+ ions with L5
Ahmed and co-workers reported a chalcone-based ligand for selective and sensitive sensing of Cd2+ ions in HEPES-buffered solution (20 mM, CH3CN:H2O, 3:7, v/v, pH 7.0) (Fig. 4.7) [16]. The probe displayed distinct optical and fluorogenic response upon interaction with Cd2+ ions. Extensive UV–visible, fluorescence, and 1H-NMR studies indicated toward a 1:1 coordination complex of the probe with Cd2+ ions, and the fluorescence enhancement was proposed to proceed via the CHEF mechanism. In addition, chromogenic and fluorogenic paper-based test kits were prepared for the selective detection of Cd2+ ions along with real sample detection in lipstick samples, brown hair coloring material, and liquid foundation makeup cream.

(adapted from [13] with permission from The Royal Society of Chemistry)
Structure of the probe L6 and its stoichiometric binding with Cd2+ ions
A benzene-based tripodal receptor was reported by Das and co-workers with naphthalene as the fluorophore unit (Fig. 4.8) [17]. The probe could selectively bind Pb2+ ions in aqueous medium at physiological pH. The 1:1 binding stoichiometry was confirmed by fluorescence titration experiments along with mass spectrometry. The binding site specificity was also studied from 1H NMR spectroscopy. The signal transduction process of the fluorescence enhancement of the probe upon addition of Pb2+ ions was proposed to proceed via the CHEF mechanism. In addition to Pb2+ ions, the probe could also detect Al3+ ions under the same experimental medium.

(adapted from [14] with permission from Elsevier)
Cartoon representation of Pb2+ ions sensing by the tripodal receptor L7
Das and co-workers reported a benzothiazole probe for fluorogenic recognition of Cd2+ in a “Turn-on” manner in aqueous medium (Fig. 4.9) [18] Fluorescence titration experiments along with mass spectrometry suggested a 1:1 coordination complex of the probe-Cd2+ ensemble.

(adapted from [14] with permission from Elsevier)
Schematic representation of selective sensing of Cd2+ ions by the probe L8
Also, 1H NMR titration experiments indicated the possible binding sites of the ligand with the metal ions. The entire process was proposed to proceed via the PET mechanism in conjunction with restriction of bond rotation of the ligand. The same probe also detected Zn2+ ions in the same medium and consecutively acted a secondary chemosensor for H2PO4− and P4O74− ions.
Lin and co-workers reported a naphthalimide-based fluorogenic probe for selective and sensitive detection of Hg2+ ions in water (Fig. 4.10) [19]. Extensive 1H NMR, mass spectrometric, emission, and absorbance experiments confirmed the 1:1 binding stoichiometry of the sensor molecule and Hg2+ ions. IR spectroscopy further delved into the binding sites of the ligand with Hg2+ ions. In addition, the detection process was reversible over several number of times and the fluorescence sensing process was viable even to the naked eye.

(adapted from [19] with permission from The Royal Society of Chemistry)
Pictorial representation of Hg2+ detection by the naphthalimide-based probe L9
A Schiff base reported by Das and co-workers functioned as a versatile fluorogenic chemosensor for a number of metal ions in exclusively different emission wavelengths in aqueous medium (Fig. 4.11) [20]. Cd2+ and Pb2+ ions were detected in a “Turn-on” fashion at wavelengths differing by at least 26 nm. The selective fluorescence signals were attributed to the CHEF mechanism in combination with ICT process. Zn2+ ions were also additionally detected by the probe in the same working medium. A 1:1 binding stoichiometry was confirmed by mass spectrometry, and an insight into the metal-binding sites of the probe was studied by theoretical calculations as well.

(adapted from [19] with permission from The Royal Society of Chemistry)
Structure and proposed binding modes of Cd2+ and Pb2+ ions with L10
3 Conclusion
Currently, the development of optical and fluorescence-based sensors for the detection of heavy metals in aqueous media remains a challenge of prime importance considering their widespread existence in the terrestrial as well as biological environment. In this mini-review, a careful insight has been provided into advances of some simple yet efficient chemosensors based on modest strategies for the selective identification of toxic metal ions in aqueous environment.
References
L. Prodi, F. Bolletta, M. Montalti, N. Zaccheroni, Coord. Chem. Rev. 205, 59–83 (2000)
E.L. Que, D.W. Domaille, C.J. Chang, Chem. Rev. 108(5), 1517–1549 (2008)
O.T. Butler, J.M. Cook, C.F. Harrington, S.J. Hill, J. Rieuwerts, D.L. Miles, J. Anal. At. Spectrom. 21, 217–243 (2006)
Z.C. Wen, R. Yang, H. He, Y.B. Jiang, Chem. Commun. 106–108 (2006)
J. Wu, W. Liu, J. Ge, H. Zhang, P. Wang, Chem. Soc. Rev. 40, 3483–3495 (2011)
D. Wu, A.C. Sedgwick, T. Gunnlaugsson, E.U. Akkaya, J. Yoon, T.D. James, Chem. Soc. Rev. 46, 7105–7123 (2017)
G. Chen, Z. Guo, G. Zeng, L. Tang, Analyst 140, 5400–5443 (2015)
H.N. Kim, W.X. Ren, J.S. Kim, J. Yoon, Chem. Soc. Rev. 41, 3210–3244 (2012)
A.P. de Silva, H.Q.N. Gunaratne, T. Gunnlaugsson, A.J.M. Huxley, C.P. McCoy, J.T. Rademacher, T.E. Rice, Chem. Rev. 97, 1515–1566 (1997)
J.F. Callan, A.P. de Silva, D.C. Magri, Tetrahedron 61, 8551–8588 (2005)
C. Kar, M.D. Adhikari, A. Ramesh, G. Das, RSC Adv. 2, 9201–9206 (2012)
R. Singh, G. Das, Analyst 144, 567–572 (2019)
D. Singhal, N. Gupta, A.K. Singh, RSC Adv. 5, 65731–65738 (2015)
R. Singh, G. Das, Sens. Actuators B Chem. 258, 478–483 (2018)
R. Singh, A. Gogoi, G. Das, RSC Adv. 6, 112246–112252 (2016)
A. Shaily, N. Kumar, Ahmed. New J. Chem. 41, 14746–14753 (2017)
B.K. Datta, C. Kar, A. Basu, G. Das, Tetrahedron Lett. 54, 771–774 (2013)
A. Gogoi, S. Samanta, G. Das, Sens. Actuators B Chem. 202, 788–794 (2014)
Y.M. Zhang, K.P. Zhong, J.X. Su, X.P. Chen, H. Yao, T.B. Wei, Q. Lin, New J. Chem. 41, 3303–3307 (2017)
S. Samanta, B.K. Datta, M. Boral, A. Nandan, G. Das, Analyst 141, 4388–4393 (2016)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Singh, R., Das, G. (2019). Towards Fluorogenic and Chromogenic Sensing of Heavy Metal Ions in Aqueous Medium: A Mini-Review. In: Singh, D., Das, S., Materny, A. (eds) Advances in Spectroscopy: Molecules to Materials. Springer Proceedings in Physics, vol 236. Springer, Singapore. https://doi.org/10.1007/978-981-15-0202-6_4
Download citation
DOI: https://doi.org/10.1007/978-981-15-0202-6_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-0201-9
Online ISBN: 978-981-15-0202-6
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)