Abstract
In this chapter, electric discharge coating (EDC) was employed for the surface modification of medical grade 316L stainless steel with hydroxyapatite nanopowder using copper as a tool electrode with reverse polarity. Experimentation was performed according to Taguchi’s L18 orthogonal array to assay the influence of input machining parameter on surface characteristics of modified surface. In vitro electrochemical corrosion analysis was executed to authenticate the enhanced corrosion resistance of the specimen. The homogenous porous surface and deposition of powder particles were examined using scanning microscopy in conjunction with the formation of bioactive and intermetallic compounds, carbides and silicides on the machined surface inspected using X-ray diffraction technique, promoting the biological compatibility with human body. Furthermore, modified surface showed better corrosion resistance with a corrosion rate of 0.0972 mm/year compared to substrate exhibited a higher corrosion rate of 1.79 mm/year. The observations validate the improved corrosion protection and bioactivity of 316L stainless steel for biomedical applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The study of biomaterials is going on since many decades. The need for safe and efficient biomaterials is rising tremendously because of increase in number of accidents causing fractured human bones, joint replacement, etc. The basic requirement of biomedical implants is the existences of proper physiological environment inside the body, strength, wear resistance, biological fixation, cell growth and corrosion resistance [1]. The surface interface and its properties determine the acceptance or rejection of the biomedical implants on the body [2,3,4].
The quality of machined surface highly affects the bioactive properties like corrosion resistance, wear resistance and cell proliferation of the metallic bio-implants [5, 6]. Success of biomaterials is based on the characteristics such as topography, chemical composition, mechanical strength, corrosion resistance and surface roughness. Hence, many surface modification and surface coating techniques are being used nowadays to enhance the required properties of biomedical implants. Modifications of biomaterials also overcome the risk of rejection due to loosening of joint, toxicity, infection within the individual providing proper cell adhesion, cell proliferations and antibacterial properties [7,8,9,10].
Among all of the biomaterials, i.e., polymeric, ceramic, natural, metallic and composites, metallic biomaterials are widely acceptable due to their mechanical properties and compatibility with the human bone. Titanium alloys, 316L stainless steel, chromium and cobalt are the metallic biomaterials; amid all, 316L stainless steel because of their easy availability, low cost, better adhesion and cell growth preferred much more [11,12,13,14,15]. But the presence of sodium, chlorine, water, saliva and amino acids within the human body disturbs the equilibrium state and consumes the metallic implant by various anodic and/or cathodic reactions [16,17,18]. As a result, metallic biomaterial implant corrodes after certain years of implantation. In order to overcome such issue, coating of bioactive layer on surface is best solution which not only evades early corrosion but also avoids release of harmful ions [19].
Electric discharge coating (EDC) is an application of well-known electrical discharge machine (EDM) by reversing the polarity during the machining process. Reverse polarity alters the flow of electrons compared to conventional process and deposits the material mixed in dielectric to modify the surface characteristics of the workpiece [20,21,22,23]. The intermetallic compounds and carbides thus formed enhance the properties of substrate biomaterial offering better adhesion and proliferation of human osteoblast-like MG-63 cells.
2 Literature Review
Among numerous studies on metallic biomaterials, Mahajan and Sidhu [24] reviewed the need for surface modification of biomaterials for the enhancement of their functionality. The surface modification of metallic biomaterials, i.e., stainless steel, titanium using EDM, helps to obtain the biocompatible surfaces, increases bond ability, etc. EDM is the method that has the ability to replicate the architecture and characteristics of the natural bones. It had great applications in biomedical fields for the enrichment of required surface characteristics. Other researchers like Prakash et al. [25] also expressed EDM as a potential and new innovative way for surface modification of various metallic implants for orthopedic applications. According to the in vitro bioactivity analysis, powder-mixed dielectric machined surface offers better cell growth than substrate and surface machined without the addition of powder in dielectric [26].
The surface of 316L stainless steel was treated by Mazur et al. [27] utilizing sol–gel technique to deposit ceramic layer of SiO2–Y2O3. Newly formed ceramic layer helped to enhance the bioactivity and corrosion resistance of 316L which was confirmed by EDS and Raman spectra analysis. Compounds like calcium (Ca) and phosphorous (P) were observed confirming the formation of apatite ceramic layer on stainless steel 316L with improved biocompatibility. Electron beam physical vapor deposition (EBPVD) was employed by Kaliaraj et al. [28] to modify 316L stainless steel surface with monoclinic (m-ZrO2) and tetragonal (t-ZrO2) phase of zirconium dioxide. They found improved cell viability during the cell culture analysis of coated samples. Superior corrosion resistance was shown by coated samples of 316L stainless steel in artificial blood plasma (ABP) as electrolyte using electrochemical test.
Abbas et al. [29] investigated the present research scenario of electrical discharge machining, suggesting PMEDM as dominating non-conventional machining process for the improvement of surface characteristics with the addition of powder in dielectric medium. Here, high MMR, better surface finish and low TWR can be obtained. The surface characteristics of aluminum powder-mixed ED machined Ti-6Al-4V was examined by Abdul-Rani et al. [30] for the formation of intermetallic compounds and carbon enriched layer on the substrate surface which helps in osseointegration and bone proliferations. Furthermore, modified surface exhibited enhanced corrosion resistance of titanium alloy.
Singh et al. [31] investigated the effect of input process parameters on MRR by machining AISI 316L stainless steel using EDM process. Current was found as the most significant factor influencing MRR. Highest MRR of 6.25 mg/min was obtained at the combination of current 28 A, Ton 90 μs, Toff 60 μs and voltage 80 V. The performance of traditional EDM process with the addition of Al powder using reverse polarity was studied by Sharma et al. [32]. Their investigation showed that various characteristics of powder like concentration, size, etc., had its dominating effect on the performance of electric discharge machine.
Karamian et al. [33] coated HAp/zircon on stainless steel 316L and concluded that powder coating helps to improve adhesion properties of metallic biomaterial. The investigation of HAp/TiO2 coating on the titanium substrate was performed by Ramires et al. [34] for improving the bioactive properties such as osseointegration and cell proliferations. Manam et al. [35] intensely reviewed the biocompatibility of metallic biomaterials, i.e., titanium alloys, stainless steel and chromium–cobalt alloys. They studied that corrosion resistance of biomaterial was an important factor. Moreover, corrosive nature causes the failure of implantation and leads to surgery. However, surface modification of biomaterials with bioactive layer offered better corrosion resistance and evaded the harmful ions to release. They concluded that metallic biomaterials could not be replaced with polymers and ceramics due to the required mechanical properties for a material to be biomaterial.
Bains et al. [36] and Long et al. [37] optimized the MRR by employing PMEDM on using copper and graphite tool with titanium and SiC-mixed dielectric. They concluded that powder concentration increases metal erosion as compared to no powder in dielectric with discharge current as one of the most significant factors followed by other variables.
In this research, EDC was employed on 316L stainless steel with hydroxyapatite nanoparticles-mixed dielectric to examine improved surface characteristics in terms of surface roughness and formation of bioactive compounds on 316L surface. Additionally, in vitro corrosion analysis was performed on machined province to scrutinize the improved corrosion resistance of HAp-coated 316L stainless steel surface.
3 Methodology
3.1 Materials
Medical grade 316L stainless steel was procured from Metline Industries, Mumbai, for the experimentation in the form of rectangular plate (size: 50 mm × 100 mm × 5 mm). Electrolytic copper tool because of its conductivity with dia. 900 μm was chosen as electrode for the ED machining of 316L SS. The compositional analysis of 316L stainless steel is listed in Table 1.
Hydroxyapatite nanopowder with average particle size 20–45 nm and purity of 99.5% mixed in dielectric medium for the surface modification of 316L stainless steel.
3.2 Design of Experiment
Taguchi’s orthogonal arrays are generally executed to reduce the number of experimental trials according to selected machining parameters. In the present work, L18 (21 × 34) was used for conducting the experiments selecting five input parameters, i.e., dielectric medium, discharge current, pulse-on-time, pulse-off-time and voltage. Minitab-17 was utilized to generate design of experiments with three levels of input parameter as shown in Table 2.
3.3 Experimentation
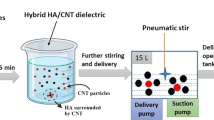
Die sinker-type electrical discharge machine with reverse polarity {workpiece as cathode (−) and electrode as anode (+)} was employed for conducting the experimentation. Out of 18 experimental runs, nine were performed in pure dielectric, i.e., EDM oil, whereas for powder-mixed experimentation, indigenously developed setup (shown in Fig. 1) consisting of stirrer and pump for proper and homogeneous mixing of HAp particles was used. Machining time for each run was fixed for 40 min with powder concentration of 15 g/l. After the ED machining, Mitutoyo SJ-400 surface roughness tester was used to measure roughness (Ra) of machined surface. Each sample measured diametrically from three locations and was averaged for further analysis.
In this experimental investigation, dielectric medium, discharge current (I), pulse-on-time (Ton), pulse-off-time (Toff) and Voltage (V) were selected as input parameters being the most common and influencing parameters during the machining process. The selected output response was surface roughness (Ra) in μm.
3.4 In Vitro Corrosion Analysis
Electrochemical corrosion test was performed to validate the enhanced corrosion resistance and subsequently low corrosion rate of modified 316L stainless steel surface with HAp particles. Ringer solution at 37 °C was used as simulated body fluid (SBF) to replicate the human body environment as electrolyte immersing 316L sample as working electrode (WE), whereas platinum wire as counter electrode (CE) and Ag/AgCl employed as reference electrode (RE). Prior to analysis, specimen was bathed in acetone and insulated with tape leaving an exposed machined area of 0.32 cm2. The insulation restricts the contribution of base metal during the electrochemical testing.
4 Results and Discussion
The experimental design matrix and surface roughness of machined specimen are shown in Table 3. The surface response table is represented according to signal-to-noise ratio (S/N) methodology—a ratio of strength of signal to the magnitude of error. S/N ratios depend on the type of responses measured such as “Larger is better” type which is given by the equation below:
where R = number of repetitions of responses; yi = value of response at ith trial.
4.1 Analysis of Variance for S/N Ratios of Surface Roughness
The surface response of machined workpiece was analyzed using analysis of variance (ANOVA) of S/N ratios as shown in Table 4 with the aid of Minitab-17 software. Momentous process parameters affecting the roughness of machined 316L stainless steel were recognized using p-value and subsequently their percentage contribution. Pulse-on-time was the most dominating parameter affecting surface roughness (contribution 33.40%) followed by discharge current (contribution 24.52%) and dielectric medium (contribution 16.81%). Consequently, the desired porous surface for proper adhesion, cell growth validating the surface modification of selected biomaterial examined in HAp powder-mixed dielectric at higher value of on-time (i.e., 90 µs) and peak current (i.e., 28 A) which concurrently endow with the desired surface topography and phase transformation of 316L stainless steel for requisite bioactivity. Figure 2 illustrates the S/N ratio plot for surface roughness.
Further, powder-mixed ED machined 316L stainless steel surface was examined for changed morphology, formation of intermetallic compounds and in vitro corrosion analysis to validate the surface modification with bioactive powder and enhanced bioactivity.
4.2 Surface Morphology of Machined Surface
Scanning electron microscopy (JSM-6610 LV Joel, Japan) was used to inspect the morphology of machined 316L specimen in hydroxyapatite nanopowder-mixed dielectric medium. Deposition of powder particles and presence of homogeneous porous structure surface encouraging cell growth, adhesion between bone and implant, and cell proliferation were revealed by SEM (Fig. 3) analysis.
4.3 Phase Transformation of Machined Surface
It was described in the previous research [38, 39] that sparks produced during ED machining changed the chemical composition of the machined surface. X-ray diffraction (XRD) technique was used to investigate the formation of intermetallic and bioactive compounds on ED machined 316L stainless steel surface (trial 17). Figure 4 witnesses the XRD pattern confirming the formation and deposition of intermetallic compounds, carbides as well as silicides on the EDMed surface. Newly formed bioactive compounds contribute for enhanced bioactivity in terms of improved corrosion resistance, proper adhesion between bone and implant, and cell growth.
4.4 In Vitro Corrosion Analysis
After the examination of porous structure and compositional analysis using SEM and XRD, respectively, electrochemical corrosion analysis was carried out to validate the response of modified surface in terms of enhanced corrosion resistance. Ringer’s solution with pH value 7.2 at 37 °C was used as simulated body fluid (SBF) to reproduce the human body environment for the current investigation.
It was evident from the Tafel plot (Fig. 5) that surface modified with HAp powder-mixed dielectric depicts higher value of corrosion potential (Ecorr = −257.810 mV) compared to bare metal possess −308.490 mV. Consequently, modified surface of 316L stainless steel showed improved corrosion rate of 0.0972 mm/year compared to substrate material with corrosion rate of 1.79 mm/year. The newly modified ED machined 316L surface exhibited improved corrosion resistance that is necessitated for better and proper bone–implant adhesion. It will avoid the release of metallic ions due to the presence of enzymes and reacting environment within the individual causing poor osseointegration and cytotoxicity.
5 Conclusions
Present work analyzed the surface modification of medical grade 316L stainless steel using electric discharge coating (EDC) in HAp powder-mixed dielectric. Based on the surface characteristics and in vitro corrosion analysis, following conclusions were drawn:
-
1.
Surface roughness directly increased with the increase in pulse-on and current applied. Desired porous surface (1.523 µm) was observed at 28A; Ton 90 µs; Toff 60 µs; and 80 V in the presence of HAp in the dielectric medium.
-
2.
SEM revealed porosity and deposition of powder particles on HAp powder-mixed dielectric machined surface.
-
3.
XRD confirmed the phase transformation of surface (trial 17) with the formation of bioactive compounds (calcium, phosphorus, calcium carbonate) and several intermetallic compounds.
-
4.
Surface machined at higher discharge current, i.e., 28 A sounds more crowded with intermetallic compounds and carbides compared to surface machined at lower value of discharge current.
-
5.
In vitro corrosion analysis exhibited improved corrosion resistance and accordingly low corrosion rate of HAp powder modified 316L stainless steel surface (0.0972 mm/year) compared to bare metal (1.79 mm/year).
-
6.
Enhanced surface characteristics and corrosion resistance validate the surface modification of 316L with HAp powder using EDC for biomedical applications.
References
Bhui AS, Singh G, Sidhu SS, Bains PS (2018) Experimental investigation of optimal ED machining parameters for Ti-6Al-4V biomaterial. FU Ser Mech Eng 16(3):337–345
Mahajan A, Sidhu SS (2018) Enhancing biocompatibility of Co-Cr alloy implants via electrical discharge process. Mater Technol 33(8):524–531
Tasnim N, Kumar A, Joddar B (2017) Attenuation of the in vitro neurotoxicity of 316L SS by graphene oxide surface coating. Mater Sci Eng C 73:788–797
Naahidi S, Jafari M, Edalat F, Raymond K, Khademhosseini A, Chen P (2013) Biocompatibility of engineered nano-particles for drug delivery. J Controlled Release 166:182–194
Shagaldi BF, Compson J (2000) Wear and corrosion of sliding counterparts of stainless-steel hip screw plates. Inj Int J Care Injured 31:85–92
Bains PS, Grewal JS, Sidhu SS, Kaur S (2017) Wear between ring and traveler: a pin-on-disc mapping of various detonation gun sprayed coatings. Mater Today Proc 4(2):369–378
Manivasagam G, Dhinasekaran D, Rajamanickam A (2010) Biomedical implants: corrosion and its prevention—a review. Rec Pat Corros Sci 2:40–54
Fu T, Wen CS, Lub J, Zhou YM, Mac SG, Dong BH, Liu BG (2012) Sol-gel derived TiO2 coating on plasma nitrided 316L stainless steel. Vacuum 86:1402–1407
Kaliaraj GS, Ramadoss A, Sundaram M, Balasubramanian S, Muthirulandi J (2014) Studies of calcium-precipitating oral bacterial adhesion on TiN, TiO2 single layer, and TiN/TiO2 multilayer-coated 316L SS. J Mater Sci 49:7172–7180
Sharifnabi A, Fathi MH, Yekta BE, Hossainalipour M (2014) The structural and bio-corrosion barrier performance of Mg-substituted fluorapatite coating on 316L stainless steel human body implant. Appl Surf Sci 288:331–340
Chang SH, Chen JZ, Hsiao SH, Lin GW (2014) Nanohardness, corrosion and protein adsorption properties of CuAlO2 films deposited on 316L stainless steel for biomedical applications. Appl Surf Sci 289:455–461
Kathuria YP (2006) The potential of biocompatible metallic stents and preventing restenosis. Mater Sci Eng A 417:40–48
Wang KK, Kim BJ, Heo II, Jung SJ, Hwang JW, Kim YR (2017) Fabrication and characterization of antimicrobial surface-modified stainless steel for bio-application. Surf Coat Technol 310:256–262
Harun WSW, Asri RIM, Romlay FRM, Sharif S, Jan NHM, Tsumori F (2018) Surface characterisation and corrosion behaviour of oxide layer for SLMed-316L stainless steel. J Alloys Compd 748:1044–1052
Bekmurzayeva A, Duncanson WJ, Azevedo HS, Kanayeva D (2018) Surface modification of stainless steel for biomedical applications: revisiting a century-old material. Mater Sci Eng C 93:1073–1089. https://doi.org/10.1016/j.msec.2018.08.049
Asri RIM, Harun WSW, Samykano M, Lah NAC, Ghani SAC, Tarlochan F, Raza MR (2017) Corrosion and surface modification on biocompatible metals: a review. Mater Sci Eng C 77:1261–1274
Bains PS, Mahajan R, Sidhu SS, Kaur S (2019) Experimental investigation of abrasive assisted hybrid EDM of Ti-6Al-4V. J Micromanuf. https://doi.org/10.1177/2516598419833498
Li W, Xin Z, Ming-hui D, Hang Z, Hong-sen Z, Bin Z, Xun-qi L, Gao-yu W (2015) Surface modification of biomedical AISI 316L stainless steel with zirconium carbonitride coatings. Appl Surf Sci 340:113–119
Kumar AM, Rajendran N (2013) Electrochemical aspects and in vitro biocompatibility of polypyrrole/TiO2 ceramic nanocomposite coatings on 316L SS for orthopedic implants. Ceram Int 39(5):5639–5650
Bains PS, Sidhu SS, Payal HS (2017) Investigation of magnetic field-assisted EDM of composites. Mater Manuf Process 33(6):670–675
Zain ZM, Ndaliman MB, Khan AA, Ali MY (2014) Improving micro-hardness of stainless steel through powder-mixed electric discharge machining. J Eng Sci 1–7
Marashi H, Sarhan AD, Hamdi M (2015) Employing Ti nano-powder dielectric to enhance surface characteristics in electrical discharge machining of AISI D2 steel. Appl Surf Sci 357:892–907
Talla G, Gangopadhayay S, Biswas CK (2016) State of art in powder-mixed electric discharge machining: a review. J Eng Manuf 231(14):2511–2526
Mahajan A, Sidhu SS (2017) Surface modification of metallic biomaterials for enhanced functionality: a review. Mater Technol 33(2):93–105
Prakash C, Kansal HK, Pabla BS, Puri S, Aggarwal A (2015) Electric discharge machining—a potential choice for surface modification of metallic implants for orthopedic applications: a review. J Eng Manuf 230(2):331–353
Prakash C, Uddin MS (2017) Surface modification of β-phase Ti implant by hydroaxyapatite mixed electric discharge machining to enhance the corrosion resistance and in-vitro bioactivity. Surf Coat Technol 326:134–145
Mazur A, Szczurek A, Checmanowski JG, Szczygiel B (2018) Corrosion resistance and bioactivity of SiO2-Y2O3 coatings deposited on 316L steel. Surf Coat Technol 350:502–510
Kaliaraj GS, Vishwakarma V, Alagarsamy K, Kamalan Kirubaharan AM (2018) Biological and corrosion behavior of m-ZrO2 and t-ZrO2 coated 316L SS for potential biomedical applications. Ceram Int 44(12):14940–14946
Abbas NM, Solomon DG, Bahari MF (2007) A review on current research trends in electrical discharge machining (EDM). Int J Mach Tools Manuf 47(7–8):1214–1228
Abdul-Rani AM, Nanimina AM, Ginta TL, Razak MA (2017) Machined surface quality in nano aluminum mixed electrical discharge machining. Proc Manuf 7:510–517
Singh G, Bhui AS, Sidhu SS (2017) Influence of input parameters on MRR of AISI-316L using tungsten electrode machined by EDM. ISBN 978-5-398-01932-2:15-19
Sharma S, Kumar A, Beri N, Kumar D (2010) Effect of aluminum powder addition in dielectric during electric discharge machining of hastelloy on machining performance using reverse polarity. Int J Adv Eng Technol 1(3):13–24
Karamian E, Motamedi MRK, Khandan A, Soltani P, Maghsoudi S (2014) An in-vitro evaluation of novel NHA/zircon plasma coating on 316L stainless steel dental implant. Prog Nat Sci Mater Int 24(2):150–156
Ramires PA, Romito A, Cosentino F, Milella E (2001) The influence of titania/hydroxyapatite composite coatings on in vitro osteoblasts behavior. Biomaterials 22(12):1467–1474
Manam NS, Harun WSW, Shri DNA, Ghani SAC, Kurniawan T, Ismail MH, Ibrahim MHI (2017) Study of corrosion in biocompatible metals for implants: a review. J Alloys Compd 70:698–715
Bains PS, Sidhu SS, Payal HS, Kaur S (2019) Magnetic field influence on surface modifications in powder mixed EDM. Silicon 11(1):415–423
Long BT, Phan NH, Cuong N, Jatti VS (2016) Optimization of PMEDM process parameter for maximizing material removal rate by Taguchi’s method. Int J Adv Manuf Technol 87(5–8):1929–1939
Sidhu SS, Bains PS (2017) Study of recast layer of particulate reinforced metal matrix composites machined by EDM. Mater Today Proc 4(2):3243–3251
Bains PS, Singh S, Sidhu SS, Kaur S, Ablyaz TR (2018) Investigation of surface properties of Al-SiC composites in hybrid electrical discharge machining. In: Futuristic composites. Springer, Singapore, pp 181–196
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Singh, G., Bhui, A.S., Sidhu, S.S., Bains, P.S., Lamichhane, Y. (2019). Surface Characteristics and In Vitro Corrosion Behavior of HAp-coated 316L Stainless Steel for Biomedical Applications. In: Bains, P., Sidhu, S., Bahraminasab, M., Prakash, C. (eds) Biomaterials in Orthopaedics and Bone Regeneration . Materials Horizons: From Nature to Nanomaterials. Springer, Singapore. https://doi.org/10.1007/978-981-13-9977-0_8
Download citation
DOI: https://doi.org/10.1007/978-981-13-9977-0_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-9976-3
Online ISBN: 978-981-13-9977-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)