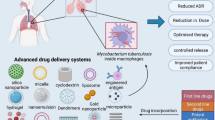
Abstract
Tuberculosis (TB) continues to be a major global challenge, claiming about two million deaths each year. The emergence of drug resistance due to high incidence of poor patient compliance has further worsened the situation. Targeted delivery of drugs to macrophage, the site of Mycobacterium tuberculosis infection and replication, has been shown to have implications as a promising option in TB treatment. A variety of biocompatible and biodegradable polymer-based carrier-based delivery systems have emerged as potential drug delivery systems (DDS). Such targeted delivery systems have been shown to have significant merits over free drug, including improved drug bioavailability, limiting adverse drug effects and requiring less frequent administration regimes and lowering drug doses. The pulmonary administration of inhalable dry powders incorporating multiple drugs has particularly exhibited encouraging results against MDR-TB, and is expected to shorten the treatment duration, thereby improving patient compliance. Recently, the administration of pulmonary drug delivery as an adjunct to existing oral treatment regimens has been shown to achieve sufficient drug concentrations in certain systemic compartments and thus further enhance treatment effectiveness. The present chapter discusses the recent research updates on carriers used in preclinical or clinical studies against TB, the challenges associated, and future perspectives.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Tuberculosis (TB) is a lifelong, devastating, chronic granulomatous disease caused by the inhalation of airborne droplet nuclei containing Mycobacterium tuberculosis (M.tb.), and it is one of the leading killers of humans worldwide. The use of antibiotics against tuberculosis (TB) in the middle of the twentieth century heralded a new era in our fight against this age-old disease. We observed a significant decrease in TB cases in the half century since the anti-TB drugs were first introduced; however, the success of the antibiotics was short-lived, with the emergence of drug-resistant strains of M.tb. In 1993, the World Health Organization (WHO) declared TB a global emergency (Zumla et al. 2014), and by 2015, 480,000 people developed multidrug-resistant TB (MDR-TB) annually (Kendall et al. 2017; Petersen et al. 2017). This staggering number that also includes individuals harboring the extensively drug-resistant TB (XDR-TB) strains known to be even more difficult to treat than MDR-TB, where in some cases, no effective treatment regimen exists. Recently, the WHO has reported 10.4 million incident cases of TB across the world in 2016, of which ~two million people die annually due to this potentially curable disease (Global TB Control 2017 Report). The only available pediatric vaccine, using Mycobacterium bovis BCG, is protective against severe forms of childhood TB, but is ineffective against TB in adults. TB has, therefore, become a global pandemic and is a priority concern across the world.
2 Pathogenesis and Immunology of TB
2.1 Progression of Disease
2.1.1 Early Events Following Infection
Infection is initiated by inhalation of aerosol droplets containing M.tb. that are expectorated by active pulmonary TB patients. These inhaled tiny droplet nuclei find their way into terminal alveoli and are readily phagocytosed by alveolar macrophages and dendritic cells. Subsequently, the mycobacteria are also reported to bind, invade, and infect non-phagocytic cells, including pneumocytes and alveolar endothelial cells (Ahmad 2010; Fernstrom and Goldblatt 2013).
M.tb. pathogenicity lies in its ability to survive and multiply within host macrophages, for which they specifically use mannose receptor (MR) and complement receptor (CR) for uptake. The bacteria engage with vesicular trafficking machinery, inhibiting phagosome–lysosome fusion (phagosomal maturation arrest) and depleting H+- ATPases from vacuolar membranes, thereby inhibiting phagosome acidification (Flannagan et al. 2012). That is, though the initial trafficking pattern of the M.tb.-containing phagosomes is normal, the arrest is marked by the absence of Rab7 in later stages, thereby inhibiting progression to phagolysosomes (Deretic et al. 1997) and promoting the intracellular survival and growth of the pathogen.
2.1.2 Disease Progression
In immunocompetent hosts, an effective immune response develops 2–8 weeks post-infection that arrests further multiplication of the bacteria. In most cases, acquired responses result in the containment of bacteria within well-organized granuloma, marked by the presence of a large number of activated macrophages, which infiltrate the region and enclose the infected cells in tubercles. These macrophages later differentiate into epithelioid cells, so called due to the resemblance of clustered macrophages to epithelial cells. Activated macrophage secretes large amounts of lytic enzyme, leading to the formation of spheroidal regions of necrotic tissue, known as the “Ghon focus.” Tubercle formation leads to hypoxic conditions within it, which, together with acidosis, kills most of the bacteria. Granuloma formation in most cases, therefore, limits infection, but does not totally eliminate it (Rom and Garay 2003). Thus, in “susceptible” persons, such as HIV patients and other immunosuppressed individuals, M.tb. can potentially become reactivated.
2.1.3 Immune Responses
Protective responses to M.tb. are complex and involve both arms of immunity: innate and acquired. M.tb. phagocytosis is typically followed by innate immune responses, including an increase in oxidative burst, reactive nitrogen intermediates (nitric oxide), phagosome acidification, and proinflammatory cytokines (Rom and Garay 2003; O’Garra et al. 2013). The classical pathway of interferon-gamma-dependent activation of macrophages by T helper 1 (Th1)-type responses is a well-established feature of cellular immunity to intracellular M.tb. infection. Virulent M.tb. strains subvert this immune response for its own survival, inducing what is known as “alternative activation” within host macrophages. MR-associated phagocytosis has been shown to be linked with activation of anti-inflammatory activity marked by “alternative activation” of macrophage, and non-activation of NADPH oxidase (Guirado et al. 2013). In susceptible individuals, the infected macrophage displays an “alternative” phenotype with diminished NO production, suppressing apoptosis (Verma et al. 2011). Such macrophages exhibit increased Th2 cytokine (IL-4, IL-13, and IL-10) production (Kahnert et al. 2006), thereby inhibiting the protective Th1 response by antagonizing IFN-γ and decreasing IL-6 and TNF-α (O’Garra et al. 2013). A great deal of work has also implicated the inhibition of autophagy as a survival strategy of M.tb. within host macrophage.
3 Different Forms of TB
While about one-third of the world population is infected with M.tb., only 10% develop active disease (Raviglione and Sulis 2016). In “susceptible” individuals, innate and acquired immune responses are insufficient to eliminate or contain the bacteria, leading to bacterial proliferation inside macrophages. In a limited number of cases, risk factors are identifiable, which include HIV/AIDS, diabetes, age, alcohol, smoking, and corticosteroid therapy. In other individuals, the immune response is sufficient to clear the infection, or arrests it in a latent state. The different forms of TB are:
Active TB
Active TB patients have rapidly multiplying bacteria and the typical symptoms of active TB, including coughing, phlegm, chest pain, weakness, weight loss, fever, chills, and night sweats. Persons with active pulmonary TB disease are the main source of disease spread via the aerosol route (Rom and Garay 2003).
Miliary TB
Unchecked multiplication of TB bacteria leads to bacterial dissemination throughout the body via the blood. Miliary TB is a rare form of active disease in which bacteria quickly spread all over the body and affect multiple organs at once. This form of TB can be rapidly fatal.
Latent TB
In some individuals, the host T cell immune response confines the pathogen to a hostile environment where it may become latent, though viable. People with latent tuberculosis infections are asymptomatic and not infectious, but can develop active TB disease in the future due to “reactivation” of latent mycobacteria.
Thus, the inhalation of mycobacteria may ultimately lead to four different infection outcomes (Rom and Garay 2003):
-
(i)
Innate response is sufficient to clear the bacteria.
-
(ii)
Active disease develops soon after infection, known as primary infection.
-
(iii)
Asymptomatic, latent infection develops.
-
(iv)
Active disease may develop many years after infection, known as reactivation, or secondary TB.
4 Drug Regimens and Current Anti-TB Therapy
When the bactericidal mechanisms of the macrophage fail to clear the intracellular bacilli, the TB patient requires treatment by the intervention of chemotherapy. In India, patients presenting with TB are treated as per the guidelines of the Revised National Tuberculosis Programme (RNTCP) (Verma et al. 2013b), with similar guidelines for other countries. The “directly observed therapy (short course)” or DOTS regimen has been demonstrated to be highly efficacious for TB treatment in various clinical settings in India. The RNTCP recommends a therapeutic regimen of a combination of drugs and dosage schedules in which two or more first-line drugs are administered for a period of no less than 6 months. The first-line drugs used in TB treatment include isoniazid [INH], rifampicin [RIF], ethambutol [EMB], and pyrazinamide [PYZ] (Tiberi et al. 2017). The second-line drugs are used for infections with tubercle bacilli resistant to first-line drugs. These include aminoglycosides [AMG], such as amikacin and kanamycin; polypeptides, such as capreomycin [CPR], viomycin, and enviomycin; fluoroquinolones, such as ciprofloxacin, ofloxacin [OFX], levofloxacin, and moxifloxacin; and thioamides, such as ethionamide, prothionamide, and cycloserine.
To overcome the rise in multidrug resistance (against first- and second-line drugs), the WHO included third-line drugs for TB treatment. These include linezolid, thioridazine, and macrolides, such as clarithromycin and thioacetazone, selected on the basis of drug susceptibility testing (DST). This further complicates treatment, requiring even longer regimens with drugs that are more expensive, less effective, and often more toxic. Rifabutin (another rifamycin) is added to the regimen if rifampicin resistance is detected with rifabutin susceptibility.
4.1 Existing TB Treatment Regimen
According to the WHO, the standard treatment regimen for drug-susceptible TB includes daily oral administration of INH, RIF, PZA, and EMB for 2 months (Pai et al. 2016). This is followed by daily administration of INH and RIF for another 4 months (WHO 2017). Daily dosing is recommended, although the 3-times weekly dosing can be used during the continuation phase under DOTS, as well as fixed-dose combinations.
The WHO strongly recommends DST (rapid and/or conventional) in all cases, and particularly for those previously treated for TB disease. While awaiting DST results, in settings with a medium or low probability of MDR-TB, retreatment cases could initially be treated with an empiric regimen, INH-RIF-PYZ-EMB-STR, for 2 months, followed by INH-RIF-PYZ-EMB for 1 month, and INH-RIF-EMB for 5 months.
For patients whose DST results are not available (a rather common phenomenon in many developing countries), a third-line regimen is recommended by the WHO. This regimen contains four drugs (an AMG, ethionamide (ETD), PYZ, and OFX) during initial phase and two drugs (ETD and OFX) during the continuation phase (Sotgiu et al. 2015).
5 Drug-Resistant Tuberculosis (MDR, XDR, and TDR-TB)
The current antibiotic treatment regimen requires a minimum of 6 months therapy that causes severe and prolonged side effects, and subsequently leads to nonadherence to the treatment regimen. Non-compliance with treatment ultimately leads to the generation of drug-resistant strains of M.tb. that not only increase the treatment regimen up to 2 years, but are also more costly to treat.
The first drug-resistant case of TB was observed in 1947 against streptomycin, and lead to the development of a regimen using multiple drugs for treatment (Kerantzas and Jacobs 2017). Multidrug-resistant (MDR) TB with resistance to INH and RIF was first reported in the 1990s (Matteelli et al. 2014). The WHO definition of extensively drug-resistant (XDR) TB involves resistance to at least RIF and INH, in addition to any fluoroquinolone, and to at least one of the three injectable second-line drugs, CPR, kanamycin, and amikacin, used in anti-TB treatment (Prasad et al. 2017). A 6-month course of standard first-line medication is ineffective in MDR-TB cases. Different combinations of second-line drugs, supported by selected first-line TB drugs, are used in patients with RIF-resistant or MDR-TB for 18 months or longer of treatment. Regardless, the success rate of XDR-TB treatment is very low, with mortality as high as >30%, as reported from treatment cohorts (WHO Global TB report 2017; Kvasnovsky et al. 2016).
Totally drug-resistant (TDR) TB is caused by M.tb. clinical strains which are resistant to all first- and all second-line drugs. It is extremely difficult to treat, but not totally impossible to treat, and thus is also referred to as extremely drug-resistant TB (XXDR-TB). Italy reported the first TDR-TB case in two patients in 2003, and India first reported four cases in 2012 (Ahmed et al. 2016). Further, in recent decades, very few anti-TB antibiotics have been approved for human use, including the newest drugs, bedaquiline and delamanid, that may be used for TDR-TB (D’Ambrosio et al. 2017). Alarmingly, both bedaquiline and delamanid have recently encountered resistant strains (Hoffmann et al. 2016), meaning that resistance has developed against every current TB antibiotic.
6 Novel Drug Delivery Systems for TB
In the last few decades, we have seen the advent of micronized carrier systems as an alternative to the conventional form of TB therapy. Several studies have shown that carrier systems incorporating single or multiple anti-TB drugs for the targeted delivery of antibiotics form an effective therapeutic approach against TB.
One of the main advantages of such particulate systems is the “intracellular delivery” of the bolus of loaded drug to macrophages, thereby directly targeting the sites of mycobacterial replication. Since such particles are rapidly phagocytosed by macrophage, they build up high intracellular drug concentrations and result in significant enhancement in antimicrobial efficacy (Sharma et al. 2001; Sen et al. 2003). These particulate drug delivery systems (DDS) are developed using biocompatible and biodegradable polymers, and have been shown to reduce the dosing frequency and days of treatment. Targeted drug delivery allows controlled release of drugs, and results in minimal host toxicity as compared to the conventional oral dosage. Therefore, while free antibiotics need to be administered daily, new formulations such as nano- or microparticles have been seen to be effective, even when administered after every few days.
6.1 Microparticles
Microparticles are spherical particles with sizes ranging from 50 nm to 2 mm, and containing a core substance.
A great deal of literature reports the entrapment of anti-TB drugs in microparticles composed of polymers, such as poly DL-lactide-coglycolic acid (PLGA) and poly DL-lactic acid (PLA) (Sharma et al. 2001), by various methods, such as solvent evaporation-double emulsification and spray-drying (Hirota and Terada 2014), leading to formation of particles containing antibiotic(s) embedded in a polymer matrix. Alternatively, the drug may be attached to the particle surface by physical adsorption or chemical reactions. The therapeutic advantages of microparticles include the following:
-
1.
Macrophages, which harbor the M.tb. cells, readily phagocytose such microparticles and thus significantly improve the uptake of the loaded drug (as compared to that by diffusion).
-
2.
The simultaneous intake of multiple drugs is recommended for TB therapy in order to prevent the emergence of drug resistance. Drugs can be easily incorporated with relatively high efficiency, and various drug release rates can be achieved via manipulations in the preparation procedure. The spray drying technology, in particular, has been widely utilized to formulate microparticles incorporating multiple hydrophilic and/or hydrophobic anti-TB drugs (Hirota and Terada 2014).
-
3.
The embedded drugs have slower release rates, and thereby longer durations of action.
-
4.
Therefore, such drug-loaded microparticles exhibit significantly greater in vitro and in vivo (in infected animal models) anti-TB activity than that observed by administration of an equivalent amount of drug(s) in soluble form (Hirota and Terada 2014).
-
5.
Such particles are more physiochemically stable, both in vitro and in vivo.
-
6.
A number of biodegradable microspheres have proved to be nontoxic, biocompatible, and non-immunogenic.
-
7.
Recent studies have shown that uptake of microparticles induces classical activation within M.tb.-infected macrophages (Verma et al. 2011).
Apart from synthetic polymers, several natural polymers, particularly saccharides, such as alginate, chitosan, and lactose, have been used to develop drug delivery systems for entrapping and delivering various therapeutic agents. Sodium alginate, a salt of alginic acid (linear copolymer of α-guluronic acid and α-mannuronic acid), has the ability to form a gel/meshwork in the presence of divalent cations, such as CaCl2. This gel is mucoadhesive, and is likely to adhere to mucosa for prolonged periods of time, thereby having the potential to release the drug in a sustained and controlled manner. Thus, alginate microparticles have been prepared as anti-TB drug carriers and studied as oral delivery systems for TB treatment (Qurrat-ul-Ain et al. 2003). In addition, leucine-containing microparticles have also found application for delivery of anti-TB drugs (Verma et al. 2013a; Garcia-Contreras et al. 2017).
Recently, the 2–4 μm hollow, porous, yeast-derived β-1, 3/1, 6 glucan particles (GPs) have been utilized for targeted payload delivery of anti-TB drugs to macrophages (Soto et al. 2010; Upadhyay et al. 2017). Particulate glucan is biodegradable and biocompatible polysaccharide that has been consumed for thousands of years, and has the GRAS (generally regarded as safe) status granted by FDA. The 𝛽-1,3-D glucan surface composition also permits its recognition by cell surface receptors on macrophages (via dectin-1 and Complement Receptor 3) and other phagocytic cells (Legentil et al. 2015). Such particles have been shown to incorporate “nanoprecipitates” or “nanoparticles” of anti-TB rifamycin drugs (RIF and RFB) within internal pore spaces, thus functioning as “nano-in-micro” particulate formulations. These particles are prepared by alkaline and acidic extraction of the cell wall of baker’s yeast (Saccharomyces cerevisiae) and have showed adequate thermal stability for pharmaceutical application.
Recently, novel carrier-free microparticles of anti-TB drugs, such as rifampicin (Parikh et al. 2014), have been developed and successfully evaluated as macrophage delivery systems against TB.
6.2 Nanotechnology for TB Treatment
Nanoparticles are colloidal structures composed of synthetic or semi-synthetic polymers. These can be formulated as monolithic nanoparticles (nanospheres) that embed the drug in the polymeric matrix, or nanocapsules, where the drug is confined within a hydrophobic or hydrophilic core surrounded by a definitive “capsule.” Nanoformulations of antibiotics enclosed within polymers, such as PLGA (Ahmad et al. 2008) and alginate (Ahmad et al. 2007) have been shown to be successful for the administration of anti-TB drugs within experimental, in vivo (animal) TB models. The use of nanotechnology in TB treatment has been expertly researched and reviewed in detail (Ahmad et al. 2007; Ahmad et al. 2011; Nasiruddin et al. 2017).
Other Nanotechnology-Based Formulations
Liposomes are concentric nano- to micro-sized vesicles in which an aqueous volume is enclosed by one or multiple phospholipid bilayers. The hydrophobic domain is utilized to entrap insoluble agents, and the core enables encapsulation of water-soluble drugs. Liposomal formulations have been developed with first- and/or second-line antibiotics and are proposed as alternative to current therapy (Shegokar et al. 2011). Intravenous administration of INH and RIF encapsulated in lung-specific stealth liposomes (liposomes with a polyethylene glycol-coated surface) showed enhanced affinity toward the lung tissue of mice and thus allowed the targeted delivery of these anti-TB drugs to lungs (Deol and Khuller 1997).
One caveat for liposome use is that, since the vesicles are degraded by intestinal lipases, they cannot be administered by oral route (Pinheiro et al. 2011). To circumvent this limitation, solid lipid nanoparticles (SLNs) and niosomes have been proposed as novel anti-TB drug delivery vehicles (Nasiruddin et al. 2017) that can be administered orally. SLNs are aqueous suspensions of nanocrystalline lipids, which have better encapsulation effectivity and increased ability to entrap hydrophobic or hydrophilic drugs when compared to liposomes and polymeric nanoparticles. While free drugs are rapidly cleared from circulation, SLN-loaded anti-TB drug(s) have been shown to have improved bioavailability and thus, are effective at reduced dosages and dosing frequency. Pandey et al. (2005) showed that 5 oral doses of drug-loaded SLNs on every tenth day were able to completely eradicate M.tb. H37Rv from the lungs and spleens of infected mice, whereas free drug needed the administration of 46 daily oral doses to achieve the same result.
Niosomes are composed of a surfactant bilayer, and are thus thermodynamically stable, colloidal, uni- or multi-lamellar (liposome-like) particles, formed by self-assembly of non-ionic surfactants and a hydrating mixture of cholesterol (Nasiruddin et al. 2017). Three polymers, Brij-35, Tween 80, and Span-80, have been used for pyrazinamide niosome formulation, of which span-80-based formulation exhibited the highest release (Thomas and Bagyalakshmi 2013).
Despite the fact that nano- and micro-particles are rapidly taken up by host macrophages, their appropriate delivery for sufficient in vivo efficacy is yet another challenge. Thus, the success of delivery vehicles is by and large dependent on their route of administration. In the following section, we will discuss the various routes of drug delivery used against TB, along with their advantages and disadvantages. We will also discuss how the pulmonary route of drug delivery could potentially minimize the evolution of resistant M.tb. strains and reduce the long treatment duration currently employed, thus aligning with the recent WHO guidance on having a shorter treatment regimen (Grace et al. 2018).
7 Drug Delivery Routes Explored in Humans
The oral route of anti-TB drug delivery is currently prescribed for long-term treatment, whereas the intravenous (IV) route is utilized where aggressive therapy is needed.
7.1 Oral Delivery
The oral route of drug administration is the most common route of anti-TB drug delivery, arising from the feasibility of long-term oral administration with high drug doses in TB-endemic regions. That being said, it becomes extremely challenging to adopt another administration route without compromising patient compliance. The prolonged treatment with multiple antibiotics leads to severe side effects, due to high systemic drug exposure and, paradoxically, sub-therapeutic drug levels in the host tissue where M.tb. resides. This is especially true for pulmonary TB, where the M.tb. hides in hard-to-penetrate lung granulomas (Muttil et al. 2009; Hickey et al. 2016) (Fig. 1a). While the second-line drugs are used in potential MDR-TB patients who do not achieve sputum conversion with first-line therapy, these antibiotics are less effective and more toxic than first-line therapy, resulting in poor patient compliance; non-compliance to the treatment regimen is regarded as the main reason for the generation of resistant M.tb. strains.
Drug concentration in various body compartments after (a) Oral delivery; (b) Parenteral delivery; and (c) Pulmonary delivery. Darker shade represents higher drug concentration. Note that the highest drug concentration in the lung is achieved by pulmonary delivery, followed by parenteral and oral delivery
7.2 Parenteral Delivery
Injectable anti-TB drugs are usually administered parenterally for second-line therapy, and include amikacin, kanamycin, and capreomycin. These agents are administered either by the intramuscular or the intravenous route. When compared to oral administration, the parenteral route achieves higher systemic drug levels (Fig. 1b), avoids first pass metabolism, and prevents GI toxicity. However, this route is also associated with painful daily injections, unacceptably high rates of ototoxicity, and renal toxicity, and requires the presence of a health care worker for drug administration. These concerns lead to poor patient compliance. In addition, parenteral administration generates needles that have the potential to be re-used if not properly disposed of (Mitragotri 2005).
7.3 Pulmonary Delivery
The pulmonary route of anti-TB drug delivery was first reported in the middle of the twentieth century, during the time when streptomycin resistance first developed (Miller et al. 1950; Hickey et al. 2016). Aerosol delivery allows for higher drug concentration in the lung, the organ that is first exposed to airborne M.tb. Since 80% of all TB cases manifested as pulmonary TB, direct lung delivery of anti-TB drugs could be a more systematic treatment strategy since it allows the drug to follow the path of M.tb. and achieve therapeutic drug levels in the niches where mycobacteria reside. As shown in Fig. 1c, aerosol delivery can potentially achieve therapeutic drug levels in the respiratory system (above MIC), and the large surface area of the lung, coupled with the thin alveolar epithelium and extensive pulmonary vascularization, allows for an overall increased systemic bioavailability of the drug (Dharmadhikari et al. 2013). The other advantages of pulmonary drug delivery include the noninvasive nature of the delivery route when compared to injection, the lower required dosages, and the potentially minimized M.tb. transmission from active TB patients to healthy individuals (Hickey et al. 2016; Pham et al. 2015). High drug concentration in the lung can also minimize M.tb. spreading to other extra-pulmonary organs from the lung. Taken together, these advantages are expected to result in better treatment outcomes, and a reduction in overall drug toxicity, when compared to oral and parenteral routes of administration. However, aerosol drug delivery alone may not be able to achieve sufficient drug concentrations in certain systemic compartments. This can be overcome by including aerosol drug delivery as an adjunct to existing oral or parenteral treatment regimens (Muttil et al. 2009; Hickey et al. 2016).
Particles below ~0.5 μm diameter are reported to be exhaled un-deposited in the lung, while those larger than 5 μm will get entangled in the upper airway and shut out by the lung’s defenses (Malcomson and Embleton 1998). Microparticles having an ideal size, specifically the median mass aerodynamic diameter (MMAD) (generally 0.5–5 μm), deposit into the deep alveoli of lungs, and can be then taken up by alveolar macrophage.
There have been occasional reports where inhaled anti-TB drugs were administered to TB patients or healthy individuals. Inhaled kanamycin was administered along with other anti-TB drugs to treat five patients with MDR-TB, and was well-tolerated, with sputum conversion reported in all patients within 60 days of treatment initiation (Turner et al. 1998). In another study, Sacks et al. treated patients harboring MDR-TB (12 patients) and drug-susceptible TB (7 patients) with inhaled aminoglycosides as an adjunct to conventional therapy. The results demonstrated that 13 of the 19 patients (6 of 7 with drug-susceptible TB, and 7 of 12 with MDR-TB) converted to smear-negative within a month of inhaled therapy (Sacks et al. 2001). These patients were smear and culture-positive after many months of treatment with conventional anti-TB therapy, demonstrating the importance of pulmonary drug delivery in achieving M.tb. clearance, especially in the lungs. In 2013, Dharmadhikari et al. conducted a phase I trial with inhaled, dry powder capreomycin as a treatment strategy against MDR-TB. Healthy adults were asked to self-administer 25, 75, 150, or 300 mg of capreomycin dry powder using a marketed dry powder inhaler (DPI) (Dharmadhikari et al. 2013). Dry powder capreomycin was well-tolerated by all subjects, with no changes in lung function observed. Further, peak and mean plasma drug concentrations were dose-proportional, and the systemic concentrations were achieved immediately after pulmonary delivery. Dry powder microparticles were thus delivered for the first time in humans as a possible treatment strategy against MDR-TB. Furthermore, dry powder microparticles, when delivered by the pulmonary route, have been studied and expertly reviewed for preclinical models (Muttil et al. 2007; Kaur et al. 2008; Muttil et al. 2009; Sharma et al. 2011; Verma et al. 2013a; Hickey et al. 2016; Garcia-Contreras et al. 2017; Parumasivam et al. 2016).
8 Challenges
Studies in the last half century have shown that the challenge of successful prolonged TB treatment with multiple high dose drugs with toxic side effects can be met by micro-particulate formulations containing multiple drugs. While pulmonary drug delivery has been shown to be a particularly effective therapeutic approach, it has some challenges that need to be overcome before it can become a mainstream treatment strategy against TB in humans, especially in resource-poor countries. The pulmonary route of drug delivery is more complex than the oral and parenteral routes, and certain considerations, such as the patient’s age, general health conditions, breathing capacity and lung physiology, formulation characteristics, inhaler performance, and the reliability of the patient to correctly and consistently use the inhaler device, are some of the concerns that need to be addressed before an inhalation product can become successful for the treatment of TB. Since TB treatment requires chronic use of multiple drugs for at least 6 months, the ability of the patient to adhere to daily inhaler use is vital. Patient compliance can be initially implemented under the guidance of a health care provider who oversees their inhaler use, thereby training and preparing the patient for good compliance in the remaining treatment duration. Further, the expectation to treat TB using pulmonary drug delivery is to shorten the treatment regimen that would further improve patient compliance (Uplekar et al. 2015).
The cost and availability of inhalers in low- and middle-income countries is another hurdle that must be overcome by governmental agencies and pharmaceutical companies. Aerosol treatment against TB has been delivered using both nebulizers and DPIs in patients. Nebulizers, usually air-jet or ultrasonic, emit micron-sized droplets of solutions or suspensions. However, the ability to formulate multiple anti-TB drugs in a single solution or suspension, the challenges of keeping them stable for at least 6 months without refrigeration, and the high cost of the devices themselves, limit their applicability for effective TB therapy in resource-poor countries (Hanif and Garcia-Contreras 2012). DPIs, on the other hand, include the dry powder formulation, usually in a capsule, and the inhaler device. In the case of DPIs, the powder formulation and device are evaluated together in clinical trials, and are considered drug products by regulatory authorities (Price et al. 2018). DPIs have the advantage of product stability over liquid formulations, given that the drug is powdered. This becomes critical in regions of the world where the temperature-controlled supply chain is inadequate (Kunda et al. 2016; Parumasivam et al. 2016). Further, dry powder formulations can potentially incorporate multiple anti-TB drugs in a single formulation, and DPI devices have increased portability over nebulizers (Chan et al. 2014; Hou et al. 2015).
Lastly, regulatory agencies around the world need to be on board for pulmonary drug delivery to become a common treatment modality against TB. The highly controlled regulatory environment, especially encountered in developed countries, has been a hindrance for the widespread use of inhaled therapy against TB. Thus, pharmaceutical companies and device manufacturers should consider the regional patient population before developing the final product. This requires sufficient clinical data to be generated using the device in the regions of the world where the treatment will ultimately be implemented. Another obstacle is that approximately 20% of TB cases are diagnosed as extrapulmonary pathologies (Lee 2015), and with inhaled therapy alone, there is a risk of inducing resistance due to sub-therapeutic drug concentrations outside of the lungs. Therefore, inhalation therapy will require the standardization of inhaled doses and the proof of systemic drug concentrations above the MIC for M.tb. (Fig. 1c) in order to convince the regulatory agencies that this novel route of drug delivery will not exacerbate drug-resistant M.tb. strains. This will require the use of multiple anti-TB drugs in a single formulation, the possible use concurrent conventional therapy, and the proper optimization of the pharmacokinetic and pharmacodynamic parameters of all delivered drugs that could potentially lead to better clinical outcomes with pulmonary administration (Hickey et al. 2016).
9 Conclusions and Future Perspective
The increasing resistance to the existing drugs, coupled with the recent acquisition of resistance to the two newest anti-TB drugs approved by the FDA for MDR-TB and XDR-TB treatment (Zumla et al. 2014), poses a serious threat to TB control across the world. The ultimate hope with pulmonary drug delivery is to implement a more-optimal treatment strategy against TB in comparison to the status quo, which has been unsuccessful in controlling M.tb. antibiotic resistance development. The highly stable, inhalable dry powder microparticles, containing multiple drugs in a single formulation, have exhibited encouraging results and possess the significant potential to address MDR-TB. Furthermore, future studies are expected to provide evidence that combining pulmonary drug delivery with conventional oral treatment could ensure that therapeutic drug concentrations are achieved in different biological compartments within the patient in order to treat both pulmonary and extra-pulmonary TB. Therefore, inhalation therapy needs to be granted the appropriate amount of attention, possibly as an adjunct therapy to conventional oral therapy, for successful translation of these preclinical studies for the effective control of TB.
References
Ahmad, S. (2010). Pathogenesis, immunology, and diagnosis of latent Mycobacterium tuberculosis infection. Clinical & Developmental Immunology, 2011, 814943.
Ahmad, Z., Sharma, S., & Khuller, G. K. (2007). Chemotherapeutic evaluation of alginate nanoparticle-encapsulated azole antifungal and antitubercular drugs against murine tuberculosis. Nanomedicine: Nanotechnology, Biology and Medicine, 3(3), 239–243.
Ahmad, Z., Pandey, R., Sharma, S., & Khuller, G. K. (2008). Novel chemotherapy for tuberculosis: Chemotherapeutic potential of econazole-and moxifloxacin-loaded PLG nanoparticles. International Journal of Antimicrobial Agents, 31, 142–146.
Ahmad, Z., Maqbool, M., & Raja, A. F. (2011). Nanomedicine for tuberculosis: Insights from animal models. International Journal of Nano Dimension, 2(1), 67–84.
Ahmed, M. M., Velayati, A. A., & Mohammed, S. H. (2016). Epidemiology of multidrug-resistant, extensively drug resistant, and totally drug resistant tuberculosis in Middle East countries. International Journal of Mycobacteriology, 5(3), 249–256.
Chan, J. G. Y., Wong, J., Zhou, Q. T., Leung, S. S. Y., & Chan, H. K. (2014). Advances in device and formulation technologies for pulmonary drug delivery. AAPS PharmSciTech, 15(4), 882–897.
D’Ambrosio, L., Centis, R., Tiberi, S., Tadolini, M., Dalcolmo, M., Rendon, A., et al. (2017). Delamanid and bedaquiline to treat multidrug-resistant and extensively drug-resistant tuberculosis in children: A systematic review. Journal of Thoracic Disease, 9(7), 2093–2101.
Deol, P., & Khuller, G. K. (1997). Lung specific stealth liposomes: Stability, biodistribution and toxicity of liposomal antitubercular drugs in mice. Biochimica et Biophysica Acta (BBA) – General Subjects, 1334(2–3), 161–172.
Deretic, V., Via, L. E., Fratti, R. A., & Deretic, D. (1997). Mycobacterial phagosome maturation, Rab proteins and intracellular trafficking. Electrophoresis, 18(14), 2542–2547.
Dharmadhikari, A. S., Kabadi, M., Gerety, B., Hickey, A. J., Fourie, P. B., & Nardell, E. (2013). Phase I, single-dose, dose-escalating study of inhaled dry powder capreomycin: A new approach to therapy of drug-resistant tuberculosis. Antimicrobial Agents and Chemotherapy, 57(6), 2613–2619.
Fernstrom, A., & Goldblatt, M. (2013). Aerobiology and its role in the transmission of infectious diseases. J Pathogens, 2013, 493960.
Flannagan, R. S., Jaumouille, V., & Grinstein, S. (2012). The cell biology of phagocytosis. Annual Review of Pathology, 7, 61–98.
Garcia-Contreras, L., Padilla-Carlin, D. J., Sung, J., VerBerkmoes, J., Muttil, P., Elbert, K., & Hickey, A. (2017). Pharmacokinetics of ethionamide delivered in spray-dried microparticles to the lungs of Guinea pigs. Journal of Pharmaceutical Sciences, 106(1), 331–337.
Grace, A. G., Mittal, A., Jain, S., Tripathy, J. P., Satyanarayana, S., Tharyan, P., & Kirubakaran, R. (2018). Shortened treatment regimens versus the standard regimen for drug-sensitive pulmonary tuberculosis. The Cochrane Library.
Guirado, E., Schlesinger, L. S., & Kaplan, G. (2013, September). Macrophages in tuberculosis: Friend or foe. In: Seminars in immunopathology (Vol. 35, No. 5, pp. 563–583). Springer: Berlin/Heidelberg.
Hanif, S. N. M., & Garcia-Contreras, L. (2012). Pharmaceutical aerosols for the treatment and prevention of tuberculosis. Frontiers in Cellular and Infection Microbiology, 2, 118.
Hickey, A. J., Durham, P. G., Dharmadhikari, A., & Nardell, E. A. (2016). Inhaled drug treatment for tuberculosis: Past progress and future prospects. Journal of Controlled Release, 240, 127–134.
Hirota, K., & Terada, H. (2014). Particle-manufacturing technology-based inhalation therapy for pulmonary diseases. In H. Ohshima & K. Makino (Eds.), Colloid and interface science in pharmaceutical research and development (Vol. 2014, Ist ed., pp. 103–119). Oxford: Elsevier.
Hoffmann, H., Kohl, T. A., Hofmann-Thiel, S., Merker, M., Beckert, P., Jaton, K., Nedialkova, L., Sahalchyk, E., Rothe, T., Keller, P. M., & Niemann, S. (2016). Delamanid and bedaquiline resistance in Mycobacterium tuberculosis ancestral Beijing genotype causing extensively drug-resistant tuberculosis in a Tibetan refugee. American Journal of Respiratory and Critical Care Medicine, 193(3), 337–340.
Hou, S., Wu, J., Li, X., & Shu, H. (2015). Practical, regulatory and clinical considerations for development of inhalation drug products. Asian Journal of Pharmaceutical Sciences, 10(6), 490–500.
Kahnert, A., Seiler, P., Stein, M., Bandermann, S., Hahnke, K., Mollenkopf, H., & Kaufmann, S. H. (2006). Alternative activation deprives macrophages of a coordinated defense program to Mycobacterium tuberculosis. European Journal of Immunology, 36(3), 631–647.
Kaur, J., Muttil, P., Verma, R. K., Kumar, K., Yadav, A. B., Sharma, R., & Misra, A. (2008). A hand-held apparatus for “nose-only” exposure of mice to inhalable microparticles as a dry powder inhalation targeting lung and airway macrophages. European Journal of Pharmaceutical Sciences, 34(1), 56–65.
Kendall, E. A., Azman, A. S., Cobelens, F. G., & Dowdy, D. W. (2017). MDR-TB treatment as prevention: The projected population-level impact of expanded treatment for multidrug-resistant tuberculosis. PLoS One, 12(3), e0172748.
Kerantzas, C. A., & Jacobs, W. R. (2017). Origins of combination therapy for tuberculosis: Lessons for future antimicrobial development and application. MBio, 8(2), e01586-16.
Kunda, N. K., Wafula, D., Tram, M., Wu, T. H., & Muttil, P. (2016). A stable live bacterial vaccine. European Journal of Pharmaceutics and Biopharmaceutics, 103, 109–117.
Kvasnovsky, C. L., Peter, C. J., & Van dW. M. L. (2016). Treatment outcomes for patients with extensively drug-resistant tuberculosis, KwaZulu-Natal and Eastern cape provinces, South Africa. Emerging Infectious Diseases, 22(9), 1529–1536.
Lee, J. Y. (2015). Diagnosis and treatment of extrapulmonary tuberculosis. Tuberculosis Respiratory Disease, 78(2), 47–55.
Legentil, L., Paris, F., Ballet, C., Trouvelot, S., Daire, X., Vetvicka, V., & Ferrières, V. (2015). Molecular interactions of β-(1→ 3)-glucans with their receptors. Molecules, 20(6), 9745–9766.
Malcomson, R. J., & Embleton, J. K. (1998). Dry powder formulations for pulmonary delivery. Pharmaceutical Science & Technology Today, 1(9), 394–398.
Matteelli, A., Roggi, A., & Carvalho, A. C. (2014). Extensively drug-resistant tuberculosis: Epidemiology and management. Clinical Epidemiology, 6, 111–118.
Miller, J. B., Abramson, H. A., & Ratner, B. (1950). Aerosol streptomycin treatment of advanced pulmonary tuberculosis in children. American Journal of Diseases of Children, 80(2), 207–237.
Mitragotri, S. (2005). Immunization without needles. Nature Reviews. Immunology, 5(12), 905–916.
Muttil, P., Kaur, J., Kumar, K., Yadav, A. B., Sharma, R., & Misra, A. (2007). Inhalable microparticles containing large payload of anti-tuberculosis drugs. European Journal of Pharmaceutical Sciences, 32(2), 140–150.
Muttil, P., Wang, C., & Hickey, A. J. (2009). Inhaled drug delivery for tuberculosis therapy. Pharmaceutical Research, 26(11), 2401–2416.
Nasiruddin, M., Neyaz, M., & Das, S. (2017). Nanotechnology-based approach in tuberculosis treatment. Tuberculosis Research and Treatment, 2017. Article ID 4920209, 12 (Review).
O’Garra, A., Redford, P. S., McNab, F. W., Bloom, C. I., Wilkinson, R. J., & Berry, M. P. (2013). The immune response in tuberculosis. Annual Review of Immunology, 31, 475–527.
Pai, M., Behr, M. A., Dowdy, D., Dheda, K., Divangahi, M., Boehme, C. C., Ginsberg, A., Swaminathan, S., Spigelman, M., Getahun, H., Menzies, D., & Raviglione, M. (2016). Tuberculosis. Nature Reviews. Disease Primers, 2, 16076. 1–23.
Pandey, R., Sharma, S., & Khuller, G. K. (2005). Oral solid lipid nanoparticle-based antitubercular chemotherapy. Tuberculosis, 85(5–6), 415–420.
Parikh, R., Patel, L., & Dalwadi, S. (2014). Microparticles of rifampicin: Comparison of pulmonary route with oral route for drug uptake by alveolar macrophages, phagocytosis activity and toxicity study in albino rats. Drug Delivery, 21(6), 406–411.
Parumasivam, T., Chang, R. Y. K., Abdelghany, S., Ye, T. T., Britton, W. J., & Chan, H. K. (2016). Dry powder inhalable formulations for anti-tubercular therapy. Advanced Drug Delivery Reviews, 102, 83–101.
Petersen, E., Maeurer, M., Marais, B., Migliori, G. B., Mwaba, P., Ntoumi, F., et al. (2017). World TB day 2017: Advances, challenges and opportunities in the “end-TB” era. International Journal of Infectious Diseases, 56, 1–5.
Pham, D. D., Fattal, E., & Tsapis, N. (2015). Pulmonary drug delivery systems for tuberculosis treatment. International Journal of Pharmaceutics, 478(2), 517–529.
Pinheiro, M., Lúcio, M., Lima, J. L., & Reis, S. (2011). Liposomes as drug delivery systems for the treatment of TB. Nanomedicine, 6(8), 1413–1428.
Prasad, R., Singh, A., Balasubramanian, V., & Gupta, N. (2017). Extensively drug-resistant tuberculosis in India: Current evidence on diagnosis & management. The Indian Journal of Medical Research, 145(3), 271–293.
Price, D. N., Kunda, N. K., & Muttil, P. (2018). Challenges associated with the pulmonary delivery of therapeutic dry powders for preclinical testing. Kona Powder and Particle Journal, 36, 2019008.
Qurrat-ul-Ain, Sharma, S., Khuller, G., & Garg, S. K. (2003). Alginate based oral drug delivery system for tuberculosis: Pharmacokinetics and therapeutic effects. The Journal of Antimicrobial Chemotherapy, 51(4), 931–938.
Raviglione, M., & Sulis, G. (2016). Tuberculosis 2015: Burden, challenges and strategy for control and elimination. Infectious Disease Reports, 8(2), 6570.
Rom, W. N., & Garay, S. M. (2003). Tuberculosis (2nd ed.). Philadelphia, PA: Lippincott Williams & Wilkins.
Sacks, L. V., Pendle, S., Orlovic, D., Andre, M., Popara, M., Moore, G., Thonell, L., & Hurwitz, S. (2001). Adjunctive salvage therapy with inhaled aminoglycosides for patients with persistent smear-positive pulmonary tuberculosis. Clinical Infectious Diseases, 32(1), 44–49.
Sen, H., Jayanthi, S., Sinha, R., Sharma, R., & Muttil, P.. (2003). Inhalable biodegradable microparticles for target-specific drug delivery in tuberculosis and a process thereof. PCT/IB03/04694.
Sharma, R., Saxena, D., Dwivedi, A. K., & Misra, A. (2001). Inhalable microparticles containing drug combinations to target alveolar macrophages for treatment of pulmonary tuberculosis. Pharmaceutical Research, 18(10), 1405–1410.
Sharma, R., Yadav, A. B., Muttil, P., Kajal, H., & Misra, A. (2011). Inhalable microparticles modify cytokine secretion by lung macrophages of infected mice. Tuberculosis, 91(1), 107–110.
Shegokar, R., Al Shaal, L., & Mitri, K. (2011). Present status of nanoparticle research for treatment of tuberculosis. Journal of Pharmacy & Pharmaceutical Sciences, 14(1), 100–116.
Sotgiu, G., Centis, R., D’ambrosio, L., & Migliori, G. B. (2015). Tuberculosis treatment and drug regimens. Cold Spring Harbor Perspectives in Medicine, 5(5), a017822.
Soto, E., Kim, Y. S., Lee, J., Kornfeld, H., & Ostroff, G. (2010). Glucan particle encapsulated rifampicin for targeted delivery to macrophages. Polymers, 2(4), 681–689.
Thomas, S., & Bagyalakshmi, J. (2013). Design, development and characterization of pyrazinamide niosomal dosage form. American Journal of PharmTech Research, 3(6), 532–544.
Tiberi, S., Scardigli, A., Centis, R., D’Ambrosio, L., Munoz-Torrico, M., Salazar-Lezama, M. A., Spanevello, A., Visca, D., Zumla, A., Migliori, G. B., & Luna, J. A. C. (2017). Classifying new anti-tuberculosis drugs: Rationale and future perspectives. International Journal of Infectious Diseases, 56, 181–184.
Turner, M. T., Haskal, R., McGowan, K., Nardell, E., & Sabbag, R. (1998). Inhaled kanamycin in the treatment of multidrug-resistant tuberculosis: A study of five patients. Infectious Diseases in Clinical Practice, 7(1), 49–53.
Upadhyay, T. K., Fatima, N., Sharma, D., Saravanakumar, V., & Sharma, R. (2017). Preparation and characterization of beta-glucan particles containing a payload of nanoembedded rifabutin for enhanced targeted delivery to macrophages. EXCLI Journal, 16, 210–228.
Uplekar, M., Weil, D., Lonnroth, K., Jaramillo, E., Lienhardt, C., Dias, H. M., et al. (2015). WHO’s new end TB strategy. The Lancet, 385, 1799–1801.
Verma, R. K., Singh, A. K., Mohan, M., Agrawal, A. K., & Misra, A. (2011). Inhaled therapies for tuberculosis and the relevance of activation of lung macrophages by particulate drug-delivery systems. Therapeutic Delivery, 2(6), 753–768.
Verma, R. K., Germishuizen, W. A., Motheo, M. P., Agrawal, A. K., Singh, A. K., Mohan, M., Gupta, P., Gupta, U. D., Cholo, M., Anderson, R., Fourie, P. B., & Fourie, P. B. (2013a). Inhaled microparticles containing clofazimine are efficacious in treatment of experimental tuberculosis in mice. Antimicrobial Agents and Chemotherapy, 57(2), 1050–1052.
Verma, R., Khanna, P., & Mehta, B. (2013b). Revised national tuberculosis control program in India: The need to strengthen. International Journal of Preventive Medicine, 4(1), 1–5.
WHO. (2017). Guidelines for treatment of drug-susceptible tuberculosis and patient care (2017 update). http://www.who.int/tb/publications/2017/dstb_guidance_2017/en/. Accessed 25 Mar 2018.
World Health Organization. Global tuberculosis report 2017. WHO/HTM/TB/2017.23. Geneva: World Health Organization. 2017, 21–63.
Zumla, A. I., Gillespie, S. H., Hoelscher, M., Philips, P. P., Cole, S. T., Abubakar, I., McHugh, T. D., Schito, M., Maeurer, M., & Nunn, A. J. (2014). New antituberculosis drugs, regimens, and adjunct therapies: Needs, advances, and future prospects. The Lancet Infectious Diseases, 14(4), 327–340.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Upadhyay, T.K., Sharma, A., Fatima, N., Singh, A., Muttil, P., Sharma, R. (2019). Targeted Delivery of Antibiotics Using Microparticles to Combat Multidrug-Resistant Tuberculosis. In: Ahmad, I., Ahmad, S., Rumbaugh, K. (eds) Antibacterial Drug Discovery to Combat MDR. Springer, Singapore. https://doi.org/10.1007/978-981-13-9871-1_20
Download citation
DOI: https://doi.org/10.1007/978-981-13-9871-1_20
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-9870-4
Online ISBN: 978-981-13-9871-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)