Abstract
Since the 1950s, reactors using algal-bacterial polycultures have been engineered by researchers to treat wastewater. The symbiotic relationship between algae and bacteria accommodates energy-efficient wastewater treatment by eliminating artificial aeration. Here, we investigated the applicability of a novel algal-based system using two polycultures to treat primary-settled municipal wastewater under field conditions in a winter climate (average daily temperature: 4–16.5 °C). The two polycultures were cultured in two closed 700L bioreactors deployed at a local wastewater treatment plant in Las Cruces, NM, United States. The Ammoniacal nitrogen and phosphate concentrations and OD750 of the two reactors were analyzed daily. The BOD5 in the reactors were analyzed once in two days. The samples from the two reactors were analyzed over 44 days from January to March in 2018. The culture in reactor 1 attained average ammoniacal nitrogen, phosphate, and BOD5 removals of 99.3%, 70.1%, and 70.0% respectively in a single step within five days. The corresponding efficiencies for reactor 2 were 44.3%, 47.5%, and 50.7%. The average batch processing times for reactor 1 and reactor 2 were 1.02 and 4.02 days respectively. This paper confirms the ability of both the polycultures to treat primary-settled wastewater in a single step.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Wastewater treatment utilities have continued to rely on energy-intensive and unsustainable technologies for removing nutrients (nitrogen and phosphorus) from wastewaters to the required discharge standards. Researchers have been looking for alternative wastewater treatment technologies to conserve energy and recover nutrients from wastewaters to render the process sustainable and cost-effective [1]. On realizing the capability of algae to uptake nutrients while growing photosynthetically, several studies have evaluated the feasibility of using algae in wastewater treatment systems. Since the premise to use an algae-bacteria mixed polyculture for wastewater treatment was tested in 1950s [2], many studies have been conducted to develop engineered systems with algae-bacteria cultures to treat industrial and domestic wastewaters [3,4,5,6]. The symbiotic relationship between algae and bacteria has made energy efficient wastewater treatment possible in solar-powered photobioreactors [1].
Microalgae and bacteria have been shown to coexist in culture while mutually benefiting each other. With the presence of sunlight, microalgae photosynthetically produce oxygen which is used by aerobic bacteria to biodegrade organic matter. The algae then consume the carbon dioxide released by bacterial respiration whereby eliminating artificial aeration [1, 6]. More recently, the ability of algal species such as Galdieria sulphuraria [7, 8] to mixotrophically metabolize organic carbon and nutrients in a single step to meet the respective discharge standards has been recognized. Since most of the ammoniacal nitrogen and phosphorus in wastewater accumulate in algal cells [2, 6], such a mixotrophic system can achieve efficient and economical wastewater treatment, while generating biomass that can be processed downstream to recover the nutrients as crop fertilizers [6] or recover energy in the form of biocrude [9, 10].
An algae-based, Photosynthetically Oxygenated Waste to Energy Recovery (POWER) system has been developed at New Mexico State University (NMSU) for energy-efficient wastewater treatment and resource recovery. An axenic culture capable of mixotrophic metabolism, G. sulphuraria, used in this system has demonstrated superior carbon and nutrient removal where the culture temperatures are near its optimal range of 27–46 °C [7, 8]. Since the temperature drops in winter, G. sulphuraria loses its treatment efficiency. Hence, finding an adequate culture replacement for winter had been a long-standing quest. The studies that have been done so far on urban wastewater (UWW) treatment with microalgae in winter climate are very limited. Therefore, a polyculture that could sustain the cold weather was selected assuming it could treat urban wastewater as efficient as by G. sulphuraria during summer.
This study evaluated the feasibility of two polycultures to accommodate winter treatment in the POWER system. The two polycultures were assumed to consist of two different algal-bacterial compositions. The specific objective of the study was to investigate the removal efficiency of dissolved organic carbon and nutrients by the two polycultures in a winter climate.
2 Materials and Methods
2.1 Microalgae Strain and Cultivation Methods
Polycultures obtained from the Fabian Garcia Research Facility (New Mexico State University) were grown in two pilot scale photobioreactors deployed at a local wastewater treatment facility, Las Cruces, NM, USA. The bioreactor was fed with primary-settled wastewater. Unlike previous studies on the cultivation of G. sulphuraria, the addition of growth mediums of any kind or enrichment of headspace with carbon dioxide was not done during this study. The initial pH in the reactors was not chemically adjusted.
2.2 Equipment Used and Fed-Batch Cultivation
The experiments were conducted in two identical enclosed photobioreactors (R1 and R2) fabricated from a thin clear polymer allowing for light penetration and distribution. The enclosed design enabled the prevention of evaporation and contamination, and conserved heat inside the reactor. The culture depth was maintained at 20 cm and circulation and mixing in the reactor were controlled by a motor-driven paddle wheel.
The reactor was operated in a fed-batch mode, with a working volume of 700L, which comprised of 400L of primary effluent and 300L of culture. Each fed-batch cycle was terminated when the reactor reached the stipulated discharge standards for all the three primary pollutants: ammoniacal nitrogen - 10 mg/L; phosphate - 1 mg/L; and BOD5 - 30 mg/L. The paddle wheel was then switched off for 24 h to settle the biomass, and 400L of the resulting supernatant was discharged and replenished with a fresh batch of 400L of primary-settled wastewater. These fed-batch experiments were conducted continuously for 44 days from January through March in 2018.
Instead of direct measurement of the dry density, the biomass concentration in the reactor was analyzed daily in terms of optical density at 750 nm wavelength (OD 750) with a HACH DR 6000 spectrophotometer. Ammoniacal nitrogen and phosphate concentrations were also analyzed daily using the same equipment following the salicylate method 8155 and the phosver3 method 8048 respectively. Dissolved BOD5 was measured every two days with the standard method 5210 B. Furthermore, the temperatures in each reactor and in the field were recorded at an interval of 15 min using Onset HOBO data loggers (UA-002-64).
2.3 Summary of Operation Conditions
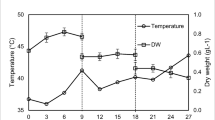
The temporal variation of daily average temperatures is illustrated in Fig. 1. The average daily temperature in the reactor and in the atmosphere ranged between 8–21 °C and 4–16.5 °C respectively. The concentrations of ammoniacal nitrogen, phosphate, and BOD5 in the primary effluent fed to the bioreactors ranged between 19.8–40.3 mg/L, 0.6–2.1 mg/L, and 39.5–70 mg/L respectively.
3 Results and Discussion
3.1 Growth, PH, and Reactor Stability
The temporal variation of biomass growth is illustrated in Fig. 2. The two reactors R1 and R2 maintained growth rates of 0.016 ± 0.001 and 0.014 ± 0.001 OD 750 nm day−1 respectively. The hypothesis test (H0: ß1 = ß2 vs. Ha: ß1 ≠ ß2) to check equality of the two slopes concluded that there is no statistically significant difference between growth rates at a significance level of 0.05 (P ≃ 0.88). However, both the reactors in the current study seemed to experience a lag phase compared to the previous results on the POWER system [11] with G. sulphuraria during summer time.
As the cultures used in this experiment endured the cold climate, stable operation of both reactors was possible. Unlike in the case of G. sulphuraria, the photobioreactors were neither supplemented with CO2 nor acidified. The pH variation in the two setups during the study period is shown in Fig. 3. As can be seen, the pH of reactor 2 was slightly higher than that of reactor 1; however, the overall pH increased in both reactors as the treatment cycle progressed. As explained by the following equation, the increase of pH can be ascribed to the uptake of photosynthetic carbon by microalgae from the surrounding which promotes the release of hydroxyl ions to the medium [12, 13].
3.2 Nutrient Removal
The temporal variation of the ammoniacal nitrogen profile during the study period is illustrated in Fig. 4. As seen in the plot, both reactors achieved the discharge standard of 10 mg/L at the end of the operation cycle. For the 8 cycles, the average volumetric nitrogen removal in reactor 1 was 5.34 ± 2.97 mg L−1 day−1 with 99.3% removal. The corresponding parameters for reactor 2 were 1.78 ± 1.03 mg L−1 day−1 and 44.3% respectively.
The temporal profile of phosphate concentration in the two reactors along with the allowable discharge limit (1 mg/L) is shown in Fig. 4. The average volumetric phosphate removal considering 8 operation cycles for reactor 1 was 0.11 ± 0.09 mg L−1 day−1 with 70.1% removal. The corresponding values for reactor 2 were 0.07 ± 0.06 mg L−1 day−1 and 47.5% respectively. The lower removal rates could be due to the lower average phosphate concentration observed during winter (1.26 mg/L) compared to that in summer (3.74 mg/L). Furthermore, the instantaneous phosphate removal rates on the initial day immediately after feeding primary effluent to reactor 1 and reactor 2 were as high as 0.92 ± 0.54 mg L−1 day−1, 0.80 ± 0.66 mg L−1 day−1 respectively. The phosphate concentration of primary wastewater dropped instantaneously by 67.8% and 52.6% on average after been mixed with the preadapted cultures in R1 and R2. This drop was calculated considering the difference of phosphate concentrations in the primary-settled wastewater and in the reactor on day 0. Apart from the dilution effect, this pronounced drop of phosphorus concentration can also be attributed to adsorption of phosphorus to the surface of cells and the surface of bioreactors [12]. Furthermore, the subsequent rise of phosphorus concentration in the reactors could partially be due to the release of phosphorus by ruptured cells as described by [12]. This phenomenon is common with old cultures, therefore, the subsequent rise of phosphorus concentrations in reactor one during the last two cycles can be explained [14] (Fig. 5).
3.3 Carbon Removal
The temporal profile of organic carbon during the study period is illustrated in Fig. 6. As can be noted, both reactors achieved the permissible discharge level by the end of the cycle time. Considering the 8 operation cycles, the average volumetric BOD removal for reactor 1 was 12.93 ± 6.53 mg L−1 day−1 with 70.0% removal; the corresponding values for reactor 2 were 6.77 ± 3.51 mg L−1 day−1 and 50.7%.
The required minimum cycle time to achieve all three discharge standards for each cycle is demonstrated in Fig. 7. The average cycle time in reactor 2 (4.02 days) appeared nearly 4-fold higher compared with that in reactor 1 (1.02 days)
4 Comparison of Previous Studies
The availability of literature for studies conducted with algal-bacterial cultures during cold climates (5–15 °C) is limited due to the widely experienced performance limitations of such systems in extreme environments. A study conducted by [15] using cyanobacterial strains isolated from the Arctic and Antarctic environments reported maximum nitrogen and phosphorus removal efficiencies of 2.3 mg NL−1d−1 and 0.07–0.15 mg PL−1d−1 at 15 °C under laboratory conditions. The maximum nitrogen removal rate reported in the above study is higher compared to the average nitrogen removal rate in reactor 2 (1.78 mg L−1 day−1). However, reactor 1 showed a higher average removal rate (5.43 mg L−1 day−1) under field conditions (average culture temperature: 8.45–21.25 °C). The average phosphate removal rates were however similar to the highest phosphorus removal rates reported by [15].
[16] reported the average batch processing times for experiments conducted in warmer temperatures (culture temperature: 24–36 °C) with G. sulphuraria Soos strain, G. sulphuraria 5587.1 and, mixed (a mixture of 5587.1 & Soos) as 4.62 days, 3.87 days and 4.16 days respectively. The batch processing time reported in reactor 2 was similar to those reported in the above study. However, the culture in reactor 1 managed to reach the discharge standards in lesser time (1.02 days) despite the cold climate.
5 Conclusion
The study evaluated the suitability of two polycultures to serve in the POWER system during cold temperatures. A summary of the research findings from January to March is documented below:
-
Average removal efficiencies of BOD5, ammoniacal nitrogen, and phosphate respectively by 70.0%, 99.3% and 70.1% by reactor 1 and 50.7%, 44.3% and 47.5% by reactor 2.
-
Both reactors remained stable despite the cold weather. reactor 1 and 2 reached the mandated discharge standards respectively in 1.02 days and 4.02 days.
-
The initial phosphate concentration in the POWER system drops rapidly. This could both be due to the dilution effect and surface adsorption.
-
Carbon dioxide and nutrients were not supplied to support the growth. Therefore, winter operation of the POWER system with the polycultures can be more economical even compared to the summer operation with G. sulphuraria.
Based on the results, a polyculture with a similar composition as in reactor 1 is recommended for winter operations of the POWER system.
References
de Godos I, Blanco S, García-Encina PA, Becares E, Muñoz R (2009) Long-term operation of high rate algal ponds for the bioremediation of piggery wastewaters at high loading rates. Bioresour Technol 100:4332–4339. https://doi.org/10.1016/J.BIORTECH.2009.04.016
Oswald WJ, Gotaas HB, Golueke CG, Kellen WR, Gloyna EF, Hermann ER (1957) Algae in waste treatment [with discussion]. Sewage Ind Wastes 29:437–457. http://www.jstor.org/stable/25033322
Muñoz R, Guieysse B (2006) Algal–bacterial processes for the treatment of hazardous contaminants: a review. Water Res 40:2799–2815. https://doi.org/10.1016/J.WATRES.2006.06.011
García J, Mujeriego R, Hernández-Mariné M (2000) High rate algal pond operating strategies for urban wastewater nitrogen removal. J Appl Phycol 12:331–339. https://doi.org/10.1023/A:1008146421368
Bordel S, Guieysse B, Muñoz R (2009) Mechanistic model for the reclamation of industrial wastewaters using algal–bacterial photobioreactors. Environ Sci Technol 43:3200–3207. https://doi.org/10.1021/es802156e
Su Y, Mennerich A, Urban B (2011) Municipal wastewater treatment and biomass accumulation with a wastewater-born and settleable algal-bacterial culture. Water Res 45:3351–3358. https://doi.org/10.1016/J.WATRES.2011.03.046
Selvaratnam T, Henkanatte-Gedera SM, Muppaneni T, Nirmalakhandan N, Deng S, Lammers PJ (2016) Maximizing recovery of energy and nutrients from urban wastewaters. Energy 104:16–23. https://doi.org/10.1016/j.energy.2016.03.102
Henkanatte-Gedera SM, Selvaratnam T, Karbakhshravari M, Myint M, Nirmalakhandan N, Van Voorhies W, Lammers PJ (2017) Removal of dissolved organic carbon and nutrients from urban wastewaters by Galdieria sulphuraria: laboratory to field scale demonstration. Algal Res 24:450–456. https://doi.org/10.1016/J.ALGAL.2016.08.001
Mehrabadi A, Craggs R, Farid MM (2015) Wastewater treatment high rate algal ponds (WWT HRAP) for low-cost biofuel production. Bioresour Technol 184:202–214. https://doi.org/10.1016/J.BIORTECH.2014.11.004
Park JBK, Craggs RJ, Shilton AN (2013) Enhancing biomass energy yield from pilot-scale high rate algal ponds with recycling. Water Res 47:4422–4432. https://doi.org/10.1016/J.WATRES.2013.04.001
Tchinda D, Henkanatte-Gedera SM, Abeysiriwardana-Arachchige ISA, Delanka-Pedige HMK, Munasinghe-Arachchige SP, Zhang Y, Nirmalakhandan N (2019) Single-step treatment of primary effluent by Galdieria sulphuraria: removal of biochemical oxygen demand, nutrients, and pathogens. Algal Res 42:101578. https://doi.org/10.1016/J.ALGAL.2019.101578
Martínez M, Sánchez S, Jiménez F, El Yousfi L, Muñoz L (2000) Nitrogen and Phosphorus removal from urban wastewater by the microalga Scenedesmus obliquus. Bioresour Technol 73:263–272. https://doi.org/10.1016/S0960-8524(99)00121-2
Axelsson L (1988) Changes in pH as a measure of photosynthesis by marine macroalgae. Mar Biol 294:287–294. https://doi.org/10.1007/BF00391314
Droop M (1975) The nutrient status of algal cells in batch culture. J Mar Biol Assoc UK 55(3):541–555. https://doi.org/10.1017/S0025315400017240
Chevalier P, Proulx D, Lessard P, Vincent WF, de la Noüe J (2000) Nitrogen and phosphorus removal by high latitude mat-forming cyanobacteria for potential use in tertiary wastewater treatment. J Appl Phycol 12:105–112. https://doi.org/10.1023/A:1008168128654
Henkanatte-Gedara S (2018) Urban wastewater treatment by Galdieria sulphuraria: single step process for dissolved organics and nutrient removal. New Mexico State University
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Abeysiriwardana-Arachchige, I.S.A., Chapman, W., Nirmalakhandan, N. (2020). Algal Wastewater Treatment: Study of Polyculture in Fed-Batch Mode in a Winter Climate. In: Dissanayake, R., Mendis, P. (eds) ICSBE 2018. ICSBE 2018. Lecture Notes in Civil Engineering , vol 44. Springer, Singapore. https://doi.org/10.1007/978-981-13-9749-3_10
Download citation
DOI: https://doi.org/10.1007/978-981-13-9749-3_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-9748-6
Online ISBN: 978-981-13-9749-3
eBook Packages: EngineeringEngineering (R0)