Abstract
Overview This chapter provides an overview and outlook of nanotechnology’s enabling roles in developing effective environmental remediation processes. Nanotechnology has the potential to substantially improve environmental remediation technologies. Here, instead of an exhaustive review of all developments related to nano-enabled environmental remediation processes/technologies, we present a brief overview and then specific comparison(s) between the two most common application approaches—individual (free) nanoparticles and those systems with integrated nanomaterials/nanotechnology. Specifically, we review examples of metal oxide nanoparticles (as nano-adsorbents) and graphene oxide enabled membranes to illustrate key technological aspects regarding their application potential in environmental remediation. Lastly, we highlight three key steps to further advance material development: the establishment of structure-property-function relationships, delineating the effects of environmental factors and the addressing of potential risk issues.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Nanotechnology is the science, engineering, and technology conducted at the nanoscale, which is typically from about 1–100 nm. Lastly, we highlight three key steps to further advance material development: the establishment of structure-property-function relationships, delineating the effects of environmental factors and the addressing of potential risk issues. Over the past two decades, with the launch of signature research initiatives such as the US National Nanotechnology Initiative (NNI), groundbreaking discoveries have been made at the nanoscale, including materials with specifically enhanced properties such as higher strength, lighter weight, increased control of the electromagnetic spectrum, greater chemical reactivity, etc. Discoveries at the nanoscale have also led to the improvement of environmental technologies, including contaminant detection, removal, and treatment processes that have remained difficult with conventional approaches. Broadly speaking, the environmental applications of nanotechnology fall into three categories: environmentally friendly and/or sustainable products (e.g., nanocatalysts for the production of solar fuels), remediation of contaminated environmental media, such as air, water, and soil, and sensors for detecting contaminants (Tratnyek and Johnson 2006). To date, a wide range of nanomaterials have been considered and evaluated, including metal (oxide) nanoparticles (e.g., Ag, Cu, TiO2, Fe0, Fe3O4, SiO2), carbon nanomaterials (e.g., fullerenes, carbon nanotubes, graphene), metal–organic frameworks (MOF), and multicomponent hybrid materials among others.
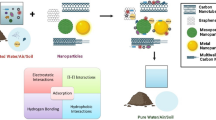
At the technology development/application level, the use of nanomaterials can be categorized via two application strategies: individual particles and incorporated systems (Fig. 1). The former describes nanomaterials applied as discrete, monodispersed particles (although aggregation of nanomaterials is usually always observed under typical environmental conditions), such as nanoscale adsorbents and/or catalysts. The later describes nanomaterials which are being incorporated/embedded into a surface or matrix. Examples include membranes that have functional nanomaterials incorporated on or in a surface and/or matrix. For the use of individual particles, applications commonly mirror the spectrum of “non-nano” strategies for contaminant remediation, but with higher reactivity and specificity. Enhanced reactivity is often attributed to larger overall surface area, greater density of reactive surface sites, and/or higher intrinsic reactivity of the reactive surface sites. For the use of nanomaterials as part of the incorporated systems, unique features of nanomaterials are being integrated into the original system, e.g., the surface coating of antimicrobial nanomaterials on membrane surface to achieve effective antifouling. Use of nanomaterials allows for new or significantly improved functions of the original system.
In this chapter, we do not present an exhaustive, detailed review of the methods and developments of applications of nanotechnology for every environmental remediation process. Instead, we provide an overview which is centered on one core question—how the use of nanomaterials enables high-performance remediation processes. Toward this, we focus on individual (discrete) nanoparticle systems and nanomaterial-incorporated systems. The discussion is mainly based on our previous research, including the development and use of metal oxide nanoparticles as nano-adsorbents and graphene oxide as a performance enabler in water treatment membranes. Finally, we provide our thoughts on advancing nanotechnology applications in environmental remediation.
2 A Tale of Two Nanotechnology Applications in Environmental Remediation
2.1 Individual Nanoparticle Systems—Nano-Adsorbents
High-capacity sorbents, enabled by engineered nanomaterials (ENMs), have wide application potential in environmental remediation, including in situ groundwater remediation, and point-of-use drinking water treatment. Sorptive remediation technologies remove contaminants by sequestration and immobilization, and can be further combined with reactive technologies to achieve enhanced degradation of contaminants. Using ENMs for the adsorptive removal of contaminants is advantageous due to enhanced capacity, selectivity, reactivity, and kinetic control (Qu et al. 2013; Alvarez et al. 2018; Mauter et al. 2018). Nanoscale sorbents have been broadly proposed and evaluated, including metal oxides (e.g., iron (Madden et al. 2006) and manganese oxides (Camtakan et al. 2012)), nanocarbons (e.g., carbon nanotube (CNT) (Yang and Xing 2010) and graphene oxide (GO) (Gao et al. 2012)), and metal–organic frameworks (MOFs) (Wang et al. 2018), among others (Shannon et al. 2008; Alvarez et al. 2018).
Among these, metal oxide nanomaterials are effective and affordable adsorbents for the removal of heavy metals, metalloids, and radionuclides, such as arsenic (As), chromium (Cr), uranium (U) (Yeap et al. 2014; Gómez-Pastora et al. 2014; Tang and Lo 2013; Qu et al. 2013; Kim et al. 2018a). Tunable size and surface structure/chemistry of metal oxide nanoparticles enable optimization of active (adsorption) sites to improve kinetics and capacities, while also allowing for selectivity (Auffan et al. 2008; Yang et al. 2008; Roduner 2006; Qu et al. 2013). For example, the functionalization of nanoparticles with specific surface coating (organic and/or inorganic) allows for extended surface area(s), enhanced selectivity, and high colloidal stability (Kim et al. 2018a; Davis et al. 1988; Moore and Shen 1983; Wang et al. 2015; Yavuz et al. 2006; Feng et al. 2012; Yantasee et al. 2007; Warner et al. 2010).
In our recent studies, a series of precisely engineered monodispersed metal oxide nanoparticles (i.e. manganese oxide (MO), iron oxide (IO), and manganese ferrite (MF)) were synthesized and systematically studied for the sorption of various contaminants (i.e. uranium, arsenic, and chromium) in water (Fig. 2) (Lee et al. 2015a, b; Li et al. 2016, 2017b; Kim et al. 2018a, b). Nanoparticles were synthesized via thermal decomposition routes to produce highly uniformed metal oxide nanoparticles with a narrow size distribution. By varying reaction temperature, time, ratio of precursor(s) to surfactant, and concentrations of coating polymer(s), various sizes and compositions of nanoparticles can be precisely controlled. For aqueous applications, such nanoparticles can be phase transferred from organic solvent(s) into water by ligand encapsulation or exchange, effectively adding a second organic layer(s) or exchanging with a single organic layer(s), which allows for a broad spectrum of surface chemistries. As we have detailed in a number of reports, sorption capacities are dependent on the nature of nanomaterials (size and composition), surface coatings (structure and functional group), and water chemistry (pH and constituent) (Lee et al. 2015a, b; Li et al. 2016, 2017b; Kim et al. 2018a, b).
(1) Core Composition: The composition of nanoscale metal oxides was found to affect the mechanism(s) and thus the capacity of decontamination and separation (Lee et al. 2015a, b; Li et al. 2016, 2017b; Kim et al. 2018b). For example, through a combination of functional group binding and U(VI) reduction, engineered manganese oxide (MO) nanoparticles and iron oxide (IO) nanoparticles displayed similar uranium sorption capacity (both around 50% wt U/wt Mn or Fe, which is 100-fold higher than their commercially available analogues). Interestingly, a higher portion of U(VI) was reduced to U(IV) on the surface of IO nanoparticles (Lee et al. 2015a; Li et al. 2016, 2017b), indicating different binding (sorption) mechanisms for these two materials (i.e. surface redox and ligand associated). In addition, IO nanoparticles can be separated via low-field magnet field due to their superparamagnetic properties (Li et al. 2016). Further, as binary and/or ternary oxide analogues seemed more effective than mono-oxide analogues (Dui et al. 2013; Wang et al. 2011), the uranium sorption capacity of manganese ferrite (MF) nanoparticles was also systematically investigated (Lee et al. 2015b). Maximum sorption capacity of MF nanoparticles increased significantly (1666.7 mg U per g nanoparticle, ca. 160 wt% loading to the nanoparticle) under optimal conditions compared to MO or IO (Lee et al. 2015b). The enhancement was in part due to the enhanced reduction from U(VI) to U(IV) at the interface between the nanocrystal and uranium ion via partial redox reactions. Uranium sorption capacity for these was found to vary among MF nanoparticles with different manganese/iron ratios, with Fe-rich MF nanoparticles demonstrating superior sorption capacity than Mn-rich MF nanoparticles (Lee et al. 2015b; Kim et al. 2018b). This can be attributed to the decreased Fe(II) availability on Mn-rich MF nanoparticles due to the formation of Mn2FeO, MnO, and Mn3O4 (Lee et al. 2015b; Krycka et al. 2013; López-Ortega et al. 2012).
(2) Size: In addition to nanoparticle composition, the size was also found to influence sorption performance (Lee et al. 2015a; Li et al. 2017b; Kim et al. 2018a). Nanoparticles with smaller size are normally considered to possess higher sorption capacities due to larger specific surface areas, which is, by in large, what we observed in our studies (Lee et al. 2015a; Li et al. 2017b). However, size might not be the only consideration when the nanoparticle surface is functionalized with ligands. For example, we observed similar arsenic and chromium sorption capacity among various sizes (i.e., 8, 12, 19, and 25 nm) of IO nanoparticles coated with cetyltrimethylammonium bromide (CTAB), which is due to higher CTAB loading (i.e., grafting density) on larger particles as a function of surface curvature dynamics (Kim et al. 2018a). As such, total sorption capacity is not exclusively dependent on particle size, as the surface area advantages of smaller particles may be negated when larger particles possess denser functional groups, underlining the importance of surface coating in determining sorption capacity (Kim et al. 2018a).
(3) Surface Coatings: Surface coating(s) properties should be taken into consideration when optimizing nanomaterial sorption performance (Lee et al. 2015a; Li et al. 2017b; Kim et al. 2018a; Li et al. 2016). For example, the uranium sorption capacity of polyethylene glycol (PEG) coated MO nanoparticles is inversely correlated to the molecular weight of the coatings (Lee et al. 2015a). PEG-200 coated nanoparticles exhibited the highest maximum sorption capacity of 227.3 mg/g compared to 142.9 mg/g for PEG-1 k and 119.0 mg/g for PEG-10 k coated MO nanoparticles, indicating that a thinner coating may allow for enhanced interfacial interactions/bindings (Lee et al. 2015a). Further, the surface coating structure and chemistry are also critical for optimized sorption performance. A bilayer coating structure of unsaturated–unsaturated carbon chains (e.g., oleic acid–oleyl phosphate) exhibited better sorption performance than those with an unsaturated-saturated carbon chain linkage (e.g., oleic acid–octadecyl phosphonic acid) (Kim et al. 2018a; Lee et al. 2015a, b), possibly due to enhanced colloidal/bilayer stability rising from additional hydrophobic and van der Waals interactions between unsaturated chains (Prakash et al. 2009). Second (outer) layer chain length also plays a role for these types of surface bilayers. Maximum uranium sorption capacity of IO nanoparticles decreased from 419 mg U/g Fe to 274 mg U/g Fe as a function of chain length, dropping from 18-carbon to 12-carbon chains, respectively (Li et al. 2017b). Lastly, the functional group(s) of surface coatings are important as they relate to charge and/or specific interactions between contaminants and coatings. For instance, positively charged CTAB coated MF nanoparticles displayed a uranium sorption capacity of 178.6 mg U/g nanoparticle while all the other MF nanoparticles with negatively charged coatings demonstrated a uranium sorption capacity at least two times greater than their CTAB counterparts (Lee et al. 2015b). Oleyl phosphate (OP) coated MF nanoparticle demonstrated the highest sorption capacities (1666.7 mg U/g nanoparticle) (Lee et al. 2015b). Similar electrostatic interaction has also been observed for arsenic/chromium sorption using IO nanoparticles with various coatings (Li et al. 2017b). Additionally, both MO and MF nanoparticles with coatings containing phosphate based functional (head) groups (i.e. sodium monododecyl phosphate, octadecylphosphonic acid, and oleyl phosphate) exhibited better sorption capacity than those coatings without phosphate, e.g., 1666.7 mg U/g nanoparticle of oleyl phosphate coated MF nanoparticle versus 909.1 mg U/g nanoparticle of oleic acid coated MF nanoparticle (Lee et al. 2015a, b). These observations underscore the importance of the well-documented uranium–phosphate interactions underpinning binding processes (Sutton and Burastero 2004; Wang et al. 2006).
(4) Water Chemistry: Apart from nanomaterial properties, including surface coatings, water chemistry (pH and constituent, etc.) is a critical variable to consider for engineering sorption processes. Sorption capacity of various contaminants (e.g., uranium/chromium/arsenic) was found to be pH dependent (Lee et al. 2015a, b; Li et al. 2016, 2017b; Kim et al. 2018a, b), as it affects surface coating properties (e.g., charge) and target contaminant speciation/complexation. For example, higher uranium sorption was observed at a slightly acidic pH (pH = 5.8), possibly due to the formation of UO22+, UO2(OH)+, and (UO2)3(OH) +5 at pH < 6, inducing more favorable electrostatic interaction between positively charged uranium species and negatively charged particles (Li et al. 2017b; Sutton and Burastero 2004; Zhao et al. 2014). Similarly, for both chromium and arsenic, the sorption was found to be higher at slightly acidic conditions due to the change of sorbate speciation and surface properties (Kim et al. 2018a). In addition to pH, ionic strength and type (e.g., Na+ and Ca2+, among others) may, in many cases, negatively influence the sorption process. The presence of ions can destabilize nanoparticles (thus inducing nanoparticle aggregation and reducing the surface area available for contaminants binding) and/or compete for available binding sites (Lee et al. 2015b; Prakash et al. 2009). As an illustration, uranium sorption capacity of MF nanoparticles decreased ca. 40% from 1250 to 714.3 mg U/g nanoparticle in typical groundwater with sodium/calcium composition of around 125 ppm (5.44 mM) sodium and 24 ppm (0.61 mM) calcium (Lee et al. 2015b).
2.2 Nanomaterial-Incorporated Systems—Graphene Oxide Enabled Membranes
A membrane is a selective barrier which allows certain feed components (e.g., water) to pass while rejecting others, which are often those with larger sizes (Zeman and Zydney 2017). Membrane separation is capable of removing a broad-spectrum of contaminants from environmental media, including water and air. Current prevailing membranes are made of various synthetic polymers, and exhibit two main technical limits. Firstly, there exists an effective trade-off between permeability and selectivity—when permeability increases, the membrane selectivity usually decreases (Sadeghi et al. 2018). However, both high permeability and selectivity are desirable. Higher permeability decreases the amount of membrane area required to treat a given amount of liquid, thereby decreasing the capital cost of membrane units, while higher selectivity results in higher purity of produced water. Secondly, membrane surfaces/processes experience fouling, especially in wastewater treatment using membrane bioreactors, where irreversible bio-fouling leads to permeability reduction that cannot be restored (Shannon et al. 2008). These issues have, in many ways, limited the technological advantages (and advancement) of membrane separations by decreasing product water quality, increasing operational costs, and shortening service lifetime.
As discussed, engineered nanomaterials (ENMs) have received substantial attention for next generation membrane applications due to their high reactivity, tunable surface properties, and tailored structures (Mauter and Elimelech 2008; Mauter et al. 2018). By taking advantage of such properties, nanomaterial integration enables the (polymeric) membranes to have tunable internal and surface structure and chemistries, underpinning the potential for higher permeability, selectivity, and enhanced antifouling performance (Alvarez et al. 2018). Considering ultrafiltration nanocomposite membranes as an example, ENMs are incorporated or assembled mainly through two strategies—impregnation (as nanofillers) or surface coating (Fig. 3). To date, a wide range of nanomaterials have been studied for these two strategies, including metal (oxide) NPs (e.g., Ag (Zodrow et al. 2009), Cu (Ben-Sasson et al. 2016), TiO2 (Razmjou et al. 2011)), carbon nanomaterials (e.g., C60 (Taurozzi et al. 2011), CNT (Sianipar et al. 2017), graphene (Jiang et al. 2016a)), metal–organic frameworks (MOF) (Sun et al. 2017), and hybrid materials (Yu et al. 2013).
Among these materials, graphene oxide (GO) can be easily fabricated with tunable size, surface chemistry, and shape at the commercial scale (Jiang et al. 2016a). GO partially remains as a one-atom-thick planar sheet with a sp2-bonded carbon structure while being derivatized with oxygen functional groups both on the basal plane (e.g., hydroxyl and epoxy groups) and at the sheet edges (e.g., carboxyl and carbonyl, etc.). Due to its unique properties and relative ease of production, it has been extensively researched for membrane applications, from ultrafiltration (UF) to reverse osmosis (RO). Below, we discuss recent progress in the applications of GO with a focus on addressing the two main drawbacks of membranes—specifically the trade-off between permeability and selectivity, and fouling.
(1) Achieving High Permeability and Selectivity
Mixed-matrix membranes (MMMs) commonly consist of a dispersed nanomaterial phase and a continuous polymer matrix, hoping to combine the high intrinsic permeability and separation properties of advanced nanomaterials with the robust processing and mechanical properties of polymers. Molecular sieving fillers with nanoscale size or nanosheet shapes (e.g., MOF nanoparticles or 2D nanosheets) were found to possibly improve both permeability and selectivity (Park et al. 2017). GO has been widely applied as nanofillers in synthesizing MMMs, including both UF and RO membranes. GO-enabled membranes can be easily synthesized by blending different amounts of GO in the membrane casting solutions (usually 0.1–6 wt% with respect to polymer) (Ganesh et al. 2013; Zhang et al. 2013; Crock et al. 2013; Wang et al. 2014).
We have summarized the existing literature regarding graphene-enabled MMMs, with most of the nanofillers as GO or its derivatives. As seen in Fig. 4, upon the incorporation of GO-based materials, the maximum water permeability can increase ca. 0.1–20 times compared with pristine membranes. For rejection (also selectivity, defined as the reciprocal of the sieving coefficient (Mehta and Zydney 2005)), it can increase at the same time (Zhang et al. 2013; Xu et al. 2014; Zinadini et al. 2014; Li et al. 2017a); or it can also decrease, showing inverse relationships between permeability and selectivity (Ma et al. 2017; Yu et al. 2013; Li et al. 2017a). Taken together, the data sets clearly shows that the mass loading and properties of nanomaterials have a large impact on membrane structure and resultant performance (Jiang et al. 2019b), and also the MMMs incorporated with GO can potentially have both high permeability and selectivity.
The change of water permeability (a) and rejection performance (b) of graphene-based MMMs compared to pristine membranes. Data was extracted from existing literature and processed (the permeability and rejection rate of pristine membranes were taken as 100%), with each line representing one study of the material variable. The Y-axis in (b) was adjusted to focus on the major changes/trends of most cases, and thus 4 cases with normalized rejection >125% (and up to 275%) were not shown with complete data sets in the figure
The impact and its extent of adding nanomaterials can be described as a function of both the properties of GO (or derivatives or hybrids) and its impacts on the membrane matrix upon incorporation. A number of studies have explained qualitatively the role of nanomaterials affecting membrane structure (e.g., surface chemistry, hydrophilicity, charge, etc.) and performance (e.g., permeability, selectivity, mechanical strength, etc.). GO has a high density of hydrophilic functional groups, which as a result improves the hydrophilicity and porosity of the membrane, thus resulting in enhanced permeability (Ma et al. 2017; Zinadini et al. 2014; Crock et al. 2013; Jiang et al. 2019b). Furthermore, such change(s) can be due to dynamic phase inversion processes which are sensitive to the presence of the nanomaterials themselves—in other words, the addition of nanomaterials will change both the kinetics and thermodynamics of membrane formation. For example, our experimental results showed that changes in the shape of graphene oxide alone can result in varied performance. And such differences can be attributed to the (more) effective dispersion/stability of crumpled GO nanoparticles in solvent (NMP) as a result of shape effects, which lowers the tipping mass percentage after which the effect of viscosity increase outweighs that of hydrophilicity increase (Jiang et al. 2019b). With the increasing molecular understanding of membranes in recent years, key design factors to disrupt the permeability–selectivity trade-off have been recognized to potentially include thin(er) selective layers, highly tuned interactions between membrane and permeants/solutes of interest (e.g., electrostatic repulsion/attraction), and properly sized pores and its distribution (Park et al. 2017). However, the fundamental insight between (addition of) nanomaterials and those factors, and the pathways which would allow for predictive frameworks remain largely outstanding.
(2) Fouling Control
For fouling control, surface hydrophilicity/charge and roughness are considered to be critical factors as they reduce favorable interactions and attachment of aqueous substances with a membrane surface. For example, the increase of hydrophilicity limits hydrophobic–hydrophobic interaction between solutes/bacteria and membrane surface. GO contains densely arranged hydrophilic functional groups, which increase membrane surface hydrophilicity (Ma et al. 2017; Zinadini et al. 2014; Crock et al. 2013). Therefore, the hydrophobic solutes are less prone to interact with the membrane surface. Moreover, enhanced surface hydrophilicity can mitigate protein adsorption because of the repulsion force(s) arising from hydrated layers on the surface which also preserves protein (tertiary) structure and thus reversible attachment (Xu et al. 2014). The incorporation of GO also reduces organic fouling by electrostatic repulsion for other foulants (e.g., humic acids, which are negatively charged at pH values from 6 to 9) (Igbinigun et al. 2016; Zhang et al. 2018; Jiang et al. 2016a). Furthermore, as surface roughness increases, faster fouling typically happens, while smoother surfaces provide less adhesion sites (Elimelech et al. 1997). GO nanosheets have been shown to form much smoother surfaces (RMS roughness decreased from 37.2 nm to 8.8 nm) when coated to PES membranes, contributing to a higher water flux recovery of 70%, compared to 28% of the unmodified ones (Igbinigun et al. 2016).
Interestingly, bio-fouling control using GO has been observed. GO can physically disrupt and chemically inactivate bacteria (i.e., via ROS), resulting in loss of cell integrity and proliferation (Liu et al. 2011; Tu et al. 2013). GO-enabled membranes have shown enhanced antimicrobial activity (against, e.g., E. Coli., M. smegmatis, and S. aureus), with <35% viable CFU after contact/inactivation experiments, compared with that of control membranes (PSF, PVDF, PTFE, and nylon) (Zou et al. 2017; Li et al. 2013; Lu et al. 2017; Kaneda et al. 2019; Perreault et al. 2013). The properties of GO nanomaterials (e.g., alignment and shape) have a large impact on fouling control efficiency. The vertically aligned GO membranes showed stronger antimicrobial activity, reducing viable E. coli cell number by 72% for cells in contact with the membrane surface compared to nonaligned GO membranes (Lu et al. 2017, 2018). The orientation-dependent cell inactivation could be related to the density of exposed GO edges with preferential orientation for bacteria inactivation (Lu et al. 2017, 2018). Our previous studies also showed that through combining GO and nano-Ag, both contact and dissolution-based antimicrobial mechanisms can be leveraged to achieve even more effective fouling control (Jiang et al. 2015, 2016b).
3 Challenges and Opportunities
The highlighted examples clearly show that the application of nanotechnology can considerably improve the performance of traditional remediation processes such as adsorption/removal and membrane separations. Some of the highest adsorption capacities reported to date were observed with the advanced metal oxide nano-adsorbents. Regardless of the way(s) engineered nanomaterials are being used—individual nanoparticles or incorporated systems, advantages at the nanoscale including high surface area and reactivity can and will be leveraged to enhance or impart additional functions, which ultimately result in the removal of contaminants with enhanced capacity, selectivity, and kinetics.
For these technologies to reach their potential, there exists a significant degree of complexity based on the numerous variations of nanomaterial properties (e.g., size of graphene oxide, or molecular weights and functional groups of surface coating), and their combinations. A small variation in one nanomaterial property can potentially result in a substantial change of function/performance (e.g., the size of graphene oxide on its toxicity (Perreault et al. 2015)). In general, for both approaches, the underlying pathway(s)—how the change(s) is transmitted from nanomaterial to eventual performance—remain poorly characterized. Notably, understanding of the interactions between the nanomaterials and the surface/matrix in incorporated systems is still a challenging prospect. This further adds to the systematic complexity and will only increase with scale.
Additionally, environmental factors, including water chemistry, foulants, etc., will have a significant impact on performance, as they affect not only the structure and properties of nanomaterials/incorporated systems but also the properties of the contaminants (e.g., chemical speciation). Overall, the studies on the effects of environmental factors have been focused on those conducted in laboratories, which largely oversimplify real-world conditions.
Lastly, for both approaches, the release and potential risks of nanomaterials should be carefully considered. However, this issue is more serious for individual particles when they are applied, as they will have higher mobility compared to those incorporated into a system.
3.1 Establishing Structure–Property–Function Relationships for Better Material Design
Despite the large volume of literature on environmental applications of nanotechnology, as described in the previous section, generalized knowledge, i.e., Structure–Property–Function relationships that relate the nanoscale structural variables, macroscale physical and chemical properties, and eventual functions, remains nascent. For example, for nano-enabled membrane separation, a systematic framework with more translatable data is needed to establish an understanding of material processing, material structure and properties, membrane applications, and their fundamental relationships (steps 1–3 in Fig. 5) (Jiang et al. 2016a). The establishment of such relationships will provide answers to a number of key questions—namely, which nanomaterial properties are desired so that the resultant MMMs can have both high permeability and selectivity? How can the nano-enabled antifouling functions be sustained? What scale-up strategies of production can be designed and optimized? And so on.

Reproduced from Ref. Jiang et al. (2016a) with permission from The Royal Society of Chemistry
A need for a systematic thinking from material processing to membrane (and also similarly, to other nanotechnology) applications.
Also, high-order correlations regarding surface coating characteristics (e.g., molecular weight, charge, etc.) and fundamental aqueous behaviors (aggregation, sedimentation, deposition) and performance (e.g., adsorption capacity and mechanism(s)) will generate knowledge that informs better design of high-performance, highly selective, “smarter” nano-adsorbents. For instance, new surface coatings could give the exact mobility and affinity of nanomaterials used for groundwater remediation applications—allowing sufficient but not over delivery of the treatment to the contaminated volume of media. Such understanding also enables more sophisticated, multifunctional nanocomposites, such as catalyzing several different pollutant reactions on the same particle, interacting with both hydrophobic and hydrophilic pollutants, or pre-concentrating and degrading high-priority pollutants using photocatalytic reactions (the so-called “bait-hook-and destroy” strategy) (Karn et al. 2009; Alvarez et al. 2018).
The nature of such structure–property–function relationships remains challenging due to the fractal complexity of the studied systems (i.e., commonly with variables differing by a few orders of magnitude) and lack of abundant, translatable, and benchmarkable data. Toward these goals, the disclosure and accessibility of research data will become necessary, in some form, if such relationships are to be established.
3.2 Delineating the Effects of Environmental Factors for Real Applications
There have yet to be many large-scale field applications or commercial products for nano-enabled environmental remediation. Among the examples to date, nanoscale zero-valent iron (nZVI) nanoparticles have been tested in a number of field applications for groundwater remediation, and for nano-enabled membranes, the incorporation of zeolite nanoparticles into polyamide layer has yielded a commercial thin-film nanocomposite membrane (LG NanoH2O Inc). In addition, novel composites that incorporate iron oxide nanoparticles in millimeter-sized polystyrene spheres are being used in meter-scale reactors to decontaminate tanning, electroplating, and mining wastewaters (Zhang et al. 2017).
For real-world applications, environmental factors are critical to consider as mentioned, in addition to the intrinsic material properties and functions. For example, the use of metal oxide nanoparticles for site remediation, usually has site-specific requirements that must be met in order for it to be effective. Compared to the common factors involved in bench-scale studies (e.g., pH, ionic strength, NOM), field applications are complicated by additional, macroscale geologic, hydrogeologic, and subsurface conditions, including soil properties, porosity, hydraulic conductivity, flow velocity, etc. (Karn et al. 2009). How bench-scale studies inform the application of nanoparticles in real environments remains unclear at this time. For instance, although being capable of producing fundamental insights, current methodologies to studying nanoparticle stability and mobility (e.g., stability studies using static and dynamic light scattering, deposition studies using quartz crystal microbalance or column experiments) may fail to provide sufficient information to interpret their behaviors in real complex conditions. Also, the transformation of nanomaterials under field conditions is a concern and is currently being evaluated. Changes induced by environmental factors such as light, oxidants, and microorganisms will likely result in chemical or biological modifications/degradation of the functionalized surface or coating of the surface (Karn et al. 2009). Our current way of technology development has been mainly targeted at one specific contaminant, which neglects the fact that many contaminants coexist and typically need to be removed at the same time. Also, almost all types of membrane fouling occur simultaneously, so it will be beneficial to look into the effects of nanotechnology on the mitigation of more than one type of fouling for the same system. It is therefore suggested that tests under real environmental conditions (e.g., real wastewater, contaminated groundwater) should be conducted in addition to classic bench-scale analyses (i.e., synthetic conditions) to assess the potential for real-world application(s).
3.3 Addressing Potential Risk Issues to Achieve Sustainability
Understanding potential ecological and health risks associated with the use of engineered nanomaterials, including in environmental applications, has been a broad, active area of research. Regardless of the application/process type, there is potential for material release into the environment and subsequent ecosystems. Despite a considerable body of related research, quantifying real risk remains a largely outstanding concern (Westerhoff et al. 2018).
The release and exposure in real (or future) scenarios are still difficult to estimate for most nanomaterials, which is further complicated by their transformation and interactions (e.g., with other pollutants) in the environment over time. To understand and quantify potential risks, mobility, bioavailability, toxicity, and persistence of manufactured nanoparticles will need to be continuously studied. Engineered nanomaterials are usually made to have enhanced, sometimes unique physical, chemical, and toxicological properties, which could pose inherit risk(s). Moreover, other than the particles themselves, their surface coating and surface reactions (e.g., ROS produced by UV irradiation) could also exert effects. Furthermore, whereas the nanoparticles themselves may not possess toxic properties, the pollutants they could carry with them may. For instance, iron-based nanoparticles may bind with metal or metalloids, thus effectively acting as a pollutant vector.
Fundamentally linking knowledge obtained in laboratory settings with the real systems will become an important research direction. The release and exposure for potentially high-concentration scenarios such as manufacturing should become a priority area of study. It also provides a venue to test new detection methods, apply precautionary/mitigation measures, consider epidemiological evaluations, and so forth (Jiang et al. 2019a).
To conclude, a number of recent studies suggest that the risks related to nanotechnology are likely to be low (at least lower than originally thought, at the current stage of material production and usage) as they relate to the environmental application. For the vast majority of exposure scenarios proposed, engineered nanomaterials are predicted to exist at low concentrations, often orders of magnitude lower than natural nanoparticles in natural waters. Furthermore, it has been reported that current engineered barriers/treatment processes, such as water treatment facilities, can effectively intercept nanomaterial transmission into drinking water/food sources (Westerhoff et al. 2018). In a similar vein, continued research on real-world material exposure is needed to provide accurate guidance on use-risk trade-offs for both specific and broad applications, and ultimate technological sustainability.
References
Alvarez PJJ, Chan CK, Elimelech M, Halas NJ, Villagrán D (2018) Emerging opportunities for nanotechnology to enhance water security. Nat Nanotechnol 13(8):634–641.
Auffan M, Rose J, Proux O, Borschneck D, Masion A, Chaurand P, Hazemann J-L, Chaneac C, Jolivet J-P, Wiesner MR (2008) Enhanced adsorption of arsenic onto maghemites nanoparticles: as (III) as a probe of the surface structure and heterogeneity. Langmuir 24(7):3215–3222
Ben-Sasson M, Lu X, Nejati S, Jaramillo H, Elimelech M (2016) In situ surface functionalization of reverse osmosis membranes with biocidal copper nanoparticles. Desalination 388:1–8.
Camtakan Z, Erenturk S, Yusan S (2012) Magnesium oxide nanoparticles: preparation, characterization, and uranium sorption properties. Environ Prog Sustain Energy 31(4):536–543
Crock CA, Rogensues AR, Shan W, Tarabara VV (2013) Polymer nanocomposites with graphene-based hierarchical fillers as materials for multifunctional water treatment membranes. Water Res 47(12):3984–3996.
Davis ME, Saldarriaga C, Montes C, Garces J, Crowdert C (1988) A molecular sieve with eighteen-membered rings. Nature 331(6158):698–699.
Dui J, Zhu G, Zhou S (2013) Facile and Economical Synthesis of Large Hollow Ferrites and Their Applications in Adsorption for As(V) and Cr(VI). ACS Appl Mater Interfaces 5(20):10081–10089.
Elimelech M, Zhu X, Childress AE, Hong S (1997) Role of membrane surface morphology in colloidal fouling of cellulose acetate and composite aromatic polyamide reverse osmosis membranes. J Membr Sci 127(1):101–109
Feng L, Cao M, Ma X, Zhu Y, Hu C (2012) Superparamagnetic high-surface-area Fe3O4 nanoparticles as adsorbents for arsenic removal. J Hazard Mater 217:439–446
Ganesh BM, Isloor AM, Ismail AF (2013) Enhanced hydrophilicity and salt rejection study of graphene oxide-polysulfone mixed matrix membrane. Desalination 313:199–207.
Gao Y, Li Y, Zhang L, Huang H, Hu J, Shah SM, Su X (2012) Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J Colloid Interface Sci 368(1):540–546
Gómez-Pastora J, Bringas E, Ortiz I (2014) Recent progress and future challenges on the use of high performance magnetic nano-adsorbents in environmental applications. Chem Eng J 256:187–204
Igbinigun E, Fennell Y, Malaisamy R, Jones KL, Morris V (2016) Graphene oxide functionalized polyethersulfone membrane to reduce organic fouling. J Membr Sci 514:518–526.
Jiang Y, Biswas P, Fortner JD (2016a) A review of recent developments in graphene-enabled membranes for water treatment. Environ Sci Water Res Technol 2(6):915–922.
Jiang Y, Liu D, Cho M, Lee SS, Zhang F, Biswas P, Fortner JD (2016b) In situ photocatalytic synthesis of Ag nanoparticles (nAg) by crumpled graphene oxide composite membranes for filtration and disinfection applications. Environ Sci Technol 50(5):2514–2521
Jiang Y, Quan X, Jiang G, Li X (2019a) Current prospective on environmental nanotechnology research in China. Environ Sci Technol 53(8):4001–4002.
Jiang Y, Zeng Q, Biswas P, Fortner JD (2019b) Graphene oxides as nanofillers in polysulfone ultrafiltration membranes: shape matters. J Membr Sci 581:453–461.
Jiang Y, Wang W-N, Liu D, Nie Y, Li W, Wu J, Zhang F, Biswas P, Fortner JD (2015) Engineered crumpled graphene oxide nanocomposite membrane assemblies for advanced water treatment processes. Environ Sci Technol 49(11):6846–6854
Kaneda M, Lu X, Cheng W, Zhou X, Bernstein R, Zhang W, Kimura K, Elimelech M (2019) Photografting graphene oxide to inert membrane materials to impart antibacterial activity. Environ Sci Technol Lett 6(3):141–147.
Karn B, Kuiken T, Otto M (2009) Nanotechnology and in Situ remediation: a review of the benefits and potential risks. Environ Health Perspect 117(12):1813–1831.
Kim C, Lee SS, Lafferty BJ, Giammar DE, Fortner JD (2018a) Engineered superparamagnetic nanomaterials for arsenic(v) and chromium(vi) sorption and separation: quantifying the role of organic surface coatings. Environ Sci Nano 5(2):556–563.
Kim C, Lee SS, Reinhart BJ, Cho M, Lafferty BJ, Li W, Fortner JD (2018b) Surface-optimized core–shell nanocomposites (Fe3O4@MnxFeyO4) for ultra-high uranium sorption and low-field separation in water. Environ Sci Nano 5(10):2252–2256.
Krycka KL, Borchers JA, Salazar-Alvarez G, López-Ortega A, Estrader M, Estrade S, Winkler E, Zysler RD, Sort J, Peiró F (2013) Resolving material-specific structures within Fe3O4| γ-Mn2O3 core|shell nanoparticles using anomalous small-angle X-ray scattering. ACS Nano 7(2):921–931
Lee SS, Li W, Kim C, Cho M, Catalano JG, Lafferty BJ, Decuzzi P, Fortner JD (2015a) Engineered manganese oxide nanocrystals for enhanced uranyl sorption and separation. Environ Sci Nano 2(5):500–508.
Lee SS, Li W, Kim C, Cho M, Lafferty BJ, Fortner JD (2015b) Surface functionalized manganese ferrite nanocrystals for enhanced uranium sorption and separation in water. J Mater Chem A 3(43):21930–21939.
Li M, Shi J, Chen C, Li N, Xu Z, Li J, Lv H, Qian X, Jiao X (2017a) Optimized permeation and antifouling of PVDF hybrid ultrafiltration membranes: synergistic effect of dispersion and migration for fluorinated graphene oxide. J Nanoparticle Res 19(3).
Li W, Troyer LD, Lee SS, Wu J, Kim C, Lafferty BJ, Catalano JG, Fortner JD (2017b) Engineering nanoscale iron oxides for uranyl sorption and separation: optimization of particle core size and bilayer surface coatings. ACS Appl Mater Interfaces 9(15):13163–13172.
Li W, Mayo JT, Benoit DN, Troyer LD, Lewicka ZA, Lafferty BJ, Catalano JG, Lee SS, Colvin VL, Fortner JD (2016) Engineered superparamagnetic iron oxide nanoparticles for ultra-enhanced uranium separation and sensing. J Mater Chem A 4(39):15022–15029.
Li Y, Yuan H, von dem Bussche A, Creighton M, Hurt RH, Kane AB, Gao H (2013) Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites. Proc Natl Acad Sci USA 110(30):12295–12300.
Liu S, Zeng TH, Hofmann M, Burcombe E, Wei J, Jiang R, Kong J, Chen Y (2011) Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS Nano 5(9):6971–6980.
López-Ortega A, Estrader M, Salazar-Alvarez G, Estradé S, Golosovsky IV, Dumas RK, Keavney DJ, Vasilakaki M, Trohidou KN, Sort J (2012) Strongly exchange coupled inverse ferrimagnetic soft/hard, Mnx Fe3–x O4/Fex Mn3–x O4, core/shell heterostructured nanoparticles. Nanoscale 4(16):5138–5147
Lu X, Feng X, Werber JR, Chu C, Zucker I, Kim JH, Osuji CO, Elimelech M (2017) Enhanced antibacterial activity through the controlled alignment of graphene oxide nanosheets. Proc Natl Acad Sci USA 114(46):E9793–E9801.
Lu X, Feng X, Zhang X, Chukwu MN, Osuji CO, Elimelech M (2018) Fabrication of a desalination membrane with enhanced microbial resistance through vertical alignment of graphene oxide. Environ Sci Technol Lett 5(10):614–620.
Ma J, Guo X, Ying Y, Liu D, Zhong C (2017) Composite ultrafiltration membrane tailored by MOF@GO with highly improved water purification performance. Chem Eng J 313:890–898.
Madden AS, Hochella MF Jr, Luxton TP (2006) Insights for size-dependent reactivity of hematite nanomineral surfaces through Cu2+ sorption. Geochim Cosmochim Acta 70(16):4095–4104
Mauter MS, Elimelech M (2008) Environmental applications of carbon-based nanomaterials. Environ Sci Technol 42(16):5843–5859
Mauter MS, Zucker I, Fo Perreault, Werber JR, Kim J-H, Elimelech M (2018) The role of nanotechnology in tackling global water challenges. Nat Sustain 1(4):166–175.
Mehta A, Zydney AL (2005) Permeability and selectivity analysis for ultrafiltration membranes. J Membr Sci 249(1–2):245–249
Moore PB, Shen J (1983) An X-ray structural study of cacoxenite, a mineral phosphate. Nature 306(5941):356–358.
Park HB, Kamcev J, Robeson LM, Elimelech M, Freeman BD (2017) Maximizing the right stuff: the trade-off between membrane permeability and selectivity. Science 356(6343).
Perreault F, De Faria AF, Nejati S, Elimelech M (2015) Antimicrobial properties of graphene oxide nanosheets: why size matters. ACS Nano 9(7):7226–7236
Perreault F, Tousley ME, Elimelech M (2013) Thin-film composite polyamide membranes functionalized with biocidal graphene oxide nanosheets. Environ Sci Technol Lett 1(1):71–76.
Prakash A, Zhu H, Jones CJ, Benoit DN, Ellsworth AZ, Bryant EL, Colvin VL (2009) Bilayers as phase transfer agents for nanocrystals prepared in nonpolar solvents. ACS Nano 3(8):2139–2146.
Qu X, Brame J, Li Q, Alvarez PJJ (2013) Nanotechnology for a safe and sustainable water supply: enabling integrated water treatment and reuse. Acc Chem Res 46(3):834–843.
Razmjou A, Mansouri J, Chen V (2011) The effects of mechanical and chemical modification of TiO2 nanoparticles on the surface chemistry, structure and fouling performance of PES ultrafiltration membranes. J Membr Sci 378(1):73–84
Roduner E (2006) Size matters: why nanomaterials are different. Chem Soc Rev 35(7):583–592
Sadeghi I, Kaner P, Asatekin A (2018) Controlling and expanding the selectivity of filtration membranes. Chem Mater 30(21):7328–7354
Shannon MA, Bohn PW, Elimelech M, Georgiadis JG, Marinas BJ, Mayes AM (2008) Science and technology for water purification in the coming decades. Nature 452(7185):301–310.
Sianipar M, Kim SH, Khoiruddin K, Iskandar F, Wenten IG (2017) Functionalized carbon nanotube (CNT) membrane: progress and challenges. RSC Adv 7(81):51175–51198.
Sun H, Tang B, Wu P (2017) Development of hybrid ultrafiltration membranes with improved water separation properties using modified superhydrophilic metal-organic framework nanoparticles. ACS Appl Mater Interfaces 9(25):21473–21484.
Sutton M, Burastero SR (2004) Uranium(VI) solubility and speciation in simulated elemental human biological fluids. Chem Res Toxicol 17(11):1468–1480.
Tang SC, Lo IM (2013) Magnetic nanoparticles: essential factors for sustainable environmental applications. Water Res 47(8):2613–2632
Taurozzi JS, Crock CA, Tarabara VV (2011) C60-polysulfone nanocomposite membranes: entropic and enthalpic determinants of C60 aggregation and its effects on membrane properties. Desalination 269(1–3):111–119.
Tratnyek PG, Johnson RL (2006) Nanotechnologies for environmental cleanup. Nano Today 1(2):44–48.
Tu Y, Lv M, Xiu P, Huynh T, Zhang M, Castelli M, Liu Z, Huang Q, Fan C, Fang H, Zhou R (2013) Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat Nanotechnol 8(8):594–601.
Wang C-M, Lee L-W, Chang T-Y, Chen Y-C, Lin H-M, Lu K-L, Lii K-H (2015) Organic–inorganic hybrid zinc phosphate with 28-ring channels. Chem A Eur J 21(5):1878–1881.
Wang D, Gilliland SE, Yi X, Logan K, Heitger DR, Lucas HR, Wang W-N (2018) Iron mesh-based metal organic framework filter for efficient arsenic removal. Environ Sci Technol 52(7):4275–4284.
Wang L, Yang Z, Gao J, Xu K, Gu H, Zhang B, Zhang X, Xu B (2006) A biocompatible method of decorporation: bisphosphonate-modified magnetite nanoparticles to remove uranyl ions from blood. J Am Chem Soc 128(41):13358–13359
Wang Y, Cheng R, Wen Z, Zhao L (2011) Synthesis and characterization of single-crystalline MnFe2O4 ferrite nanocrystals and their possible application in water treatment. Eur J Inorg Chem 19:2942–2947.
Wang Z, Teychene B, Chalew TE, Ajmani GS, Zhou T, Huang H, Wu X (2014) Aluminum-humic colloid formation during pre-coagulation for membrane water treatment: mechanisms and impacts. Water Res 61:171–180.
Warner CL, Addleman RS, Cinson AD, Droubay TC, Engelhard MH, Nash MA, Yantasee W, Warner MG (2010) High-Performance, superparamagnetic, nanoparticle-based heavy metal sorbents for removal of contaminants from natural waters. ChemSusChem 3(6):749–757
Westerhoff P, Atkinson A, Fortner J, Wong MS, Zimmerman J, Gardea-Torresdey J, Ranville J, Herckes P (2018) Low risk posed by engineered and incidental nanoparticles in drinking water. Nat Nanotechnol 13(8):661–669.
Xu Z, Zhang J, Shan M, Li Y, Li B, Niu J, Zhou B, Qian X (2014) Organosilane-functionalized graphene oxide for enhanced antifouling and mechanical properties of polyvinylidene fluoride ultrafiltration membranes. J Membr Sci 458:1–13.
Yang HG, Sun CH, Qiao SZ, Zou J, Liu G, Smith SC, Cheng HM, Lu GQ (2008) Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 453(7195):638
Yang K, Xing B (2010) Adsorption of organic compounds by carbon nanomaterials in aqueous phase: polanyi theory and its application. Chem Rev 110(10):5989–6008.
Yantasee W, Warner CL, Sangvanich T, Addleman RS, Carter TG, Wiacek RJ, Fryxell GE, Timchalk C, Warner MG (2007) Removal of heavy metals from aqueous systems with thiol functionalized superparamagnetic nanoparticles. Environ Sci Technol 41(14):5114–5119
Yavuz CT, Mayo J, William WY, Prakash A, Falkner JC, Yean S, Cong L, Shipley HJ, Kan A, Tomson M (2006) Low-field magnetic separation of monodisperse Fe3O4 nanocrystals. Science 314(5801):964–967
Yeap SP, Leong SS, Ahmad AL, Ooi BS, Lim J (2014) On size fractionation of iron oxide nanoclusters by low magnetic field gradient. J Phys Chem C 118(41):24042–24054
Yu L, Zhang Y, Zhang B, Liu J, Zhang H, Song C (2013) Preparation and characterization of HPEI-GO/PES ultrafiltration membrane with antifouling and antibacterial properties. J Membr Sci 447:452–462.
Zeman LJ, Zydney AL (2017) Microfiltration and ultrafiltration: principles and applications. CRC Press
Zhang J, Xu Z, Mai W, Min C, Zhou B, Shan M, Li Y, Yang C, Wang Z, Qian X (2013) Improved hydrophilicity, permeability, antifouling and mechanical performance of PVDF composite ultrafiltration membranes tailored by oxidized low-dimensional carbon nanomaterials. J Mater Chem A 1(9).
Zhang W, Cheng W, Ziemann E, Be’er A, Lu X, Elimelech M, Bernstein R (2018) Functionalization of ultrafiltration membrane with polyampholyte hydrogel and graphene oxide to achieve dual antifouling and antibacterial properties. J Membr Sci 565:293–302.
Zhang X, Cheng C, Qian J, Lu Z, Pan S, Pan B (2017) Highly efficient water decontamination by using sub-10 nm FeOOH confined within millimeter-sized mesoporous polystyrene beads. Environ Sci Technol 51(16):9210–9218.
Zhao Y, Li J, Zhao L, Zhang S, Huang Y, Wu X, Wang X (2014) Synthesis of amidoxime-functionalized Fe3O4@ SiO2 core–shell magnetic microspheres for highly efficient sorption of U (VI). Chem Eng J 235:275–283
Zinadini S, Zinatizadeh AA, Rahimi M, Vatanpour V, Zangeneh H (2014) Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J Membr Sci 453:292–301.
Zodrow K, Brunet L, Mahendra S, Li D, Zhang A, Li Q, Alvarez PJ (2009) Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res 43(3):715–723.
Zou F, Zhou H, Jeong DY, Kwon J, Eom SU, Park TJ, Hong SW, Lee J (2017) Wrinkled surface-mediated antibacterial activity of graphene oxide nanosheets. ACS Appl Mater Interfaces 9(2):1343–1351.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Jiang, Y., Peng, B., Wan, Z., Kim, C., Li, W., Fortner, J. (2020). Nanotechnology as a Key Enabler for Effective Environmental Remediation Technologies. In: Jiang, G., Li, X. (eds) A New Paradigm for Environmental Chemistry and Toxicology. Springer, Singapore. https://doi.org/10.1007/978-981-13-9447-8_12
Download citation
DOI: https://doi.org/10.1007/978-981-13-9447-8_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-9446-1
Online ISBN: 978-981-13-9447-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)