Abstract
Microbes are widely utilized as workhorses for production of numerous valuable metabolites with utility in different sectors including food, energy, environment, agriculture, health, and disease management. Microbes are capable of synthesizing spectrum of structurally divergent compounds, which are even difficult to be prepared by chemical synthesis. Microbes synthesize many metabolites of interest naturally as an intermediate/product of their metabolic pathways, or another approach is redesigning/creating biosynthetic pathways in microbes for production of non-native target metabolite from simple and cheap substrates not only at laboratory scale but also at the industrial scale. Advancements in genetic engineering, genome sequencing, transcriptomics, proteomics, and computational approaches have revolutionized the identification of novel bioactive molecules of microbial origin and redesigning of microbial biosynthetic pathways. The present book chapter focuses primarily on microbial products including enzymes, proteins, chemicals, and secondary metabolites as well as their production strategies, their application in various fields, and development of engineered microbes as production factories.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Microbes like bacteria, fungi, yeast, and microalgae are the prolific source of large number of valuable natural compounds of commercial and therapeutic interest. Microbes are capable of synthesizing structurally divergent compounds. According to a recent report by Business Communication Company (BCC), the global market of microbes and microbial products would reach to $302.4 billion by 2023 from $186.3 billion in 2018 (McWilliams 2012). The microbial products are comprised of either the whole microbial cells or the metabolites derived from the microbes. Various products including pharmaceuticals, bulk and fine chemicals, metabolites, proteins, nutraceuticals, biofuels, antibiotics, bioplastics, food supplements, and biofertilizers are produced using biocatalytic processes, microbial cell factories, or cell-free processes (Schmidt-Dannert 2017). The producer microbes are identified through various approaches followed by establishment of microbial growth as well as production parameters for target molecule under laboratory conditions and further optimizations for large-scale production of target molecule in fermenters. The microbial production platforms are successfully becoming an effective alternative to traditional chemical synthesis due to various advantages offered by them such that microbial biosynthesis does not require heavy metals, solvents, strong acids, or bases unlike chemical synthesis, enzymes exhibit broader substrate specificity resulting in lesser by-products, natural synthetic pathways are already available for some compound with complex structures, engineering of biosynthetic pathways can further improve yield, and productivity of the compound of interest or novel pathways can be constructed in host microbe (Du et al. 2011).
The advances in recombinant DNA technology have prompted the development of microbial systems for bio-manufacturing of various valuable chemicals and natural products (Chemier et al. 2009). Microbes with well-studied genetics, physiology, and biochemistry like Escherichia coli and Saccharomyces cerevisiae are commonly used as bio-production platform. Pseudomonas putida, Bacillus, Cyanobacteria, and Streptomyces species have also been used for biosynthesis of target compounds (Chemier et al. 2009). Metabolic engineering and synthetic biology approaches have significantly contributed in development of engineered microbes for production of various useful compounds from simple and cheap substrates not only at laboratory scale but also at the industrial scale (Jullesson et al. 2015). Computational softwares are tremendously used in metabolic engineering to extract the information from big datasets as well as to assist in designing and optimizing the novel pathways in microbes (Reed et al. 2011).
15.2 Microbial Enzymes
The enzymes are biological molecules, usually proteinaceous in nature with the exception of ribozymes (catalytic RNA molecules), and they play crucial role in different stages of metabolism or biochemical reactions as bio-catalysts (Cech and Bass 1986; Gurung et al. 2013). Enzymes possess several features that make them attractive candidates for various applications such that they enhance the rate of reaction under mild physico-chemical conditions without being consumed, they are non-toxic, and they exhibit remarkable chemoselectivity, enantioselectivity, regioselectivity, and substrate specificity.
15.2.1 Potential Sources of Enzymes
Nature contributes an extensive amount of enzyme resources. In the beginning of enzyme biotechnology era, the plant tissues and animals were the most important sources of enzymes. However, currently microbes represent the largest and useful sources of many enzymes (Demain and Adrio 2008; Volesky et al. 1984). Most of enzymes which are used commercially are obtained from aerobic strains. Majority of microbial enzymes are derived from Aspergillus, Bacillus, Streptomyces, and Saccharomyces species (Headon and Walsh 1994). Microbes are usually preferred over plants and animals as a source of enzymes because they represent amicable and economical way for enzyme production in short time, microbes have shorter generation time and genetic manipulation can be easily performed, microbial enzyme expression is controllable, microbial enzymes are more stable as well as active, and production in larger quantities can be achieved (Anbu et al. 2015; Gurung et al. 2013).
Microbial enzymes are obtained from different microorganisms. For example, proteases of commercial applicability are produced mainly by bacteria species such as Pseudomonas, Clostridium, and Bacillus and also by some fungal species (Nigam 2013). Studies on enzyme isolation, their characterization, and production on bench and pilot scale are continuously increasing. Owing to their commercial applications, the market for industrial enzymes is widespread (Sanchez and Demain 2017; Adrio and Demain 2014). The market for industrial enzymes will reach to nearly $6.2 billion by 2020 with annual growth rate (CAGR) of 7% (Singh et al. 2016b). In general, numerous microbial enzymes are already being exploited in many different industrial processes.
15.2.2 Microbial Enzyme Production
Microbes produce vast variety of enzymes but the absolute amount of produced enzyme differs markedly even between the strains of same microbial species. Thus, for the production of the desired enzyme for commercial applications, the strain that exhibit highest yield is ideally selected (Underkofler et al. 1958). The enzyme-based product, which is newly introduced in market, can become a commercial success if it has a large existing market share and if it is economically viable. For the successful development of a commercial enzyme process, various requirements should be fulfilled including the ability of producer microbe to grow at a rapid rate on an inexpensive medium, production of the enzymes in high yields as well as at high concentration, minimal generation of enzyme contaminants and other metabolites in the fermentation of broth, the possibility to grow the microbe on a concentrated medium in a dense culture which improves the enzyme productivity in fermenters, and easy as well as inexpensive recovery of the enzyme from the culture media (Headon and Walsh 1994; Volesky et al. 1984).
Generally, the production of the desired enzymes begins with the screening of the microbes present in the collected environmental samples to identify the producer strain using suitable selection procedures. It is followed by optimization of the culture conditions, physico-chemical properties, and process parameters to maximize the production of target enzyme. The screening processes on laboratory scale focuses on the search for a high titre enzyme-producing microorganism, and they are usually labour intensive, monotonous, and time consuming (Yoo et al. 2017). The advent of genetic engineering approaches facilitated the cloning of the gene encoding for the target enzyme in microbes with defined growth conditions, with controllable gene expression, and with GRAS status (generally recognized as safe), leading to impressive enzyme yields. The construction of metagenomic library by cloning of total isolated DNA from environmental samples in suitable vector system, and subsequent function-based screening is another powerful approach that allows to explore the potential of biological diversity in different ecosystems for the identification of target enzyme (Thies et al. 2016). This approach circumvents the need of culturing and isolation of individual microbe in laboratory (Guazzaroni et al. 2015). Another approach to obtain superior enzyme producer strain is mutagenesis where the microbial cultures are exposed to mutagenic agents like chemicals, heat, and radiations. The screening for survival of cells is then performed to select the strain that can overproduce the target enzyme (Ghazi et al. 2014).
For production of target enzyme, the producer microbes are cultivated by inoculation of the pure culture into the suitable sterile medium. Submerged fermentation and solid-state fermentation (SSF) are the methods used for the enzyme cultivation (Renge et al. 2012). In submerged fermentation, the microorganisms are cultivated in a closed vessel (fermenter) containing liquid nutrient media and a high concentration of oxygen. The growing microbes release the target enzyme in extracellular environment i.e. in fermentation broth. The biomass is then removed from fermentation broth by centrifugation and the enzymes in the broth are then concentrated by evaporation of media, membrane filtration, or crystallization. This approach was used traditionally to prepare the target enzymes due to easy handling and ability to control physico-chemical factors (Mrudula and Murugammal 2011). In solid-state fermentation, microbes are cultivated on a solid substrate like wheat bran, wheat straw, and rice straw. This method is used for the cultivation of fungi such as Aspergillus and Penicillium to obtain enzymes such as amylase, proteases, and pectinases (Volesky et al. 1984).
15.2.3 Applications of Microbial Enzymes
The demand of microbial enzymes in various industries is expanding rapidly. Their application in few sectors is summarized.
15.2.3.1 Industrial Application
Microbial enzymes are used in various industrial applications including production of pharmaceuticals or pharmaceutically important intermediates, leather processing, textile industry, and paper and pulp, detergents, and biofuel production. In laundry detergents, proteases are extensively used to remove the proteinaceous dirt from the fabric. Proteolytic enzymes in many commercially available detergents are derived from the Bacillus species (Kumar et al. 2008). Other enzymes are also used in combination with proteases to improve the cleaning performance of the detergent, which includes lipases, amylases, and cellulases to remove fats or oils, remove starch residues, and brighten colour, respectively (Hasan et al. 2010). Several active pharmaceutical ingredients are being generated using the enzymes because of their remarkable specificity and selectivity. Carbonyl reductases have been used to obtain an intermediate for synthesis of blockbuster drugs and statins, by reduction of ethyl 4-chloro-3-oxobutanoate (COBE) to ethyl (S)-4-chloro-3-hydroxybutanoate ((S)-CHBE) (Xu et al. 2016). Atorvastatin which is an important ingredient of Lipitor, a cholesterol-lowering drug, has also been shown to produce through enzymatic synthesis (Bornscheuer et al. 2012). The commercial manufacturing of telaprevir, boceprevir, and esomeprazole drugs against hepatitis C virus involved in the oxidase-catalysed desymmetrization (Li et al. 2012).
Tyrosine phenol lyase expressed in Erwinia herbicola cells has been used to produce L-3,4-dihydroxyphenylalanine (L-DOPA), a drug for treatment of Parkinson’s disease (Patel 2008). Most of the enzymes used in textile industry are hydrolases like cellulases, pectinases, laccases, amylases, and catalases. These enzymes are being used as a substitute of stone wash, in bio-finishing, in bio-scouring, and in improving the look of material (Doshi and Shelke 2001). The involvement of lipases, cellulases, and xylanases has been reported for bioethanol production by decomposition of lignocellulosic material and also synthesis of fatty acid methyl esters (Liew et al. 2014). In leather industry, proteases and lipases are involved at different stages of leather processing. They are used in curing, soaking, dehairing, degreasing, tanning, and waste processing of leather (Choudhary et al. 2004).
15.2.3.2 Food
Microbial enzymes are significantly used in processing of food products such as cheese, beer, bread, and soft drinks, and the use of enzymes in manufacturing is increasing (Fernandes and Carvalho 2017). Amylases from the malted cereal, bacterial, or fungal sources are added to flour at the bakery and mill (Taylor and Richardson 1979). Another example of a microbial enzyme used in food industry is microbial transglutaminase which catalyses isopeptide bond formation between proteins. This property is widely used in manufacturing cheese and other dairy products, meat processing, manufacturing bakery products, and producing edible films (Kieliszek and Misiewicz 2014). Proteases are used in meat tenderization, ripening of cheese, and milk coagulation (Aruna et al. 2014). Lipases are also used in cheese flavour development and improving its texture. They are also in used in flavour development in butter and improving the shelf life of baking products (Aravindan et al. 2007). Galactosidases are used in lactose hydrolysis of milk-based products for lactose-intolerant people, in preparation of prebiotic food ingredient like galacto-oligosaccharides, and in lactose hydrolysis in whey (Rosenberg 2006).
15.2.3.3 Medicines
Therapeutic enzymes derived from microbial sources are used to treat various diseases. Nattokinase from Bacillus subtilis decreases the blood coagulation and removes existing thrombus. It is also used to decrease the lipids that can increase the chances of cardiovascular disease (Banerjee et al. 2004; Milner 2008). Streptokinase and urokinase are used for dissolving the blood clots in blocked blood vessels (Banerjee et al. 2004; Olson et al. 2011). Collagenases have been used to assist in healing skin burns and tumours in combination with antibiotics (Ostlie et al. 2012). In dental hygiene, enzymes like dextranase and cariogenanase from Penicillium funiculosum and Bacillus sp. are, respectively, used to reduce plaques and dental carries. Toothpastes containing a mixture of enzymes from Aspergillus niger and Aspergillus oryzae reduce calculus and soft accretions (Singh et al. 2016a). Tyrosine hydroxylase is responsible for catalysing the conversion of L-tyrosine to L-dopa, which is a useful agent in the treatment of Parkinson’s disease (Taylor and Richardson 1979).
15.2.4 Strategies for Enhancing Applicability of Existing Microbial Enzymes
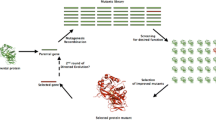
In spite of the significant advances in screening and selection approaches for identifying the novel enzymes to combat the ever-increasing industrial demands, there still remains the need of efficient ways to obtain enzymes with better catalytic performance for relevant industrial processes. In this connection, protein engineering strategies have been devised to improve the efficiency of existing enzymes (Kaushik et al. 2018). Protein engineering focuses on tailoring enzymes to overcome inherent shortcomings in existing enzymes like low activity, lack of specificity, and low stability or to introduce new functionalities. One of the protein engineering approaches is directed evolution or in vitro evolution, which mimics natural evolution process and does not require detailed knowledge on structure, function, and mechanistic aspects of target enzyme (Chen et al. 2012). It involves exposure of the gene encoding for the enzyme of interest to iterative rounds of random mutagenesis resulting in construction of library of gene variants; the resulting library is then screened for the variant that exhibits desired level of improvement (Chen and Arnold 1993). The process of directed evolution basically relies on effective mutagenesis method that generates significant genetic diversity and a robust screening method that leads to identification of the enzyme variant with desired catalytic characteristics as compared to wild-type enzyme.
The genetic diversity can be introduced by random mutagenesis methods like use of mutator strains, UV irradiation, chemical mutagenesis, error-prone PCR, and sequence saturation mutagenesis (SeSAM), or it can be introduced by gene recombination methods like DNA shuffling and oligonucleotide primer-based methods (Labrou 2010). Another protein engineering approach is rational redesign, which involves use of sequence and structure-based information with computational modelling to predict the hotspot residues which on mutagenesis are likely to result in improved enzyme functionalities. This approach dramatically reduces the library size and subsequently eliminates need of high-throughput screening methods. A semi-rational approach involving both the components of random and rational mutagenesis to design smart libraries with small size and high quality has shown to be practically more effective in generating tailor-made enzymes for specific needs (Lutz 2010). In recent years, engineering of access tunnel residues in enzymes with buried catalytic site has become an attractive approach to alter the enzyme properties. Access tunnels are the transport pathways that connect the buried active site of the enzyme to the exterior environment and allow the access or egress of substrates, reactive intermediates, solvents, ions, and products to the catalytic site (Damborsky et al. 2010; Timmis et al. 2010). Modification of the access tunnel lining residues doesn’t affect the architecture of catalytic site and thus increases the chances of getting functional clones with tailored properties. This strategy has been applied on several enzymes with buried active site to improve their catalytic properties (Kaushik et al. 2018; Prokop et al. 2012; Sandström et al. 2012). De novo protein design is another strategy that can allow to introduce new catalytic functions in protein scaffold such that de novo enzymes have been successfully designed that can catalyse the Diels-Alder and Kemp elimination reactions (Blomberg et al. 2013; Siegel et al. 2010). Recently, possibility to introduce de novo functional tunnels in existing protein has been demonstrated to facilitate creation of better and efficient enzymes (Brezovsky et al. 2016).
15.3 Proteins
The word protein is derived from the Greek word ‘protos’ that means first or ‘protieos’ which means primary (Aronson 2012). Proteins are the primary constituent of living things and are part of the molecular machinery in living organisms. They form the fundamental basis of the structure and function of life. Peptides and proteins are polymers of amino acids. They are the products of translation of mRNA within the living cell (Berg et al. 2002; Nelson et al. 2008). Variety of proteins/peptides derived from microbial systems have direct implication in production of vaccines, as therapeutic agents and as food supplements (Akash et al. 2015).
15.3.1 Microbial Proteins and Their Utility in Vaccine Production
A vaccine is a biological substance that stimulates the active acquired immune system of the body to act against a particular germ, thereby preventing the disease caused by it. Microbial surface proteins are associated with pathogenesis and thus represents major target for vaccine development. The commercially available vaccines contain attenuated pathogenic microbes or the microbial antigenic protein (Table 15.1). Various in silico tools have been developed by researchers so as to rapidly identify the surface proteins which can possibly display antigenic properties (Giombini et al. 2010). Approaches like whole-genome sequencing, labeling of surface proteins by selective biotinylation of whole bacteria, identification of immunogenic proteins from pathogens on protein microarrays, and enzymatic shaving of surface of bacteria with proteases have made identification of the surface antigens easier for vaccine development (Grandi 2010).
Strategies used in the production of vaccines are attenuation of the live pathogenic microbe, structural vaccinology, reverse vaccinology, epitope mapping, recombinant protein synthesis, and microbial cell-surface display. Structural vaccinology or structure-based antigen design involves the use of high-resolution structural analysis in distinguishing structural components of the antigen that elicit protective and disease-enhancing immunity (Dormitzer et al. 2008). This strategy has effectively guided design of engineered RSV F subunit antigen against respiratory syncytial virus, GBS (group B Streptococcus) pilus-based fusion protein, and an improved MenB (serogroup B meningococcus) single-domain fHbp (factor H-binding protein) antigen against meningitis (Dormitzer et al. 2012). Reverse vaccinology uses whole genome sequencing and immunological information of the pathogen to identify the suitable candidate vaccine antigens (Sette and Rappuoli 2010). Bexsero™ is a meningococcal group B vaccine that was developed through reverse vaccinology (Del Tordello et al. 2017). Recombinant protein subunit vaccines have been formulated with the help of protein antigens synthesized with heterologous host cells including Escherichia coli, Saccharomyces cerevisiae, and Pichia pastoris and mammalian cells. To design new recombinant protein production strategies, the gene sequence should be optimized to be stably expressed in the recombinant host cell. Optimizing culture conditions and induction protocols increases recombinant protein yields and it has been demonstrated in cultures of both P. pastoris and E. coli (Bill 2015). For posttranslational modifications such as glycosylation of the expressed protein, baculoviral system in insect cells is ideal (Demain and Vaishnav 2009). Microbial cell surface display is another strategy, which deals with expressing the protein of interest as a fusion to various anchoring motifs like surface proteins or their fragments. The host strain selected for display must be compatible with the protein of interest being displayed with minimal activity of proteases and should be able to cultivate without lysis (Lee et al. 2003). This approach has been used for the development of live vaccine where the heterologous epitopes were exposed on human commensal or attenuated pathogenic bacterial cells to evoke antibody responses specific to the antigen (Lee et al. 2000; Liljeqvist et al. 1997). More strategies for vaccine production are being developed for optimum yield in research laboratories globally in an effort to reduce the manufacturing costs, and microbes as hosts for the production of vaccine would be very advantageous in achieving this goal.
15.3.2 Toxins and Antimicrobial Peptides
15.3.2.1 Toxins
Microbial toxins are poisons produced biologically by either bacteria or fungi. They function as autonomous molecules, attacking specific cells in an organism by punching holes into the cell membranes or modifying intracellular components. Some bacteria secrete toxins into their surroundings to overcome host defence and are responsible for the symptoms of bacterial infections (de Wit 2013). Microbial toxins are typically soluble, stable, non-volatile, and highly bioactive compounds that may have cytotoxic, inflammatory, immunosuppressive, and carcinogenic effects (Korkalainen et al. 2017). Bacterial toxins are classified into two endotoxins and exotoxins while fungal toxins are classified into peptidic toxins and non-peptidic toxins. Despite of their detrimental effects, the toxins have been used as therapeutics, cosmetic agents, and adjuvants or drug delivery agents (Fabbri et al. 2008) (Table 15.2).
15.3.2.2 Antimicrobial Peptides and Proteins
Antimicrobial peptides and proteins (AMPs) or host defence peptides (HDP) are a diversified group of very small, normally positively charged molecules composed of varying number of amino acids. Multicellular organisms produce them as a first line of defence. They are used by unicellular organisms to compete for nutrients with other organisms. AMPs can be classified based on various parameters such as biological activity, 3D structure, and peptide family (Wang 2015). In 1939, Rene Dubos discovered and isolated the first microbial peptidic antibiotic Gramicidin from Bacillus brevis (renamed as Brevibacillus brevis) (Dubos and Cattaneo 1939). Since then, new AMPs are being discovered and their biochemical aspects were studied to shed light on their mechanism of action as well as their potential in clinical therapeutics. Some of these AMPs are listed in Table 15.3. Existing AMPs are being genetically engineered to create recombinant peptides with greater potency against infectious microorganisms. Thus, they represent attractive alternative to antibiotics in controlling pathogenic microbes and maintenance of human lifespan.
15.3.3 Microbial Proteins as a Food and Feed Source
Proteins are a dietary requirement for both humans and domesticated animals. Nutritious food is required in bulk quantities for livestock and pisciculture industry, both of which are among the major sources of proteins for humans. The concerns about future food security are raising due to rapidly increasing human population which is expected to reach ten billion in 2050 as per the United Nations report. Sustainable manufacturing of proteins in bulk will reduce the strain on the environment to provide sufficient nutritious food for the maintenance of these industries. Microbial proteins also known as single-cell proteins (SCP) can be a solution to this perplexing problem as bacteria already have high protein content and multiply exponentially using low-cost substrates under optimal conditions. SCP is a protein source from microbial cultures such as bacteria, yeast, filamentous fungi, and algae with the potential to be animal feed as well as human protein supplements. They are either dehydrated microbial cell culture or purified proteins derived from microbial cell culture (Ugbogu and Ugbogu 2016).
Some SCPs that are available commercially or under study are indicated in Table 15.4. SCPs offer various advantages: they contain high protein content (60–82% of dry cell weight) along with other nutrients, they are good source of essential amino acids such as lysine and methionine which are limited in most plant- and animal-based foods (Suman et al. 2015), the microbes have rapid generation time, they are genetically modifiable (e.g. for composition of amino acids), and they require less space as compared to conventional agriculture. However, SCPs have some disadvantages like high nucleic acid content, accumulation of uric acid crystals caused by bacterial SCPs leading to gout, possibility of allergic reactions with fungal SCPs as mycotoxins are allergens, and slow digestibility due to rigid cell wall. Currently SCPs are produced using solid-state fermentation (Jaganmohan et al. 2013). Recent advances in fermentation, extraction, downstream processing techniques, and optimization of substrates/conditions resulted in large-scale production of protein biomass. Production and marketing of a wider range of SCPs could be a promising step to alleviate food shortage and malnutrition.
15.3.4 Microbial Factories for Production of Recombinant Proteins
Microbes represent convenient system for production of proteins which are difficult to obtain from their native sources (Ferrer-Miralles et al. 2009). The use of microbes for protein production has increased in recent times due to the low cost, high productivity, and rapid use (Terpe 2006). A range of microbes including bacteria such as Escherichia coli and Bacillus megaterium, filamentous fungi such as Aspergillus niger and Trichoderma reesei, and yeast such as Saccharomyces cerevisiae and Pichia pastoris are exploited as recombinant cell factories. The first licensed protein drug successfully produced by recombinant DNA technology was human insulin in E. coli by Genentech and was commercialized by Eli Lilly in 1982. At present, nearly 400 drugs out of approved 650 protein drugs are produced by recombinant technologies (Sanchez-Garcia et al. 2016).
Recombinant protein production involves manipulation of the gene expression system of microbes with the aim of producing large amounts of recombinant protein tailored for a specific function. For a microbe to express foreign protein, the gene encoding the protein of interest is cloned into an expression vector with a suitable promoter gene and then introduced into the microbe. If the gene contains introns, it is cloned from a cDNA library as bacteria cannot excise introns. The plasmid is then transformed into a suitable host that is able to produce the desired protein. The transformed strain is transferred to liquid media and cultured. At a specific stage of growth, a chemical inducer triggers the promoter of the expression vector and induces expression of recombinant gene. The polypeptide produced folds into the recombinant protein of interest, which can be further purified by suitable purification approaches. The production of the target protein can also be scaled up from initial batch cultures to stirred tank bioreactors on fed-batch regimens to manufacture large protein biomass, which is then released and purified (Overton 2014).
E. coli has been one of the most commonly employed microbial cell factories for heterologous expression, and it has been used for the production of 30% of recombinant proteins approved by the FDA (Rosano and Ceccarelli 2014). It has been used for producing a range of biopharmaceuticals ranging from growth hormones (Goeddel et al. 1980; Olson et al. 1981), growth factors (Kwong et al. 2016), peptides (Zorko and Jerala 2010), and therapeutic proteins (Mane and Tale 2015). However, the major hurdles in exploiting E. coli as an expression host include inclusion body formation due to aggregation of overexpressed protein. Proteins derived from eukaryotes often undergo posttranslational modifications to achieve proper folding, but E. coli lacks such system and thus recombinant proteins expressed in E. coli microenvironment does not fold properly or misfolding occurs (Sharma and Chaudhuri 2017). The membrane proteins and the proteins with molecular weight more than 60 kDa are also difficult to express in E. coli. Toxic nature of heterologous protein and instability of the plasmid are other obstacles affecting successful expression in E. coli. Saccharomyces cerevisiae is another conventionally used host for recombinant protein production. Other non-conventional yeasts are Hansenula polymorpha, Pichia pastoris, and Yarrowia lipolytica (Kim et al. 2015).
The dominant role of yeast is seen in production of human blood proteins (Martinez et al. 2012), insulin analogues, and hepatitis vaccine (Wang et al. 2017). Efforts are being done to improve the titre, rate, and yield of the yeast cell factory through rational metabolic engineering in Saccharomyces cerevisiae. Multiple-genome integration was observed to be an ideal approach for generating stable strains with high copy numbers of heterologous genes. Strong glycolytic promoters (PGK1p, TPI1p, ADH1p) and inducible promoters have been developed to induce heterologous protein expression at various levels as the glycosylation capability of yeast is inappropriate for human proteins (Hou et al. 2012; Wang et al. 2017). One of the bottlenecks of protein production in yeasts is the protein secretory machinery, which may not be able to handle a high flux of proteins requiring specific posttranslational modification. This can result in missorting where the heterologous protein is targeted to the vacuole for degradation instead of being secreted. The use of systems biology integrates large-scale datasets (-omics) with mathematical modelling to direct metabolic engineering and site-directed mutagenesis towards overcoming the limitations of the protein secretion machinery (Martínez et al. 2012; Wang et al. 2017).
Information obtained using systems biology involving the study of the transcriptomics, proteomics, metabolomics, and metabolic flux analysis of P. pastoris, a methylotrophic yeast, is being utilized to enhance protein folding and secretion as well as engineer the recombinant protein process towards maximizing the yield and improving the yeast strain (Zahrl et al. 2017). High-throughput screening of improved strains with high protein yield in S. cerevisiae and P. pastoris specific to the target protein is the final step in the development of recombinant strains (Ahmad et al. 2014; Wang et al. 2017). Filamentous fungi are other candidates for recombinant protein production. They have mostly been used as robust cell factories for producing pharmaceutically relevant enzymes. Examples of recombinant enzymes are catalase, glucose oxidase, and phytase from Aspergillus niger and cellulose and xylanase from Trichoderma reesei (Archer 2000). Filamentous fungi have enormous potential in efficient large-scale production of recombinant proteins as they are cheap to cultivate and downstream processing is easier as the proteins are secreted through hyphae (Nevalainen and Peterson 2014). Numerous efforts have been made to develop filamentous fungi as a host for recombinant proteins, but further improvement is required for the expression of wider range of heterologous proteins. To achieve this, proteome profile of filamentous fungi like recombinant strains of Aspergillus nidulans is being performed to identify the bottlenecks in heterologous protein expression (Zubieta et al. 2018). These findings help us to understand the mechanisms underlying protein production and to rationally manipulate target genes for the improvement of fungal strains.
15.4 Secondary Metabolites
Secondary metabolites derived from microbes represent the important group of compounds with a wide range of applications. The term secondary metabolite has been introduced by Bu’LocK in 1961. Secondary metabolites are the low-molecular-weight products with no direct involvement in physiology and development of microbe but may render several benefits to the organism (Bu’Lock 1961). For instance, antibiotics are one of the well-known secondary metabolites, which confer selective growth advantage and better survival ability to the host microbe. Other examples of secondary metabolite from microbial origin with varied biological functions include antibiotics, alkaloids, pigments, antitumour agents, toxins, growth promoters, carotenoids, and enzyme inhibitors.
15.4.1 Microbial Source of Secondary Metabolites
Secondary metabolites or small molecule natural products are synthesized by prokaryotes like bacteria to eukaryotes like fungi, plants, and animals, although the secondary metabolite producing ability is unevenly distributed. Secondary metabolites are formed by the biosynthetic pathways which branch off from the primary metabolic pathways. Secondary metabolism in fungi occurs during stationary phase in the liquid cultures and is often linked to the onset of morphological developments in surface-grown cultures. Similarly, in bacteria the secondary metabolites are formed during the late growth phase. Nearly 20,000 so-called microbial secondary metabolites are known (Marinelli 2009). Among prokaryotes, the filamentous actinomycetes species has been reported to produce over 10,000 bioactive compounds, streptomyces produces 7600 compounds, and rare actinomycetes produces nearly 2500 bioactive compounds, and they produce 45% of known bioactive microbial metabolites, representing the largest producer group (Bérdy 2005). Streptomyces is the largest antibiotic-producing genus and it alone provides more than 60% of the antibiotics (Esnault et al. 2017).
The genome sequencing of model actinomycete Streptomyces coelicolor A3(2) led to identification of more than 20 gene clusters capable for coding the secondary metabolites (Bentley et al. 2002). The gene clusters (polyketide synthases type I and II, nonribosomal peptide synthetases) were found in its genome. This strain also produces metabolites like methylenomycin, prodigiosin, actinorhodin, and a calcium-dependent antibiotic. The microbes with lesser ability to produce secondary metabolites include mycoplasma, mycoplasmatales, and spirotheces. Among the eukaryotic fungi, ascomycetes and endophytic fungal species are frequent producers, while yeasts, phycomycetes, and slime moulds are less frequent producers. The fungal bioactive compounds constitute 38% of known microbial products (Bérdy 2005). It has been shown that a large number of microbial species that cannot grow under standard laboratory conditions, known as ‘unculturable’ strains, can also be potential source of novel secondary metabolites. Development of methods to culture such microbes would further allow the exploitation of microbial diversity to produce interesting metabolites (Lewis et al. 2010; Newman 2016).
15.4.2 Approaches for Isolation and Identification of Bioactive Secondary Metabolites
In 1929, the serendipitous discovery of antibiotic penicillin G from Penicillium notatum (Fleming 1929) established the therapeutic potential of this fungal secondary metabolite and further expedited the exploration of novel bioactive metabolites. Since then various microbial metabolites have been isolated including β-lactams, aminoglycosides, glycopeptides, tetracyclines, and cephalosporins. The classical approach leading to the antibiotic discovery was based on the growth inhibition of target microbes. However, in recent times the screening methods based on growth inhibition has turned out to be unsuccessful in identifying new antibiotics. This propelled the development of modern methodologies and techniques to accelerate the discovery process (Davies 2011).
15.4.2.1 Isolation of Secondary Metabolite Producing Microbes and Strain Improvement
The screening of microbial fermentation extracts to identify biologically active compound was practiced previously. For successful screening, the selection of growth conditions that can initiate the synthesis of secondary metabolites in microbes and the bioassays or analytical methods that allow detection of the secondary metabolite are the general requirements. Once the desired strain that can overproduce a particular compound is isolated, the next step involves improving the concentration of the compound. It may be achieved by optimization of the culture conditions like medium composition, pH, temperature, agitation, and aeration. Various additives can also be tested in culture media as limiting precursors of desired compound; e.g. lysine is added to the culture media as a precursor and cofactor to enhance the production of cephamycin by Streptomyces clavuligerus (Demain 1998; Gonzalez et al. 2003; Khetan et al. 1999).
The advent of recombinant DNA techniques led to manipulation and improvement of microbial strain for enhanced production of target secondary metabolite. In classical genetics, mutations are introduced randomly or on rational basis followed by screening/selection to identify the mutants with desired improvements (Sharma et al. 2014). The random screening method requires the limited knowledge of genetics, biochemistry, and physiology of biosynthetic pathway. On the other hand, rational screening requires basic knowledge of pathway regulation and product metabolism. For example, Streptomyces hygroscopicus mutant strain producing higher titre of rapamycin was obtained after mutagenesis and screening of parent culture (Cheng et al. 2001).
15.4.2.2 Mining Microbial Genomes for New Natural Products
The whole genome sequencing enabled rapid identification of the producer strains. Only specific regions of genome, namely, biosynthetic gene clusters, are involved in formation of valuable bioactive molecules. These gene clusters encode for proteins, which participate in synthesis of bioactive molecule using building blocks derived from primary metabolism. The ribosomal peptide synthetases and polyketide synthases have particularly much attention in recent years as they account for majority of structurally diverse, clinically and commercially important molecules (Naughton et al. 2017). Recently, microbial genome sequencing analysis has revealed the presence of numerous cryptic or orphan gene clusters which are responsible for production of a number of unknown secondary metabolites (Chiang et al. 2009). Various strategies have been devised to identify the metabolic products of the microbial cryptic gene clusters. It includes isotopic tracer technique, in vitro reconstitution, sequence analysis to predict physico-chemical properties of product, gene knockout or comparative metabolic profiling, and heterologous expression of cryptic gene cluster (Bentley 1999; Challis 2008; Davati and Habibi Najafi 2013). Web-based platforms like antiSMASH 2.0 (Blin et al. 2013), ClustScan (Starcevic et al. 2008), and CLUSEAN (Weber et al. 2009) have also been developed to automate the identification and characterization of bioactive secondary metabolites.
15.4.2.3 Metabolic Engineering
Metabolic engineering is the approach to modify the existing metabolic pathway or combining the pathways or enzymes from different host to single microbe with an objective of improved production of target compound or to produce new compounds in host cells from simple, inexpensive starting material (Keasling 2010). The important design parameters in production of secondary metabolite are yield and productivity. Thus, in optimizing the production of microbial metabolite, the primary aim is to enhance the metabolic flux towards the compound of interest and to minimize the flux towards the by-products. Increasing the flux towards the product increases both the overall productivity and yield (Nielsen 1998). Metabolic engineering has been successfully applied for the efficient production of amino acids like L-threonine and L-valine, antimalarial drugs like artemisinin, anticancer drugs like taxol, antibiotics like β-lactams and cephalosporins, and benzylisoquinoline alkaloids (Davati and Habibi Najafi 2013; Minami et al. 2008).
15.4.3 Biosynthesis of Secondary Metabolites and Its Regulation
The secondary metabolite production is not only strain dependent but it is also influenced by diverse regulatory conditions like growth stage, optimum supply of nutrients, and the regulatory effects imparted by them (Liu et al. 2013). The production of particular secondary metabolite initiates due to the recognition of specific signal, transduction of this signal to generate the required regulators followed by regulator-mediated activation of biosynthetic gene cluster to produce the secondary metabolite, and then transport of the produced metabolite (Chang and Stewart 1998). The physiological regulation for production of secondary metabolites usually differs with the kind of microbe and metabolic pathway involved. It has been shown that when antibiotic-producing strain like streptomyces are cultivated under conditions that leads to nutritional stress, the stationary growth phase conforms to the onset of biosynthesis of secondary metabolite (Bibb 2005). Nutrients in culture media have been reported to be exerting their regulatory effects by activating or repressing the transcription factors and regulatory proteins.
Fine-tuning of optimal concentration of carbon source in medium is an important parameter to balance the qualitative production of the secondary metabolite and growth of the microbe. Presence of glucose as a carbon source usually improves the growth of the host but could interfere with the production of varied secondary metabolites like cephalosporin, alkaloids, and actinomycin. However, in some cases glucose acts as a good substrate for growth and differentiation as well as for the secondary metabolite production like aflatoxin (Luchese and Harrigan 1993). Glucose in high concentration of 100 g/L maximizes the production of the anticapsin by Streptomyces griseoplanus. The type of nitrogen sources employed in the medium affects the secondary metabolic pathways differently. Ammonium ions cause inhibition of novobiocin, cephamycin, and rifamycin production (Aharonowitz 1980). The biosynthesis of gibberellins by the fungus Gibberella fujikuroi was shown to be suppressed by the presence of ammonium ions and glucose as well (Brückner 1992). L-amino acids were found to positively influence the production of actinomycin D by Streptomyces parvulus (Bennett et al. 1977). The type of L-amino acids added to synthetic media strongly influenced the production of mycotoxins like emodin, catenarin, and islandicin by isolates of Pyrenophora tritici-repentis from wheat (Bouras et al. 2016). The concentration of inorganic phosphate that favours growth of the microbes generally exerts negative control on synthesis of secondary metabolites. However, in some cases high phosphate concentration is well tolerated for production of secondary metabolite as reported in the case of avermectin biosynthesis by Streptomyces avermitilis (Čurdová et al. 1989). Secondary metabolite production also requires trace elements like manganese, iron, and zinc, although their required optimal concentration may vary depending on the metabolite to be produced.
15.4.4 Applications of Secondary Metabolites
Secondary metabolites are valuable compounds with a wide range of applications (Williams et al. 1989). The microbial secondary metabolites are now progressively used as drugs for the treatment of various diseases in place of synthetic drugs. They are widely used as uterocontractants, anti-inflammatory agent, anticancer drug, cholesterol-lowering agent, hypotensive agent, immunosuppressant, antibacterial/antifungal agent, and antiparasitic agent (Gonzalez et al. 2003). They are also being used for non-medical applications like weed management and plant growth regulation (Cutler 1995; Sadia et al. 2015).
Secondary metabolites in addition to their known activities have also shown alternative activities, and thus they have been unexpectedly used as possible solution to other diseases for which the effective treatment is not available. β-Lactams are known for their antibiotic action, and their derivatives have also displayed antitumour prodrug activity (Xing et al. 2008). Prodigines, pigmented antibiotics, display antifungal, antiprotozoal, antimalarial, anticancer, and immunosuppressive activities in addition to their antibiotic activity (Williamson et al. 2006). Squalestatin, a fungal metabolite known for lowering the cholesterol by inhibiting 3-hydroxy-3-methylglutaryl-CoA reductase enzyme of cholesterol biosynthesis pathway, has been identified as a potential drug against prion disease (Bate et al. 2004). Thus, exploring the new functions of existing secondary metabolites along with speeding up the process of identification of the novel secondary metabolites can allow the better targeting of the diseases for which currently no effective solutions are present (Vaishnav and Demain 2011).
15.5 Valuable Chemicals
Numerous chemicals are used in everyday life to serve various purposes such that they act as drugs for treating diseases, as fertilizers, as disinfectants, as industrial solvents, as pest control agents, and as health or hygiene products. These chemicals are produced using defined chemical synthesis reactions where simple chemicals are reacted to generate target products. The chemicals can be categorized into bulk chemicals, fine chemicals, and speciality chemicals. Bulk or commodity chemicals are produced on large scales and used as intermediates for production of other chemicals. Fine chemicals are produced as pure chemical substance in small quantities unlike bulk chemicals and are often used for production of speciality chemicals such as agrochemicals and pharmaceuticals.
15.5.1 Microbial Platform for Production of Bio-based Chemicals
The need of improved biotechnological processes for production of target chemicals is increasing with each passing year owing to the limited fossil resources and serious climate changes (Wu et al. 2018). The popularity of microbial systems as a tool for biological synthesis of chemicals is gaining momentum as they can produce a variety of complex molecules, and they require relatively less energy resources as compared to chemical synthetic techniques, thus making it a feasible option to produce fine chemicals. Many fine chemicals have been found to be ideal for microbial biosynthesis as they are intermediates or products of the natural metabolic pathways of various microbes. Industries that benefit from microbial biosynthesis include food, agriculture, chemical, pharmaceutical, and cosmetics (Gurung et al. 2013). The natural biosynthetic pathway in a microbial cell can also be modified by combining various approaches to produce target chemicals.
-
1.
Enzymatic synthesis of fine chemicals where enzymes with or without coenzymes convert the substrate to the chemical of interest. The genes responsible for expressing the enzymes capable of catalysing the bio-based reaction are identified and isolated. The computational tools are used to mine genome and transcriptome data to identify novel biosynthetic pathways and enzymes (Lautru et al. 2005; Zhao et al. 2013). The identified enzymatic synthesis system is introduced into microbial cell to create a microbial cell factory.
-
2.
Metabolic engineering is used to increase yield as well as productivity by redesigning the existing biosynthetic pathway to optimize the production of target compound. Various tools used in designing metabolic pathways are biochemical network integrated computational explorer (BNICE), RetroPath, GEM-Path, OptStrain, and DESHARKY (Chae et al. 2017). Flux balance analysis is a method that indicates how gene deletion and expression can be manipulated to distribute carbon towards chemicals of interest without blocking or reducing cell proliferation. This is a standard method to optimize metabolic pathways (Orth et al. 2010).
-
3.
Genetic manipulation according to the redesigned pathway map obtained by computer simulation can be performed to give a relatively efficient recombinant strain of the selective microorganism. This involves heterologous expression, overexpression, downregulation, deletion, or mutation of the gene of interest.
Despite of various advantages offered by microbial systems for bio-based production of chemicals and other valuable materials, their potential could not be fully exploited as new alternative energy sources are coming to existence. Moreover, higher production cost of bio-products, lower yields, relatively decreased efficiency of bioprocesses as compared to chemical processes, and longer production periods due to slow microbial growth are other factors hindering the development of bio-based products at commercial scale (Chen 2012).
15.5.2 Bio-manufacturing of Bulk and Speciality Chemicals
The production of bulk chemicals is primarily driven by petrochemical feedstocks. However, as demand for bio-based chemicals is increasing, the chemical processes are being replaced with microbial catalysts and improved fermentation methods. Thus, the possibilities to utilize renewable resources for sustainable production of commodity chemicals are rapidly progressing in the current scenario (Hermann et al. 2007). Bio-based production of several commodity chemicals including alcohols, organic acids, amino acids, aromatic amines, diols, polyhydroxyalkanoates, and polysaccharides through fermentation has been successfully reported (Table 15.3). In parallel to fermentation approaches, system metabolic engineering has also been successfully used in production of commodity chemicals like amino acids (Ma et al. 2017). Such engineering strategies have been applied mainly in Corynebacterium glutamicum and Escherichia coli for amino acid production. Dedicated attempts are being made by researchers worldwide to construct novel pathways in microbes for bio-manufacturing of target bulk chemicals (Shin et al. 2013) (Table 15.5).
Like bulk chemicals, the fine chemicals were also conventionally produced by energy-intensive multistep chemical processes that resulted in high levels of wastes and by-products. However, the efforts are being made to exploit biological routes for chemical production on par with chemical synthetic techniques. The fine chemicals are synthesized by microbes, either as products of their natural metabolic pathways or by genetically engineering their metabolic pathways to produce the desired product (Hara et al. 2014). A range of speciality chemicals like isoprenoids, flavonoids, alkaloids, aromatic compounds, polyphenols, peptides, drugs, organic acids, and oligosaccharides has been reported to be produced by microbes using synthetic biology principles. The production strategy of few chemicals in microbial systems has been summarized.
15.5.2.1 Artemisinin
The antimalarial drug artemisinin is a sesquiterpene lactone with an endoperoxide bridge. It is naturally produced by Artemisia annua (sweet wormwood) (Liu et al. 2006; Rathod et al. 1997). However, the methods for extraction of artemisinin were not economical and resulted in insufficient production levels. This led to development of recombinant strains as microbial factories to produce artemisinic acid, which is a precursor of artemisinin. This precursor was then converted to artemisinin by following synthetic organic chemistry steps (Paddon and Keasling 2014). In one of the studies, E. coli strain was engineered to synthesize the precursor amorphadiene by introduction of heterologous, high-flux isoprenoid pathway from S. cerevisiae to E. coli (Martin et al. 2003). The pathway genes were coexpressed with a codon modified amorphadiene synthase (Martin et al. 2003) resulting in a recombinant strain that could produce amorphadiene up to 24 mg/L. In a follow-up study, a higher yield of amorphadiene was achieved by utilizing a two-phase partitioning bioreactor (TPPB) strategy that resulted in efficient separation of amorphadiene from the fermentation broth (Newman et al. 2006). Much later, production of artemisinic acid at gram scale (25 g/L) was achieved by optimizing the expression of CYP71AV1:CPR1 along with co-expression of cytochrome b5 and two dehydrogenases (Paddon et al. 2013).
15.5.2.2 γ-Aminobutyric Acid (GABA)
GABA, a non-protein amino acid, is synthesized by microbes, plants, and animals. It acts as an inhibitory neurotransmitter in the central nervous system of mammals and as a stimulant for immune cells (Dhakal et al. 2012). In microbes, it is involved in spore germination in the case of B. megaterium and N. crassa (Foerster and Foerster 1973; Schmit et al. 1975), while it provides resistance to acidic pH in L. lactis, E. coli, and other microbes (Castanie-Cornet et al. 1999; Sanders et al. 1998). It has a wide application in food, cosmetic, and pharmaceutical industry. The biosynthetic route of GABA involves a single-step reaction involving decarboxylation of glutamate to GABA, catalysed by glutamate decarboxylase (GAD) (Ueno 2000). The main GABA-producing microbes are lactic acid bacteria (LAB) (Dhakal et al. 2012). Corynebacterium glutamicum expressing Escherichia coli glutamate decarboxylase (GAD) has been engineered for production of GABA, and in order to further enhance its production, protein kinase G has been disrupted resulting in increased intracellular concentration of glutamate precursor and eventually improved yield of GABA (Okai et al. 2014).
15.5.2.3 Resveratrol
Resveratrol (trans-3,5,4′-trihydroxystilbene) is a plant-derived polyphenol that is present in red wine. It is used as an antioxidant, in cosmetic and food industry and as therapeutic agent due to its anticarcinogenic, anti-inflammatory, anti-diabetic, and anti-ageing properties (Beekwilder et al. 2006; Mei et al. 2015). As such, production of polyphenols in microbes is a challenging task due to antibacterial and antifungal activity of these compounds (Daglia 2012). However, still metabolic engineering principles have been utilized to produce such compounds via microbial systems. Engineered E. coli and S. cerevisiae strains expressing 4-coumarate:coenzyme A ligase from tobacco and stilbene synthase from grapes has been developed to achieve resveratrol accumulations in the culture medium by supplying p-coumaric acid as a precursor molecule. These engineered strains showed relatively low production titres (Beekwilder et al. 2006). Another research group investigated various constructs for resveratrol synthesis, different E. coli strains, promoters and gene expression combinations, sequence, and structure analysis to achieve high titres (g/l) of resveratrol from biotransformation of p-coumaric acid (Lim et al. 2011).
15.5.2.4 Cinnamic Acid
Cinnamic acid is a phenylpropanoid acid, which is used as a cinnamon flavouring agent, in high performance thermoplastics, as precursor for chemical compounds, and as nutraceutical and pharmaceutical products (Vargas-Tah and Gosset 2015). It can be obtained by either chemical synthesis or by extraction from source plant. It can also be produced by engineered microbes like Escherichia coli, Streptomyces lividans, Saccharomyces cerevisiae, and Pseudomonas putida. Genes encoding phenylalanine ammonia-lyase (PAL) and tyrosine ammonia-lyase (TAL) have been expressed in E. coli and S. cerevisiae to allow the conversion of L-phenylalanine and L-tyrosine to cinnamic acid and p-hydroxycinnamic acids (p-coumaric acid) (Vannelli et al. 2007). Pseudomonas putida S12 strain was engineered for conversion of p-hydroxycinnamic acid from glucose (Nijkamp et al. 2007). The heterologous expression of PAL encoding gene from Streptomyces maritimus in Streptomyces lividans resulted in production of cinnamic acid from glucose with maximum titre of 450 mg/L (Noda et al. 2011).
15.6 Conclusion
Microbes play a significant role in maintaining the ecological sustainability. They synthesize a wide range of products like antibiotics, toxins, antimicrobial peptides or proteins, and enzymes that help them to thrive in the varied environmental conditions and provide them an ability to compete with other species in their ecological niche. These products are valuable due to their application in industrial bioprocesses, as they are used in nutraceuticals, in agriculture for production of drugs or vaccine, and for generation of clean fuel and bioremediation. For instance, enzymes derived from microbial source have potential applicability in different fields as they are used in pharmaceutical industry, in processing of food products, as therapeutic agents, and in production of biofuels and bioplastics. In order to further enhance their usefulness, protein engineering methods are being employed to generate custom-made biocatalysts for the desired processes.
Microbial surface proteins with antigenic properties represent major target for generation of vaccines, and various strategies have been devised to utilize microbial systems for production of recombinant vaccines to decrease the production costs. Antimicrobial peptides derived from microbes are another group of interesting biomolecules with therapeutic applications owing to their utility as alternative to antibiotics. Similarly, microbial toxins produced by bacteria or fungi are utilized in cosmetic industry, as therapeutic agent, and for drug delivery. Microbial proteins or whole microbial cells are used as food source and feed supplement. A variety of bioactive secondary metabolites have been derived from microbes. The discovery of new bioactive compounds has been achieved by advent of modern techniques like genome sequencing, metabolic engineering, proteomics, and advance computational tools. Metabolic engineering and synthetic biology principles have been successfully employed to develop the engineered microbial strains as cell factories for heterologous expression of recombinant proteins and bio-based production of bulk and speciality chemicals. The natural biosynthetic pathways can be either fine-tuned or novel pathways can be assembled in host microbe to optimize the production of the target compounds. In conclusion, microbes share a major role in bio-production of valuable chemicals, toxins, metabolites, proteins, and peptides with broad scope of applications.
References
Abou-Zeid A, Fouad M, Yassein M (1978) Microbiological production of acetone-butanol by Clostridium acetobutylicum. Zentralbl Bakteriol Parasitenkunde Infektionskr Hyg Zweite Naturwiss Abt Mikrobiol Landwirtsch Technol Umweltschutzes 133:125–134
Adrio JL, Demain AL (2014) Microbial enzymes: tools for biotechnological processes. Biomol Ther 4:117–139
Ageitos J, Sánchez-Pérez A, Calo-Mata P, Villa T (2017) Antimicrobial peptides (AMPs): ancient compounds that represent novel weapons in the fight against bacteria. Biochem Pharmacol 133:117–138
Agnandji ST et al (2017) Safety and immunogenicity of rVSVΔG-ZEBOV-GP Ebola vaccine in adults and children in Lambaréné, Gabon: a phase I randomised trial. PLoS Med 14:e1002402
Aharonowitz Y (1980) Nitrogen metabolite regulation of antibiotic biosynthesis. Ann Rev Microbiol 34:209–233
Ahmad M, Hirz M, Pichler H, Schwab H (2014) Protein expression in Pichia pastoris: recent achievements and perspectives for heterologous protein production. Appl Microbiol Biotechnol 98:5301–5317
Akash MSH, Rehman K, Tariq M, Chen S (2015) Development of therapeutic proteins: advances and challenges. Turk J Biol 39:343–358
Anbu P, Gopinath SC, Chaulagain BP, Tang T-H, Citartan M (2015) Microbial enzymes and their applications in industries and medicine. Biomed Res Int 2015:816419
Andrade MR, Costa JA (2007) Mixotrophic cultivation of microalga Spirulina platensis using molasses as organic substrate. Aquaculture 264:130–134
Aravindan R, Anbumathi P, Viruthagiri T (2007) Lipase applications in food industry. Indian J Biotechnol 6:141–158
Archer DB (2000) Filamentous fungi as microbial cell factories for food use. Curr Opin Biotechnol 11:478–483
Aronson SM (2012) A proliferation of pro-words Rhode island. Med J 95:371
Aruna K, Shah J, Birmole R (2014) Production and partial characterization of alkaline protease from Bacillus tequilensis strains CSGAB0139 isolated from spoilt cottage cheese. Int J Appl Biol Pharm 5:201–221
Bachran C, Leppla SH (2016) Tumor targeting and drug delivery by anthrax toxin. Toxins 8:197
Baldauf KJ, Royal JM, Hamorsky KT, Matoba N (2015) Cholera toxin B: one subunit with many pharmaceutical applications. Toxins 7:974–996
Banerjee A, Chisti Y, Banerjee U (2004) Streptokinase—a clinically useful thrombolytic agent. Biotechnol Adv 22:287–307
Bate C, Salmona M, Diomede L, Williams A (2004) Squalestatin cures prion-infected neurons and protects against prion neurotoxicity. J Biol Chem 279:14983–14990
Bechinger B (1997) Structure and functions of channel-forming peptides: magainins, cecropins, melittin and alamethicin. J Membr Biol 156:197–211
Beekwilder J, Wolswinkel R, Jonker H, Hall R, de Vos CR, Bovy A (2006) Production of resveratrol in recombinant microorganisms. Appl Environ Microbiol 72:5670–5672
Bennett WM, Singer I, Golper T, Feig P, Coggins CJ (1977) Guidelines for drug therapy in renal failure. Ann Intern Med 86:754–783
Bentley R (1999) Secondary metabolite biosynthesis: the first century. Crit Rev Biotechnol 19:1–40
Bentley SD et al (2002) Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141
Bérdy J (2005) Bioactive microbial metabolites. J Antibiot 58:1
Berg JM, Tymoczko JL, Stryer L (2002) Protein structure and function. In: Biochemistry, 5th edn. W. H. Freeman & Co Ltd, New York
Bibb MJ (2005) Regulation of secondary metabolism in streptomycetes. Curr Opin Microbiol 8:208–215
Biebl H, Menzel K, Zeng AP, Deckwer WD (1999) Microbial production of 1, 3-propanediol. Appl Microbiol Biotechnol 52:289–297
Bill RM (2015) Recombinant protein subunit vaccine synthesis in microbes: a role for yeast? J Pharm Pharmacol 67:319–328
Blin K, Medema MH, Kazempour D, Fischbach MA, Breitling R, Takano E, Weber T (2013) antiSMASH 2.0—a versatile platform for genome mining of secondary metabolite producers. Nucleic Acids Res 41:W204–W212
Blomberg R et al (2013) Precision is essential for efficient catalysis in an evolved Kemp eliminase. Nature 503:418
Bornscheuer U, Huisman G, Kazlauskas R, Lutz S, Moore J, Robins K (2012) Engineering the third wave of biocatalysis. Nature 485:185
Bouras N, Holtz MD, Aboukhaddour R, Strelkov SE (2016) Influence of nitrogen sources on growth and mycotoxin production by isolates of Pyrenophora tritici-repentis from wheat. Crop J 4:119–128
Brezovsky J et al (2016) Engineering a de novo transport tunnel. ACS Catal 6:7597–7610
Brückner B (1992) Regulation of gibberellin formation by the fungus Gibberella fujikuroi. In: Secondary metabolites: their function and evolution. Wiley, Chichester, pp 129–143
Bu’Lock J (1961) Intermediary metabolism and antibiotic synthesis. In: Advances in applied microbiology, vol 3. Elsevier, pp 293–342
Burroughs JR, Anderson RL (2015) Cosmetic botulinum toxin applications: general considerations and dosing. In: Pearls and pitfalls in cosmetic oculoplastic surgery. Springer, New York, pp 393–394
Castanie-Cornet M-P, Penfound TA, Smith D, Elliott JF, Foster JW (1999) Control of acid resistance in Escherichia coli. J Bacteriol 181:3525–3535
Cech TR, Bass BL (1986) Biological catalysis by RNA. Annu Rev Biochem 55:599–629
Chae S, Hwang E, Shin H-S (2006) Single cell protein production of Euglena gracilis and carbon dioxide fixation in an innovative photo-bioreactor. Bioresour Technol 97:322–329
Chae TU, Choi SY, Kim JW, Ko Y-S, Lee SY (2017) Recent advances in systems metabolic engineering tools and strategies. Curr Opin Biotechnol 47:67–82
Challis GL (2008) Mining microbial genomes for new natural products and biosynthetic pathways. Microbiology 154:1555–1569
Chandola TR et al (2017) ROTAVAC® does not interfere with the immune response to childhood vaccines in Indian infants: a randomized placebo controlled trial. Heliyon 3:e00302
Chang C, Stewart RC (1998) The two-component system: regulation of diverse signaling pathways in prokaryotes and eukaryotes. Plant Physiol 117:723–731
Chemier JA, Fowler ZL, Koffas MA, Leonard E (2009) Trends in microbial synthesis of natural products and biofuels. Adv Enzymol Relat Area Mol Biol 76:151
Chen G-Q (2012) New challenges and opportunities for industrial biotechnology. Microb Cell Factories 11:111
Chen K, Arnold FH (1993) Tuning the activity of an enzyme for unusual environments: sequential random mutagenesis of subtilisin E for catalysis in dimethylformamide. Proc Natl Acad Sci 90:5618–5622
Chen MM, Snow CD, Vizcarra CL, Mayo SL, Arnold FH (2012) Comparison of random mutagenesis and semi-rational designed libraries for improved cytochrome P450 BM3-catalyzed hydroxylation of small alkanes. Protein Eng Des Sel 25:171–178
Cheng YR, Huang J, Qiang H, LIN WL, Demain AL (2001) Mutagenesis of the rapamycin producer Streptomyces hygroscopicus FC904. J Antibiot 54:967–972
Chiang Y-M, Lee K-H, Sanchez JF, Keller NP, Wang CC (2009) Unlocking fungal cryptic natural products. Nat Prod Commun 4:1505
Choudhary R, Jana A, Jha M (2004) Enzyme technology applications in leather processing. Indian J Chem Technol 11:659–671
Čurdová E, Jechová V, Zima J, Vaněk Z (1989) The effect of inorganic phosphate on the production of avermectin in Streptomyces avermitilis. J Basic Microbiol 29:341–346
Cutler HG (1995) Microbial natural products that affect plants, phytopathogens, and certain other microorganisms. Crit Rev Plant Sci 14:413–444
Daglia M (2012) Polyphenols as antimicrobial agents. Curr Opin Biotechnol 23:174–181
Damborsky J, Chaloupkova R, Pavlova M, Chovancova E, Brezovsky J (2010) Structure–function relationships and engineering of haloalkane dehalogenases. In: Handbook of hydrocarbon and lipid microbiology. Springer, Berlin/Heidelberg, pp 1081–1098
Davati N, Habibi Najafi MB (2013) Overproduction strategies for microbial secondary metabolites: a review. Int J Life Sci Pharma Res 3:23–27
Davies J (2011) How to discover new antibiotics: harvesting the parvome. Curr Opin Chem Biol 15:5–10
Del Tordello E, Rappuoli R, Delany I (2017) Reverse vaccinology: exploiting genomes for vaccine design. In: Human vaccines. Elsevier, pp 65–86
Demain AL (1998) Microbial natural products: alive and well in 1998. Nat Biotechnol 16:3
Demain AL, Adrio JL (2008) Contributions of microorganisms to industrial biology. Mol Biotechnol 38:41
Demain AL, Vaishnav P (2009) Production of recombinant proteins by microbes and higher organisms. Biotechnol Adv 27:297–306
Dhakal R, Bajpai VK, Baek K-H (2012) Production of GABA (γ-aminobutyric acid) by microorganisms: a review. Braz J Microbiol 43:1230–1241
Dien BS, Nichols NN, O’bryan PJ, Bothast RJ (2000) Development of new ethanologenic Escherichia coli strains for fermentation of lignocellulosic biomass. Appl Biochem Biotechnol 84:181–196
Dormitzer PR, Ulmer JB, Rappuoli R (2008) Structure-based antigen design: a strategy for next generation vaccines. Trends Biotechnol 26:659–667
Dormitzer PR, Grandi G, Rappuoli R (2012) Structural vaccinology starts to deliver. Nat Rev Microbiol 10:807
Doshi R, Shelke V (2001) Enzymes in textile industry-an environment-friendly approach. Indian J Fibre Text Res 26:202–205
Du J, Shao Z, Zhao H (2011) Engineering microbial factories for synthesis of value-added products. J Ind Microbiol Biotechnol 38:873–890
Dubos RJ (1939) Studies on a bactericidal agent extracted from a soil bacillus: I. Preparation of the agent. Its activity in vitro. J Exp Med 70:1
Dubos RJ, Cattaneo C (1939) Studies on a bactericidal agent extracted from a soil bacillus: III. Preparation and activity of a protein-free fraction. J Exp Med 70:249–256
Esnault C et al (2017) Strong antibiotic production is correlated with highly active oxidative metabolism in Streptomyces coelicolor M145. Sci Rep 7:200
Essig A et al (2014) Copsin, a novel peptide-based fungal antibiotic interfering with the peptidoglycan synthesis. J Biol Chem 289:34953–34964. M114. 599878
Fabbri A, Travaglione S, Falzano L, Fiorentini C (2008) Bacterial protein toxins: current and potential clinical use. Curr Med Chem 15:1116–1125
Fernandes P, Carvalho F (2017) Microbial enzymes for the food industry. In: Biotechnology of microbial enzymes. Elsevier, Amsterdam, pp 513–544
Ferrer-Miralles N, Domingo-Espín J, Corchero JL, Vázquez E, Villaverde A (2009) Microbial factories for recombinant pharmaceuticals. Microb Cell Factories 8:17
Fleming AG (1929) Responsibilities and opportunities of the private practitioner in preventive medicine. Can Med Assoc J 20:11
Foerster CW, Foerster HF (1973) Glutamic acid decarboxylase in spores of Bacillus megaterium and its possible involvement in spore germination. J Bacteriol 114:1090–1098
Foster JA, Wulc AE, Straka D, Cahill KV, Czyz C, Tan J (2018) Cosmetic uses of botulinum toxin. In: Manual of oculoplastic surgery. Springer, New York, pp 165–172
Franzoi M, van Heuvel Y, Thomann S, Schurch N, Kallio PT, Venier P, Essig A (2017) Structural insights into the mode of action of the peptide antibiotic copsin. Biochemistry 56:4992–5001
Garodia S, Naidu P, Nallanchakravarthula S (2017) QUORN: an anticipated novel protein source
Ghazi S, Sepahy AA, Azin M, Khaje K, Khavarinejad R (2014) UV mutagenesis for the overproduction of xylanase from Bacillus mojavensis PTCC 1723 and optimization of the production condition. Iran J Basic Med Sci 17:844
Giombini E, Orsini M, Carrabino D, Tramontano A (2010) An automatic method for identifying surface proteins in bacteria: SLEP. BMC Bioinformatics 11:39
Goeddel DV et al (1980) Human leukocyte interferon produced by E. coli is biologically active. Nature 287:411
Gonzalez JB, Fernandez F, Tomasini A (2003) Microbial secondary metabolites production and strain improvement. Indian J Biotechnol 2:322–333
Grandi G (2010) Bacterial surface proteins and vaccines. F1000 Biol Rep 2:36
Guazzaroni ME, Silva-Rocha R, Ward RJ (2015) Synthetic biology approaches to improve biocatalyst identification in metagenomic library screening. Microb Biotechnol 8:52–64
Gurung N, Ray S, Bose S, Rai V (2013) A broader view: microbial enzymes and their relevance in industries, medicine, and beyond. Biomed Res Int 2013:329121
Hara KY, Araki M, Okai N, Wakai S, Hasunuma T, Kondo A (2014) Development of bio-based fine chemical production through synthetic bioengineering. Microb Cell Factories 13:173
Hasan F, Shah AA, Javed S, Hameed A (2010) Enzymes used in detergents: lipases. Afr J Biotechnol 9:4836–4844
Haugh M, Gresset-Bourgeois V, Macabeo B, Woods A, Samson SI (2017) A trivalent, inactivated influenza vaccine (Vaxigrip®): summary of almost 50 years of experience and more than 1.8 billion doses distributed in over 120 countries. Expert Rev Vaccines 16:545–564
Headon D, Walsh G (1994) The industrial production of enzymes. Biotechnol Adv 12:635–646
Hermann B, Blok K, Patel MK (2007) Producing bio-based bulk chemicals using industrial biotechnology saves energy and combats climate change. Environ Sci Technol 41:7915–7921
Hodgson J (1994) Bulk amino–acid fermentation: technology and commodity trading. Nat Biotechnol 12:152
Hou J, Tyo KE, Liu Z, Petranovic D, Nielsen J (2012) Metabolic engineering of recombinant protein secretion by Saccharomyces cerevisiae. FEMS Yeast Res 12:491–510
Ivers LC et al (2015) Immunogenicity of the bivalent oral cholera vaccine Shanchol in Haitian adults with HIV infection. J Infect Dis 212:779–783
Jaganmohan P, Daas BP, Prasad S (2013) Production of single cell protein (SCP) with Aspergillus terreus using solid state fermentation. Eur J Biol Sci 5:38–43
Jullesson D, David F, Pfleger B, Nielsen J (2015) Impact of synthetic biology and metabolic engineering on industrial production of fine chemicals. Biotechnol Adv 33:1395–1402
Kataoka M, Sasaki M, Hidalgo A-RG, Nakano M, Shimizu S (2001) Glycolic acid production using ethylene glycol-oxidizing microorganisms. Biosci Biotechnol Biochem 65:2265–2270
Kaushik S et al (2018) Impact of the access tunnel engineering on catalysis is strictly ligand-specific. FEBS J 285:1456–1476
Keasling JD (2010) Manufacturing molecules through metabolic engineering. Science 330:1355–1358
Keating GM (2016) Shingles (herpes zoster) vaccine (zostavax®): a review in the prevention of herpes zoster and postherpetic neuralgia. BioDrugs 30:243–254
Kennedy SB et al (2017) Phase 2 placebo-controlled trial of two vaccines to prevent Ebola in Liberia. N Engl J Med 377:1438–1447
Khan AI, Islam MT, Qadri F (2017) Safety of oral cholera vaccines during pregnancy in developing countries. Hum Vaccin Immunother 13:2245–2246
Khetan A, Malmberg LH, Kyung YS, Sherman DH, Hu WS (1999) Precursor and cofactor as a check valve for cephamycin biosynthesis in Streptomyces clavuligerus. Biotechnol Prog 15:1020–1027
Kieliszek M, Misiewicz A (2014) Microbial transglutaminase and its application in the food industry. A review. Folia Microbiol 59:241–250
Kim H, Yoo SJ, Kang HA (2015) Yeast synthetic biology for the production of recombinant therapeutic proteins. FEMS Yeast Res 15:1–16
Korkalainen M et al (2017) Synergistic proinflammatory interactions of microbial toxins and structural components characteristic to moisture-damaged buildings. Indoor Air 27:13–23
Kumar D, Savitri TN, Verma R, Bhalla T (2008) Microbial proteases and application as laundry detergent additive. Res J Microbiol 3:661–672
Kwong KW, Sivakumar T, Wong W (2016) Intein mediated hyper-production of authentic human basic fibroblast growth factor in Escherichia coli. Sci Rep 6:33948
Labrou NE (2010) Random mutagenesis methods for in vitro directed enzyme evolution. Curr Protein Pept Sci 11:91–100
Lacadena J et al (2007) Fungal ribotoxins: molecular dissection of a family of natural killers. FEMS Microbiol Rev 31:212–237
Lautru S, Deeth RJ, Bailey LM, Challis GL (2005) Discovery of a new peptide natural product by Streptomyces coelicolor genome mining. Nat Chem Biol 1:265–269
Lee J-S, Shin K-S, Pan J-G, Kim C-J (2000) Surface-displayed viral antigens on Salmonella carrier vaccine. Nat Biotechnol 18:645
Lee SY, Choi JH, Xu Z (2003) Microbial cell-surface display. Trends Biotechnol 21:45–52
Levin MJ, Buchwald UK, Gardner J, Martin J, Stek JE, Brown E, Popmihajlov Z (2018) Immunogenicity and safety of zoster vaccine live administered with quadrivalent influenza virus vaccine. Vaccine 36:179–185
Lewis K, Epstein S, D’Onofrio A, Ling LL (2010) Uncultured microorganisms as a source of secondary metabolites. J Antibiot 63:468
Li T et al (2012) Efficient, chemoenzymatic process for manufacture of the boceprevir bicyclic [3.1. 0] proline intermediate based on amine oxidase-catalyzed desymmetrization. J Am Chem Soc 134:6467–6472
Li X-F et al (2018) Development of a chimeric Zika vaccine using a licensed live-attenuated flavivirus vaccine as backbone. Nat Commun 9:673
Liew WH, Hassim MH, Ng DK (2014) Review of evolution, technology and sustainability assessments of biofuel production. J Clean Prod 71:11–29
Liljeqvist S, Samuelson P, Hansson M, Nguyen TN, Binz H, Ståhl S (1997) Surface display of the cholera toxin B subunit on Staphylococcus xylosus and Staphylococcus carnosus. Appl Environ Microbiol 63:2481–2488
Lim CG, Fowler ZL, Hueller T, Schaffer S, Koffas MA (2011) High-yield resveratrol production in engineered Escherichia coli. Appl Environ Microbiol 77:3451–3460. https://doi.org/10.1128/AEM.02186-10
Link E et al (1992) Tetanus toxin action: inhibition of neurotransmitter release linked to synaptobrevin proteolysis. Biochem Biophys Res Commun 189:1017–1023
Liu C, Zhao Y, Wang Y (2006) Artemisinin: current state and perspectives for biotechnological production of an antimalarial drug. Appl Microbiol Biotechnol 72:11–20
Liu G, Chater KF, Chandra G, Niu G, Tan H (2013) Molecular regulation of antibiotic biosynthesis in Streptomyces. Microbiol Mol Biol Rev 77:112–143
Luchese RH, Harrigan W (1993) Biosynthesis of aflatoxin—the role of nutritional factors. J Appl Bacteriol 74:5–14
Lutz S (2010) Beyond directed evolution—semi-rational protein engineering and design. Curr Opin Biotechnol 21:734–743
Ma Q et al (2017) Systems metabolic engineering strategies for the production of amino acids. Synth Syst Biotechnol 2:87–96
Mahan KM et al (2018) Production of single cell protein from agro-waste using Rhodococcus opacus. J Ind Microbiol Biotechnol 45(9):795–801
Mane P, Tale V (2015) Overview of microbial therapeutic enzymes. Int J Curr Microbiol Appl Sci 4:17–26
Marinelli F (2009) From microbial products to novel drugs that target a multitude of disease indications. Methods Enzymol 458:29–58
Martin VJ, Pitera DJ, Withers ST, Newman JD, Keasling JD (2003) Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat Biotechnol 21:796
Martínez JL, Liu L, Petranovic D, Nielsen J (2012) Pharmaceutical protein production by yeast: towards production of human blood proteins by microbial fermentation. Curr Opin Biotechnol 23:965–971
Masuyer G, Conrad J, Stenmark P (2017) The structure of the tetanus toxin reveals pH-mediated domain dynamics. EMBO Rep 18:1306–1317
Mazet I, Vey A (1995) Hirsutellin A, a toxic protein produced in vitro by Hirsutella thompsonii. Microbiology 141:1343–1348
McWilliams A (2012) Microbial products: technologies, applications and global markets. BCC Research
Mei Y-Z, Liu R-X, Wang D-P, Wang X, Dai C-C (2015) Biocatalysis and biotransformation of resveratrol in microorganisms. Biotechnol Lett 37:9–18
Milner M (2008) Nattokinase: clinical updates from doctors support its safety and efficacy. FOCUS Allergy Res Group News: Lett
Minami H, Kim J-S, Ikezawa N, Takemura T, Katayama T, Kumagai H, Sato F (2008) Microbial production of plant benzylisoquinoline alkaloids. Proc Natl Acad Sci 105:7393–7398
Mishra B, Reiling S, Zarena D, Wang G (2017) Host defense antimicrobial peptides as antibiotics: design and application strategies. Curr Opin Chem Biol 38:87–96
Morris AP, Estes MK (2001) VIII. Pathological consequences of rotavirus infection and its enterotoxin. Am J Physiol Gastrointest Liver Physiol 281:G303–G310
Moyes DL et al (2016) Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 532:64
Mrudula S, Murugammal R (2011) Production of cellulase by Aspergillus niger under submerged and solid state fermentation using coir waste as a substrate. Braz J Microbiol 42:1119–1127
Nagasawa T, Nakamura T, Yamada H (1990) Production of acrylic acid and methacrylic acid using Rhodococcus rhodochrous J1 nitrilase. Appl Microbiol Biotechnol 34:322–324
Nakamura J, Hirano S, Ito H, Wachi M (2007) Mutations of the Corynebacterium glutamicum NCgl1221 gene, encoding a mechanosensitive channel homolog, induce L-glutamic acid production. Appl Environ Microbiol 73:4491–4498
Naughton LM, Romano S, O’Gara F, Dobson AD (2017) Identification of secondary metabolite gene clusters in the Pseudovibrio genus reveals encouraging biosynthetic potential toward the production of novel bioactive compounds. Front Microbiol 8:1494
Nayeem M, Chauhan K, Khan S, Rattu G, Dhaka RK, Sidduqui H (2017) Optimization of low-cost substrate for the production of single cell protein using Kluyveromyces marxianus. Pharma Innov J 6:22–25
Nelson DL, Lehninger AL, Cox MM (2008) Lehninger principles of biochemistry. Macmillan
Nevalainen H, Peterson R (2014) Making recombinant proteins in filamentous fungi-are we expecting too much? Front Microbiol 5:75
Newman DJ (2016) Predominately uncultured microbes as sources of bioactive agents. Front Microbiol 7:1832
Newman JD et al (2006) High-level production of amorpha-4, 11-diene in a two-phase partitioning bioreactor of metabolically engineered Escherichia coli. Biotechnol Bioeng 95:684–691
Nielsen J (1998) The role of metabolic engineering in the production of secondary metabolites. Curr Opin Microbiol 1:330–336
Nigam PS (2013) Microbial enzymes with special characteristics for biotechnological applications. Biomol Ther 3:597–611
Nijkamp K, Westerhof RM, Ballerstedt H, De Bont JA, Wery J (2007) Optimization of the solvent-tolerant Pseudomonas putida S12 as host for the production of p-coumarate from glucose. Appl Microbiol Biotechnol 74:617–624
Noda S et al (2011) Cinnamic acid production using Streptomyces lividans expressing phenylalanine ammonia lyase. J Ind Microbiol Biotechnol 38:643–648
Okai N, Takahashi C, Hatada K, Ogino C, Kondo A (2014) Disruption of pknG enhances production of gamma-aminobutyric acid by Corynebacterium glutamicum expressing glutamate decarboxylase. AMB Express 4:20
Olombrada M, Martínez-del-Pozo Á, Medina P, Budia F, Gavilanes JG, García-Ortega L (2014) Fungal ribotoxins: natural protein-based weapons against insects. Toxicon 83:69–74
Olson KC et al (1981) Purified human growth hormone from E. coli is biologically active. Nature 293:408
Olson DM et al (2011) A qualitative assessment of practices associated with shorter door-to-needle time for thrombolytic therapy in acute ischemic stroke. J Neurosci Nurs 43:329–336
Orth JD, Thiele I, Palsson BØ (2010) What is flux balance analysis? Nat Biotechnol 28:245
Ostlie DJ et al (2012) Topical silver sulfadiazine vs collagenase ointment for the treatment of partial thickness burns in children: a prospective randomized trial. J Pediatr Surg 47:1204–1207
Overton TW (2014) Recombinant protein production in bacterial hosts. Drug Discov Today 19:590–601
Paddon CJ, Keasling JD (2014) Semi-synthetic artemisinin: a model for the use of synthetic biology in pharmaceutical development. Nat Rev Microbiol 12:355
Paddon CJ et al (2013) High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 496:528
Pasricha S, Pearson J (2016) Lifting the veil on fungal toxins. Nature Publishing Group
Patel RN (2008) Synthesis of chiral pharmaceutical intermediates by biocatalysis. Coord Chem Rev 252:659–701
Prado-Rubio OA, Jørgensen JB, Jørgensen SB (2010) Systematic model analysis for single cell protein (scp) production in a u-loop reactor. In: Computer aided chemical engineering, vol 28. Elsevier, Amsterdam, pp 319–324
Prokop Z, Gora A, Brezovsky J, Chaloupkova R, Stepankova V, Damborsky J (2012) Engineering of protein tunnels: keyhole-lock-key model for catalysis by the enzymes with buried active sites, vol 3. Wiley-VCH, Weinheim
Quesada-Moraga E, Alain V (2004) Bassiacridin, a protein toxic for locusts secreted by the entomopathogenic fungus Beauveria bassiana. Mycol Res 108:441–452
Rao MB, Varma A, Deshmukh SS (2010) Production of single cell protein, essential amino acids, and xylanase by Penicillium janthinellum. BioResources 5:2470–2477
Raspor P, Goranovič D (2008) Biotechnological applications of acetic acid bacteria. Crit Rev Biotechnol 28:101–124
Rathod PK, McErlean T, Lee P-C (1997) Variations in frequencies of drug resistance in Plasmodium falciparum. Proc Natl Acad Sci 94:9389–9393
Ray G, Noori MT, Ghangrekar M (2017) Novel application of peptaibiotics derived from Trichoderma sp. for methanogenic suppression and enhanced power generation in microbial fuel cells. RSC Adv 7:10707–10717
Reed JL, Senger RS, Antoniewicz MR, Young JD (2011) Computational approaches in metabolic engineering. J Biomed Res 2010
Renge V, Khedkar S, Nandurkar NR (2012) Enzyme synthesis by fermentation method: a review. Sci Rev Chem Comm 2:585e590
Rodríguez-Zavala J, Ortiz-Cruz M, Mendoza-Hernández G, Moreno-Sánchez R (2010) Increased synthesis of α-tocopherol, paramylon and tyrosine by Euglena gracilis under conditions of high biomass production. J Appl Microbiol 109:2160–2172
Rosano GL, Ceccarelli EA (2014) Recombinant protein expression in Escherichia coli: advances and challenges. Front Microbiol 5:172
Rosenberg ZM-M (2006) Current trends of β-galactosidase application in food technology. J Food Nutr Res 45:47–54
Sadia S, Qureshi R, Khalid S, Nayyar BG, Zhang JT (2015) Role of secondary metabolites of wild marigold in suppression of Johnson grass and Sun spurge. Asian Pac J Trop Biomed 5:733–737
Sanchez S, Demain AL (2017) Useful microbial enzymes—an introduction. In: Biotechnology of microbial enzymes. Elsevier, Amsterdam, pp 1–11
Sanchez-Garcia L, Martín L, Mangues R, Ferrer-Miralles N, Vázquez E, Villaverde A (2016) Recombinant pharmaceuticals from microbial cells: a 2015 update. Microb Cell Factories 15:33
Sanders JW, Leenhouts K, Burghoorn J, Brands JR, Venema G, Kok J (1998) A chloride-inducible acid resistance mechanism in Lactococcus lactis and its regulation. Mol Microbiol 27:299–310
Sandström AG, Wikmark Y, Engström K, Nyhlén J, Bäckvall JE (2012) Combinatorial reshaping of the Candida antarctica lipase A substrate pocket for enantioselectivity using an extremely condensed library. Proc Natl Acad Sci 109:78–83
Schmidt-Dannert C (2017) The future of biologically inspired next-generation factories for chemicals. Microb Biotechnol 10:1164–1166
Schmit J, Edson CM, Brody S (1975) Changes in glucosamine and galactosamine levels during conidial germination in Neurospora crassa. J Bacteriol 122:1062–1070
Sette A, Rappuoli R (2010) Reverse vaccinology: developing vaccines in the era of genomics. Immunity 33:530–541
Sharma N (2016) How does recent understanding of molecular mechanisms in botulinum toxin impact therapy? In: Botulinum toxin therapy manual for dystonia and spasticity. InTech open. doi:https://doi.org/10.5772/66696
Sharma A, Chaudhuri TK (2017) Revisiting Escherichia coli as microbial factory for enhanced production of human serum albumin. Microb Cell Factories 16:173
Sharma A, Kumari N, Menghani E (2014) Bioactive secondary metabolites: an overview. Int J Sci Eng Res 5:1395–1407
Sharma S et al (2018) Immunogenicity and safety of the first indigenously developed Indian tetravalent influenza vaccine (split virion) in healthy adults ≥18 years of age: a randomized, multicenter, phase II/III clinical trial. Hum Vaccin Immunother 14(6):1362–1369
Shin JH, Kim HU, Kim DI, Lee SY (2013) Production of bulk chemicals via novel metabolic pathways in microorganisms. Biotechnol Adv 31:925–935
Siegel JB et al (2010) Computational design of an enzyme catalyst for a stereoselective bimolecular Diels-Alder reaction. Science 329:309–313
Singh Saharan B, Grewal A, Kumar P (2014) Biotechnological production of polyhydroxyalkanoates: a review on trends and latest developments. Chin J Biol 2014:802984
Singh HB, Jha A, Keswani C (2016a) Intellectual property issues in biotechnology. CABI, Wallingford/Boston
Singh R, Kumar M, Mittal A, Mehta PK (2016b) Microbial enzymes: industrial progress in 21st century. 3 Biotech 6:174
Stanley M (2007) Prevention strategies against the human papillomavirus: the effectiveness of vaccination. Gynecol Oncol 107:S19–S23
Starcevic A, Zucko J, Simunkovic J, Long PF, Cullum J, Hranueli D (2008) ClustScan: an integrated program package for the semi-automatic annotation of modular biosynthetic gene clusters and in silico prediction of novel chemical structures. Nucleic Acids Res 36:6882–6892
Stewart G, Panchal CJ, Russell I, Sills AM (1983) Biology of ethanol-producing microorganisms. Crit Rev Biotechnol 1:161–188
Suman G, Nupur M, Anuradha S, Pradeep B (2015) Single cell protein production: a review. Int J Curr Microbiol App Sci 4(9): 251–262
Tan JP, Md. Jahim J, Wu TY, Harun S, Kim BH, Mohammad AW (2014) Insight into biomass as a renewable carbon source for the production of succinic acid and the factors affecting the metabolic flux toward higher succinate yield. Ind Eng Chem Res 53:16123–16134
Taylor MJ, Richardson T (1979) Applications of microbial enzymes in food systems and in biotechnology. In: Advances in applied microbiology, vol 25. Elsevier, Burlington, pp 7–35
Terpe K (2006) Overview of bacterial expression systems for heterologous protein production: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol 72:211
Thies S et al (2016) Metagenomic discovery of novel enzymes and biosurfactants in a slaughterhouse biofilm microbial community. Sci Rep 6:27035
Timmis KN, McGenity T, Van Der Meer JR, de Lorenzo V (2010) Handbook of hydrocarbon and lipid microbiology, vol DLII. Springer, Berlin, p 4716
Ueno H (2000) Enzymatic and structural aspects on glutamate decarboxylase. J Mol Catal B Enzym 10:67–79
Ugbogu E, Ugbogu O (2016) A review of microbial protein production: prospects and challenges. Fuw Trends Sci Technol J 1:182–185
Underkofler L, Barton R, Rennert S (1958) Production of microbial enzymes and their applications. Appl Microbiol 6:212
Vaishnav P, Demain AL (2011) Unexpected applications of secondary metabolites. Biotechnol Adv 29:223–229
Vandenbergh PA (1993) Lactic acid bacteria, their metabolic products and interference with microbial growth. FEMS Microbiol Rev 12:221–237
Vandenberghe LP, Soccol CR, Pandey A, Lebeault J-M (1999) Microbial production of citric acid. Braz Arch Biol Technol 42:263–276
Vannelli T, Qi WW, Sweigard J, Gatenby AA, Sariaslani FS (2007) Production of p-hydroxycinnamic acid from glucose in Saccharomyces cerevisiae and Escherichia coli by expression of heterologous genes from plants and fungi. Metab Eng 9:142–151
Vargas-Tah A, Gosset G (2015) Production of cinnamic and p-hydroxycinnamic acids in engineered microbes. Front Bioeng Biotechnol 3:116
Verier A, Chenal A, Babon A, Ménez A, Gillet D (2006) Engineering of bacterial toxins for research and medicine. In: The comprehensive sourcebook of bacterial protein toxins, 3rd edn. Elsevier, Amsterdam/Boston, p 991–1007
Volesky B, Luong JH, Aunstrup K (1984) Microbial enzymes: production, purification, and isolation. Crit Rev Biotechnol 2:119–146
Wang G (2015) Improved methods for classification, prediction, and design of antimicrobial peptides. In: Computational peptidology. Springer, New York/Heidelberg, pp 43–66
Wang G, Huang M, Nielsen J (2017) Exploring the potential of Saccharomyces cerevisiae for biopharmaceutical protein production. Curr Opin Biotechnol 48:77–84
Weber T, Rausch C, Lopez P, Hoof I, Gaykova V, Huson D, Wohlleben W (2009) CLUSEAN: a computer-based framework for the automated analysis of bacterial secondary metabolite biosynthetic gene clusters. J Biotechnol 140:13–17
Wee Y-J, Kim J-N, Ryu H-W (2006) Biotechnological production of lactic acid and its recent applications. Food Technol Biotechnol 44:163–172
Williams DH, Stone MJ, Hauck PR, Rahman SK (1989) Why are secondary metabolites (natural products) biosynthesized? J Nat Prod 52:1189–1208
Williamson NR, Fineran PC, Leeper FJ, Salmond GP (2006) The biosynthesis and regulation of bacterial prodiginines. Nat Rev Microbiol 4:887
de Wit PJ (2013) Microbial toxins in the green world. FEMS Microbiol Rev 37:1–2
Wu F, Cao P, Song G, Chen W, Wang Q (2018) Expanding the repertoire of aromatic chemicals by microbial production. J Chem Technol Biotechnol 93:2804–2816
Xing B, Rao J, Liu R (2008) Novel beta-lactam antibiotics derivatives: their new applications as gene reporters, antitumor prodrugs and enzyme inhibitors. Mini Rev Med Chem 8:455–471
Xu Q, Tao W-Y, Huang H, Li S (2016) Highly efficient synthesis of ethyl (S)-4-chloro-3-hydroxybutanoate by a novel carbonyl reductase from Yarrowia lipolytica and using mannitol or sorbitol as cosubstrate. Biochem Eng J 106:61–67
Yang X, Yousef AE (2018) Antimicrobial peptides produced by Brevibacillus spp.: structure, classification and bioactivity: a mini review. World J Microbiol Biotechnol 34:57
Yoo YJ, Feng Y, Kim Y-H, Yagonia CFJ (2017) Fundamentals of enzyme engineering. Springer, p X, 209
Zahrl RJ, Peña DA, Mattanovich D, Gasser B (2017) Systems biotechnology for protein production in Pichia pastoris. FEMS Yeast Res 17:fox068
Zhao S et al (2013) Discovery of new enzymes and metabolic pathways by using structure and genome context. Nature 502:698
Zheng P, Dong J-J, Sun Z-H, Ni Y, Fang L (2009) Fermentative production of succinic acid from straw hydrolysate by Actinobacillus succinogenes. Bioresour Technol 100:2425–2429
Zhu X, Siegert M, Yates MD, Logan BE (2015) Alamethicin suppresses methanogenesis and promotes acetogenesis in bioelectrochemical systems. Appl Environ Microbiol 81:3863–3868
Zorko M, Jerala R (2010) Production of recombinant antimicrobial peptides in bacteria. In: Antimicrobial peptides. Springer, pp 61–76
Zubieta MP, Contesini FJ, Rubio MV, Gonçalves AESS, Gerhardt JA, Prade RA, Damasio ARL (2018) Protein profile in Aspergillus nidulans recombinant strains overproducing heterologous enzymes. Microb Biotechnol 11:346–358
Acknowledgement
SK is thankful to the Department of Biotechnology (DBT) (BT/P19588/BIC/101/425/2016) for their financial assistance and National Institute of Pharmaceutical Education and Research (NIPER), Guwahati, for providing the necessary support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Johnson, A., Deshmukh, P., Kaushik, S., Sharma, V. (2019). Microbial Bio-production of Proteins and Valuable Metabolites. In: Singh, D., Gupta, V., Prabha, R. (eds) Microbial Interventions in Agriculture and Environment. Springer, Singapore. https://doi.org/10.1007/978-981-13-8391-5_15
Download citation
DOI: https://doi.org/10.1007/978-981-13-8391-5_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-8390-8
Online ISBN: 978-981-13-8391-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)



