Abstract
Paroxysmal nocturnal hemoglobinuria (PNH) is a nonmalignant, clonal hematopoietic stem cell disorder, which is characterized by features of hemolysis, thrombosis, and bone marrow (BM) failure. The genetic mechanism behind PNH is a somatic mutation in a gene known as phosphatidylinositol glycan class A (PIGA), present on chromosome “X.” PIGA gene is required for the synthesis of glycosylphosphatidylinositol (GPI), the anchor through which many proteins are attached to the cell membrane. These proteins are collectively known as GPI-anchored proteins (GPI-APs) [1, 2]. Among these GPI-APs are CD55 and CD59, the two important complement regulatory proteins. Absence of these leads to complement-mediated red blood cell lysis, one of the characteristic features of PNH [3, 4]. The term “PNH,” however, appears imprecise as only a fraction of patients presents with hemoglobinuria, which is also not always nocturnal.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a nonmalignant, clonal hematopoietic stem cell disorder, which is characterized by features of hemolysis, thrombosis, and bone marrow (BM) failure. The genetic mechanism behind PNH is a somatic mutation in a gene known as phosphatidylinositol glycan class A (PIGA), present on chromosome “X.” PIGA gene is required for the synthesis of glycosylphosphatidylinositol (GPI), the anchor through which many proteins are attached to the cell membrane. These proteins are collectively known as GPI-anchored proteins (GPI-APs) [1, 2]. Among these GPI-APs are CD55 and CD59, the two important complement regulatory proteins. Absence of these leads to complement-mediated red blood cell lysis, one of the characteristic features of PNH [3, 4]. The term “PNH,” however, appears imprecise as only a fraction of patients presents with hemoglobinuria, which is also not always nocturnal.
It is a well-known fact that BM failure syndrome, especially aplastic anemia (AA), has a frequent association of PNH. A fair proportion of AA patients, as high as 70%, have been found to harbor a PNH clone, depending upon the sensitivity of the screening method used [5]. The clone size in these patients is usually much smaller as compared to those seen in classic PNH patients [6, 7]. However, a proportion of these patients may exhibit the clonal expansion and can progress to clinical hemolytic PNH. Hence, a careful screening to detect the presence of PNH clone in these cases is of utmost importance. The drug eculizumab, a humanized monoclonal antibody which inhibits the action of complement C5, has been a boon for PNH patient. Before its advent, a fair proportion of patients (~35%) used to die within 5 years of diagnosis even with the administration of the best available treatment option [8]. This drug significantly reduces the symptoms associated with PNH and the transfusion requirement of these patients. It has been shown to improve the life expectancy as well as the quality of life of PNH patient. Eculizumab (Soliris, Alexion Pharmaceuticals) is one of the costliest drugs of the world, and this fact again underscores the utility of an accurate clone size estimation [PNH clone in MDS and other diseases need to be also included here].
Presence of PNH clones in myelodysplastic syndrome is also a well-established fact. With a stringent flow cytometry-based screening, about 35% of refractory anemia can show the presence of PNH clone, and there can be a clonal expansion of PNH in the cases with MDS. It has now been well established that PNH is more frequently noted in MDS patients who present with BM failure. These patients with an associated PNH clone are less likely to transform into leukemia and have a better prognosis [9,10,11].
There are also reports of finding a PNH clone in JAK2-mutated myeloproliferative neoplasm [12]. However, their exact significance as well as follow-up of the clone sizes is largely unknown.
2 Historical Perspective of PNH
Some of the landmark developments related to PNH are summarized as follows:
1882: Paul Strubing from Germany first identified PNH as an entity, separate from paroxysmal cold hemoglobinuria (PCH) and march hemoglobinuria. He concluded that sleep played an important role in hemolysis. Accumulation of lactic acid and carbon dioxide because of slowing of the circulation during sleep was reasoned for this phenomenon. According to him, normal RBCs were resistant to acidic conditions; while, the defective RBCs were sensitive and got lysed [13].
Early twentieth Century: Marchiafava and Micheli got interested in this disease, and PNH got its eponym as Marchiafava–Micheli disease [14].
1911: Dutch physician Hijmans van den Berg first suggested the role of a plasma factor, now known as complement, in hemolysis of PNH patients. It was shown that when the RBCs from PNH patients were suspended in serum of normal subjects or patients’ own serum, they got lysed because of the presence of complements in the serum [15].
1928: Dutch physician Ennekin first used the term “paroxysmal nocturnal hemoglobinuria” [16].
1937: Thomas Hale Ham reported that treatment of PNH patients with sodium bicarbonate reduces hemoglobinemia and hemoglobinuria. He performed some in vitro tests by acidifying the plasma with CO2 or lactic acid and putting the patients’ RBCs in it. This test remained one of the most common and useful tests for screening of PNH. He observed that there was rapid hemolysis when the serum or plasma was acidified. This hemolysis could be inhibited by adding sodium bicarbonate. An important additional information was that the red cells from blood group “O” volunteers did not hemolyze when resuspended in patients’ serum or plasma. He thus concluded that the main defect lied in the red cells of these patients, whereas the factor for the lysis is present in plasma of all individuals. He also noted that the lysis was not noted when the acidified serum was heated at 60 degrees and thus concluded that a thermolabile factor (complement) was responsible for the hemolysis [17, 18].
1939: Ham and Dingle published a landmark paper titled “Certain immunological aspects of the hemolytic mechanisms with special reference to serum complement” that had repercussion on the PNH research for next five decades [19].
1954: Pillemer identified the properdin system (the alternative complement pathway system) which was later confirmed to be involved in PNH pathogenesis [20].
1963: Dacie identified the phenotypic mosaicism in PNH RBC [21].
1966: Rosse developed an assay to test the complement sensitivity of PNH RBCs, better known as complement lysis sensitivity assay. Based on this test, he concluded that PNH mostly has a mosaic of two abnormal populations of RBC, which were later known as type II and type III cells [22].
1969: Aster and Enright demonstrated that platelets and neutrophils from PNH patients are abnormally sensitive to complement-mediated lysis [23]. Hoffmann showed the presence of factors that inhibited complement-mediated hemolysis in human RBC stroma [24, 25].
1970: Studies by Oni and colleagues indicated the monoclonal nature of the complement-sensitive erythrocytes [26].
1974: Hoffmann used the name decay accelerating factor (DAF) describing the functional property of the substance that enhanced the decay rate of classic pathway C3 convertase. This is now commonly known as CD55 [27].
1979: Stern and Rosse demonstrated the mosaic nature of granulocytes, similar to RBCs [28].
1983: Pangburn et al. demonstrated functional as well as immunochemical evidence of DAF deficiency in PNH [29].
1986: It was first hypothesized that on the hematopoietic cells of PNH, all the deficient proteins are GPI anchored, and all the GPI-APs are deficient in case of PNH [30].
1987: The complex structure of the GPI anchor was fully elucidated. It was suggested that the expression of the GPI-AP on cell surface would require multiple enzymes and cofactors.
I989: Holguin and colleagues reported the isolation membrane inhibitor of reactive lysis from the cell surface of normal human RBCs. This substance is now commonly known as CD59 [31].
1993: Kinoshita and colleagues suggested the defect in a common gene in the complementation class A. This gene was named as phosphatidylinositol glycan A (PIGA). Due to a defect in this gene, PNH cells fail to synthesize N-acetylglucosamine phosphatidylinositol [32].
Early 1990s: Flow cytometric detection of cells lacking the expression of GPI AP.
2000: Use of fluorescent aerolysin (FLAER) for better sensitive detection of PNH clones using flow cytometry [33].
2008: Two hit concept in PNH, first hit leading to PIGA mutation, the second hit usually an epigenetic phenomenon giving survival advantage for the proliferation of PNH clones [34].
2010: ICCS guideline for flow cytometry-based detection of PNH clones [35].
2012: Practical guidelines for high sensitivity analysis and monitoring of PNH clones by flow cytometry was published [36].
3 Diagnostic Modalities for PNH
3.1 In Vitro Methods Demonstrating Complement-Mediated Hemolysis
Introduction of these tests started with the discovery that the lysis of RBC in PNH is dependent on a thermolabile serum factor (now known as a complement). Increased susceptibility of RBC lysis with activated complement could be demonstrated by a variety of tests like acidified serum [18], thrombin [37], cold antibody lysis [38], sucrose [39], cobra venom [40], and inulin [41]. Acidified serum, cobra venom, and inulin activate the complement via the alternative pathway; whereas cold antibody test and thrombin test activate the complement via the classic pathway. In sucrose lysis tests, the low ionic strength leads to nonspecific binding of IgG to the cell surface and subsequent activation of complement via classic as well as an alternate pathway. Among the abovementioned tests, the Hams acidified serum lysis and sucrose lysis tests were most commonly used. But these have now become obsolete owing to their cumbersome nature, low sensitivity, specificity, and not being quantitative. The classic Hams test is negative if <5% type III PNH cells are present. Additionally, it may give false positivity in CDA type II (HEMPAS). Similarly, false positivity of sucrose lysis test can be seen in megaloblastic anemia, autoimmune hemolytic anemia, etc. The complement lysis tests developed by Rosse and colleagues could give an idea of the quantity and type of hemolytic RBCs [42, 43]. They showed that PNH was a heterogeneous disease with three populations of red cells. These were cells with normal sensitivity (type I), cells of medium sensitivity (type II, which are 3–5 times more sensitive), and very sensitive cells (which are 10–15 times more sensitive than the normal cells, type III).
3.2 Methods Utilizing Hemagglutinition and Gel Card System
This method was utilized in the 1980s when antibodies against CD55 (DAF) and CD59 (MIRL) were used to see the RBC agglutinition in the microcolumns of the gel card. Normal RBCs would agglutinate, while the RBCs deficient in CD55/CD59 would not agglutinate [44]. The commercially available kits have micro typing cards containing sephacryl and anti-mouse antibody from rabbit within a gel matrix. Erythrocyte suspension in low ionic strength buffer is added to three microtubes; followed by anti-CD55 antibody, anti-CD59 antibody, and a negative control in these three tubes and labeling them, respectively. The card is centrifuged for 10 min. PNH cells lacking CD55 or CD59 epitopes settle down at the bottom, whereas normal cells expressing CD55 and CD59 clump on top of the sephacryl gel. This test is easier to perform compared with complement-based tests. However, certain limitations include its poor sensitivity, which has been reported as 2–10% of type III RBCs, and not being quantitative [45].
3.3 Flow Cytometry-Based Methods
Flow cytometric methods have been in vogue for PNH clone detection since the late 1980s and early 1990s. Classically, they utilize fluorescently tagged antibodies against the GPI-APs. Numerous GPI-APs have been identified in different cell lineages that can be targeted for this purpose (Fig. 6.1). Absent expression of these antigens (GPI-AP) suggests a diagnosis of PNH. More recently, FLAER has been used, which directly binds to the GPI anchor. Use of FLAER has markedly increased the sensitivity of flow-based assay [46].
4 Flow Cytometry-Based PNH Testing
Flow cytometric detection of differential expression of DAF (CD55) on the normal and PNH RBCs can be traced back to 1985 [47]. The flow cytometric methods for the detection of GPI-linked antibody have now become the method of choice. Since the clinical manifestation of PNH predominantly involved complement-mediated hemolysis, all the initial research studies were focused on finding the proteins involved it. CD55 (DAF) was identified first, which was named after the functional property of this material to enhance the rate at which the activity of a classic pathway C3 convertase diminished [25]. Subsequently, it was realized that DAF cannot be the sole mechanism responsible for all the abnormalities of the membrane attack complex (MAC). Hence, search for substances which regulated the MAC led to the identification of a protein from the surface of normal erythrocytes that inhibited reactive lysis of PNH erythrocytes [31]. As soon as the deficiencies of CD55 and CD59 in PNH were confirmed, their absence found using flow cytometry techniques was being used to define a PNH clone. Sooner it was noted that a few patients in spite of being deficient for CD55 or CD59 did not have any manifestation of PNH. It was subsequently realized that these were some familial cases of isolated CD55 or CD59 deficiency. Hence, it was recommended to use both CD55 and CD59. True PNH cases used to be deficient for both these antibodies, in contrast to familial cases where RBC was deficient for either CD55 or CD59. The simple flow cytometric assays using CD55- and CD59-based approaches, however, are neither sensitive nor accurate, rendering them inadequate to detect small clone size which is frequently seen in AA and MDS. Hence, development and validation of standardized methodologies using other robust GPI-linked reagents were required.
4.1 Specimen Consideration
The preferred and widely accepted sample for the flow cytometric testing of PNH is ethylene-diamine-tetra-acetic acid (EDTA) anticoagulated peripheral blood. However, other anticoagulants like heparin and acid citrate dextrose (ACD) can also be used. BM is not preferred owing to variable expression of antibodies used for screening in different stages of RBC and WBC development. However, some of the newer antibodies used like FLAER and CD157 do not have a much variable expression on different stages of cells.
The sample should be processed preferably within 48 h. However, inevitable delay in the RBC analysis can be overcome by storing the sample in the refrigerator at 4 °C for a period of 7 days. Long-term storage of RBC can be done by freezing the sample in 25% dextrose. For WBC screening, sample storage may lead to change in scatter properties, ultimately leading to difficulty in gating the polymorphs and monocytes. Hence, it is recommended to complete the analysis within 48 h.
4.2 Cell Lineages to be Screened for PNH
Screening of PNH clone and accurate estimation of the clone size in red cells is of utmost importance, as the clone size directly correlates with hemolysis and thrombosis, the main clinical manifestation of this disease. However, isolated testing of RBCs is never recommended because of underestimation of exact clone size owing to an ongoing hemolysis and subsequent transfusion. Hence, WBC (granulocytes and monocytes) screening is recommended with RBC screening and a comparison of RBC and WBC clone sizes provides useful clinical information. It may be a frequent observation to have a PNH clone in WBCs without PNH RBC clone. But, the vice versa is almost never seen. In our own experience of FLAER-based PNH screening in leukocytes, almost all the cases with a PNH clone size of less than 2% in WBC did not show any PNH RBC clone [7]. Screening of lymphocytes is not recommended owing to their long life as well as variable expression of the GPI-linked antigens on lymphoid cells.
4.3 Analysis of RBC
CD59 is expressed at a more abundant level as compared to CD55. Additionally, CD55 does not provide a good separation of type II cell population. Hence, for the single reagent analysis, CD59 is preferred over CD55.
One of the important considerations in RBC analysis is frequent aggregate formation on the addition of antibodies. Hence extra wash steps and frequent vortexing/racking are required to disaggregate the RBC clumps. It is also recommended to dilute the antibodies to an extent which gives sufficient positive signals without producing much of RBC clumps. The use of IgM antibody is to be avoided. Strategies which include both CD55 and CD59 should be carried out after a careful titration of antibodies. Antibodies should be diluted, and the lowest concentration that gives acceptable positive signals should be used.
CD235a (glycophorin A) which is used to gate the RBCs in a high sensitivity analysis causes significant aggregation of RBC, owing to its very high surface density. Aggregation is typically higher with the PE conjugates as compared to FITC. In general, a much higher dilution of this antibody is required for PNH RBC analysis, as compared to those used for BM leukemia immunophenotyping. In our practice, the CD235a-FITC reagent from BD Biosciences (San Jose, CA, USA) is diluted 1:200 times before adding 10 microliters of the diluted form for gating RBC. It is thus stressed that each laboratory should carefully evaluate the optimal dilution of antibodies, so that acceptable signals are noted with very limited aggregation (Fig. 6.2).
Gating and Analysis: RBCs can be identified in a log-scaled FSC vs. SSC scatterplot. When no lysis is used, almost all the cells seen in the scatterplot are RBCs. The tube should be vortexed vigorously before acquisition, in order to disaggregate the RBC clumps. Voltage and threshold should be adjusted so that the RBCs are seen in the center of the scatterplot. For high sensitivity analysis, it is recommended to use a gating marker (CD235a—glycophorin A). This is done to get a pure population of RBCs and exclude the platelet clumps, which could falsely get included in FSC vs. SSC gate. As mentioned above, adding CD235 may significantly increase the aggregation of RBCs and hence optimum dilution is required. Gated RBCs are displayed on the single parameter histogram of CD55 or CD59. A negative control consisting of CD235a-stained RBC is only used to identify the expected position of type III RBCs. Normal RBCs represent the type I cells, while type II cells are represented in area between type I and type III cells. Dots plots provide better representation of small PNH clones, which are usually not very well seen with one dimensional histogram. RBCs are the easiest targets for a high sensitivity analysis, especially in cases of AA, where getting 2.5 lakh leukocytes may be difficult.
4.4 Leukocyte Assay
Analyses of neutrophils and monocytes are widely used for accessing the clone size. However, there are instances where a sufficient number of monocytes or neutrophils are difficult to acquire. Lymphocytes are not used for screening purpose owing to their long life as well as variable expression of the GPI-linked antigens on their surface.
For dealing with leukocytes, getting rid of RBCs by lysing them is an important step in cell processing. Mostly, the stain-lyse-wash method is preferred, where lysis can be performed by any commercially available or in-house method that does not compromise the scatter properties. For screening of leukocytes in cases of pancytopenia (AA or myelodysplastic syndrome), it may be necessary to prelyse the red cells taking more samples. In these situations, especially for high sensitivity PNH screening, a bulk lysis with 500 μL of the sample using formalin-free ammonium chloride-based lysing agent is preferred.
Since the leukocyte screening grew out of the experience from RBC staining, earlier CD59 and CD55 were the commonly used markers. Slowly, the knowledge about other GPI-linked leukocyte surface antigens grew, and these were explored for PNH testing using flow cytometry. CD66b, CD24, and CD16 were commonly used for granulocytes, while CD14 was commonly used as a monocytic marker. CD48 and CD157 were some of the other used markers, but their experience was limited.
5 FLAER as Marker for Both Granulocyte and Monocyte
Discovery of FLAER had a great impact on leukocyte screening for PNH. It was noted that the binding of aerolysin, a channel-forming toxin from bacterium Aeromonas hydrophila, is determined by the presence of GPI anchors of membrane glycoproteins. Hence, PNH cells which were deficient in GPI anchor were not lysed by this bacterium. Aerolysin is secreted as an inactive form, pro-aerolysin, activation of which requires a proteolytic cleavage of the C-terminal peptide [48, 49]. FLAER is a mutant form of pro-aerolysin, labeled with a fluorophore Alexa Fluor 488. It has the binding property but lacks the channel-forming property of active aerolysin. Peripheral blood nucleated cells are able to bind to FLAER because they are able to convert pro-aerolysin to aerolysin by using proteases like furin present on their cell surface. RBCs lack these proteases and hence are unable to bind [33, 49]. FLAER is manufactured by Pinewood Scientific (Vancouver, BC, Canada). For some years after its discovery, its wide use was limited owing to its lyophilized preparation, which was inconvenient to use as well as unstable. Later, more stable reagents were available. Nowadays the liquid form of this reagent is available, that has better stability and storage requirement comparable to commonly used antibodies. However, there are still some countries where this reagent is not available. Screening of PNH clone in leukocytes using FLAER is currently the gold standard for flow cytometry-based screening (Fig. 6.3).
5.1 CD157 as Marker for Both Granulocyte and Monocyte
CD157 has been identified as one of the promising reagents which is expressed on both polymorphs and monocytes [50]. CD157 is also known as bone marrow stromal cell antigen 1 (BST 1). It is a single-chain GPI-AP which is helpful in pre-B cell growth. It is expressed on B cell progenitors, T progenitors, granulocytes, monocytes, and some other cells as well. It is structurally and functionally similar to CD38.
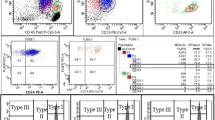
Sutherland et al. reported the use of CD157 as a common PNH marker and demonstrated its utility in a two-tube four-color as well as single-tube five-color combinations in FLAER-based PNH screening [51]. It has been found as a sensitive marker with a low background rate in normal sample [52]. CD157-based PNH screening was found to be very precise with minimal inter-assay and intra-assay variations [52, 53]. It provided a better separation between the PNH-negative and PNH-positive populations. Additionally, there was a better identification of type II PNH clones compared to CD24/CD14-based assay (Fig. 6.4). It can be used in a single-tube five-color combination and can replace the two-tube four-color assay, thus leading to reduction in both reagent and technical time cost. Replacing the single-tube six-color assay will not reduce the cost of technical time, but will definitely reduce the reagent (antibody) cost.
Some of the papers as well as verbal communication with some of the researchers have shown that there are cases which show a subset of granulocytes and monocytes population to be negative for CD157 but express FLAER. Hence, these were not considered as the true PNH clones. The exact reason for this phenomenon has not yet been established. But, the phenotypic polymorphism has been suggested as one of the probable reason. In our own experience, we had one such case out of some 200 odd cases that were screened using FLAER/CD157 in a single-tube five-color combination (Fig. 6.5). Recently, one of the papers has highlighted the importance of CD157 in a non-FLAER-based screening [54]. They confirmed a good performance characteristic and agreement between the FLAER- and non-FLAER-based approaches, using CD157. They highlighted its importance in a cost-effective analysis especially in those areas of the world where FLAER is not available. However, taking in view the selective absence of CD157 in a proportion of cases, replacement of FLAER does not seem to be a wise step at this point of time.
A proportion of gated polymorphs (shown by arrow) showing loss of CD157 expression, but are positive for FLAER, indicating them to be normal cells. These cells are seen to co-express FLAER and CD16 (another PNH marker) at normal level. The true PNH clone is negative for FLAER, CD157, as well as CD16 (shown in blue)
Gating and Analysis: For routine sensitivity analysis, FSC vs. SSC or CD45 vs. SSC analysis is sufficient for gating granulocytes and monocytes. However, in cases of MDS where the scatter properties are altered, gating the granulocytes and separating them from monocytes may be difficult. Getting a pure population based on CD45 vs. SSC plot may be difficult for monocytes as well, especially if they are in low number. Hence, for better delineation and for high sensitivity analysis, use of gating markers like CD15 for granulocytes and CD33/CD64 for monocytes is recommended. CD64 has been found to be a better marker as compared to CD33 to get a purer population of monocytes [7]. For routine analysis, acquisition of 5000–10,000 gated population is sufficient, but for high sensitivity analysis, 2.5 lakh events are recommended. This may be difficult for monocytes especially in cases with pancytopenia, and hence a lower number of monocytes is acceptable.
6 Routine Sensitivity Versus High Sensitivity Analysis
A routine sensitivity analysis is one which is sufficient enough for detecting PNH clones at 1% level, while high sensitivity analysis should be able to detect the clone sizes of 0.01%. For classic PNH patients, who present with features of hemolysis or thrombosis, a routine analysis may be sufficient enough to detect the presence of PNH clone. However, in patients with AA and MDS who usually do not have the clinical features of hemolysis, the clone size is small and in a majority of patients less than 1%. In these cases, a routine sensitivity analysis will miss the PNH clone detection in a majority of cases. Identifying these minor clones are important as ~25% of these patients may show expansion of clone size and may later present with clinical PNH.
For routine analysis, RBCs are gated on log-scaled FSC vs. SSC plot. The use of CD235a as a gating marker is optional and not mandatory. But for high sensitivity analysis, use of CD235a as a gating marker for RBCs is mandatory. For gating the leukocytes, CD45 vs. side scatter may be sufficient to gate the granulocytes as well as monocytes in a routine sensitivity analysis. However, for high sensitivity analysis, it is desirable to use a gating marker (CD15 for granulocytes and CD33/CD64 for monocytes); to get a pure population, FLAER plus one other informative reagent is mandatory for identifying a PNH clone. About 5000–10,000 gated events may be sufficient for routine sensitivity analysis, but for high sensitivity analysis, 2.5 lakhs of gated population is recommended [35].
7 Quality Assurance for High Sensitivity Analysis
7.1 Instrument Setup
As with any other high sensitivity analysis of rare events, an optimum instrument setup is required. The PMT voltage setup, scatter properties, and optimal fluorescence compensation are required. PMT voltage is set using unstained cells and making them lie on the scale in the negative area. It is cross-checked using the bright antibody-fluorochrome conjugate, usually CD3, ensuring that CD3-positive T cells do not go off scale and CD3-negative B cells are properly on the scale in the negative region and not crushed against the axis. If required, the PMT voltage is lowered down to bring the positive signal on the scale. Fluorochrome compensations can be set using the beads or the cells. If antibody capture beads are used, these are stained with the same antibody-fluorochrome conjugate which is to be used in the panel, except for FLAER, which can be replaced by CD3-AF488. Using cells, the compensation can be set up, staining the lymphocytes in different tubes with CD3 in different fluorochromes. The compensation can be cross-checked by staining the cells using antibody cocktails.
7.2 Choosing the Correct Reagent Conjugates and Clones
FLAER and CD24/CD157 are the most commonly used combination for the screening of granulocytes, while FLAER and CD14/CD157 are the most commonly used combination for monocytes [36, 51]. Available literature has some recommendations for the reagents and fluorochrome conjugates, which can be used in 4, 5, or 6 color combinations [35, 55]. These reagents and clones have extensively been checked to be preferred over other clone and conjugate combination. These are mentioned in Tables 6.1, 6.2, 6.3, 6.4, and 6.5 [55]. If some laboratory wants to use its own combination, it should be thoroughly checked for the proper functioning of these reagents.
7.3 Frequency of PNH-Positive Cells in Normal Samples
At least 10–20 peripheral blood samples from healthy volunteers should be subjected to PNH testing to determine the background level of PNH-positive cells. Studies have shown an average of 0.0004% PNH-type RBCs, 0.0013% PNH-type granulocytes, and 0.0033% PNH-type monocytes in normal individuals [36]. If the assay is fully optimized, the laboratory cutoff can be determined using mean value + 4 standard deviation from these normal samples.
7.4 Sensitivity of the Assay Using Dilution/Spiking Experiments
Fresh PNH-positive sample can be diluted in a normal peripheral blood sample. A serial tenfold dilution yielding 1:10, 1:100, 1:1000, and 1:10,000 should be tested for the linearity of dilution and size of PNH clone. It is desirable to get 100 PNH (FLAER-negative CD14/CD24 or CD157-negative) events. However, for higher dilutions, 30–50 clustered events or one million total gated leukocytes may be acceptable (Fig. 6.6).
7.5 Inter-Assay and Intra-Assay Variability
This can be tested by running the same stained tube multiple times (minimum three times) for intra-assay variability testing or staining the same sample multiple times in separate tubes and analyzing each tube individually. This should be done with samples having a wide range of PNH clone size. A higher variation may be observed in cases with lower clone sizes as compared to those having a higher clone size. Any variation of less than 10% is acceptable.
7.6 External Quality Assurance Program
College of American Pathologists in North America and the United Kingdom National External Quality Assessment Service in Europe provide the external quality assurance program. The former provides EQA for RBC analysis only while the latter is for leukocyte immunophenotyping. It has been demonstrated that stabilized whole blood sample is suitable for the currently recommended high sensitivity analysis. It has also been highlighted that using the carefully selected conjugates and standardized protocol can lead to reduced variation around the median even for experienced laboratories [56]. A recent study has demonstrated that using same standardized four-color assay on fresh samples had good precision and reproducibility performance. It also had a good correlation and agreement between centers (both novice and experienced) for all target PNH clone sizes [57]. Whatever be the approach, it is important to have continuing education for standardized testing and reporting of PNH. Exchange of samples between the laboratories is also recommended as one of the important steps for standardizing the testing of this rare condition.
8 Data Interpretation and Reporting
Clinical PNH cases, who present with hemolysis, usually have large PNH clone sizes. The PNH clone size in AA is usually significantly lower in comparison with classic PNH group. Routinely, these cases have a clone size of less than 10%, with most of them showing a minor clone of less than 1%. The clinical significance of this small size clone is controversial. But it is important to detect PNH clones in AA because of three important reasons. (1) Some studies, but not all, have shown that AA with the presence of PNH clone had a better response to immunosuppressive therapy [59,60,60]. (2) Finding a PNH clone virtually rules out other causes of inherited BM failure syndrome. Studies have shown that none of the cases of AA with PNH clone exhibit increased chromosome breakage [7, 46]. (3) A fair proportion of these patients may show an increase in clone size and progression of these subclinical PNH cases to frank clinical hemolytic PNH. Use of immunosuppressive therapy in the form of ATG may also lead to an increase in clone size [61].
Some of the cases of AA may have a large clone size of more than 50% [7, 46, 61]. Interestingly, even in the presence of a large clone size, many of these patients do not show clinical or laboratory evidence of hemolysis. The probable reason for this would be a reduced absolute mass of newly formed RBCs/reticulocytes (as a result of marrow hypoplasia in AA), which are the most sensitive cells to complement-mediated lysis. In contrast, classic PNH cases have more of these neocytes (as a result of compensatory erythropoiesis) and thus more features of hemolysis.
The PNH clone size of leukocytes is usually greater than that of RBCs. This can be explained by two mechanisms: one being the complement-mediated lysis of RBC and the other being the dilution of the PNH-positive RBCs following transfusion. However, RBC analysis is useful as RBC clone size directly correlates with the clinical features. Additionally, RBC staining is better in demonstrating the partial antigen deficiency as compared to the granulocytes. There is no specific cutoff, but cases with more than 20% type III RBCs are likely to be symptomatic. Cases with large type II and absence of significant type III populations show reticulocytosis with elevated LDH levels but less hemolysis [35]. Monitoring RBC clone is also important in cases with eculizumab therapy, as the drug prevents the complement-mediated hemolysis and hence the size of RBC clone increases with ongoing therapy.
Among the leukocytes, the reliability of neutrophils in assessing the clone sizes is better than monocytes, owing to the relatively lower number of monocytes that could be acquired and analyzed. But, for the cases where a sufficient number of monocytes could be analyzed, the clone size of monocytes is usually found to be greater than neutrophils [7, 36, 46]. There have been reports by some authors documenting that the analysis of monocytes and reticulocytes gives the best estimation of clone sizes in patients with small PNH clone [62].
It is of utmost importance to avoid the term negative or positive in the reports to avoid confusion (the cells “Positive” for GPI markers are actually “negative” for PNH and vice versa). Hence it is better to use the term “PNH clones detected” and “no PNH clones detected.” The total proportion of abnormal cells (type II + type III) should be mentioned for each lineage of cells analyzed. For RBCs, it is important to give information about the type II clone sizes. The importance of type II clone size for leukocytes is not well understood. Repeat testing is recommended for cases of AA. However, there is no requirement of repeat testing for the patient with unexplained thrombosis or hemolysis, where no PNH clone is detected. The small clone size should include a language in the report that should make clear that this is not the same as a diagnosis of hemolytic PNH. A mention of the antibody cocktail used along with the sensitivity of the assay should also be incorporated in the report.
For PNH detection using high sensitivity analysis, where small clones are detected, it is important to follow the rule of two. PNH clone should be detected in two lineages (RBCs, granulocyte or monocytes) using two gating markers for leukocytes (including CD45 and one other, usually CD15 for granulocyte and CD33/CD64 for monocytes) and two PNH markers (including FLAER and one other, usually CD24 for granulocyte and CD14 for monocytes). For RBCs, CD235 is the only gating marker and CD59 is the only PNH marker. When using separate tubes for granulocytes, monocytes, and RBCs, it is recommended that a high sensitivity screening for RBC and granulocytes should be done first, followed by a high sensitivity monocyte assay for all cases which contain PNH RBCs or granulocyte [36]. Since we perform a common five-color/six-color assays for granulocyte and monocyte, our strategy is to perform high sensitivity screening for leukocytes first. This is followed by high sensitivity screening of RBC for the cases which demonstrate PNH granulocytes or monocytes.
9 Conclusion
PNH is a clonal, non-neoplastic stem cell disorder with varied clinical manifestation. Its association with AA and MDS has been well established. The diagnosis can be established through various techniques, but flow cytometry is the preferred one owing to its sensitivity, specificity, and ability to accurately provide the clone size. Discovery of FLAER has been a major breakthrough, and currently, FLAER-based flow cytometric analysis is the “gold standard” diagnostic modality for PNH. CD157 also seems to be a promising reagent that can target both granulocyte and monocytes. Recent guidelines have been established for the diagnosis and monitoring of PNH using flow cytometry by International Clinical Cytometry Society (ICCS). It was followed by practical guidelines for high sensitivity analysis of PNH clones, where specific reagent cocktails and analytical strategies were directly addressed. Following these guidelines will lead to standardized comparable results across various laboratories. These guidelines do not prevent the laboratories from making the modification, but these should be extensively validated. Given the rarity of this disease and presence of small size clones in BM failure (AA and MDS) cases, it is important to address all the quality assurance steps for improving the detection of PNH clones. Data interpretation should be diligently done, with the generation of simple but informative reports, avoiding the terms that can create confusion.
References
Nagarajan S, Brodsky RA, Young NS, Medof ME. Genetic defects underlying paroxysmal nocturnal hemoglobinuria that arises out of aplastic anemia. Blood. 1995;86:4656–61.
Takahashi M, Takeda J, Hirose S, Hyman R, Inoue N, Miyata T, et al. Deficient biosynthesis of N-acetylglucosaminyl-phosphatidylinositol, the first intermediate of glycosyl phosphatidylinositol anchor biosynthesis, in cell lines established from patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1993;177:517–21.
Medof ME, Kinoshita T, Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984;160:1558–78.
Rollins SA, Sims PJ. The complement-inhibitory activity of CD59 resides in its capacity to block incorporation of C9 into membrane C5b-9. J Immunol. 1990;144:3478–83.
Mukhina GL, Buckley TJ, Barber JP, Jones RJ, Brodsky RA. Multilineage glycophosphatidylinositol anchor deficient haematopoeisis in untreated aplastic anemia. Br J Haematol. 2001;115:476–82.
Sachdeva MUS, Varma N, Chandra D, Bose P, Malhotra P, Varma S. Multiparameter FLAER-based flow cytometry for screening of paroxysmal nocturnal hemoglobluniera enhances detection rate in patients with aplastic anemia. Ann Hematol. 2015;94(5):721–8.
Rahman K, Gupta R, Yadav G, Hussein N, Singh MK, Nitynanand S. Fluorescent Aerolysin (FLAER)-based paroxysmal nocturnal hemoglobinuria (PNH) screening: a single center experience from India. Int J Lab Hematol. 2017;39(3):261–71.
Hillmen P, Lewis SM, Bessler M, et al. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333(19):1253–8.
Wang SA, Pozdnyakova O, Jorgensen JL, Medeiros LJ, Stachurski D, Anderson M, et al. Detection of paroxysmal nocturnal hemoglobinuria clones in patients with myelodysplastic syndromes and related bone marrow diseases, with emphasis on diagnostic pitfalls and caveats. Haematologica. 2009;94:29–36.
Iwanaga M, Furukawa K, Amenomori T, Mori H, Nakamura H, Fuchigami K, et al. Paroxysmal nocturnal hemoglobinuria clones in patients with myelodysplastic syndromes. Br J Haematol. 1998;102:465–74.
Young NS. Paroxysmal nocturnal hemoglobinuria and myelodysplastic sydromes: clonal expansion of PIG-A-mutant hematopoietic cells in bone marrow failure. Haematologica. 2009;94(1):3–7.
Sugimori C, Padron E, Caceres G, Shain K, Sokol L, Zhang L, et al. Paroxysmal nocturnal hemoglobinuria and concurrent JAK2V617F mutation. Blood Cancer J. 2012;2(3):e63.
Strubing P. Paroxysmale Ha¨moglobinurie. Deutsch Medicinische Wochenschrift. 1882;8:17–21.
Marchiafava E, Nazari A. Nuovo conttributo allo studiodegli itteri cronici emolitici. Il Policlinico. Sezion Med. 1911;18:241–8.
van den Bergh HAA. Ictére hémolytique avec criseshé moglobinuriques. Fragilité globulaire. Rev Med. 1911;31:63–9.
Crosby WH. Paroxysmal nocturnal hemoglobinuria. A classic description by Paul Strübing in 1882 and a bibliography of the disease. Blood. 1951;6:270–84.
Ham TH. Chronic hemolytic anemia with paroxysmal nocturnal hemoglobinuria. A study of the mechanism of hemolysis in relation to acid-base equilibrium. N Engl J Med. 1937;217:915–7.
Ham TH. Studies on destruction of red blood cells. Chronic hemolytic anemia with paroxysmal nocturnal hemoglobinuria: an investigation of the mechanism of hemolysis, with observations of five cases. Arch Intern Med. 1939;64:1271–305.
Ham TH, Dingle JH. Studies on destruction of red blood cells. II. Chronic hemolytic anemia and paroxysmal nocturnal hemoglobinuria: certain immunological aspects of the hemolytic mechanism with special reference to serum complement. J Clin Investig. 1939;18:657–72.
Pillemer L, Blum L, Lepow IH, Ross OA, Todd EW, Wardlaw AC. The properdin system and immunity. Demonstration and isolation of a new serum protein, properdin, and its role in immune phenomena. Science. 1954;120:279–85.
Dacie JV. Paroxysmal nocturnal haemoglobinuria. Proc R Soc Med U S A. 1963;56:587–96.
Rosse WF, Dacie JV. Immune lysis of normal human and paroxysmal nocturnal hemoglobinuria (PNH) redcells. The sensitivity of PNH red cells to lysis by complement and specific antibody. J Clin Investig. 1966;45:736–48.
Aster RH, Enright SE. A platelet and granulocyte membrane defect in paroxysmal nocturnal hemoglobinuria: usefulness for the detection of platelet antibodies. J Clin Investig. 1969;48:1199–210.
Hoffmann EM. Inhibition of complement by a substrate isolated from human erythrocytes. Extraction from human erythrocyte stomata. Immunochemistry. 1969;6:391–403.
Hoffmann EM. Inhibition of complement by a substrate isolated from human erythrocytes. Studies on the site and mechanism of action. Immunochemistry. 1969;6:405–19.
Oni SB, Osunkoya BO, Luzzatto L. Paroxysmalnocturnal hemoglobinuria: evidence for monoclonal origin of abnormal red cells. Blood. 1970;36:145–52.
Hoffmann E, Cheng W, Tomeu E, Renk C. Resistance of sheep erythrocytes to immune lysis by treatment of the cells with a human erythrocyte extract: studies on the site of inhibition. J Immunol. 1974;113:1501–9.
Stern M, Rosse WF. Two populations of granulocytes in paroxysmal nocturnal hemoglobinuria. Blood. 1979;53:928–34.
Pangburn MK, Schreiber RD, Müller-Eberhard HJ. Deficiency of an erythrocyte membrane protein with complement regulatory activity in paroxysmal nocturnal hemoglobinuria. Proc Natl Acad Sci U S A. 1983;80:5430–4.
Davitz MA, Low MG, Nussenzweig V. Release of decay accelerating factor from the cell membrane by phosphatidylinositol-specific phospholipase C. J Exp Med. 1986;163:1150–61.
Holguin MH, Fredrick LR, Bernshaw NJ, Parker CJ. Isolation and characterization of a protein from normal erythrocytes that inhibits reactive lysis of the erythrocytes of paroxysmal nocturnal hemoglobinuria. J Clin Investig. 1989;84:7–17.
Miyata T, Takeda J, Iida J, Yamada N, Inoue N, Takahashi M, et al. The cloning of PIG-A, a component in the early step in GPI-anchor synthesis. Science. 1993;259:1318–20.
Brodsky RA, Mukhina G, Li S, Nelson KL, Chiurrazi PL, Buckley T, et al. Improved detection and characterization of paroxysmal nocturnal hemoglobinuria using florescent aerolysin. Am J Clin Pathol. 2000;114:459–66.
Bessler M, Hiken J. The pathophysiology of disease in patients with paroxysmal nocturnal hemoglobinuria. Hematology Am Soc Hematol Educ Program. 2008;2008:104–8.
Borowitz MJ, Craig FE, DiGuiseppe JA. On behalf of the Clinical Cytometry Society et al Guidelines for the diagnosis andmonitoring of paroxysmal nocturnal hemoglobinuria and related disorders by flow cytometry. Cytometry B Clin Cytom. 2010;78:211–30.
Sutherland DR, Keeney M, Illingworth A. Practical guidelines for high sensitivity detection and monitoring of paroxysmal nocturnal hemoglobinuria clones by flow cytometry. Cytometry B. 2012;82B:195–208.
Crosby WH. Paroxysmal nocturnal hemoglobinuria. A specific test for the disease based ability of thrombin to activate the hemolytic factor. Blood. 1950;5:843–6.
Dacie JV, Lewis SM, Tills D. Comparative sensitivity of erythrocytes in paroxysmal nocturnal hemoglobinuria to hemolysis by normal serum and by high titre cold antibody. Br J Hematol. 1960;6:362–71.
Hartman RC, Jenkins DE Jr, Arnold AB. Diagnostic specificity of sucrose hemolysis tests for paroxysmal nocturnal hemoglobinuria. Blood. 1970;35:462–75.
Kabaksi T, Rosse WF, Logue GL. The lysis of paroxysmal nocturnal hemoglobinuria red cells by serum and cobra factor. Br J Haematol. 1972;23:693–705.
Brubaker LH, Schaberg DR, Jefferson DH, Mengel CE. A potential rapid screening test for paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1973;288:1059–60.
Rosse WF, Dacie JV. The role of complement in the sensitivity of the paroxysmal nocturnal hemoglobinuria red cell to immune lysis. Bibl Haematol. 1965;23:11–8.
Rosse WF. Variations in the red cells in paroxysmal nocturnal haemoglobinuria. Br J Haematol. 1973;24:327–42.
Navenot JM, Bernard D, Petit-Frioux Y, Loirat MJ, Guimbretière J, Muller JY, Blanchard D. Rapid diagnosis of paroxysmal nocturnal hemoglobinuria by gel test agglutination. Rev Fr Transfus Hemobiol. 1993;36(2):135–47.
Gupta R, Pandey P, Choudhry R, Kashyap R, MehrotraM NS, Nityanand S. A prospective comparison of four techniques for diagnosis of paroxysmal nocturnal haemoglobinuria. Int J Lab Hematol. 2007;29:119–26.
Sachdeva MU, Varma N, Chandra D, Bose P, Malhotra P, Varma S. Multiparameter FLAER-based flow cytometry for screening of paroxysmal nocturnal hemoglobinuria enhances detection rates in patients with aplastic anemia. Ann Hematol. 2015;94:721–8.
Kinoshita T, Medof ME, Silber R, Nussenzweig V. Distributionof decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1985;162:75–92.
Diep DB, Nelson KL, Raja SM, Pleshak EN, Buckley TJ. Glycosylphosphatidylinositol anchors of membrane glycoproteins are binding determinants for the channel-forming toxin aerolysin. J Biol Chem. 1998;273:2355–60.
Sutherland DR, Kuek N, Davidson J, et al. Diagnosing PNH with FLAER and multiparameter flow cytometry. Cytometry B Clin Cytom. 2007;72:167–77.
Hernandez-Campo PM, Almeida J, Sanchez ML, Malvezzi M, Orfao A. Normal patterns of expression of glycosylphosphatidylinositol anchored proteins on different subsets of peripheral blood cells: a frame of reference for the diagnosis of paroxysmal nocturnal hemoglobinuria. Cytometry B. 2006;70B:71–81.
Sutherland DR, Acton E, Keeney M, Davis BH, Illingworth A. Use of CD157 in FLAER based assay for high sensitivity PNH granulocyte and PNH monocyte detection. Cytometry Part B. 2014;86B:44–55.
Rahman K, Gupta R, Harankhedkar S, Gupta T, Sarkar MK, Nityanand S. Utility of CD157 as a common leukocytes marker for paroxysmal nocturnal hemoglobinuria screening in a single tube five color combination. Indian J Hematol Blood Transfus. 2018;34(2):304–9. https://doi.org/10.1007/s12288-017-0867-z.
Marinov I, Kohutova M, Tkakova V, Pesek A, Cermak J, Cetkovsky P. Clinical relevance of CD157 for rapid and cost effective simultaneous evaluation of PNH granulocyte and monocytes by low cytometry. Int J Lab Hematol. 2015;37:231–7.
Marinov I, Illingworth AJ, Benko M, Sutherland DR. Performance characteristics of a non-fluorescent aerolysin-based paroxysmal nocturnal hemoglobinuria (PNH) assay for simultaneous evaluation of PNH neutrophils and PNH monocytes by flow cytometry, following published PNH guidelines. Cytometry B Clin Cytom. 2018;94(2):257–63. https://doi.org/10.1002/cyto.b.21389.
Keeney M, Illingworth A, Sutherland DR. Paroxysmal nocturnal hemoglobinuria assessment by flow cytometric analysis. Clin Lab Med. 2017;37(4):855–67. https://doi.org/10.1016/j.cll.2017.07.007.
Fletcher M, Sutherland DR, Whitby L, et al. Standardizing leucocyte PNH clonedetection: an international study. Cytometry B Clin Cytom. 2014;86(5):311–8.
Marinov I, Kohoutova M, Tkacova V, et al. Intra- and interlaboratory variability of paroxysmal nocturnal hemoglobinuria testing by flow cytometry following the2012 practical guidelines for high-sensitivity paroxysmal nocturnal hemoglobinuria testing. Cytometry B Clin Cytom. 2013;84(4):229–36.
Scienberg P, Wu CO, Numez O, Young NS. Predictive response of immunosuppressive therapy and survival in severe aplastic anemia. Br J Hematol. 2009;144:206–16.
Sugimori C, Chuhjo T, Feng X, Yamazaki H, Takami A, Teramura M, Mizoguchi H, Omine M, Nakao S. Minor population of CD55-CD59-blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107:1308–14.
Yoshida N, Yagasaki H, Takahashi Y, Yamamoto T, Liang J, Wang Y, Tanaka M, Hama A, Nishio N, Kobayashi R, Hotta N, Asami K, Kikuta A, Fukushima T, Hirano N, Kojima S. Clinical impact of HLA-DR15, a minor population of paroxysmal nocturnal haemoglobinuria-type cells, and an aplastic anaemia-associated autoantibody in children with acquired aplastic anaemia. Br J Haematol. 2008;142:427–35.
Scheinberg P, Marte M, Nunez O, Young NS. Paroxysmal nocturnal hemoglobinuria clones in severe aplastic anemia patients treated with horse anti-thymocyte globulin plus cyclosporine. Haematologica. 2010;95:1075–80.
Hochsmann B, Rojewski M, Schrezenmeier H. Paroxysmal nocturnal hemoglobinuria (PNH): higher sensitivity and validity in diagnosis and serial monitoring by flow cytometric analysis of reticulocytes. Ann Hematol. 2011;90:887–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Rahman, K., Chandra, D. (2019). Historical Investigations and Advances in Flow Cytometry-Based Tests in Paroxysmal Nocturnal Hemoglobinuria. In: Saxena, R., Pati, H. (eds) Hematopathology. Springer, Singapore. https://doi.org/10.1007/978-981-13-7713-6_6
Download citation
DOI: https://doi.org/10.1007/978-981-13-7713-6_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-7712-9
Online ISBN: 978-981-13-7713-6
eBook Packages: MedicineMedicine (R0)