Abstract
The reproductive success of plants is often dependent on their flowering time being adapted to the territorial environment, in which gravity remains constant. Whether plants can follow the same rule to determine their flowering time under microgravity in space is unknown. Here, a 12-day mission on orbiter Chinese SJ-10 recoverable microgravity experimental satellite (SJ-10 satellite) carried long-day-flowering Arabidopsis thaliana and short-day-flowering rice (Oryza sativa), and transgenic Arabidopsis plants engineered with a transgene composed of a heat shock-inducible promoter (HSP) linked to the green fluorescence protein (GFP) reporter gene and FLOWERING LOCUS T (FT) gene. The plants were used to examined FT gene expression patterns in space to address the effects of microgravity on flowering induction. In addition, application of GFP technique for FT visualization on the SJ-10 satellite in this study is also introduced. Finally, a comprehensive analysis of global gene expression of leaves of Arabidopsis and rice grown in space under a long-day (LD) and a short-day (SD) conditions, respectively, was carried out to understand effects of microgravity on photoperiodic flowering induction at molecular level. Our results showed that microgravity apparently down-regulated expression of GIGANTEA (GI), which is involved in circadian clock functions. Furthermore, possible key points of microgravity responses in the main photoperiod pathways, GI-CO-FT module in Arabidopsis or GI-Hd1-Hd3a module in rice, are also discussed with regard to potential future spaceflight experiment opportunities.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Plants respond sensitively to many environmental stimuli, such as light, temperature, nutritional conditions and gravity. Among of these environmental signals, gravity is one that remains constant during plant lifetime. Removal of gravitational acceleration by spaceflight caused a wide range of morphogenesis and cellular and molecular changes in plants (Cogoli and Gmünder 1991; Hampp et al. 1997; Paul et al. 2013; Hoson 2014; De Micco et al. 2011; Zheng et al. 2015; Zheng 2018). For example, under microgravity conditions, plants exhibit spontaneous curvatures or changes in growth direction (Driss-Ecole et al. 2008; Soga et al. 2014), increased guttation (Wang et al. 2018), reduced growth and photosynthetic rate of wheat seedlings (Tripathy et al. 1996; Levinskikh et al. 2000), alteration in chloroplast morphology and chlorophyll a/b ratio in Brassica plants (Adamchuk et al. 1999; Jiao et al. 1999, 2004), and swollen mitochondria with an electron-dense matrix and well-developed cristae (Popova 2003). Changes that occur in the spaceflight environment at the genetic (DNA and RNA) and biochemical (protein) levels have recently been intensively studied. Informative results from several recent space flight experiments indicated that microgravity induced a wide range of alterations in gene expression and protein synthesis, including calcium-, lipid, and auxin-mediated signaling, the cell membrane biosynthesis, the total metabolism, responses to stress, and protein synthesis (Tan et al. 2011; Zheng et al. 2015; Zhang et al. 2015; Fengler et al. 2015). Expression of an Adh/GUS reporter gene in Arabidopsis indicated that spaceflight affected stress signal perception and transduction (Paul et al. 2001). The etiolated seedlings grown in space displayed significant changes in the expression of genes associated with drought stress, wounding, and calcium- and auxin-mediated signaling and cell wall development (Paul et al. 2012). In one of our pervious studies, comparison of the proteomic profiles of Arabidopsis callus growing under microgravity conditions with the controls on-board centrifuge (1 × g of the control) in the Chinese spacecraft SZ-8 flight indicated that significant differences were identified in the content of 45 proteins participating in a wide range of cellular processes, including general responses to stresses, carbohydrate metabolism, protein synthesis and degradation, intracellular transport, signaling, and the biosynthesis of the cell membrane (Zhang et al. 2015). The expression of peroxidase and cell wall remodeling genes associated with root hair development was repressed under microgravity (Kwon et al. 2015). These results indicated the plasticity of plant growth and development in response to microgravity, and different tissues or organs might exhibit different responses to microgravity.
The life cycle of higher plants has three mutually distinct development stages: the embryogenetic, vegetative, and reproductive stages. Plants undergo a major physiological change when they transition from vegetative growth to reproductive development. The onset of reproductive development is a pivotal switch in the life of plants. Plant reproduction in the spaceflight environment is of great interest. Not only does it help us study fundamental questions regarding the role of gravity in normal development, but also higher plants could supply food and fiber, purify ambient air, and recycle human waste and water in a closed environmental life support system envisioned for long-duration spaceflight. Most of previous space experiments focused on response of plant vegetative stage to short-term microgravity (Kiss et al. 1998; Driss-Ecole et al. 2008; Kordyum 2014; Kordyum and Chapman 2017), but very little is known about the reproductive stage (Link et al. 2003; Merkys and Laurinavicius 1983; Musgrave et al. 1997). Plants frequently died in the transition from the vegetative to the reproductive stage in early attempts (Halstead and Dutcher 1987; Mashinsky et al. 1994). Even when Arabidopsis plants were pre-grown to the flowering stage on Earth and allowed to for seeds on orbit only 55% of seeds were fertile (27% aborted and 18% had non-viable embryos). The first successful complete plant life cycle under microgravity occurred in 1983 with Arabidopsis thaliana on board Salyut 7 (Merkys and Laurinavicius 1983). The second successful experiment was performed with Brassica rapa on board the Mir space station (Musgrave et al. 2000; Kuang et al. 2000). The seeds obtained in this experiment were healthy and viable, but less protein, fewer cotyledon cell, and aberrant deposition of starch grains. Wheat crop under the same environment on board Mir was failed to develop seeds (Strickland et al. 1997). These experiments indicated that ethylene concentration in the growth chambers was considered responsible for influence of plant reproduction. As international space station (ISS) was established, more long-term experiments on growing plant for a full life cycle were carried out in space. The first seed-to-seed experiment on board ISS was performed in the advanced astroculture plant growth unit, which could provide nutrients to the plant and control soil moisture, light, air temperature, humidity, ethylene, and CO2 level (Link et al. 2003). Since then, advances in plant culture technology have led to more frequent successful seed set in space (Sychev et al. 2007; Link et al. 2014; Yano et al. 2013). However, seeds developed in space are still often of lower quality in comparison with control seeds formed in normal gravity conditions (De Micco et al. 2014; Link et al. 2014). Seeds produced in space exhibited delayed embryo development, alteration of storage reserves, delay in starch use in cotyledons and decreased cotyledon cell number (Kuang et al. 2000; Musgrave et al. 2000; Link et al. 2014). Flower development is crucial to ensure successful plant reproduction and seed yield. However, most of information is available on final seed development in space (Kuang et al. 2000; Musgrave et al. 2000; Link et al. 2014), very little is known about the effects of microgravity on the transition from vegetative to reproductive stages. In the SJ-10 satellite experiment, we examined the effects of microgravity in space on the induction of flowering of two different photoperiodic response plants, Arabidopsis thaliana and rice. The molecular mechanisms that control the transition from vegetative growth to reproductive development under microgravity were also studied. Here, detail experimental designs and operations of the spaceflight experiment on the SJ-10 satellite to investigate plant flowering are introduced.
2 SJ-10 Satellite Space Experiment
2.1 Experimental Design
Arabidopsis thaliana and rice were used as plant materials in Chinese SJ-10 satellite (April 6–18, 2016) to investigate the effect of microgravity on the photoperiod controlling flowering induction. According to the response of plants to light during the induction of flowering, they can be classified as LD plants that induce flowering when day length exceeds a certain threshold, SD plants that flower when days are short and nights are long, and day neutral plants whose flowering is not dependent on the length of the day. Arabidopsis thaliana is LD plant, which floral transition is promoted under the LD (16 h light) condition in comparison with those under the SD (8 h light) condition. Rice is SD plant in which flowering is faster under SD (i.e., 8 h light) than that under the LD (i.e., 16 h light) condition.
To address the possibility that microgravity affect photoperiodic flowering and to assess whether the movement of FT from leaf to shoot apex under microgravity was different from the control on ground condition, we have designed an experiment by comparing LD flowering Arabidopsis thaliana and SD flowering rice on board SJ-10 satellite with their controls on ground. In addition, we used the advantages of heat shock (HS)-inducible gene switch and developed transgenic Arabidopsis containing the HSP gene promoter linked to the GFP reporter gene (pHSP:: GFP) and the FT gene (pHSP::FT). The expression of pHSP::GFP and pHSP::FT in Arabidopsis leaves were induced by half hour 37 °C heating under the SD condition and then expression of GFP in these leaves were monitored by a plant GFP imager. At the same time, time-lapse images also documented the effect of microgravity on the flowering induction of Arabidopsis and rice plants under a LD (16 h light/8 h dark) and a SD (8 h light/16 h dark) conditions, respectively (Fig. 1). After SJ-10 satellite returned to Earth, the Plant Growth Box (PGB) was unloaded and the root modules-grown plants were harvested approximately 4 h after landing. Samples from four different root modules were collected and fixed with fixation solution immediately and stored at 4 °C, and transported to the laboratory for analysis.
Chinese SJ-10 satellite experimental setup. 15-day old Arabidopsis thaliana and 30-day old rice plants were loaded into sterile PGB approximately 24 h prior to launch on SJ-10 satellite. The PGB provided two photoperiodic conditions: LD (16 h light/8 h dark) and SD (16 h dark/8 h light) conditions. Arabidopsis “Columbia” was used as wild-type and transgenic plant expressing pHSP::GFP and pHSP::FT was also carried to observe in orbitor induction of FT in leaves by heating 37 °C for half hour (red asterisk indicates)
The PGB used for this experiment had a growing area of 0.013 m2 and a height of 19 cm. It consisted of four different systems, including growth compartments, illumination system, photograph system, a temperature controlling system and a heater (Fig. 2). The growth compartments included four root modules and relate growth space. Seedlings of Arabidopsis and rice were set in the root modules containing commercially available vermiculite immersed by a medium containing macronutrient as described by Haughn and Sommerville (1986) in the PGB to investigate the effect of microgravity on the course from vegetative stage to reproductive stage and the flowering on the orbit. Illumination was provided by light banks of LED lamp (red: white, 1:2) on a 16-h photoperiod (LD) and an 8-h photoperiod (SD), respectively. Temperature and humidity were recorded every 1 min during flight. Light levels were also recorded with the same frequency. These data were used to set the ground control in a control growth chamber. Ethylene concentration in the growth chamber was regulated by a chemical regent (Zheng et al. 2008) for removal of virtually all released ethylene. Photographic equipments was used to record images of Arabidopsis and rice plants. Images were recorded by three automatic, preprogrammed cameras. Of two cameras were used for photographed plants under the LD and the SD conditions, respectively (Fig. 3). One was a GFP imager for GFP signal recorded. The photographs of plants were taken at 2-h intervals and 4 images every day for GFP signals. Temperature system was regulated by a control box to keep temperature and air movement in the box. A heater was designed to induce the HSP promoter controlling expression of GFP and FT genes by locally heating leaves to 37 °C for half hour.
The PGB was used in Chinese SJ-10 satellite. a The detail mechanical set up for the PGB, consisted of four growth modules including two root modules for Arabidopsis and two modules for rice; three cameras, two cameras were used for monitoring plant growth under the LD (cam 1) and the SD (cam 2) conditions, respectively; the other one was used to photograph GFP fluorescence (cam F); one heater was used to increase local temperature on leaves to activate the HSP promoter and induce expression of FT and GFP genes. b The PGB used in this experiment. c and d Two example experimental equipment, which were used as root modules to culture Arabidopsis and rice, respectively
Two representative images of plants grown the LD (a) and the SD (b) condition, respectively, on board the SJ-10 satellite. The images were transmitted from space cameras at day 10 after launching. The plants samples included rice wild-type, Arabidopsis wild-type (WT) and transgenic plants (pHSP::GFP; pHSP::FT)
This heating induced a local activation of the HSP promoter. We then monitored gene expression in the induced leaves with GFP imager camera (cam F) and development of inflorescence shoot with cameras (cam 1 and cam 2, Fig. 2). Rice “d18h” was used as wild-type. The plants were grown in the PGB in space for about 12 days. Samples from the orbiter was harvested and fixation with RNAlater solution 4 h after landing, immediately stored at 4 °C, and transported to the laboratory for analysis.
2.2 Arabidopsis thaliana Flowering in Space
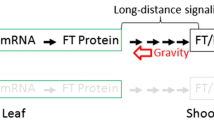
Flowering time in Arabidopsis thaliana is dependent on the length of the day with the LD (16 h light) in general promoting floral transition compared to the SD (8 h light). The phenomenon that plant flowering in response to day length is dubbed photoperiod response, which has been observed to be perceived in the leaves from which the long-distance signal called the florigen is transmitted to the shoot apex to induce flowering. The studies on florigen have been recently made tremendous progress due to identification of numerous genes involved in flowering regulation (reviewed by Jung et al. 2017). A protein called FT in Arabidopsis was proven to be established as florigen, which is contributing to the floral induction by acting as a long-distance signal between leaves and the shoot meristem. FT is genetically down-stream of CONSTANS (CO), which expression is under the control of the circadian clock with a phase of 24 h. The CO-FT module is conserved in both LD and SD plants (Srikanth and Schmid 2011). The FT protein act as a systemic inducer of flowering that is expressed in the companion cells of the phloem and exported to the phloem sieve elements in leaves from where it is transported to the shoot apex. This process includes at least three critical steps in florigen signaling: (1) exportation from companion cells to the sieve element; (2) transport from leaves to the shoot apex through phloem; (3) activation of its effector genes at the shoot apex that trigger subsequent flower development.
Gravity and light are two of most important environmental factors to regulate plant growth and development on earth. Effects of light on the regulation of plant flowering have been extensively studied (Putterill et al. 2004; Srikanth and Schmid 2011). However, gravity is permanent and always present in a constant direction and magnitude on earth, there is no way to eliminate the influence of gravity on plants on the surface of earth. Plants have utilized gravity as the most stable and reliable signal to direct their developmental processes. With the increased interest of plants in space, gravity cannot be ignored in studies of plant developmental processes, including plant flowering control. On board the Chinese SJ-10 satellite, the effects of microgravity on the flowering induction of LD Arabidopsis were investigated (Fig. 4). The FT protein act as a systemic inducer of flowering that is expressed in leaves from where it is transported to the shoot apex. To make sure we can induce expression of FT at the early time during flight, we generated pHSP::FT, pHSP::GFP fusion construct under control of the promoter of the HSP gene. We heated leaves of Arabidopsis plants grown under SD condition on SJ-10 satellite to 37 °C. This heating induced a local activation of the HSP promoter. We then monitored GFP gene expression in the induced leaves and compared the flowering time of the heating treated plants with control (untreated plants) under the SD and LD both in space and on ground (Fig. 3). These results indicated that flowering time of plants grown in space was much longer than those on ground, and suggested that microgravity could affect movement of the FT protein from leaves to the shoot apex.
Subsequent phases during life cycle of Arabidopsis thaliana. a Schematic diagram illustrating the phases those are more likely sensitive to microgravity. The framed region in red dotted line indicates that samples grown in space on board SJ-10 satellite and the Arabidopsis flowering time pathway could be modified under microgravity. b Preculture of Arabidopsis plants in green house for about 20 days before take-off. c Top view of 20-day old plants, which were used as samples in SJ-10 experiment. Seedlings at day 25 and day 32 after germination grown in green house on ground were also showed as controls to those grown in space
The evaluation of genome-wide patterns of native gene expression within Arabidopsis (wild-type) leaves grown under the LD and the SD condition, respectively, on board the SJ-10 satellite were preformed by comparing with their controls on ground with utilizing the Affymetrix Arabidopsis ATH1 genome array of 22,746 genes. 4432 genes in the LD plants and 2572 genes in the SD plants were observed to be differentially expressed between microgravity samples and their ground controls (1 × g), of which 2959 and 1099 genes were specific to the LD and the SD condition, respectively (Fig. 5a). GO classification of differential expression genes (DEGs) indicated that the function of genes in response to light in chloroplast was apparently affected among the top 10 of GO term enrichments (Fig. 5b, c). Genes involved in controlling Arabidopsis flowering, including GI, CCA1, LHY, PCC1, ELF4, BT3 and ADG1, were detected to alter expression under microgravity (Fig. 5d). According to these results, we proposed a model to address microgravity involved in Arabidopsis photoperiodic flowering pathway. In this model, microgravity inhibited expression of GI, which plays a key role in control of FT expression. In addition, microgravity enhanced expression of CCA1 and LHY, which can regulate expression of GI.
Microgravity regulated genes in Arabidopsis leaves grown in space under different photoperiod conditions in comparison with their controls on ground. a Vennen diagram of transcriptome data. b and c GO classification of differential expression of genes (DEGs) of microgravity response genes under the LD and the SD conditions, respectively. d Expression profile of photoperiod controlling flowering under microgravity (μg) in comparison with their controls on ground (1 × g) under the LD and the SD conditions, respectively. Yellow indicates an increase in expression, blue indicates a decrease in expression. Scale bar shows log2 fold changes (FC). e Proposed model of the influence of microgravity involved in photoperiod pathway in Arabidopsis flowering controlling. In this model, microgravity inhibited expression of GI, which plays a key role in control of FT expression. In addition, microgravity enhanced expression CCA1 and LHY, which can regulate expression of GI
2.3 Rice Flowering in Space
Like Arabidopsis, the life cycle of rice has also three mutually distinct developmental stages: the embryogenetic, vegetative, and reproductive stages (Fig. 6a). The vegetative stage can be further divided into the juvenile and adult phases. Importantly, plants can initiate flowering only during the adult phase, endogenous and environmental factors, which affect the change of juvenile-to-adult phase, are considered as key players in regulating plant development (Tanaka et al. 2011). Rice is a facultative SD plant, and molecular genetic studies have identified the major genes involved in SD flowering. Recent progress in genome analysis has provided a strategy for analyzing the genetic control of flowering in rice. Several studies have demonstrated that the structure of gene involved in the photoperiodic response of flowering in rice showed remarkable similarity to those in Arabidopsis. For example, heading date 1 (Hd1), a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CO. Hd3a, which was proven to be established as florigen, functions as FT in Arabidopsis.
Subsequent phases during life cycle of rice. a Schematic diagram illustrating the phases those are more likely sensitive to microgravity. The framed region in red dotted line indicated that samples grown in space on board SJ-10 satellite and the rice flowering time pathway which could be modified under microgravity. b Preculture of Arabidopsis plants in green house for about 30 days before take-off
On SJ-10 satellite space experiment, we focused on the transition vegetative-reproductive phase of rice. 30-day old seedlings were selected as samplers for space experiment (Fig. 5b). After grown in space on the SJ-10 satellite for about 12 days, the plants began flowering under the SD condition but not under the LD. Genome-wide patterns of gene expression within rice leaves grown under the LD and the SD condition, respectively, on board the SJ-10 satellite were preformed by the mapping of mRNAseq reads to the rice genome. Genes of very low aboundance were removed from the analysis, leaving a final number of 20,929 genes. 13,908 genes in the LD plants and 7136 genes in the SD plants were observed to be differentially expressed between microgravity (μg) samples and their ground controls (1 × g), of which 10,532 and 3760 genes were specific to the LD and the SD condition, respectively (Fig. 7a). GO classification of DEGs indicated that the genes function in regulated reproductive process were among the top 20 enrichment list (Fig. 7b, c). The expression of genes involved in controlling rice flowering, including OsPhyB, OsGI, OsCOP1, OsFCA, Os FTL1, OsFTL2, OsFTL3, WD domain, OsFBO0 and OsFBO9, were altered under microgravity (Fig. 7d). According to these results, we proposed a model to address microgravity involved in rice photoperiodic flowering pathway. In this model, microgravity affected the signaling cascades of photoperiodic flowering of rice is indicated. Expression of GI and OsPhyB was down-regulated by microgravity. In addition, OsCOP1 was also slightly down-regulated, but RFT1 and F-box protein genes, such as OsFBO8 and OsFBO9, were up-regulated to controlling rice flowering under microgravity.
Microgravity regulated genes in rice leaves grown in space under different photoperiod conditions in comparison with their controls on ground. a Vennen diagram of transcriptome data. b and c GO classification of differential expression of genes (DEGs) of microgravity response genes under the LD and the SD conditions, respectively. d Expression profile of photoperiod controlling flowering under microgravity (μg) in comparison with their controls on ground (1 × g) under the LD and the SD conditions, respectively. Yellow indicates an increase in expression, blue indicates a decrease in expression. Scale bar shows log2 fold changes (FC). e Proposed model of the influence of microgravity involved in photoperiod pathway in rice flowering controlling. In this model, microgravity affected the signaling cascades of photoperiodic flowering is indicated. Expression of GI and phytochrome B (OsPhyB) was down-regulated by microgravity. In addition, the expression of OsCOP1 was also slightly down-regulated, but the expression of RFT1 and F-box protein genes, such as OsFBO8 and OsFBO9, were up-regulated to controlling rice flowering under microgravity
2.4 Application of GFP Technique for FT Visualization on the SJ-10 Satellite
The GFP camera in the PGB (Fig. 2) was the first generation of hardware designed to collect GFP expression data in real-time aboard a spaceflight experiment. A system of blue-light emitting diodes (LEDs) for excitation of GFP and a specially designed narrow band pass filter with a spectrum spread of λ 475/40 nm. Imaging is accomplished with a 10 mega pixel CMOS imager that can capture the leaves of seedlings grown in the CC in a 1280 × 1024 pixel image and these images then were transferred into on-board 3 mega pixel video chips before processing and storage on a local SD card. Telemetry was used to transmit GFP fluorescence images by issuing the commands to perform the functions of taking and downloading images from the ground controlling computer. In addition, the functions of imager and lighting can be modified by uploading commands.
One of the goals of our SJ-10 satellite experiment was to address specifically the possibility that microgravity affect photoperiod induced flowering and to assess whether the movement of FT from leaf to shoot apex under microgravity was similar to control terrestrial transformation of FT. Heat shock (HS)-inducible gene expression systems can respond to spatial information provided by localized heating, which is easy to handle in space experiment. On the SJ-10 satellite experiments, we used the advantages of HS-inducible gene switch and developed transgenic Arabidopsis containing the HSP gene promoter linked to the GFP reporter gene and the FT gene, respectively. The expression of pHSP::GFP and pHSP::FT were by locally heating Arabidopsis leaves grown in space at 37 °C for half hour under the SD condition (Fig. 8a, b). After the HSP promoter was activated by local heating the leaves, expression of FT gene was monitored by observed the GFP influorescence produced in the induced leaves through the GFP imager camera. The images downloaded from the SJ-10 satellite plants showed that a strong GFP influorescence appeared in the heating treated leaves (Fig. 8d) in comparison with those untreated leaves in the same condition (Fig. 8c). This confirmed that this heat shock system was effective to induce expression of goal genes (i.e., GFP and FT) and useful to monitor activation of genes under microgravity in space.
Arabidopsis plants distribution in a root module and a representative fluorescence image of leaves before and after heating treated. a An example of the SJ-10 satellite plants distributed in a root module, including two wild-type (WT) plants and two transgenic plants, pHSP::FT, pHSP::GFP. b The framed region in dotted lines indicated the heating treated area. c The fluorescence image of the framed region before the heating treatment. d The fluorescence image of transgenic plants (pHSP::FT; pHSP::GFP) under the SD (8 h light/16 h dark) condition on board the SJ-10 satellite in the framed region 2 h after half hour 37 °C heating treatment
3 Conclusion and Outlook
One of the key developmental processes for plant produce seeds is the differentiation of the shoot apical meristem into a floral meristem. This process has been proven to be regulated by both endogenous and environmental factors. Gravity and light are considered as two of the most important environmental factors that control plant flowering development. In recent years, the studies on the effect of light on plant development have been tremendous progress due to identification of numerous genes involved in flowering regulation, but whether plants can follow the same rule to determine their flowering time under microgravity in space is unknown. Although numerous attempts have been made to grow a plant through a complete life cycle in space, apparently no published information exists concerning the flowering control of plant in space. In this study, we successfully grew two different type photoperiodic response model plants, the LD flowering Arabidopsis and the SD flowering rice in space on board SJ-10 satellite for 12 days. FT gene expression patterns in Arabidopsis leaves were also observed by a GFP imager. Finally, transcriptomes of Arabidopsis and rice response to microgravity under the LD and the SD conditions were obtained. Our results indicated that GI could be a key gene for both Arabidopsis and rice adaptation to microgravity in adjusting photoperiod pathways, GI-CO-FT module in Arabidopsis or GI-Hd1-Hd3a module in rice. As we know, this is the first time that examination of plant flowering control in space at molecular level.
In the future, long-term space experiments from successive generations and a systematic analysis of regulatory networks at the molecular level is needed to understand the mechanism of plant flowering control under microgravity condition in space. On board the Chinese space lab TG-2, we have extended to study the effect of microgravity on flowering control of Arabidopsis and rice, which were grown in space from seed to seed. The results will be reported in the future paper. The genes involved microgravity response identified in space experiments so far represent only a very small part of the plant genome and many other microgravity-responsive genes, including those in regulating plant flowering, will likely be identified in the future. For example, genes that control the activities of the pathways in the regulation of flowering timing in plants under microgravity will be very interested, because they will enable the manipulation of flowering time in crop species, which could have a major impact on the production of plants in bioregenerative life support system (BLSS). The energy using in plant cultivation on aboard spacecrafts such as space shuttle and space station, and even the future space farm must rely on solar battery or fuel cell, which will be severely restricted on board long-term missions. How to increase of production efficiency of plant will be the most important task in setting up BLSS. A acceleration of flowering time in seed crops, such as rice and wheat, while prevention of bolting in vegetable plants, such as sugarbeet, many Brassica species, potato, spinach, and lettuce would significantly improve yield. Thus, control of flowering time by manipulation will be clearly an important biotechnological method to increase the productive of BLSS.
Abbreviations
- BLSS:
-
Bioregenerative life support system
- CO:
-
CONSTANS
- DEGs:
-
Differential expression genes
- FT:
-
FLOWERING LOCUS T
- GFP:
-
Green fluorescence protein
- GI:
-
GIGANTEA
- Hd1:
-
Heading date 1
- HSP:
-
Heat shock-inducible promoter
- ISS:
-
International Space Station
- LEDs:
-
Light emitting diodes
- LD:
-
Long-day
- PGB:
-
Plant Growth Box
- SD:
-
Short-day
- SJ-10 satellite:
-
SJ-10 recoverable microgravity experimental satellite
- WT:
-
Wild-type
References
Adamchuk NI, Mikhaylenko NF, Zolotareva EK et al (1999) Spaceflight effects on structural and some biochemical parameters of Brassica rapa photosynthetic apparatus. J Gravit Physiol 6:95–96
Cogoli A, Gmünder FK (1991) Gravity effects on single cells: techniques, findings and theory. Adv Space Biol Med 1:183–248
De Micco V, Arena C, Pignalosa D, Durante M (2011) Effects of sparsely and densely ionizing radiation on plants. Rad Env Biophy 50:1–19
De Micco V, De Pascale S, Paradiso R et al (2014) Microgravity effects on different stages of higher plant life cycle and completion of the seed-to-seed cycle. Plant Biology 16(suppl.1):31–38
Driss-Ecole D, Legue V, Carnero-Diaza E et al (2008) Gravisensitivity and automorphogenesis of lentil seedling roots grown on board the international space station. Physiol Plant 134:191–201
Fengler S, Spirer I, Neef M, Ecke M, Nieselt K, Hampp R (2015) A whole-genome microarray study of Arabidopsis thaliana semisolid callus cultures exposed to microgravity and nonmicrogravity related spaceflight conditions for 5 days on board of Shenzhou 8. BioMed Res Int Article ID 547495
Halstead TW, Dutcher FR (1987) Plants in space. Ann Rev Plant Physiol 38:317–345
Hampp R, Hoffmann E, Schönherr K et al (1997) Fusion and metabolism of plant cells as affected by microgravity. Planta 203:S42–53
Haughn GW, Sommerville C (1986) Sulfonylurea-resistant mutants of Arabidopsis thaliana. Mol Gen Gen 204:430–434
Hoson T (2014) Plant growth and morphogenesis under different gravity conditions: relevance to plant life in space. Life 4:205–216
Jiao S, Hilaire E, Paulsen AQ et al (1999) Ultrastructural observation of altered chloroplast morphology in space-grown Brassica rapa cotyledons. J Gravit Physiol 6:93–94
Jiao S, Hilaire E, Paulsen AQ et al (2004) Brassica rapa plants adapted to microgravity with reduced photosystem I and its photochemical activity. Physiol Plant 122:281–290
Jung C, Pillen K, Staiger D et al (2017) Editorial: recent advances in flowering time control. Front Plant Sci 7:2011
Kiss JZ, Katembe WJ, Edelmann RE (1998) Gravitropism and development of wild-type and starch-deficient mutants of Arabidopsis during spaceflight. Physiol Plant 102:493–502
Kordyum EL (2014) Plant cell gravisensitivity and adaptation to microgravity. Plant Biol 16(S1):79–90
Kordyum EL, Chapman DK (2017) Plants and microgravity: patterns of microgravity effects at the cellular and molecular levels. Cyt Gen 51:108–116
Kuang A, Xiao Y, Mcclure G et al (2000) Influence of microgravity on ultrastructure and storage reserves in seeds of Brassica rapa L. Ann Bot 85:851–859
Kwon T, Sparks JA, Nakashima J et al (2015) Transcriptional response of Arabidopsis seedlings during spaceflight reveals peroxidase and cell wall remodeling genes associated with root hair development. Amer J Bot 102:21–35
Levinskikh MA, Sychew VN, Derendyaeva TA et al (2000) Analysis of the spaceflight effects on growth and development of super dwarf wheat grown on the space station Mir. J Plant Physiol 156:522–529
Link BM, Durst SJ, Zhou W et al (2003) Seed-to-seed growth of Arabidopsis thaliana on the international space station. Adv Space Res 31:2237–2243
Link BM, Busse JS, Stankovic B (2014) Seed-to-seed-to-seed growth and development of Arabidopsis in microgravity. Astrobiology 14:866–875
Mashinsky A, Ivanova I, DerendyaevaT et al (1994) ‘‘From seed-to-seed’’ experiment with wheat plants under space-flight conditions. Adv Space Res 14:13–19
Merkys AL, Laurinavicius RS (1983) Complete cycle of individual development of Arabidopsis thaliana (L.) Heynh. Plants on board the Salyut-7 orbital station. Dokladi Akademii Nauk SSSR 271:509–512
Musgrave ME, Kuang A, Matthews SW (1997) Plant reproduction during spaceflight: importance of the gaseous environment. Planta 203:S177–S184
Musgrave ME, Kuang A, Xia Y et al (2000) Gravity independence of seed-to-seed cycling in Brassica rapa. Planta 210:400–406
Paul A-L, Daugherty CJ, Bihn EA et al (2001) Transgene expression patterns indicate that spaceflight affects stress signal perception and transduction in Arabidopsis. Plant Physiol 126:613–621
Paul A-L, Amalfitano CE, Ferlr J (2012) Plant growth strategies are remodeled by spaceflight. BMC Plant Biol 12:232
Paul A-L, Wheeler RM, Levine HG et al (2013) Fundamental plant biology enabled by the space shuttle. Amer J Bot 100:226–234
Popova AF (2003) Comparative characteristic of mitochondria ultrastructural organization in Chalorella cells under altered gravity conditions. Adv Space Res 31:2253–2259
Putterill J, Laurie R, Macknight R (2004) It’s time to flower: the genetic control of flowering time. BioEssays 26:363–373
Soga K, Club B, Kurita A et al (2014) Growth and morphogenesis of Azui bean seedlings in space during SSAF2013 program. Biol Sci Space 28:6–11
Srikanth A, Schmid M (2011) Regulation of flowering time: all roads lead to rome. Cell Mol Life Sci 68:2013–2037
Strickland DT, Campbell WF, Salisbury FB et al (1997) Morphological assessment of reproductive structures of wheat grown on Mir. Gravit Space Biol Bull 11:14
Sychev VM, Levinskikh MA, Gostimsky SA, Bingham GE, Podolsky IG (2007) Space flight effects on consecutive generations of peas grown onboard the Russian segment of the international space station. Acta Astronaut 60:426–432
Tan C, Wang H, Zhang Y et al (2011) A proteomic approach to analyzing responses of Arabidopsis thaliana root cells to different gravitational conditions using an agravitropic mutant, pin2 and its wild type. Proteome Sci 9:72
Tanaka N, Itoh H, Sentoku N et al (2011) The COP1 ortholog PPS regulates the juvenile-adult and vegetative-reproductive phase changes in rice. Plant Cell 23:2143–2154
Tripathy BC, Brown CS, Levine HG et al (1996) Growth and photosynthetic responses of wheat plants grown in space. Plant Physiol 110:801–806
Wang L, Han F, Zheng HQ (2018) Photoperiod-controlling guttation and growth of rice seedlings under microgravity on board Chinese spacelab TG-2. Microgravity Sci Tech. https://doi.org/10.1007/s12217-018-9644-3
Yano S, Kasahara H, Masuda D, Tanigaki F, Shimazu T, Suzuki H, Karahara I, Soga K, Hoson T, Tayama I, Tsuchiya Y, Kamisaka S (2013) Improvements in and actual performance of the plant experiment unit onboard Kibo, the Japanese experiment module on the international space station. Adv Space Res 51:780–788
Zhang Y, Wang L, Xie J et al (2015) Differential protein expression profiling of Arabidopsis thaliana callus under microgravity on board the Chinese SZ-8 spacecraft. Planta 241:475–488
Zheng HQ (2018) Flowering in space. Microgravity Sci Tech. https://doi.org/10.1007/s12217-018-9626-5
Zheng HQ, Wang H, Wei N et al (2008) Live imaging technique for studies of growth and development of Chinese cabbage under microgravity in a recoverable satellite (SJ-8). Microgravity Sci Tech 20:137–143
Zheng HQ, Han F, Le J (2015) Higher plants in space: microgravity perception, response, and adaptation. Microgravity Sci Tech 27:377–386
Acknowledgements
The authors are indebted to Prof. W. R. Hu for suggestion in space experiment design and Prof. Tao Zhang’s group for PGB construction. This work was supported by the National natural fund joint fund project (U1738106), the Strategic Pioneer Projects of CAS (XDA15013900), the National Natural Science Foundation of China (31670864), the China Manned Space Flight Technology project TG-2 and the National Science Foundation for Young Scientists of China (31500687).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Science Press and Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Zheng, H., Wang, L.H., Xie, J.Y. (2019). Flowering of Arabidopsis and Rice in Space. In: Duan, E., Long, M. (eds) Life Science in Space: Experiments on Board the SJ-10 Recoverable Satellite. Research for Development. Springer, Singapore. https://doi.org/10.1007/978-981-13-6325-2_8
Download citation
DOI: https://doi.org/10.1007/978-981-13-6325-2_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-6324-5
Online ISBN: 978-981-13-6325-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)