Abstract
Particles dispersed in a solution are constantly moving in Brownian motion. Since this Brownian motion depends on the velocity of the particle size, the size can be estimated by observing the fluctuation corresponding to this velocity. By obtaining the autocorrelation function of the observed fluctuation, the particle size and distribution can be obtained. Dynamic light scattering (DLS) uses the above principle and provides size information of a wide range of particles easily and accurately. Since the size information of particles, molecular aggregates, and emulsion droplet is extremely important, DLS is widely preferred by many colloid researchers. However, data analysis of DLS is based on the assumption that the particles are uniform and spherical. It should also be noted that the obtained size includes a solvated layer around the particles. Due to its nature, other equipment must be used to obtain detailed information such as the shape. The principle and detailed analysis method by DLS measurement will be described in this section.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
Particles (micelles, microparticles, etc.) in colloidal dimension size (c.a. 1 μm to 1 nm) are constantly moving in solutions due to translational diffusion in Brownian motion. When light is irradiated, the fluctuation of the scattering intensity that depends on the particle size in the solution can be observed. In other words, small particles diffuse quickly and the scattering intensities fuctuate quickly, while large particles fluctuate slowly. By analyzing the autocorrelation based on this difference variation, the diffusion coefficient can be obtained, and from this the particle size can be obtained. Therefore, dynamic light scattering (DLS) is a measurement that can easily obtain the size information for a molecular aggregate formed by surfactants (typically micelles), microparticles, etc.
10.2 What You Get
-
1.
Measurement of a wide range of particle sizes from 1 nm to 5 μm
-
2.
Noncontact measurement using light, allowing undamaged sample measurement
-
3.
Particle size, if the viscosity and refractive index of solvent are known
10.3 Essentials and Tips
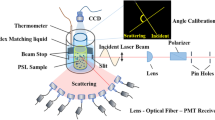
Figure 10.1 shows the principle of dynamic light scattering measurement and a simple process to determine the particle size. As mentioned in the introduction section, the particles are constantly diffusing in the solution in Brownian motion. Therefore, time-dependent spatial position change of the particles causes the variation of the light scattering intensity due to interference. By analyzing autocorrelation from the temporal fluctuation of the scattering intensity, the diffusion coefficient is obtained. Assuming that the molecules are spherical and uniform, the hydrodynamic radius can be determined by the Stokes-Einstein equation (Eq. 10.1):
-
D: Diffusion coefficient
-
K B: Boltzmann constant
-
T: Temperature
-
η: Viscosity
-
d: Hydrodynamic diameter
From the particle size information, the formation of molecular aggregate can be confirmed.
10.4 Understanding Your Data
10.4.1 Quality of Autocorrelation Function Graph
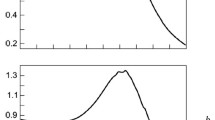
It is common to evaluate the particle size from the calculated size distribution graph (right bottom in Fig. 10.1); the autocorrelation function graph must be evaluated carefully. Therefore, it is important to judge whether the autocorrelation function graph is eligible for the analysis. Figure 10.2 shows examples of autocorrelation function graphs, and their interpretation are explained as follows:
The autocorrelation curve in Fig. 10.2a has decays with two inflection points, indicating the existence of two different size particles in the sample with fast attenuation due to small particles and slow attenuation due to large particles.
The autocorrelation curve in Fig. 10.2b stays unchanged for a while and then attenuates, though it would not completely decay even after a long time period. This curve indicates the influence of scattering by considerably large particles due to contamination of bubbles and dust. If such a curve is seen, re-measuring is required after a centrifugation or filtering process.
An irregular function is observed early in the attenuation curve in Fig. 10.2c. This phenomenon is often observed when the sole solvent does not have particles. Therefore, curve type in Fig. 10.2c indicates that the particle sizes are too small or the particle concentration is too dilute. The laser intensity must be increased to increase the scattered light intensity from the small particles or to increase particle concentration.
10.4.2 Types of Particle Size Distribution
There are three types of size distribution obtained from DLS: scatter intensity, volume, and number distribution. Figure 10.3 shows the difference between volume and number distribution. The scattered light intensity depends on the particle volume. The large particles give strong scattered intensity even in small amounts because the radius cubed equals the volume. Hence, it is important to understand what the objective information required from DLS measurement is, and volume distribution is often used for molecular aggregates by surfactants, while number distribution is used for bulky monomers and solid microparticles.
10.5 What to Look Out for
-
1.
Data analysis of DLS is based on the assumption that the particles are uniform and spherical. Because of its feature, other equipment is required to obtain detailed information such as the shape. Additionally, it is important to keep in mind that the obtained size includes a solvated layer around the particles.
-
2.
The viscosity and refractive index of the solution are required for accurate measurement, since Stokes-Einstein equation (Eq. 10.1) is used for analysis.
-
3.
When foreign substances (dust, bubbles, etc.) enter the measurement sample, a stronger scattering intensity than the target particle is detected. Therefore, it is important to prevent contamination of foreign substances by using a filter.
-
4.
When the sample solution is cloudy at high concentration, it is affected by multiple scattered lights. Therefore, it is preferable that the sample concentration is as low as possible.
10.6 Useful Hints
Finally, NICOMP will be described among a number of dynamic light scattering equipment in here. The instrument is depicted in Fig. 10.4. NICOMP has flexible customizability and is also equipped with automatic sample changer. Additionally, its unique analysis software “NICOMP algorithm” is included for particle size analysis, so accurate measurement samples with multiple peaks can be performed. Figure 10.5 shows the results of mixed samples with different size particles, i.e., 93 and 150 nm, using NICOMP. Despite the relatively small size differences, NICOMP can provide accurate particle size and distribution.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Misono, T. (2019). Dynamic Light Scattering (DLS). In: Abe, M. (eds) Measurement Techniques and Practices of Colloid and Interface Phenomena. Springer, Singapore. https://doi.org/10.1007/978-981-13-5931-6_10
Download citation
DOI: https://doi.org/10.1007/978-981-13-5931-6_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-5930-9
Online ISBN: 978-981-13-5931-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)