Abstract
An important feature of the gastrointestinal (GI) muscularis externa is its ability to generate phasic contractile activity. However, in some GI regions, a more sustained contraction, referred to as “tone,” also occurs. Sphincters are muscles oriented in an annular manner that raise intraluminal pressure, thereby reducing or blocking the movement of luminal contents from one compartment to another. Spontaneous tone generation is often a feature of these muscles. Four distinct smooth muscle sphincters are present in the GI tract: the lower esophageal sphincter (LES), the pyloric sphincter (PS), the ileocecal sphincter (ICS), and the internal anal sphincter (IAS). This chapter examines how tone generation contributes to the functional behavior of these sphincters. Historically, tone was attributed to contractile activity arising directly from the properties of the smooth muscle cells. However, there is increasing evidence that interstitial cells of Cajal (ICC) play a significant role in tone generation in GI muscles. Indeed, ICC are present in each of the sphincters listed above. In this chapter, we explore various mechanisms that may contribute to tone generation in sphincters including: (1) summation of asynchronous phasic activity, (2) partial tetanus, (3) window current, and (4) myofilament sensitization. Importantly, the first two mechanisms involve tone generation through summation of phasic events. Thus, the historical distinction between “phasic” versus “tonic” smooth muscles in the GI tract requires revision. As described in this chapter, it is clear that the unique functional role of each sphincter in the GI tract is accompanied by a unique combination of contractile mechanisms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Gastrointestinal
- Tone
- Interstitial cells of Cajal
- Myofilament sensitization
- Electromechanical coupling
- Sphincter
- Slow wave
- Membrane potential
- Motility
- ANO1
1 Anatomical and Functional Features of Gastrointestinal Sphincters
1.1 Lower Esophageal Sphincter
Anatomy and pressure. The lower esophageal sphincter (LES) is a specialized region of smooth muscle located at the junction of the esophagus and stomach (Fig. 2.1a). However, the precise morphological features of the LES are still controversial. Historically, the LES was described as consisting of semicircular “clasp” muscle fibers near the lesser curvature of the stomach and obliquely oriented “sling” muscle fibers near the fundus [1,2,3,4,5,6,7,8,9,10]. However, it is now recognized that distal circular muscle fibers of the esophagus also contribute to the high-pressure zone [11]. Indeed, there is likely overlap in the literature between muscles described as “clasp” vs. distal esophageal circular muscle fibers. A recent three-dimensional high-resolution pressure profile of the human esophagogastric junction (EGJ) has been undertaken [12, 13]. These studies conclude that the LES is a noose-like structure consisting of oblique distal esophageal circular muscle fibers that cross with sling fibers at the angle of His (i.e., the acute angle at the junction of the esophagus and fundus) producing “purse strings” that raise luminal pressure when contracted.
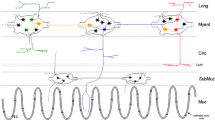
Anatomical location of gastrointestinal sphincters. Highlighted in each diagram is a portion of the circular muscle layer with arrows indicating the location of the sphincter. (a) The lower esophageal sphincter (LES) is located at the junction between the esophagus and the stomach. It is comprised of oblique distal esophageal and clasp fibers near the lesser curvature of the stomach (left). These fibers combine with oblique sling fibers at the angle of His (right). The LES in combination with the crural diaphragm constitute the esophagogastric junction (EGJ). (b) The pyloric sphincter (PS) is located at the junction of the distal antrum and proximal duodenum. It is a thickening of the circular muscle layer. (c) The ileocecal sphincter is located at the junction of the distal ileum and proximal cecum. It is created by a folding over of the circular muscle layer that protrudes into the cecum. (d) The internal anal sphincter (IAS) is a thickening of the circular muscle layer at the distal extremity of the gastrointestinal tract. It is surrounded by the external anal sphincter which also extends distal to the IAS
The LES passes through the crural diaphragm en route to the stomach; thus, the EGJ is considered to include both the LES and the crural diaphragm [14]. Importantly, the high-pressure zone generated by the EGJ is responsible for preventing the reflux of gastric contents into the esophagus. Values for EGJ pressure at end expiration (considered to be exclusively due to the LES) range from 15 to 35 mmHg in humans [13, 15, 16] and similar pressures have been reported for rat [17, 18]. Interestingly, LES pressure is higher near the angle of His and encompasses a wider portion of the esophagus near the lesser curvature [2, 13, 19,20,21].
Neural innervation. Extrinsic neural regulation of the LES is largely mediated by vagal preganglionic cholinergic neurons. These neurons innervate the LES at the level of the myenteric plexus and activate either excitatory or inhibitory motor neurons depending upon the neural pathway involved [22,23,24,25,26]. Stimulation of excitatory motor neurons significantly increases LES pressure [25] while atropine decreases LES pressure [27, 28]. On the other hand, some studies have reported that vagotomy increases LES pressure suggesting a predominance of inhibitory neural pathways [25]. When all neural influences are eliminated there is still elevated pressure at the LES indicating that spontaneous tone generation plays a role in this region [25, 29]. Purines, nitric oxide (NO), and vasoactive intestinal peptide (VIP) each participate in inhibitory neural responses in the LES, with NO being the most important [5, 7, 9, 30, 31]. Sympathetic nerves arising from the celiac ganglia also innervate the LES but their actions appear to be modest since LES pressure is unchanged following sympathectomy [25, 32].
1.1.1 In Vitro Contractile and Electrical Activity
Contraction. Muscle strips isolated from the LES spontaneously develop tone in every species examined (e.g., monkey LES, Fig. 2.2a). In vitro studies of human muscles report that tone in clasp muscle is twice that of sling muscle and ten times greater than that of esophageal body muscles [5]. Similar differences are apparent for clasp and sling muscles in cat [8, 33]. The greater tone developed by muscles of the LES than the esophageal body is in agreement with the high-pressure zone noted for the LES in vivo [13, 25]. However, the greater tone developed by clasp muscle compared to sling muscle cannot explain the greater pressure measured in vivo near the sling side of the LES [2, 19,20,21]. It is possible that clasp muscle receives greater neural inhibition than sling muscle in vivo since inhibitory nerves produce greater inhibition of contraction of clasp than of sling muscles in vitro [5, 9]. Morphological differences in the composition of smooth muscle at the clasp and sling side of the LES have also been proposed to be an important determinant of the circumferential pressure gradient [12, 13].
Spontaneous contractile activity generated by strips of the monkey lower esophageal sphincter (LES ) and internal anal sphincter (IAS) following dissection in cold (4 °C) Krebs bicarbonate Ringers solution and placement in a warm (37 °C) isolated tissue bath. An initial stretch was applied to both muscles at the arrows. As ionic gradients are reestablished tone and phasic activity develop. Contractile activity in the LES is predominantly tonic (a, Keef and Cobine, unpublished observation) while phasic contractions are superimposed upon tone in the IAS (b [115])
Electrical activity. The membrane potential of the human and opossum LES is approximately 8–10 mV more depolarized than that of the esophageal body (i.e., ~−40 vs. −50 mV) while clasp and sling muscle membrane potentials are not different [5, 9, 34, 35]. In addition, continuous spike potentials have been observed in the opossum LES but not in the esophageal body [35, 36] (Fig. 2.3). Spikes as well as 70% of tone are abolished by the L-type calcium (CavL) channel blocker nifedipine suggesting that electromechanical coupling mechanisms significantly contribute to tone generated in this muscle [35].
Spontaneous spiking activity recorded from the opossum lower esophageal sphincter [35]. (a) Example of a single spike potential. Top: Membrane potential initially undergoes a slow depolarization followed by a rapid upstroke and repolarization. Bottom: Plot of the change in voltage with time (dV/ dt) during this recording. The maximum rate of depolarization during the spike was ~0.3 V/s. (b) Examples of two patterns of continuous spiking activity shown at a slower sweep speed. Spikes occurred either upon a constant resting membrane potential (upper trace) or on occasion, superimposed upon slow oscillations (3–5/min) of resting membrane potential (lower trace)
1.2 Pyloric Sphincter
Anatomy, function, and in vivo pressure. The pyloric sphincter (PS) is located at the gastroduodenal junction (GDJ) and is composed of the thickened circular muscle layer of the pylorus (Fig. 2.1b). The PS plays an important role in regulating the passage of chyme from the stomach to the small intestine. After ingested food has been broken down and the peristaltic contractions of the stomach have lessened, the PS relaxes allowing the passage of small volumes of chyme into the duodenum [37]. A feedback mechanism from the duodenum closes the PS to limit the amount of incoming chyme. The question of whether the PS exists in a state of tonic contracture at rest is controversial [29]. The pressure within the pylorus has been reported to be ≤10 mmHg between meals [38,39,40] suggesting minimal tone. However an in vivo pressure study of the dog PS described phasic pressure waves (1–5 cpm) superimposed upon elevated basal pressure [41]. The amplitude of phasic events was decreased by addition of atropine and enhanced by tetrodotoxin (TTX) indicating that this activity was modulated by neural input. The discrepancies between Allescher et al. [41] and others may arise from differences in the stage of gastric emptying and the nutrient content of the chyme in the duodenum [42,43,44,45]. For example, pyloric pressure is elevated after ingestion of a meal [40].
Neural innervation. Extrinsic neural regulation of the PS is similar to that of the LES, i.e., it is mediated by vagal preganglionic cholinergic neurons. It also receives sympathetic input from the celiac ganglia. Vagal preganglionic cholinergic neurons innervate the PS at the level of the myenteric plexus and give rise to either excitatory or inhibitory motor responses depending upon the neural pathway activated. Excitatory responses are predominantly cholinergic in nature [41, 46,47,48] whereas inhibitory responses are due to the release of purines, NO, and VIP [46, 47, 49, 50]. Despite innervating the pylorus, sympathetic nerves do not appear to play an important role in excitatory motor responses, rather they appear to modulate cholinergic pathways [51, 52].
1.2.1 In Vitro Contractile and Electrical Activity
Contraction. In vitro studies of the PS describe slow frequency (i.e., ~1–3 cycles per minute, cpm) phasic contractions [53,54,55,56] (Fig. 2.4). Unlike other sphincters, little tone is observed in the PS under “resting” conditions. However, tone is generated in the rat pylorus in response to the NO synthase (NOS)-inhibitor L-NAME suggesting ongoing suppression of tonic activity via nitric oxide (NO) [56]. CavL channel antagonists abolish phasic contractions in the PS indicating an important role for these channels in this activity [57].
Spontaneous contractile activity observed in isolated strips of the rabbit pyloric sphincter (PS) [53]. (a) Low-frequency phasic contractions were observed in the PS without tone. Addition of erythromycin 50 μM (arrow) abolished these contractions. (b) In a different muscle strip erythromycin 50 μM (arrow) was applied in the presence of tetrodotoxin (1 μM). In this case, contractile inhibition was abolished. The authors concluded that erythromycin acts predominantly by stimulating motilin receptors on inhibitory motor neurons [53]
Electrical activity. A number of intracellular microelectrode measurements from isolated strips of the PS have identified slow waves [54, 58,59,60,61]. Studies of the dog PS indicate that slow waves arise at the myenteric edge of the circular muscle layer and conduct toward the lumen, disappearing before reaching the submucosal edge [59]. The average frequency of slow waves recorded from isolated strips of the PS is 0.5–1 cpm [58, 59]. However, when electrical activity is recorded from muscle segments that include the distal antrum, the PS slow wave frequency is entrained by the higher frequency of slow waves in the distal antrum [58, 59]. Figure 2.5 shows how slow waves generated by stimulation of the distal antrum conduct across the distal antrum/PS/proximal duodenum. These topographical recordings indicate that slow waves can conduct to the PS but that much of this region is electrically quiescent under these conditions.
Topography of slow wave activity recorded from cells in the circular muscle layer with microelectrodes across the width of the pyloric canal [59]. Slow waves were repetitively evoked in the antral region and recorded from cells throughout the surface of the preparation. Slow waves spread throughout the circular layer of the terminal antrum (TA) but petered out within the pyloric ring (PR) and proximal duodenum (PD). Much of the circular muscle of the pylorus was electrically quiescent
Slow waves in the PS arise from a relatively negative resting level, i.e., −70 mV in the guinea pig and −63 mV and −61 mV in the myenteric and submucosal regions of the dog PS, respectively [58, 62]. However, there appears to be species-dependent differences in the role of CavL channels since slow waves are unaffected by nifedipine in the guinea pig PS [58], whereas both the amplitude and duration of slow waves is substantially reduced in the dog PS [59]. Activity in the pylorus is electrically isolated from that of the duodenum and occurs in the absence of nerves [59].
1.3 Ileocecal Sphincter
1.3.1 Anatomy, Function, and In Vivo Pressure
The ileocecal sphincter (ICS) is a papillose structure located at the junction between the ileum and cecum (Fig. 2.1c). It has also been referred to as ileocolonic sphincter or valve. The ICS is ~4 cm wide in humans and is composed of two circular muscle layers, an internal one that is continuous with the circular muscle layer of the ileum and an external one that is continuous with the circular muscle layer of the cecum. There is also a single longitudinal muscle layer interposed between the two circular muscle layers [29, 63]. Functionally the ICS plays a role in facilitating the passage of luminal contents from the ileum to the colon. Perhaps more importantly, it plays a role in preventing the reflux of colonic contents, specifically bacteria, into the small intestine [64].
Manometry studies performed on human subjects report a 10–20 mmHg pressure zone over a distance of ~4 cm within the ICS [65, 66]. A similar high-pressure zone is present in dog, i.e., ~26 mmHg over 3 cm [67, 68]. However, there is controversy regarding this pressure zone in vivo since other studies have failed to detect it [69]. In some studies phasic pressure waves (4–8/min) have been observed superimposed upon tone [66]. The differences between these studies may arise from differences in recording techniques, as it is difficult to measure ICS pressure and tone noninvasively. Furthermore, discrepancies may also arise from differences in the fed-state of the subject. Greater tone and phasic activity are noted in the human ICS immediately after a meal versus during the interdigestive phase [66, 70]. Colonic distension results in increased ICS tone and larger amplitude pressure waves in both human and dog [66, 71], a reflex that will limit the reflux of material from the colon back into the ileum.
1.3.2 Neural Innervation
The ICS is innervated by sympathetic (via superior and inferior mesenteric ganglia) and parasympathetic (via vagus nerve) neural pathways. Tone in the ICS can occur in the absence of excitatory neural input although stimulation of sympathetic and cholinergic pathways enhance tone [72,73,74,75,76]. Non-adrenergic non-cholinergic (NANC ) inhibitory responses are also present in the ICS [76]. A number of studies have identified an important role for NO in inhibitory motor innervation to the ICS [73, 77,78,79,80]. However, much less is known about the possible role of purines and VIP. Studies of the dog ICS have reported inhibitory junction potentials consisting of NOS- and apamin-sensitive components [73] while immunohistochemical studies have identified VIP in a subset of neurons in the myenteric ganglia [81]. Thus, VIP and purines likely play some role in the ICS but further studies are required.
1.3.3 In Vitro Contractile and Electrical Activity
Contraction. Most in vitro studies of the ICS report tone although not in every muscle strip examined [29, 76, 82, 83]. Spontaneous phasic contractions are also observed but again not in every muscle strip. Phasic activity is typically superimposed upon tone [29, 76, 79, 82,83,84] (Fig. 2.6). Interestingly, addition of the COX-2 inhibitor indomethacin to mechanically quiescent strips of the guinea pig ICS increases tone generation as well as phasic contractions suggesting that ongoing prostaglandin synthesis suppresses contractile activity [83]. Although the ICS generates tone, this activity represents only 10–20% of maximum contraction [82, 83]. CavL channel blockers have limited effects upon tone (i.e., reducing tone by less than half), while phasic contractions are abolished [82, 84].
Spontaneous contractile activity observed in isolated strips of the human ileocecal sphincter [82]. Tone was consistently observed in muscles (left and right trace) but in some cases phasic contractions were superimposed upon tone (right). Acetylcholine caused concentration-dependent contraction (left) whereas gastrin releasing peptide abolished phasic activity as well as most tonic contraction
Electrical activity. Resting membrane potential in the guinea pig ICS is more depolarized than that of the ileum or cecum i.e., ~−43 mV (ICS) vs. −58 mV (ileum) and −62 mV (cecum) [76], whereas in the dog ICS it is more negative (i.e., −55 mV) [73]. Differences in resting membrane potential between species may explain why spike-like activity is reported in some species (cat and guinea pig ICS [76, 83, 85]) but not in the dog [73] (Fig. 2.7). However, even in species with spikes, this activity is not observed in all muscle strips. Thus, the variability in contractile behavior (Fig. 2.6) likely reflects the variability in electrical events between muscle strips from the same animal. Indeed, in the only study in which membrane potential and contraction were simultaneously recorded, it was shown that spike potentials were associated with phasic contractile activity [85] (Fig. 2.7a).
Sample traces of spontaneous electrical activity recorded in the ileocecal sphincter (ICS) of several animal species. (a) Simultaneous recording (sucrose gap) of contractile (upper) and electrical (lower) activity in the cat ICS reveals occasional spike discharge associated with phasic contractions [85]. (b) Microelectrode recording of periodic bursts of spikes in the guinea pig ICS. Shown is an excerpt taken from the original figure [76]. (c) Microelectrode recording of periodic fluctuations in membrane potential in the dog ICS. Electrical field stimulation (EFS ; 3 pulses, 20 Hz) gave rise to a fast cholinergic excitatory junction potential followed by a slower inhibitory junction potential due in part to nitric oxide [73]
1.4 Internal Anal Sphincter
Anatomy and in vivo pressure. The internal anal sphincter (IAS) is a thickening of the circular muscle layer at the distal end of the gastrointestinal tract (Fig. 2.1d). Circular and longitudinal muscle layers are separated from one another by a sparsely occupied connective tissue space [86, 87]. Surrounding the IAS is a second sphincter composed of skeletal muscle (i.e., the external anal sphincter). The IAS is responsible for >70% of resting anal pressure [88, 89] and is important for maintaining continence. Anal pressure in humans averages 75–90 mmHg [90,91,92], making pressure at this sphincter substantially greater than other GI sphincters. In contrast, resting anal pressure in rodents is significantly less than in humans (i.e., 15–21 mmHg) [93,94,95].
Neural innervation. The IAS receives sympathetic nerve fibers that arise from the inferior mesenteric ganglion and travel in the lumbar colonic nerves (LCN) and hypogastric nerves (HGN) or from the pelvic plexus. High spinal anesthesia or cutting the HGN and/or LCN significantly reduces IAS tone in a number of species suggesting that they exert a tonic excitatory influence on the IAS [96,97,98,99,100,101,102]. Varicose sympathetic fibers are located throughout the circular muscle layer of the monkey IAS and their activation doubles tone in vitro [87]. Since anal pressure is maintained in the absence of sympathetic nerves [103], they are not essential for tone generation. However, given the strength of this motor pathway, they likely assist in maintaining continence under some circumstances.
The IAS is also innervated by enteric inhibitory motor neurons that participate in the rectoanal inhibitory reflex and in defecation [87, 95, 104]. In contrast to the LES and ICS, the IAS lacks a myenteric plexus [87, 105]. Nonetheless, the circular muscle layer is richly innervated by NADPH/nNOS positive neurons that arise from cell bodies located within the rectal myenteric plexus and project distally [87, 104]. Nitric oxide plays a prominent role as an inhibitory neurotransmitter in the human and monkey IAS while purinergic transmission is absent [106, 107]. In contrast, both nitrergic and purinergic transmission are present in rodents [108,109,110,111,112]. VIP also significantly contributes to inhibitory neuromuscular transmission in the IAS [113].
1.4.1 In Vitro Contractile and Electrical Activity
Contraction. IAS muscle strips develop tone as well as phasic contractile activity in vitro independent of nerves [29, 87, 114,115,116,117] (Fig. 2.2b). Tone, and the frequency of phasic contractions, declines in the proximal direction [105, 114, 118]. In all species examined, contractile activity is highly sensitive to CavL channel blockers such as nifedipine [111, 115,116,117]. Indeed, the IC50 for nifedipine in the mouse IAS is 0.03 μM [119].
Electrical activity. Resting membrane potential in the IAS (i.e., −43 to −49 mV) is significantly less negative than in the predominantly phasic rectum (5 mV gradient from IAS to distal rectum in mouse [105]; 20 mV gradient from IAS to proximal rectum in dog [118]). Early sucrose gap recordings identified slow waves and associated phasic contractions in the cat IAS [29] while subsequent intracellular microelectrode studies have identified slow waves in dog, monkey, and mouse IAS [105, 110, 114, 118, 120, 121] (Fig. 2.8). The frequency of slow waves in the IAS ranges from ~45–80 cpm in mouse to ~15–30 cpm in dog and monkey IAS. Slow waves in the IAS differ from those of the intestine in that they lack an initial rapid upstroke (i.e., 1–10 V/s maximum dV/dt for intestine [122, 123] versus 10–100 mV/s maximum dV/dt for the IAS [110]). Instead, IAS slow waves reach peak depolarization after 0.38–0.7 s in mouse and after 1–2 s in dog and monkey. Spikes (260–800 mV/s) of variable amplitude are sometimes superimposed upon slow waves [110]. The amplitude and frequency of slow waves does not vary across the thickness of the IAS circular muscle layer. Indeed, even subsections of this layer exhibit the same electrical activity [118]. Thus, pacemaker potentials appear to be generated throughout the muscle thickness rather than being localized to one specific region (e.g., myenteric or submucosal edge). In contrast, slow wave frequency declines in the proximal direction [105, 114, 118].
Sample traces of slow wave activity recorded from the internal anal sphincter (IAS) of several animal species (Adapted from [121]). (a) Monkey, (b) Canine, (c) Mouse. Note the significantly higher frequency of slow waves in the mouse IAS
2 Role of ICC in the Control of Electrical and Contractile Activity in Sphincters
2.1 Role of ICC in the Generation of Spontaneous Contractile Activity
Historically, spontaneous contractile activity in the GI tract was believed to arise directly from the properties of smooth muscle cells, hence the name “myogenic.” However, since the identification of interstitial cells of Cajal (ICC) in the GI tract and recognition that ICC generate pacemaker potentials [124] it is now widely accepted that spontaneous phasic contractile activity is mediated in large part by slow waves arising from pacemaker-type ICC. Pacemaker ICC are predominantly located at the myenteric (ICC -MY) and submucosal (ICC -SM) surfaces of the circular muscle layer. A second population of ICC has also been identified within the circular and longitudinal muscle layers. These cells are referred to as “intramuscular” ICC (ICC -IM) and they are known to play an important role in excitatory and inhibitory neuromuscular transmission in the GI tract [125]. The morphology of ICC subtypes also differs, with ICC-MY and ICC-SM generally being highly branched stellate cells while ICC-IM are usually spindle-shaped. Pacemaker ICC are electrically coupled to one another and to smooth muscle cells allowing slow waves to conduct into the muscularis in a coordinated fashion [126, 127]. On the other hand, ICC-IM are interspersed within the muscularis, and run parallel to smooth muscle cells. ICC-IM are also electrically coupled to smooth muscle cells forming a syncytium that permits transmission from nerve, through ICC, to adjacent smooth muscle cells [125]. In contrast to this functional difference between ICC subtypes in the intestine, recent studies suggest that some ICC-IM may instead serve as pacemaker cells in the IAS giving rise to tone development (see below).
2.2 ICC in Gastrointestinal Sphincters
ICC have been identified in each of the GI sphincters discussed in this chapter (Fig. 2.9). However, there are significant differences in their morphology and distribution. The distribution of ICC in the ICS [128] resembles that of the colon [129] in that ICC-MY, ICC-SM, and ICC-IM are all represented (Fig. 2.9a). However, slow waves like those described in the large and small intestine [130, 131] are absent and phasic contractions are associated with periodic spiking activity (Fig. 2.7). Furthermore, tone in the ICS is relatively insensitive to blockade by dihydropyridines [82, 84]. Thus, the role of ICC in the ICS likely diverges from that of intestine. The distribution of ICC in the remaining three sphincters differs from that of the ICS. Although the antral end of the pylorus contains ICC-MY, ICC-SM, and ICC-IM, the density of ICC-MY and ICC-SM declines distally approaching undetectable levels at the PS while ICC-IM persist (Fig. 2.9b, d) [132]. Since low-frequency slow waves (i.e., 0.5–1/min) occur in isolated strips of the PS [58, 59], some pacemaker-type ICC-MY likely persist in this region. The LES and IAS resemble one another in that ICC-MY and ICC-SM are absent while having a dense population of ICC-IM (LES [129, 133]; IAS [86, 87, 105] Fig. 2.9f). Interestingly, whereas ICC-IM in the monkey LES are spindle shaped (Fig. 2.9c [129]), those of the monkey and dog IAS are highly branched (Fig. 2.9e [86, 87]). Thus, unlike most of the GI tract, the morphology of ICC-IM in the monkey and dog IAS resembles that of pacemaker ICC. ICC in the dog IAS have also been shown to form gap junctions with one another and with smooth muscle cells [86]. Because the dog and monkey IAS both have slow waves (Fig. 2.8) and “pacemaker like” ICC (Fig. 2.9e), we have proposed that ICC-IM are responsible for the generation of pacemaker potentials in the IAS [87]. Our recent studies with the Kit-GCaMP3+ mouse, a transgenic mouse that expresses the calcium-sensitive fluorophore GCaMP3 in ICC, further support this hypothesis. These studies have revealed that adjacent GCaMP3+ cells generate synchronized whole-cell phasic calcium events at the slow wave frequency [134, 135].
Immunohistochemical labeling of interstitial cells of Cajal (ICC) in various gastrointestinal sphincters and animals. (a) Longitudinal section of the guinea pig ileocecal sphincter (valve) showing ICC (Kit, green) and nerves (PGP, red). ICC are located at the submucosal and myenteric surfaces as well as within the muscle layer. Some ICC are in close association to nerves. The terminal ileum immediately adjacent to the sphincter contains a thickened circular muscle layer [128]. (b) A longitudinal section of the rat pyloric sphincter (PS) and adjacent regions immunostained for Kit. In the distal antrum (left), ICC are concentrated at the myenteric plexus (arrows) surrounding ganglia. ICC-IM are also scattered in both circular and longitudinal muscle layers. There is also a concentration of ICC at the submucosal border (small arrows). In the pyloric region (middle), ICC-MY are markedly decreased in a segment ~4 mm in width while ICC-IM are abundant and diffusively distributed. In the proximal duodenum (right), ICC-MY are present (arrows) except for the first 1–2 mm, where ICC are evenly distributed in the whole musculature and not concentrated at the level of the myenteric plexus. From 3–4 mm onward in the duodenum, ICC are present at the deep muscular plexus and submucosa. Background staining of the mucosa is due to endogenous biotin within the mucosal glands only [132]. (c) Kit labeling (green) of ICC-IM in a 100 μm cryostat section cut parallel to the circular muscle fibers in the monkey lower esophageal sphincter (LES) [129]. (d) Whole mount preparation of the rat pyloric sphincter labeled for Kit (green). ICC-MY are absent from this region. Instead, there are many bipolar-shaped ICC-IM running parallel to circular muscle fibers of the pyloric sphincter (left) whereas a loose network of ICC can be seen at the beginning of the proximal duodenum (right) [132]. (e) Ten micron optical section taken from a whole mount preparation of the monkey internal anal sphincter cut parallel to circular muscle fibers. Highly branched stellate ICC-IM are seen at this focal plane as well as throughout each muscle bundle within the circular muscle layer [87]. (f) Longitudinal section of the smMHCCre-eGFP mouse anorectum. Kit+ ICC (red) were identified using immunohistochemical techniques while smooth muscle cells were identified through expression of green fluorescent protein (green) [105]. ICC-SM and ICC-MY are apparent along the submucosal and the myenteric edges of the circular muscle layer in rectum respectively but disappear before reaching the distal IAS. In contrast, ICC-IM are present throughout the CM layer of the rectum and IAS. Spindle-shaped ICC-IM can also be seen in the longitudinal muscle layer. Division of the CM layer into bundles (green) can clearly be seen within the IAS. Images are composites of three images taken at 10×. CM circular muscle, ICC interstitial cells of Cajal, IAS internal anal sphincter, IM intramuscular, LM longitudinal muscle, MY myenteric, SM submucosa
2.3 Role of Chloride Channels in the Generation of Slow Waves and the Control of Membrane Potential in Gastrointestinal Sphincters
Intracellular chloride concentration in smooth muscle cells is significantly greater than in skeletal muscle with an estimated chloride reversal potential between −20 and −30 mV [135,136,138]. Thus, chloride channels have long been proposed as contributors to setting the level of resting membrane potential in smooth muscle. More recently, deep sequencing of smooth muscle cells of the mouse large and small intestine has identified transcript for voltage-gated Cl− channels, as well as several members of the anoctamin/TMEM16 (ANO1) family of Ca2+-activated Cl− channels [139]. The resting membrane potential of LES is ~10 mV less negative than the esophageal body [5, 9, 34, 35] and early studies suggested that this was due to a greater chloride conductance in LES cells [140]. Subsequent studies have supported this proposal by showing that chloride channel blockers (i.e., niflumic acid, 9-Anthracene carboxylic acid) hyperpolarize membrane potential, abolished action potentials, and greatly reduced tone in the LES [35, 141].
In the LES studies described above, the cell type responsible for generating chloride currents was not addressed. More recently it has been shown that intestinal ICC also express some voltage-gated Cl− channels as well as Ca2+-activated Cl− channels in the ANO channel family [142]. In particular, expression levels of ANO1 in ICC far exceeds that of smooth muscle cells. There is now substantial evidence that ANO1 in ICC plays a central role in the generation of slow waves as discussed in the other GI chapter in this book. Briefly, in pacemaker ICC, intermittent release of calcium from the endoplasmic reticulum results in activation of ANO1 and spontaneous transient inward currents (STICs). STIC generated membrane depolarization in turn activates voltage-dependent calcium channels giving rise to slow waves that conduct into adjacent smooth muscle cells via gap junctions [143].
Since the IAS exhibits slow waves and phasic contractile activity while only ICC-IM are present, we hypothesized that ICC-IM are responsible for the generation of pacemaker potentials in this muscle. Thus, we investigated whether ANO1 channels in ICC-IM have a similar role to that described for pacemaker ICC in the intestine. These studies revealed that: (1) Ano1 gene expression is 26× greater in ICC-IM than in smooth muscle cells. (2) Gene expression levels of Ano1 in whole IAS and rectum are the same. (3) ANO1 protein expression is resolvable in ICC but not in smooth muscle cells. (4) ANO1 channel antagonists abolish slow waves, phasic contractions, and tone as well as causing a small hyperpolarization of resting membrane potential. These data all support the hypothesis that ICC-IM are responsible for the generation of slow waves in the IAS and that ANO1 channels play a central role in this process [119]. Equivalent experiments have not been undertaken in the LES.
2.4 Limitations of the W/Wv Mouse for Examining the Role of ICC in Sphincters
The W/Wv mouse has been used in a number of studies to examine the role of ICC in the generation of pacemaker potentials [144]. Kit-labeling of ICC-IM is not resolvable in the IAS, LES, and PS of the W/Wv mouse [144,145,147] while membrane potential is ~4 mV more polarized [110, 146]. In the IAS tone and slow waves persist in the W/Wv mouse [110] while tone is significantly reduced in the LES [146]. These data do not strongly support a role for ICC-IM in the generation of tone and slow waves in the IAS. The W/Wv mouse is not a quantitative Kit knockout since the W kit allele is null while the WV allele has reduced function [148]. Thus, Kit labeling of ICC throughout the GI tract is “patchy” with Kit resolvable in some regions but not others [149]. Likewise, the degree of functional knockout differs between regions [144]. Indeed, differences in the degree of functional knockout have also been reported for the same region by different groups (e.g., LES [146, 150]). It is possible that functional ICC-IM persist in the W/Wv mouse IAS even though Kit protein is no longer resolvable with immunohistochemistry. Alternatively, since the reduced Kit function in the W/Wv mouse is constitutive, it is possible that some gain of function occurs during development in other cell types (i.e., smooth muscle cells, PDGFRα+ cells) that compensates for the loss of ICC. Indeed, ICC, PDGFRα+ cells, and smooth muscle cell all have a common precursor, namely, gut mesenchymal cells [150,151,152,153,155]. Because of these issues, the W/Wv mouse is not ideal for examining the role of ICC-IM in the generation of slow waves in the IAS. Our observations that Kit-GCaMP3+ cells generate phasic calcium events at the slow wave frequency [134, 135] as well as our studies linking ANO1 to ICC-IM and slow wave generation [119] all provide evidence that ICC-IM generate slow waves in the IAS.
3 Specific Mechanisms Proposed for the Generation of Tone in GI Sphincters
In Part I we briefly summarized the anatomy and physiological behavior of the four main sphincters in the GI tract. A general conclusion of this section is that there is stronger evidence supporting a role for spontaneous tone generation in the LES, ICS, and IAS than for the PS. In the following section (Part III) we examine specific mechanisms proposed to underlie tone generation in these GI sphincters.
3.1 Mechanism #1: Tone May Arise from the Summation of Asynchronous Phasic Activity Generated by Adjacent Muscle Bundles that are not Highly Coupled to one Another
It has long been known that skeletal postural muscles can maintain tone through the asynchronous firing of motor units [156]. Specifically, the asynchronous contractile activity generated by different motor units sum to maintain tone. It is possible that similar events contribute to tone generation in the GI tract. In this case however the “motor unit” corresponds to a small bundle of electrically coupled smooth muscle cells isolated from adjacent muscle bundles. This division of muscle into small bundles differs somewhat from the classic definition of smooth muscle as either “multiunit” (i.e., each cell electrically isolated from its neighbors) or “single unit” (i.e., all cells electrically coupled to neighbors). In fact, most GI muscles fall somewhere between these extremes since they are composed of muscle bundles of various sizes as well as varying degrees of electrical coupling between bundles. In the large and small intestine muscle bundles are generally quite large and cells within them are highly coupled. The activity of the bundles is coordinated by pacemaker ICC located at the myenteric and/or submucosal edge of the muscle layer. In contrast, the IAS is subdivided into numerous smaller “minibundles,” each separated by wide connective septa (Fig. 2.10 [86, 87]). ICC-IM and nerves are located within each minibundle. This provides a means for eliciting spontaneous activity within the minibundle (i.e., ICC-IM) and a mechanism (i.e., nerves) to coordinate the activity of all minibundles [87]. To conclude that the electrical activity of minibundles in large animals is asynchronous will require dual microelectrode recordings and/or studies examining the spread of calcium transients across the tissue. However, there is one study that has been undertaken on a smaller, simpler muscle, i.e., the mouse IAS. The mouse IAS is also composed of minibundles but in this case there are only six to ten bundles and each bundle spans the entire width of the circular muscle layer. Dual microelectrode measurements in this tissue have shown that slow waves are only coordinated across a few muscle bundles longitudinally and ~20% of the circumference [105]. Thus, even in the mouse IAS, the circular muscle layer does not behave as a “single unit” but rather as a composite of minibundles that are only loosely coupled to one another supporting mechanism #1.
Comparison of the organization of muscle bundles in the monkey IAS (left) and rectum (right) at the same magnification. Thin (3 μm) cross sections of the internal anal sphincter (IAS ) and rectum were stained with Masson’s trichrome to visualize both smooth muscle (red/purple) and connective tissue (blue). (a) Numerous minibundles (red) are present in the IAS separated by connective tissue septa (blue). (b) The rectal wall thickness is thinner than the IAS thus at this magnification it is possible to see the entire width of the circular muscle (CM) layer as well as some longitudinal muscle (LM). Muscle bundles are again observed in the rectum but in this case each bundle is larger, spanning the entire CM width and overall, there is less connective tissue within and between muscle bundles [87]
3.2 Mechanism #2: Tone May Arise from the Accumulation of Intracellular Calcium in the Smooth Muscle Cytoplasm Because Calcium Entry via High-Frequency Slow Waves Outpaces Calcium Efflux/Uptake Mechanisms
Drawing again from the behavior of skeletal muscle, it is known that the characteristics of contraction in skeletal muscle changes with the frequency of stimulation. Thus, as stimulus frequency increases, contractile amplitude increases until a point is reached at which contraction ceases to return to baseline between stimuli. This condition, referred to as “partial (incomplete) tetanus,” occurs because there is insufficient time between stimuli for calcium removal to return intracellular calcium ([Ca2+]i) to subthreshold levels [157]. The pattern of contractile activity in the IAS resembles partial tetanus in that it is composed of phasic contractions superimposed upon tone. Recently, a transgenic mouse has become available that expresses the calcium-sensitive fluorophore GCaMP3 in smooth muscle cells (SM-GCaMP3). This makes the SM-GCaMP3 mouse a valuable tool for evaluating the possible role of mechanism #2 in tone generation. Transient changes in [Ca2+]i are observed in smooth muscle cells of the SM-GCaMP3 mouse IAS and these occur at the same frequency as slow waves (Fig. 2.11). Stimulation of inhibitory motor neurons (which hyperpolarizes membrane potential and abolishes almost all contractile activity [109, 110]) reveals that [Ca2+]i between calcium transients is significantly elevated. Furthermore, the average time constant for the decline of [Ca2+]i following each calcium transient is ~0.3–0.5 s. Consequently, there is insufficient time for calcium to reach subthreshold levels before the arrival of the next calcium transient [135] (Fig. 2.11). This observation provides evidence for mechanism #2 in the mouse IAS. Such a mechanism is also likely to contribute to tone generation in the opossum LES where ongoing spike discharge occurs at a high frequency (Fig. 2.3).
Calcium transients recorded at the distal end of the internal anal sphincter (IAS ) of the SM-GCaMP3 mouse. Upper trace: Spatiotemporal map of the spontaneous activity in a 33 s recording taken from a 100 × 200 micron region of interest (ROI) of the distal IAS. A calcium trace taken from this ROI shows the changes in [Ca2+]i as a function of time. Electrical stimulation of nerves (EFS , 5 Hz, 10 s) was applied in the presence of atropine to eliminate cholinergic transmission. EFS blocked all phasic activity as well as reducing basal calcium levels. The time constant for calcium removal (tau = 0.35 s) was estimated by fitting the decline of individual calcium transients to a simple exponential function from peak to baseline. At the end of the stimulus train a large rebound event was observed before the pattern returned to control levels [134, 135]
In contrast to the above examples, the frequency of slow waves in the IAS of larger animals such as dog and monkey is significantly lower than mouse (see Fig. 2.8). However, the rate of slow wave depolarization and repolarization is also slower in these muscles resulting in the membrane potential remaining above the CavL channel threshold for a longer period of time (Fig. 2.12). Thus, the predominant factor keeping [Ca2+]i above threshold in these muscles is likely the extended period of time that calcium entry continues with each slow wave rather than a partial tetanus type mechanism. To further evaluate the relationship between slow waves and tone generation will require addressing the following questions: (1) Is [Ca2+]i also tonically elevated in the IAS of animals with low-frequency slow waves? (2) To what extent are there differences in the characteristics of CavL channels and/or calcium uptake/efflux mechanisms between species? (3) To what extent are there differences in the cell type and/or ionic conductances that are responsible for slow wave generation between species?
Comparison of slow waves recorded from the mouse and monkey IAS. Slow waves differed in several ways including: (1) Slow wave frequency is significantly less in the monkey IAS than in the mouse IAS. (2) The rate of depolarization and repolarization is significantly slower in the monkey IAS than in the mouse IAS. (3) “Resting” membrane potential is more depolarized in the monkey IAS than in the mouse IAS. Because of these differences, each slow wave in the monkey IAS spends a greater amount of time above the activation threshold for CavL channels than a slow wave in the mouse IAS (Adapted from [121])
3.3 Mechanism #3: Tone May Arise from Continuous Calcium Entry into Smooth Muscle Cells via Window Current
Voltage-dependent calcium channels , in particular CavL channels, play an important role in delivering calcium to the cytoplasm for contraction in most GI muscles, including the sphincters covered in this section. Both the activation and inactivation of CavL channels are voltage-dependent processes with maximum current occurring around 0 mV and threshold current appearing around −50 to −45 mV [157,158,160]. At potentials between these two extremes inactivation is incomplete and some sustained calcium current can occur which is referred to as “window current” [159,160,162]. Window current has long been associated with the sustained contraction that follows exposure of smooth muscles to elevated extracellular potassium solution. In phasic muscles without tone, slow waves arise periodically from a relatively polarized level of membrane potential (e.g., −78 mV for canine colon at the submucosal edge [130]), CavL channels open during slow wave depolarization and close as the membrane potential falls below their activation threshold. However, the level of membrane potential between slow waves or spikes in the LES and IAS is less polarized lying within the activation threshold for CavL channels. Hence some contribution from window current (mechanism #3) is anticipated.
A further consideration with regard to the relationship between membrane potential and tone relates to the actions of nitrergic nerves. Most GI muscles are under tonic inhibition via the spontaneous release of NO from inhibitory motor neurons. NO hyperpolarizes sphincters [73, 163, 164] and blockade of tonic NO release from nerves causes depolarization [73, 111]. Hence, removal of NO (by blocking either NOS or neural activity) results in increased phasic contractions as well as tone [7, 111, 164,165,166,168]. Likewise, eliminating guanylate cyclase in ICC and smooth muscle cells (SMC/ICC-GCKO) has been shown to result in a 2.5-fold increase in LES pressure [169].
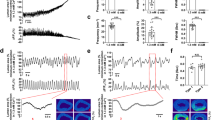
The monkey IAS is under strong tonic nitrergic inhibition. Thus blockade of NO release from nerves either with TTX or with the NOS inhibitor L-NNA causes increased tone as well as a decrease in phasic contractile activity (Fig. 2.13a). These changes are accompanied by depolarization and a reduction in the amplitude of slow waves (Fig. 2.13b). Under these depolarizing conditions, Ca2+ entry associated with CavL channel window current will result in more tonic contracture. The effects of NOS blockade can be reversed either by addition of an NO donor such as sodium nitroprusside (Fig. 2.13a, c, d) or by hyperpolarizing the muscle with a potassium channel opener such as pinacidil (Fig. 2.13e) [120]. Thus, tonic release of NO from inhibitory motor neurons appears to play an important role in determining the electromechanical mechanisms underlying contractile activity in the monkey IAS. In the presence of NO, membrane potential is more polarized, and calcium delivery involves more phasic electrical events (i.e., slow waves). On the other hand, when NO is removed and membrane potential is more depolarized, continuous entry of calcium via window current plays a greater role (mechanism #3).
Examples of the electrical and contractile changes that occur in the monkey IAS with blockade of nitric oxide synthase with L-NNA, or following addition of the nitric oxide donor sodium nitroprusside (SNP) or the KATP activator pinacidil. (a) Tone and phasic activity are observed under control conditions in this muscle. Tone is significantly increased with addition of L-NNA while phasic activity is reduced. The effect of L-NNA was reversed by addition of increasing concentrations of SNP while the highest concentration abolished all contractile activity. (b) Electrical recording from a different muscle strip showing slow waves occurring in the absence of L-NNA (left). Following L-NNA addition membrane potential depolarized and slow wave amplitude was reduced (right). (c) Addition of 0.03 μM SNP in the continued presence of L-NNA restores control slow wave activity and returns membrane potential to the control level. (d) Addition of a higher concentration of SNP (0.1 μM) in another muscle strip caused hyperpolarization and a transient period of larger amplitude slow waves followed by further hyperpolarization and blockade of slow waves (L-NNA present throughout). (e) Addition of pinacidil to another muscle in the presence of L-NNA also resulted in hyperpolarization and the return of larger amplitude slow waves [120] (a–d Adapted from [121])
Besides blocking nitrergic inhibition, tone can also be generated by increasing extracellular potassium ([K]o) leading to membrane depolarization. Thus, when [K]o is raised, the normally phasically active canine gastric antrum generates tone with superimposed phasic contractions [170]. Likewise raising (K)o in the guinea pig ICS increases tone while lowering [K]o diminishes tone [76, 83].
3.4 Mechanism #4 Tone May Arise from Sensitization of the Myofilaments to Calcium
Spontaneous contractile activity in GI smooth muscles is dependent upon a rise in [Ca2+]i. Furthermore, calcium entry through CavL channels plays an important role in this process. The first three mechanisms considered above describe processes linking CavL channels to tone generation in sphincters. The fourth mechanism instead addresses how tone in sphincters may result from a greater sensitivity of the myofilaments to calcium.
Smooth muscle myosin is composed of two heavy chains forming head and tail regions and two 20-kD regulatory light chains (MLC20 ). The phosphorylation of MLC20 at S19 by myosin light chain kinase (MLCK ) stimulates myosin ATPase activity, cross-bridge cycling, and contraction [170,171,172,174]. MLCK is activated by a calcium-calmodulin complex that forms when [Ca2+]i increases. Contraction is terminated when myosin light chain phosphatase (MLCP) dephosphorylates MLC20. The extent of smooth muscle contraction therefore depends upon a balance between the activities of MLCK and MLCP. MLCP is composed of a catalytic subunit (protein phosphatase 1, or PP1), a regulatory subunit (myosin phosphatase target subunit 1, or MYPT1), and a 20-kD subunit (M20) of unknown function. The regulatory subunit MYPT1 facilitates the ability of PP1 to dephosphorylate MLC20 by serving as a scaffolding protein [174,175,176,177,178,179,181].
Several different second messenger pathways have been shown to inhibit MLCP resulting in greater phorphorylation of MLC20 at a given [Ca2+]i, i.e., “calcium sensitization” [176,177,179, 181,182,184]. One such pathway involves phosphorylation of MYPT1 at threonine 696 (T696) and 853 (T853). Recent studies suggest that T696 phosphorylation is responsible for direct inhibition of MYPT1 whereas T853 phosphorylation causes disassembly of the myosin-MLCP complex [185]. An important regulator of MYPT1 phosphorylation is Ras homolog gene family, member A (RhoA). RhoA is a small, monomeric G-protein that is activated when GDP is exchanged for GTP. Activated RhoA in turn activates Rho-associated protein kinase 2 (ROCK2 ). In many smooth muscles, the RhoA/ROCK2 pathway is activated by G-protein coupled receptors resulting in phosphorylation of T696 and T853, decreased MLCP activity and calcium sensitization [176, 183, 186, 187]. However, since tone in sphincters arises spontaneously, the more relevant issue is whether constitutive phosphorylation of T696 and T853 also occurs. There is general agreement that constitutive T696 and T853 phosphorylation occurs in both phasic and tone-generating muscles [181, 188] and that constitutive T853 phosphorylation is dependent upon phosphorylation by ROCK2 [176, 183, 185,186,188]. However, there are reports of ROCK2-independent phosphorylation of T696 [185, 187,188,190]. Since there are other kinases in smooth muscle cells that can phosphorylate T696 [191], ROCK2-independent T696 phosphorylation likely involves one or more of these other kinases.
Recently a conditional MYPT1 KO mouse (MYPT1SM−/−) has become available to further examine the role of MYPT1 and calcium sensitization in the regulation of smooth muscle contraction. Surprisingly, the contractile activity of smooth muscles in the MYPT1SM−/− mouse is only modestly different from that of WT mice [192] including the tone-generating IAS [193]. However, loss of MYPTI in the MYPT1SM−/− mouse has also been shown to result in a reduction in PP1 levels, possibly because the cellular stability of PP1 depends upon MYPT1 [188, 194]. In addition, regulation of MLCP activity by MYPT1 may be shared with the closely related MYPT1 family member myosin binding subunit 85 (MS85) so that loss of one regulator may be compensated for by the other [195]. Given the complexity of molecular pathways that regulate MLCP activity, it appears premature to discount a possible role for calcium sensitization in tone development in the IAS based upon the MYPT1SM−/− mouse [193].
The extent to which myofilament sensitization contributes to tone generation in sphincters likely differs between sphincters as well as between species. The relative ability of CavL channel blockers to abolish tone provide us with some preliminary information regarding the possible mechanisms contributing to tone generation since electromechanical coupling pathways are sensitive to these antagonists while myofilament sensitization pathways are not. The extreme example in this regard is the mouse fundus which is entirely insensitive to dihydropyridines. Biochemical studies have shown that tone in the fundus is generated in large part by the RhoA/ROCK2 pathway [190]. Phasic contractions in the PS are abolished by dihydropyridines [57] while phasic activity and >90% of tone are eliminated in the IAS [115]. These data clearly suggest an important role for electromechanical coupling in the PS and IAS. In contrast, dihydropyridines are less effective at blocking tone in the LES (i.e., 72% inhibition [35]) and least effective in the ICS (i.e., <50% inhibition [82, 84]). From these observations, it is tempting to suggest that the most prominent role for myofilament sensitization is in the ICS followed by the LES and then the IAS. Unfortunately, little attempt has been made to examine myofilament sensitization in the ICS while only limited work has been completed on the LES (see below).
The most comprehensive examination of calcium sensitization pathways in a sphincter are studies of rat and human GI muscles by Rattan and coworkers [195,196,197,199]. These studies revealed significantly greater RhoA, ROCK2, and T696 phosphorylation levels in the tone-generating IAS than in rectum, the latter being predominantly a phasic muscle with significantly less tone. ROCK2 antagonists also abolished T696 phosphorylation and were more potent antagonists of contraction in the IAS than in other phasic muscles. From these data, the authors concluded that tone in the IAS is due to greater calcium sensitization via the ROCK2/MYPT1 pathway (i.e., mechanism #4). We reexamined this topic by comparing various proteins in the RhoA/ROCK2 pathway in the monkey IAS and rectum. Our results are in general agreement with those of Rattan et al. in that T696 and T853 phosphorylation and ROCK2 expression levels were all greater in the IAS than in rectum. However, we noted little difference in the potency of ROCK2 inhibitors between muscles [200]. Likewise, little difference has been noted in the potency of ROCK2 inhibitors in the phasically active mouse colon versus the tone-generating fundus [190]. Taken together, these data suggest that some degree of tonic myofilament sensitization is likely to be present in both phasic and tone-generating muscles [191], a concept that is further developed in the summary section.
Another inhibitor of MLCP is C-kinase (PKC ) potentiated protein phosphatase-1 Inhibitor protein-17 (CPI-17). Phosphorylation of CPI-17 at threonine 38 (T38) results in a conformational change that permits docking of CPI-17 with PP1 resulting in MLCP inhibition [201]. In smooth muscle, phosphorylation of CPI-17 is predominantly mediated by PKC or ROCK2 [191]. The degree to which PKC-mediated Ca2+ sensitization occurs has been correlated with CPI-17 expression levels in the smooth muscle [202, 203]. Constitutive phosphorylation of CPI-17 is generally considered to be low in smooth muscles compared with constitutive MYPT1 phosphorylation [190, 203,204,205,206,207,208,209,211]. In studies of the rat and human IAS and rectum CPI-17 levels and constitutive T38 phosphorylation were reported to be greater in the IAS than in rectum. Nonetheless the authors concluded that calcium sensitization is predominantly mediated by the RhoA/ROCK2 /MYPT1 pathway, in part because of the limited ability of PKC blockers to reduce tone. PKC inhibitors also have very limited effects on basal tone in the LES whereas ROCK2 inhibitors produce full relaxation [212, 213], again suggesting a more important role for the RhoA/ROCK2 pathway in calcium sensitization in sphincters than PKC/CPI-17. It should be noted that MYPT1 and CPI-17 phosphorylation were not measured in the LES studies.
NO-mediated desensitization. The discussion thus far has focused on how calcium sensitization might give rise to tone in sphincters. However, as previously described, it is known that contractile activity in sphincters is subject to tonic inhibition due to the release of NO from inhibitory motor neurons [7, 111, 164,165,166,168]. NO binds to guanylyl cyclase leading to increased formation of cGMP and activation of cGMP-dependent protein kinase ( PKG). PKG in turn is linked to calcium desensitization in part through phosphorylation of RhoA at serine 188 (S188). This prevents RhoA binding to ROCK2, thereby reducing MYPT1 phosphorylation and restoring MLCP activity [213,214,215,216,217,219]. Increased cGMP has also been associated with increased phosphorylation of MYPT1 at serine 695 (S695) leading to a reduction in T696 phosphorylation, again restoring MLCP activity [220, 221] although this effect was not observed in cerebral artery [222]. Telokin is another candidate for NO-mediated disinhibition of MLCP. Telokin is a small protein with partial sequence homology to MLCK. Following a rise in cGMP, telokin becomes phosphorylated at serine 13 (S13) leading to stabilization of the unphosphorylated state of MLC20 and relaxation [223]. Studies of a telokin-deficient mouse have reported increased MLCP activity and decreased cGMP-mediated relaxation in ileum [224], further supporting a role for telokin in NO-mediated contractile inhibition.
Most studies examining the role of MLCP phosphorylation and calcium sensitization are undertaken in the presence of NOS inhibitors and/or neural blockers. This approach is likely to underestimate MLCP activity in vivo and consequently overestimates the degree to which calcium sensitization participates in tone generation. Indeed, our contractile and microelectrode measurements in the monkey IAS further emphasize the substantial changes that occur in the pattern of spontaneous electrical and contractile activity following NOS blockade (Fig. 2.13). When tonic neural inhibition is present, there is less tone while phasic electrical and contractile activity are enhanced. In contrast, following NOS blockade, the membrane depolarizes, slow waves and phasic contractile activity are reduced, and tone is increased. Thus, NO can have profound effects upon both electromechanical coupling mechanisms and myofilament sensitization. Tonic neural inhibition may therefore provide a system in which contractile activity in vivo is predominantly controlled by electromechanical mechanisms which are better suited to rapid moment to moment adjustments whereas calcium sensitization represents a means for retaining contracture if neural pathways are compromised.
4 Summary
The LES, PS, ICS, and IAS each serve to restrict the movement of luminal contents between adjacent regions of the GI tract. However, the mechanisms by which this function is achieved differ significantly between sphincters. In the first section, we reviewed the basic anatomy and physiology of the various sphincters. A general conclusion of this section is that there is stronger evidence supporting a role for spontaneous tonic contraction in the LES, ICS, and IAS than for the PS. In the second section, we examined the general relationship of contractile and electrical activity in the GI tract to ICC along with the possible role of ICC-IM in the generation of tone in sphincters. In the third section, four different mechanisms were considered that have been proposed to give rise to tone generation in sphincters. Several of these mechanisms are likely to participate in tone generation although the contribution of each will differ between sphincters as well as between species. Both the IAS and LES exhibit ongoing phasic electrical activity while the IAS in particular generates substantial phasic contractile activity as well. Two different mechanisms have been discussed (mechanism #1 and #2) that result in tone generation from phasic electrical events. For this reason, the conventional description of the IAS as a “purely tonic muscle” is mistaken [225]. Instead the IAS is best considered as a phasically active muscle that generates tone. The final figure in this chapter (Fig. 2.14) presents a summary diagram that depicts how electromechanical coupling mechanisms (#1–3) and calcium sensitization (mechanism #4) might interface with one another to generate tone versus phasic contractions in the IAS and rectum, respectively. To further advance this field it is important that future studies consider the entire spectrum of mechanisms that may interact with one another to achieve the final contractile status of each GI sphincter.
Cartoon depicting the possible interactions occurring between electromechanical coupling, calcium sensitization and contraction in the IAS and rectum (Adapted from [121]). The predicted relationships between contraction and intracellular calcium concentration (pCa) in the IAS (left) and rectum (middle) are shown. The IAS curve is drawn to the left of the rectum because of greater constitutive MYPT1 phosphorylation (i.e., calcium sensitization, mechanism #4). Thus, for a given pCa there will be more contraction in the IAS than in rectum. Tone is observed in the IAS since resting membrane potential is more depolarized and slow wave frequency is greater [105, 118] resulting in a larger, more sustained increase in CavL channel activity and pCa (dot, mechanism #2 and #3). Each IAS slow wave causes a further increase in pCa along the pCa/contraction curve (black arrow) resulting in phasic contractions superimposed upon tone. Asynchrony between muscle bundles will dampen the average fluctuation in pCa in whole tissue resulting in smaller amplitude phasic contractions and greater tone (mechanism #1). In contrast to the IAS, resting membrane potential in rectum is more polarized and slow waves occur at a lower frequency. Thus, basal pCa is lower in rectum (dot at the base of the middle curve). Each slow wave causes a large transient increase in pCa (dashed arrow, middle curve) and a phasic contraction followed by return of pCa to low levels. If depolarization in the rectum is sustained in some manner (e.g., by elevating extracellular potassium concentration) then tone will develop (e.g. [170]). Blocking CavL channels (x) with nifedipine causes pCa to fall to low levels in both the IAS and rectum abolishing tone and phasic contractions. Blocking ROCK2 (y) with an inhibitor such as Y27632 causes the entire IAS pCa/contraction relationship to shift to the right (zero sensitization curve) eliminating tone with little change in pCa. The pCa/contraction relationship for rectum will also shift to the right with ROCK2 inhibition since these inhibitors also reduce contraction and constitutive MYPT1 phosphorylation in rectum [200]
Clinical Relevance. Changes in the structure and mechanical properties of sphincters can result in a number of different pathological conditions that diminish their ability to carry out normal functions. For example, a reduction in the ability of the lower esophageal/sling/clasp region to contract leads to a return of gastric contents to the esophagus resulting in gastroesophageal reflux disease (i.e., GERD) [11]. Likewise, stenosis of the PS can lead to vomiting, delayed emptying, and gastroparesis [226]. Failure of the ICS to adequately contract can result in an increase in the return of colonic contents to the small intestine giving rise to bacterial overgrowth [64, 227]. Finally, inadequate contraction of the IAS contributes to fecal incontinence [228]. In some cases, sphincter malfunction has been associated with loss of ICC [226, 228,229,231]. However, since different ICC populations perform different functional roles (e.g., pacemaking versus neuromuscular transmission) additional studies are required before a definitive relationship is established between ICC loss and disease. Clearly, establishing the basic mechanisms that underlie sphincter motility is an important first step in advancing our understanding of the etiology of disease states that compromise sphincter function.
References
Liebermann-Meffert D, Allgower M, Schmid P, Blum AL. Muscular equivalent of the lower esophageal sphincter. Gastroenterology. 1979;76:31–8.
Preiksaitis HG, Tremblay L, Diamant NE. Nitric oxide mediates inhibitory nerve effects in human esophagus and lower esophageal sphincter. Dig Dis Sci. 1994;39:770–5.
Brookes SJ, Chen BN, Hodgson WM, Costa M. Characterization of excitatory and inhibitory motor neurons to the guinea pig lower esophageal sphincter. Gastroenterology. 1996;111:108–17.
Yuan S, Costa M, Brookes SJ. Neuronal pathways and transmission to the lower esophageal sphincter of the guinea pig. Gastroenterology. 1998;115:661–71.
Lecea B, Gallego D, Farre R, Opazo A, Auli M, Jimenez M, Clave P. Regional functional specialization and inhibitory nitrergic and nonnitrergic coneurotransmission in the human esophagus. Am J Physiol Gastrointest Liver Physiol. 2011;300:G782–94.
Farre R, Auli M, Lecea B, Estrada O, Sunol X, Clave P. Mechanisms controlling function in the clasp and sling regions of porcine lower oesophageal sphincter. Br J Surg. 2007;94:1427–36.
Gonzalez AA, Farre R, Clave P. Different responsiveness of excitatory and inhibitory enteric motor neurons in the human esophagus to electrical field stimulation and to nicotine. Am J Physiol Gastrointest Liver Physiol. 2004;287:G299–306.
L’Heureux MC, Muinuddin A, Gaisano HY, Diamant NE. Feline lower esophageal sphincter sling and circular muscles have different functional inhibitory neuronal responses. Am J Physiol Gastrointest Liver Physiol. 2006;290:G23–9.
Zhang Y, Mashimo H, Paterson WG. Regional differences in nitrergic innervation of the smooth muscle of murine lower oesophageal sphincter. Br J Pharmacol. 2008;153:517–27.
Liu JF, Lu HL, Wen SW, Wu RF. Effects of acetylcholine on sling and clasp fibers of the human lower esophageal sphincter. J Gastroenterol Hepatol. 2011;26:1309–17.
Miller L, Vegesna A, Ruggieri M, Braverman A. Normal and abnormal physiology, pharmacology, and anatomy of the gastroesophageal junction high-pressure zone. Ann N Y Acad Sci. 2016;1380:48–57.
Zifan A, Kumar D, Cheng LK, Mittal RK. Three-dimensional myoarchitecture of the lower esophageal sphincter and esophageal hiatus using optical sectioning microscopy. Sci Rep. 2017;7:13188.
Mittal RK, Zifan A, Kumar D, Ledgerwood-Lee M, Ruppert E, Ghahremani G. Functional morphology of the lower esophageal sphincter and crural diaphragm determined by three-dimensional high-resolution esophago-gastric junction pressure profile and CT imaging. Am J Physiol Gastrointest Liver Physiol. 2017;313:G212–9.
Mittal RK, Balaban DH. The esophagogastric junction. N Engl J Med. 1997;336:924–32.
Cowgill SM, Bloomston M, Al-Saadi S, Villadolid D, Rosemurgy AS. Normal lower esophageal sphincter pressure and length does not impact outcome after laparoscopic Nissen fundoplication. J Gastrointest Surg. 2007;11:701–7.
Richter JE, Wu WC, Johns DN, Blackwell JN, Nelson JL III, Castell JA, Castell DO. Esophageal manometry in 95 healthy adult volunteers. Variability of pressures with age and frequency of “abnormal” contractions. Dig Dis Sci. 1987;32:583–92.
Jiang Y, Bhargava V, Lal HA, Mittal RK. Variability in the muscle composition of rat esophagus and neural pathway of lower esophageal sphincter relaxation. Am J Physiol Gastrointest Liver Physiol. 2011;301:G1014–9.
Vitaic S, Stupnisek M, Drmic D, Bauk L, Kokot A, Klicek R, Vcev A, Luetic K, Seiwerth S, Sikiric P. Nonsteroidal anti-inflammatory drugs-induced failure of lower esophageal and pyloric sphincter and counteraction of sphincters failure with stable gatric pentadecapeptide BPC 157 in rats. J Physiol Pharmacol. 2017;68:265–72.
Richardson BJ, Welch RW. Differential effect of atropine on rightward and leftward lower esophageal sphincter pressure. Gastroenterology. 1981;81:85–9.
Schulze K, Dodds WJ, Christensen J, Wood JD. Esophageal manometry in the opossum. Am J Phys. 1977;233:E152–9.
Vicente Y, Da RC, Yu J, Hernandez-Peredo G, Martinez L, Perez-Mies B, Tovar JA. Architecture and function of the gastroesophageal barrier in the piglet. Dig Dis Sci. 2001;46:1899–908.
Rattan S, Goyal RK. Effect of nicotine on the lower esophageal sphincter. Studies on the mechanism of action. Gastroenterology. 1975;69:154–9.
Behar J, Kerstein M, Biancani P. Neural control of the lower esophageal sphincter in the cat: studies on the excitatory pathways to the lower esophageal sphincter. Gastroenterology. 1982;82:680–8.
Biancani P, Zabinski M, Kerstein M, Behar J. Lower esophageal sphincter mechanics: anatomic and physiologic relationships of the esophagogastric junction of cat. Gastroenterology. 1982;82:468–75.
Goyal RK, Chaudhury A. Physiology of normal esophageal motility. J Clin Gastroenterol. 2008;42:610–9.
Rossiter CD, Norman WP, Jain M, Hornby PJ, Benjamin S, Gillis RA. Control of lower esophageal sphincter pressure by two sites in dorsal motor nucleus of the vagus. Am J Phys. 1990;259:G899–906.
Miller LS, Vegesna AK, Brasseur JG, Braverman AS, Ruggieri MR. The esophagogastric junction. Ann N Y Acad Sci. 2011;1232:323–30.
Opie JC, Chaye H, Fraser GC. Fundoplication and pediatric esophageal manometry: actuarial analysis over 7 years. J Pediatr Surg. 1987;22:935–8.
Papasova M. Sphincteric function. In: Handbook of physiology: the gastrointestinal system. Washington, DC: American Physiological Society; 1989. p. 987–1024.
Imaeda K, Cunnane TC. Electrophysiological properties of inhibitory junction potential in murine lower oesophageal sphincter. J Smooth Muscle Res. 2003;39:119–33.
Tottrup A, Svane D, Forman A. Nitric oxide mediating NANC inhibition in opossum lower esophageal sphincter. Am J Phys. 1991;260:G385–9.
Kwiatek MA, Kahrilas PJ. Physiology of the LES. Dis Esophagus. 2012;25:286–91.
Muinuddin A, Ji J, Sheu L, Kang Y, Gaisano HY, Diamant NE. L-type Ca(2+) channel expression along feline smooth muscle oesophagus. Neurogastroenterol Motil. 2004;16:325–34.
Papasova M, Milousheva E, Bonev A, Boev K, Kortezova N. On the changes in the membrane potential and the contractile activity of the smooth muscle of the lower esophageal and ileo-caecal sphincters upon increased K in the nutrient solution. Acta Physiol Pharmacol Bulg. 1980;6:41–9.
Zhang Y, Miller DV, Paterson WG. Opposing roles of K(+) and Cl(−) channels in maintenance of opossum lower esophageal sphincter tone. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1226–34.
Asoh R, Goyal RK. Electrical activity of the opossum lower esophageal sphincter in vivo. Its role in the basal sphincter pressure. Gastroenterology. 1978;74:835–40.
Camilleri M. Integrated upper gastrointestinal response to food intake. Gastroenterology. 2006;131:640–58.
Indireshkumar K, Brasseur JG, Faas H, Hebbard GS, Kunz P, Dent J, Feinle C, Li M, Boesiger P, Fried M, et al. Relative contributions of “pressure pump” and “peristaltic pump” to gastric emptying. Am J Physiol Gastrointest Liver Physiol. 2000;278:G604–16.
Friedenberg FK, Palit A, Parkman HP, Hanlon A, Nelson DB. Botulinum toxin A for the treatment of delayed gastric emptying. Am J Gastroenterol. 2008;103:416–23.
Desipio J, Friedenberg FK, Korimilli A, Richter JE, Parkman HP, Fisher RS. High-resolution solid-state manometry of the antropyloroduodenal region. Neurogastroenterol Motil. 2007;19:188–95.
Allescher HD, Daniel EE, Dent J, Fox JE, Kostolanska F. Extrinsic and intrinsic neural control of pyloric sphincter pressure in the dog. J Physiol. 1988;401:17–38.
Keinke O, Ehrlein HJ. Effect of oleic acid on canine gastroduodenal motility, pyloric diameter and gastric emptying. Q J Exp Physiol. 1983;68:675–86.
Keinke O, Schemann M, Ehrlein HJ. Mechanical factors regulating gastric emptying of viscous nutrient meals in dogs. Q J Exp Physiol. 1984;69:781–95.
Rao SS, Safadi R, Lu C, Schulze-Delrieu K. Manometric responses of human duodenum during infusion of HCl, hyperosmolar saline, bile and oleic acid. Neurogastroenterol Motil. 1996;8:35–43.
Deane AM, Besanko LK, Burgstad CM, Chapman MJ, Horowitz M, Fraser RJ. Modulation of individual components of gastric motor response to duodenal glucose. World J Gastroenterol. 2013;19:5863–9.
Vogalis F, Sanders KM. Excitatory and inhibitory neural regulation of canine pyloric smooth muscle. Am J Phys. 1990;259:G125–33.
Tomita R, Tanjoh K, Fujisaki S, Fukuzawa M. The role of nitric oxide (NO) in the human pyloric sphincter. Hepato-Gastroenterology. 1999;46:2999–3003.
Tomita R, Igarashi S, Fijisaki S, Koshinaga T, Tanjoh K. Regulation of enteric nervous system in the proximal and distal parts of the normal human pyloric sphincter—in vitro study. Hepato-Gastroenterology. 2007;54:1289–92.
Allescher HD, Tougas G, Vergara P, Lu S, Daniel EE. Nitric oxide as a putative nonadrenergic noncholinergic inhibitory transmitter in the canine pylorus in vivo. Am J Phys. 1992;262:G695–702.
Deloof S, Croix D, Tramu G. The role of vasoactive intestinal polypeptide in the inhibition of antral and pyloric electrical activity in rabbits. J Auton Nerv Syst. 1988;22:167–73.
Deloof S. Sympathetic control of antral and pyloric electrical activity in the rabbit. J Auton Nerv Syst. 1988;22:1–10.
Allescher HD, Ahmad S, Kostolanska F, Kwan CY, Daniel EE. Modulation of pyloric motor activity via adrenergic receptors. J Pharmacol Exp Ther. 1989;249:652–9.
Parkman HP, Pagano AP, Ryan JP. Erythromycin inhibits rabbit pyloric smooth muscle through neuronal motilin receptors. Gastroenterology. 1996;111:682–90.
Yuan SY, Costa M, Brookes SJ. Neuronal control of the pyloric sphincter of the guinea-pig. Neurogastroenterol Motil. 2001;13:187–98.
Mandrek K, Kreis S. Regional differentiation of gastric and of pyloric smooth muscle in the pig: mechanical responses to acetylcholine, histamine, substance P, noradrenaline and adrenaline. J Auton Pharmacol. 1992;12:37–49.
Ishiguchi T, Takahashi T, Itoh H, Owyang C. Nitrergic and purinergic regulation of the rat pylorus. Am J Physiol Gastrointest Liver Physiol. 2000;279:G740–7.
Milenov K, Golenhofen K. Differentiated contractile responses of gastric smooth muscle to substance P. Pflugers Arch. 1983;397:29–34.
Domae K, Hashitani H, Suzuki H. Regional differences in the frequency of slow waves in smooth muscle of the guinea-pig stomach. J Smooth Muscle Res. 2008;44:231–48.
Sanders KM, Vogalis F. Organization of electrical activity in the canine pyloric canal. J Physiol. 1989;416:49–66.
Van Helden DF, Imtiaz MS. Ca2+ phase waves: a basis for cellular pacemaking and long-range synchronicity in the guinea-pig gastric pylorus. J Physiol. 2003;548:271–96.
Van Helden DF, Imtiaz MS, Nurgaliyeva K, von der WP, Dosen PJ. Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. J Physiol. 2000;524(Pt 1):245–65.
Vogalis F, Publicover NG, Hume JR, Sanders KM. Relationship between calcium current and cytosolic calcium in canine gastric smooth muscle cells. Am J Phys. 1991;260:C1012–8.
Cserni T, Paran S, Kanyari Z, O’Donnell AM, Kutasy B, Nemeth N, Puri P. New insights into the neuromuscular anatomy of the ileocecal valve. Anat Rec (Hoboken). 2009;292:254–61.
Phillips SF, Quigley EM, Kumar D, Kamath PS. Motility of the ileocolonic junction. Gut. 1988;29:390–406.
Cohen S, Harris LD, Levitan R. Manometric characteristics of the human ileocecal junctional zone. Gastroenterology. 1968;54:72–5.
Dinning PG, Bampton PA, Kennedy ML, Kajimoto T, Lubowski DZ, de Carle DJ, Cook IJ. Basal pressure patterns and reflexive motor responses in the human ileocolonic junction. Am J Phys. 1999;276:G331–40.
Quigley EM, Phillips SF, Dent J, Taylor BM. Myoelectric activity and intraluminal pressure of the canine ileocolonic sphincter. Gastroenterology. 1983;85:1054–62.
Quigley EM, Dent J, Phillips SF. Manometry of canine ileocolonic sphincter: comparison of sleeve method to point sensors. Am J Phys. 1987;252:G585–91.
Nasmyth DG, Williams NS. Pressure characteristics of the human ileocecal region—a key to its function. Gastroenterology. 1985;89:345–51.
Quigley EM, Phillips SF, Dent J. Distinctive patterns of interdigestive motility at the canine ileocolonic junction. Gastroenterology. 1984;87:836–44.
Quigley EM, Phillips SF, Cranley B, Taylor BM, Dent J. Tone of canine ileocolonic junction: topography and response to phasic contractions. Am J Phys. 1985;249:G350–7.
Pelckmans PA, Van Maercke YM, De Maeyer MH, Herman AG, Verbeuren TJ. Cholinergic and adrenergic contractile properties of the canine ileocolonic junction. J Pharmacol Exp Ther. 1990;254:158–64.
Ward SM, McKeen ES, Sanders KM. Role of nitric oxide in non-adrenergic, non-cholinergic inhibitory junction potentials in canine ileocolonic sphincter. Br J Pharmacol. 1992;105:776–82.
Rubin MR, Fournet J, Snape WJ Jr, Cohen S. Adrenergic regulation of ileocecal sphincter function in the cat. Gastroenterology. 1980;78:15–21.
Rubin MR, Cardwell BA, Ouyang A, Snape WJ Jr, Cohen S. Effect of bethanechol or vagal nerve stimulation on ileocecal sphincter pressure in the cat. Gastroenterology. 1981;80:974–9.
Kubota M. Electrical and mechanical properties and neuro-effector transmission in the smooth muscle layer of the guinea-pig ileocecal junction. Pflugers Arch. 1982;394:355–61.
Boeckxstaens GE, Pelckmans PA, Bult H, De Man JG, Herman AG, Van Maercke YM. Non-adrenergic non-cholinergic relaxation mediated by nitric oxide in the canine ileocolonic junction. Eur J Pharmacol. 1990;190:246.
Boeckxstaens GE, De Man JG, Pelckmans PA, Herman AG, Van Maercke YM. Alpha 2-adrenoceptor-mediated modulation of the nitrergic innervation of the canine isolated ileocolonic junction. Br J Pharmacol. 1993;109:1079–84.
McKirdy HC, Marshall RW, Taylor BA. Control of the human ileocaecal junction: an in vitro analysis of adrenergic and non-adrenergic non-cholinergic mechanisms. Digestion. 1993;54:200–6.
Leelakusolvong S, Sarr MG, Miller SM, Phillips SF, Bharucha AE. Role of extrinsic innervation in modulating nitrergic transmission in the canine ileocolonic region. Am J Physiol Gastrointest Liver Physiol. 2002;283:G230–9.
Ward SM, Xue C, Sanders KM. Localization of nitric oxide synthase in canine ileocolonic and pyloric sphincters. Cell Tissue Res. 1994;275:513–27.
Vadokas B, Ludtke FE, Lepsien G, Golenhofen K, Mandrek K. Effects of gastrin-releasing peptide (GRP) on the mechanical activity of the human ileocaecal region in vitro. Neurogastroenterol Motil. 1997;9:265–70.
Kubota M, Ito Y, Domae M. Actions of prostaglandins and indomethacin on the electrical and mechanical properties of smooth muscle cells of the guinea-pig ileocecal junction. Pflugers Arch. 1982;394:347–54.
Papasova M, Boev K, Bonev A, Milusheva E. Relationship between the changes in the membrane potential and the contraction of the smooth muscles of the lower oesophageal sphincter and the ileocaecal sphincter. Agressologie. 1981;22:205–8.
Papasova M, Milousheva E, Bonev A, Gachilova S. Specific features in the electrical and contractile activities of the gastro-intestinal sphincters. Acta Physiol Pharmacol Bulg. 1980;6:19–27.
Horiguchi K, Keef KD, Ward SM. Distribution of interstitial cells of Cajal in tunica muscularis of the canine rectoanal region. Am J Physiol Gastrointest Liver Physiol. 2003;284:G756–67.
Cobine CA, Hennig GW, Bayguinov YR, Hatton WJ, Ward SM, Keef KD. Interstitial cells of Cajal in the cynomolgus monkey rectoanal region and their relationship to sympathetic and nitrergic nerves. Am J Physiol Gastrointest Liver Physiol. 2010;298:G643–56.
Rao SS, Meduri K. What is necessary to diagnose constipation? Best Pract Res Clin Gastroenterol. 2011;25:127–40.
Bharucha A. Anorectal disorders. In: Spiller R, Grundy D, editors. Pathophysiology of the enteric nervous system: a basis for understanding functional diseases. Oxford: Wiley Blackwell Publishing; 2004. p. 161–75.
Lee YY, Erdogan A, Rao SS. High resolution and high definition anorectal manometry and pressure topography: diagnostic advance or a new kid on the block? Curr Gastroenterol Rep. 2013;15:360.
Seong MK, Park UC, Jung SI. Determinant of anal resting pressure gradient in association with continence function. J Neurogastroenterol Motil. 2011;17:300–4.
Noelting J, Ratuapli SK, Bharucha AE, Harvey DM, Ravi K, Zinsmeister AR. Normal values for high-resolution anorectal manometry in healthy women: effects of age and significance of rectoanal gradient. Am J Gastroenterol. 2012;107:1530–6.
de Lorijn F, de Jonge WJ, Wedel T, Vanderwinden JM, Benninga MA, Boeckxstaens GE. Interstitial cells of Cajal are involved in the afferent limb of the rectoanal inhibitory reflex. Gut. 2005;54:1107–13.
De Godoy MA, Rattan S. Angiotensin-converting enzyme and angiotensin II receptor subtype 1 inhibitors restitute hypertensive internal anal sphincter in the spontaneously hypertensive rats. J Pharmacol Exp Ther. 2006;318:725–34.
Stebbing JF. Nitric oxide synthase neurones and neuromuscular behaviour of the anorectum. Ann R Coll Surg Engl. 1998;80:137–45.
Hedlund H, Fasth S, Hulten L. Efferent sympathetic nervous control of rectal motility in the cat. Acta Physiol Scand. 1984;121:317–24.
Garrett JR, Howard ER, Jones W. The internal anal sphincter in the cat: a study of nervous mechanisms affecting tone and reflex activity. J Physiol. 1974;243:153–66.
Frenckner B, Ihre T. Influence of autonomic nerves on the internal and sphincter in man. Gut. 1976;17:306–12.
Carlstedt A, Nordgren S, Fasth S, Appelgren L, Hulten L. Sympathetic nervous influence on the internal anal sphincter and rectum in man. Int J Colorectal Dis. 1988;3:90–5.
Carlstedt A, Fasth S, Hulten L, Nordgren S. The sympathetic innervation of the internal anal sphincter and rectum in the cat. Acta Physiol Scand. 1988;133:423–31.
Mizutani M, Neya T, Ono K, Yamasato T, Tokunaga A. Histochemical study of the lumbar colonic nerve supply to the internal anal sphincter and its physiological role in dogs. Brain Res. 1992;598:45–50.
Brading AF, Ramalingam T. Mechanisms controlling normal defecation and the potential effects of spinal cord injury. Prog Brain Res. 2006;152:345–58.
Rattan S. Sympathetic (adrenergic) innervation modulates but does not generate basal tone in the internal anal sphincter smooth muscle. Gastroenterology. 2008;134:2179–81.
O’Kelly TJ. Nerves that say NO: a new perspective on the human rectoanal inhibitory reflex. Ann R Coll Surg Engl. 1996;78:31–8.
Hall KA, Ward SM, Cobine CA, Keef KD. Spatial organization and coordination of slow waves in the mouse anorectum. J Physiol. 2014;592:3813–29.
McKechnie M, Harvey N, Cobine C, Keef K. Comparison of inhibitory motor innervation in the primate and mouse internal anal sphincter. Gastroenterology. 2008;134(4):A686.
O’Kelly T, Brading A, Mortensen N. Nerve mediated relaxation of the human internal anal sphincter: the role of nitric oxide. Gut. 1993;34:689–93.
McDonnell B, Hamilton R, Fong M, Ward SM, Keef KD. Functional evidence for purinergic inhibitory neuromuscular transmission in the mouse internal anal sphincter. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1041–51.
Cobine CA, Sotherton AG, Peri LE, Sanders KM, Ward SM, Keef KD. Nitrergic neuromuscular transmission in the mouse internal anal sphincter is accomplished by multiple pathways and post-junctional effector cells. Am J Physiol Gastrointest Liver Physiol. 2014;307:G1057–72.
Duffy AM, Cobine CA, Keef KD. Changes in neuromuscular transmission in the W/W(v) mouse internal anal sphincter. Neurogastroenterol Motil. 2012;24:e41–55.
Opazo A, Lecea B, Gil V, Jimenez M, Clave P, Gallego D. Specific and complementary roles for nitric oxide and ATP in the inhibitory motor pathways to rat internal anal sphincter. Neurogastroenterol Motil. 2011;23:e11–25.
Rae MG, Muir TC. Neuronal mediators of inhibitory junction potentials and relaxation in the guinea-pig internal anal sphincter. J Physiol Lond. 1996;493:517–27.
Keef KD, Saxton SN, McDowall RA, Kaminski RE, Duffy AM, Cobine CA. Functional role of vasoactive intestinal polypeptide in inhibitory motor innervation in the mouse internal anal sphincter. J Physiol. 2013;591:1489–506.
Kubota M, Suita S, Szurszewski JH. Membrane properties and the neuro-effector transmission of smooth muscle cells in the canine internal anal sphincter. J Smooth Muscle Res. 1998;34:173–84.
Cobine CA, Fong M, Hamilton R, Keef KD. Species dependent differences in the actions of sympathetic nerves and noradrenaline in the internal anal sphincter. Neurogastroenterol Motil. 2007;19:937–45.
Cook TA, Brading AF, Mortensen NJ. Effects of nifedipine on anorectal smooth muscle in vitro. Dis Colon Rectum. 1999;42:782–7.
Jonas-Obichere M, Scholefield JH, Acheson A, Mundey M, Tyler H, Wilson VG. Comparison of the effects of nitric oxide donors and calcium channel blockers on the intrinsic myogenic tone of sheep isolated internal anal sphincter. Br J Surg. 2005;92:1263–9.
Mutafova-Yambolieva VN, O’Driscoll K, Farrelly A, Ward SM, Keef KD. Spatial localization and properties of pacemaker potentials in the canine rectoanal region. Am J Physiol Gastrointest Liver Physiol. 2003;284:G748–55.
Cobine CA, Hannah EE, Zhu MH, Lyle HE, Rock JR, Sanders KM, Ward SM, Keef KD. ANO1 in intramuscular interstitial cells of Cajal plays a key role in the generation of slow waves and tone in the internal anal sphincter. J Physiol. 2017;595:2021–41.
Harvey N, McDonnell B, McKechnie M, Keef K. Role of L-type calcium channels, membrane potential and nitric oxide in the control of myogenic activity in the primate internal anal sphincter. Gastroenterology. 2008;134(4):A63.
Keef KD, Cobine CA. Control of motility in the internal Anal Sphincter. J. Neurogastroenterol Motil. 2019, March 2 [ePub ahead of print] PMID 30827084.
Ward SM, Sanders KM. Upstroke component of electrical slow waves in canine colonic smooth muscle due to nifedipine-resistant calcium current. J Physiol Lond. 1992;455:321–37.
Kito Y, Mitsui R, Ward SM, Sanders KM. Characterization of slow waves generated by myenteric interstitial cells of Cajal of the rabbit small intestine. Am J Physiol Gastrointest Liver Physiol. 2015;308:G378–88.
Sanders KM, Kito Y, Hwang SJ, Ward SM. Regulation of gastrointestinal smooth muscle function by interstitial cells. Physiology (Bethesda). 2016;31:316–26.
Ward SM, Sanders KM. Involvement of intramuscular interstitial cells of Cajal in neuroeffector transmission in the gastrointestinal tract. J Physiol. 2006;576:675–82.
Komuro T, Seki K, Horiguchi K. Ultrastructural characterization of the interstitial cells of Cajal. Arch Histol Cytol. 1999;62:295–316.
Sanders KM, Koh SD, Ro S, Ward SM. Regulation of gastrointestinal motility—insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol. 2012;9:633–45.
Miyamoto-Kikuta S, Ezaki T, Komuro T. Distribution and morphological characteristics of the interstitial cells of Cajal in the ileocaecal junction of the guinea-pig. Cell Tissue Res. 2009;338:29–35.
Blair PJ, Bayguinov Y, Sanders KM, Ward SM. Interstitial cells in the primate gastrointestinal tract. Cell Tissue Res. 2012;350:199–213.
Smith TK, Reed JB, Sanders KM. Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. Am J Phys. 1987;252:C215–24.
Ward SM, Harney SC, Bayguinov JR, McLaren GJ, Sanders KM. Development of electrical rhythmicity in the murine gastrointestinal tract is specifically encoded in the tunica muscularis. J Physiol Lond. 1997;505(Pt 1):241–58.
Wang XY, Lammers WJ, Bercik P, Huizinga JD. Lack of pyloric interstitial cells of Cajal explains distinct peristaltic motor patterns in stomach and small intestine. Am J Physiol Gastrointest Liver Physiol. 2005;289:G539–49.
Farre R, Wang XY, Vidal E, Domenech A, Pumarola M, Clave P, Huizinga JD, Jimenez M. Interstitial cells of Cajal and neuromuscular transmission in the rat lower oesophageal sphincter. Neurogastroenterol Motil. 2007;19:484–96.
Cobine C, Foulkes H, Sanders K, Baker S, Keef K. Visualization of pacemaker activity in intramuscular ICC in the internal anal sphincter. Neurogastroenterol Motil. 2016;28(Suppl 1)):11–2.
Keef K, Ward S, Cobine C. Evidence supporting a pivotal role for intramuscular interstitial cells of Cajal in the generation of pacemaker activity, phasic contractions and tone in the internal anal sphincter. Transl Androl Urol. 2016;5(Suppl 2):S346.
Aickin CC, Brading AF. Measurement of intracellular chloride in guinea-pig vas deferens by ion analysis, chloride efflux and micro-electrodes. J Physiol. 1982;326:139–54.
Kitamura K, Yamazaki J. Chloride channels and their functional roles in smooth muscle tone in the vasculature. Jpn J Pharmacol. 2001;85:351–7.
Leblanc N, Ledoux J, Saleh S, Sanguinetti A, Angermann J, O’Driscoll K, Britton F, Perrino BA, Greenwood IA. Regulation of calcium-activated chloride channels in smooth muscle cells: a complex picture is emerging. Can J Physiol Pharmacol. 2005;83:541–56.
Lee MY, Park C, Berent RM, Park PJ, Fuchs R, Syn H, Chin A, Townsend J, Benson CC, Redelman D, et al. Smooth muscle cell genome browser: enabling the identification of novel serum response factor target genes. PLoS One. 2015;10:e0133751.
Daniel EE, Taylor GS, Holman ME. The myogenic basis of active tension in the lower esophageal sphincter. Gastroenterology. 1976;70:874.
Zhang Y, Paterson WG. Role of sarcoplasmic reticulum in control of membrane potential and nitrergic response in opossum lower esophageal sphincter. Br J Pharmacol. 2003;140:1097–107.
Lee MY, Ha SE, Park C, Park PJ, Fuchs R, Wei L, Jorgensen BG, Redelman D, Ward SM, Sanders KM, et al. Transcriptome of interstitial cells of Cajal reveals unique and selective gene signatures. PLoS One. 2017;12:e0176031.
Sanders KM, Zhu MH, Britton F, Koh SD, Ward SM. Anoctamins and gastrointestinal smooth muscle excitability. Exp Physiol. 2012;97:200–6.
Sanders KM, Ward SM. Kit mutants and gastrointestinal physiology. J Physiol. 2007;578:33–42.
Ward SM, Sanders KM. Interstitial cells of Cajal: primary targets of enteric motor innervation. Anat Rec. 2001;262:125–35.
Zhang Y, Carmichael SA, Wang XY, Huizinga JD, Paterson WG. Neurotransmission in lower esophageal sphincter of W/Wv mutant mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G14–24.
Cobine CA, Hennig GW, Kurahashi M, Sanders KM, Ward SM, Keef KD. Relationship between interstitial cells of Cajal, fibroblast-like cells and inhibitory motor nerves in the internal anal sphincter. Cell Tissue Res. 2011;344:17–30.
Nocka K, Tan JC, Chiu E, Chu TY, Ray P, Traktman P, Besmer P. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. EMBO J. 1990;9:1805–13.
Iino S, Horiguchi S, Horiguchi K, Nojyo Y. Interstitial cells of Cajal in the gastrointestinal musculature of W mutant mice. Arch Histol Cytol. 2007;70:163–73.
Ward SM, Morris G, Reese L, Wang XY, Sanders KM. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115:314–29.
Kluppel M, Huizinga JD, Malysz J, Bernstein A. Developmental origin and Kit-dependent development of the interstitial cells of cajal in the mammalian small intestine. Dev Dyn. 1998;211:60–71.
Vanderwinden JM, Rumessen JJ, de Kerchove dA Jr, Gillard K, Panthier JJ, De Laet MH, Schiffmann SN. Kit-negative fibroblast-like cells expressing SK3, a Ca2+-activated K+ channel, in the gut musculature in health and disease. Cell Tissue Res. 2002;310:349–58.
Carmona R, Cano E, Mattiotti A, Gaztambide J, Munoz-Chapuli R. Cells derived from the coelomic epithelium contribute to multiple gastrointestinal tissues in mouse embryos. PLoS One. 2013;8:e55890.
Bourret A, Chauvet N, de Santa BP, Faure S. Colonic mesenchyme differentiates into smooth muscle before its colonization by vagal enteric neural crest-derived cells in the chick embryo. Cell Tissue Res. 2017;368:503–11.
Kurahashi M, Niwa Y, Cheng J, Ohsaki Y, Fujita A, Goto H, Fujimoto T, Torihashi S. Platelet-derived growth factor signals play critical roles in differentiation of longitudinal smooth muscle cells in mouse embryonic gut. Neurogastroenterol Motil. 2008;20:521–31.
Martini F. Muscle tissue. In: Anatomy and physiology. San Francisco, CA: Pearson Education, Inc; 2005. p. 209–40.
Caputo C, Edman KA, Lou F, Sun YB. Variation in myoplasmic Ca2+ concentration during contraction and relaxation studied by the indicator fluo-3 in frog muscle fibres. J Physiol. 1994;478(Pt 1):137–48.
Lang RJ. The whole-cell Ca2+ channel current in single smooth muscle cells of the guinea-pig ureter. J Physiol. 1990;423:453–73.
Sims SM. Calcium and potassium currents in canine gastric smooth muscle cells. Am J Phys. 1992;262:G859–67.
Fleischmann BK, Murray RK, Kotlikoff MI. Voltage window for sustained elevation of cytosolic calcium in smooth muscle cells. Proc Natl Acad Sci U S A. 1994;91:11914–8.
Langton PD, Standen NB. Calcium currents elicited by voltage steps and steady voltages in myocytes isolated from the rat basilar artery. J Physiol. 1993;469:535–48.
Imaizumi Y, Muraki K, Takeda M, Watanabe M. Measurement and simulation of noninactivating Ca current in smooth muscle cells. Am J Phys. 1989;256:C880–5.
Bayguinov O, Sanders KM. Role of nitric oxide as an inhibitory neurotransmitter in the canine pyloric sphincter. Am J Phys. 1993;264:G975–83.
Farre R, Auli M, Lecea B, Martinez E, Clave P. Pharmacologic characterization of intrinsic mechanisms controlling tone and relaxation of porcine lower esophageal sphincter. J Pharmacol Exp Ther. 2006;316:1238–48.
Middleton SJ, Cuthbert AW, Shorthouse M, Hunter JO. Nitric oxide affects mammalian distal colonic smooth muscle by tonic neural inhibition. Br J Pharmacol. 1993;108:974–9.
Mule F, D’Angelo S, Amato A, Contino I, Serio R. Modulation by nitric oxide of spontaneous mechanical activity in rat proximal colon. J Auton Pharmacol. 1999;19:1–6.
Fox-Threlkeld JE, Woskowska Z, Daniel EE. Sites of nitric oxide (NO) actions in control of circular muscle motility of the perfused isolated canine ileum. Can J Physiol Pharmacol. 1997;75:1340–9.
Yamato S, Spechler SJ, Goyal RK. Role of nitric oxide in esophageal peristalsis in the opossum. Gastroenterology. 1992;103:197–204.
Groneberg D, Zizer E, Lies B, Seidler B, Saur D, Wagner M, Friebe A. Dominant role of interstitial cells of Cajal in nitrergic relaxation of murine lower oesophageal sphincter. J Physiol. 2015;593:403–14.
Bauer AJ, Sanders KM. Gradient in excitation-contraction coupling in canine gastric antral circular muscle. J Physiol Lond. 1985;369:283–94.
Kamm KE, Stull JT. Regulation of smooth muscle contractile elements by second messengers. Annu Rev Physiol. 1989;51:299–313.
Nagai R, Kuro-o M, Babij P, Periasamy M. Identification of two types of smooth muscle myosin heavy chain isoforms by cDNA cloning and immunoblot analysis. J Biol Chem. 1989;264:9734–7.
Kelley CA, Takahashi M, Yu JH, Adelstein RS. An insert of seven amino acids confers functional differences between smooth muscle myosins from the intestines and vasculature. J Biol Chem. 1993;268:12848–54.
White S, Martin AF, Periasamy M. Identification of a novel smooth muscle myosin heavy chain cDNA: isoform diversity in the S1 head region. Am J Phys. 1993;264:C1252–8.
Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol (Lond). 2000;522(Pt 2):177–85.
Somlyo AP, Somlyo AV. Signal transduction through the RhoA/Rho-kinase pathway in smooth muscle. J Muscle Res Cell Motil. 2004;25:613–5.
Matsumura F, Hartshorne DJ. Myosin phosphatase target subunit: many roles in cell function. Biochem Biophys Res Commun. 2008;369:149–56.
Hartshorne DJ, Ito M, Erdodi F. Role of protein phosphatase type 1 in contractile functions: myosin phosphatase. J Biol Chem. 2004;279:37211–4.
Grassie ME, Moffat LD, Walsh MP, MacDonald JA. The myosin phosphatase targeting protein (MYPT) family: a regulated mechanism for achieving substrate specificity of the catalytic subunit of protein phosphatase type 1delta. Arch Biochem Biophys. 2011;510:147–59.
Velasco G, Armstrong C, Morrice N, Frame S, Cohen P. Phosphorylation of the regulatory subunit of smooth muscle protein phosphatase 1M at Thr850 induces its dissociation from myosin. FEBS Lett. 2002;527:101–4.
Gao N, Chang AN, He W, Chen CP, Qiao YN, Zhu M, Kamm KE, Stull JT. Physiological signalling to myosin phosphatase targeting subunit-1 phosphorylation in ileal smooth muscle. J Physiol. 2016;594:3209–25.
Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–58.
Dimopoulos GJ, Semba S, Kitazawa K, Eto M, Kitazawa T. Ca2+-dependent rapid Ca2+ sensitization of contraction in arterial smooth muscle. Circ Res. 2007;100:121–9.
Kitazawa T. G protein-mediated Ca(2)+-sensitization of CPI-17 phosphorylation in arterial smooth muscle. Biochem Biophys Res Commun. 2010;401:75–8.
Khasnis M, Nakatomi A, Gumpper K, Eto M. Reconstituted human myosin light chain phosphatase reveals distinct roles of two inhibitory phosphorylation sites of the regulatory subunit, MYPT1. Biochemistry. 2014;53:2701–9.
Wang T, Kendig DM, Smolock EM, Moreland RS. Carbachol-induced rabbit bladder smooth muscle contraction: roles of protein kinase C and Rho kinase. Am J Physiol Renal Physiol. 2009;297:F1534–42.
Mori D, Hori M, Murata T, Ohama T, Kishi H, Kobayashi S, Ozaki H. Synchronous phosphorylation of CPI-17 and MYPT1 is essential for inducing Ca(2+) sensitization in intestinal smooth muscle. Neurogastroenterol Motil. 2011;23:1111–22.
Tsai MH, Chang AN, Huang J, He W, Sweeney HL, Zhu M, Kamm KE, Stull JT. Constitutive phosphorylation of myosin phosphatase targeting subunit-1 in smooth muscle. J Physiol. 2014;592:3031–51.
Woodsome TP, Polzin A, Kitazawa K, Eto M, Kitazawa T. Agonist- and depolarization-induced signals for myosin light chain phosphorylation and force generation of cultured vascular smooth muscle cells. J Cell Sci. 2006;119:1769–80.
Bhetwal BP, An CL, Fisher SA, Perrino BA. Regulation of basal LC20 phosphorylation by MYPT1 and CPI-17 in murine gastric antrum, gastric fundus, and proximal colon smooth muscles. Neurogastroenterol Motil. 2011;23:e425–36.
Perrino BA. Calcium sensitization mechanisms in gastrointestinal smooth muscles. J Neurogastroenterol Motil. 2016;22:213–25.
He WQ, Qiao YN, Peng YJ, Zha JM, Zhang CH, Chen C, Chen CP, Wang P, Yang X, Li CJ, et al. Altered contractile phenotypes of intestinal smooth muscle in mice deficient in myosin phosphatase target subunit 1. Gastroenterology. 2013;144:1456–65–1465.e1–5.
Zhang CH, Wang P, Liu DH, Chen CP, Zhao W, Chen X, Chen C, He WQ, Qiao YN, Tao T, et al. The molecular basis of the genesis of basal tone in internal anal sphincter. Nat Commun. 2016;7:11358.
Scotto-Lavino E, Garcia-Diaz M, Du G, Frohman MA. Basis for the isoform-specific interaction of myosin phosphatase subunits protein phosphatase 1c beta and myosin phosphatase targeting subunit 1. J Biol Chem. 2010;285:6419–24.
Gao N, Tsai MH, Chang AN, He W, Chen CP, Zhu M, Kamm KE, Stull JT. Physiological vs. pharmacological signalling to myosin phosphorylation in airway smooth muscle. J Physiol. 2017;595:6231–47.
Patel CA, Rattan S. Cellular regulation of basal tone in internal anal sphincter smooth muscle by RhoA/ROCK. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1747–56.
Patel CA, Rattan S. Spontaneously tonic smooth muscle has characteristically higher levels of RhoA/ROK compared with the phasic smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2006;291:G830–7.
Rattan S, Patel CA. Selectivity of ROCK inhibitors in the spontaneously tonic smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2008;294:G687–93.
Rattan S, Singh J. RhoA/ROCK pathway is the major molecular determinant of basal tone in intact human internal anal sphincter. Am J Physiol Gastrointest Liver Physiol. 2012;302:G664–75.
Cobine C, Bhetwal B, Stever J, Keef K, Perrino B. Comparison of proteins involved in the regulation of myosin light chain (MLC(20)) phosphorylation in the monkey IAS and rectum. Neurogastroenterol Motil. 2013;25(Suppl 1)):43.
Eto M. Regulation of cellular protein phosphatase-1 (PP1) by phosphorylation of the CPI-17 family, C-kinase-activated PP1 inhibitors. J Biol Chem. 2009;284:35273–7.
Hirano K. Current topics in the regulatory mechanism underlying the Ca2+ sensitization of the contractile apparatus in vascular smooth muscle. J Pharmacol Sci. 2007;104:109–15.
Woodsome TP, Eto M, Everett A, Brautigan DL, Kitazawa T. Expression of CPI-17 and myosin phosphatase correlates with Ca(2+) sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J Physiol. 2001;535:553–64.
Bhetwal BP, Sanders KM, An C, Trappanese DM, Moreland RS, Perrino BA. Ca2+ sensitization pathways accessed by cholinergic neurotransmission in the murine gastric fundus. J Physiol. 2013;591:2971–86.
Bhetwal BP, An C, Baker SA, Lyon KL, Perrino BA. Impaired contractile responses and altered expression and phosphorylation of Ca(2+) sensitization proteins in gastric antrum smooth muscles from ob/ob mice. J Muscle Res Cell Motil. 2013;34:137–49.
Huang J, Zhou H, Mahavadi S, Sriwai W, Lyall V, Murthy KS. Signaling pathways mediating gastrointestinal smooth muscle contraction and MLC20 phosphorylation by motilin receptors. Am J Physiol Gastrointest Liver Physiol. 2005;288:G23–31.
Ihara E, Moffat L, Ostrander J, Walsh MP, MacDonald JA. Characterization of protein kinase pathways responsible for Ca2+ sensitization in rat ileal longitudinal smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2007;293:G699–710.
Hersch E, Huang J, Grider JR, Murthy KS. Gq/G13 signaling by ET-1 in smooth muscle: MYPT1 phosphorylation via ETA and CPI-17 dephosphorylation via ETB. Am J Physiol Cell Physiol. 2004;287:C1209–18.
Ohama T, Hori M, Sato K, Ozaki H, Karaki H. Chronic treatment with interleukin-1beta attenuates contractions by decreasing the activities of CPI-17 and MYPT-1 in intestinal smooth muscle. J Biol Chem. 2003;278:48794–804.
Ohama T, Hori M, Fujisawa M, Kiyosue M, Hashimoto M, Ikenoue Y, Jinno Y, Miwa H, Matsumoto T, Murata T, et al. Downregulation of CPI-17 contributes to dysfunctional motility in chronic intestinal inflammation model mice and ulcerative colitis patients. J Gastroenterol. 2008;43:858–65.
Shimomura A, Ohama T, Hori M, Ozaki H. 17Beta-estradiol induces gastrointestinal motility disorder by decreasing CPI-17 phosphorylation via changes in rho-family G-protein Rnd expression in small intestine. J Vet Med Sci. 2009;71:1591–7.
Harnett KM, Cao W, Biancani P. Signal-transduction pathways that regulate smooth muscle function I. Signal transduction in phasic (esophageal) and tonic (gastroesophageal sphincter) smooth muscles. Am J Physiol Gastrointest Liver Physiol. 2005;288:G407–16.
Sims SM, Chrones T, Preiksaitis HG. Calcium sensitization in human esophageal muscle: role for RhoA kinase in maintenance of lower esophageal sphincter tone. J Pharmacol Exp Ther. 2008;327:178–86.
Himpens B, Matthijs G, Somlyo AP. Desensitization to cytoplasmic Ca2+ and Ca2+ sensitivities of guinea-pig ileum and rabbit pulmonary artery smooth muscle. J Physiol. 1989;413:489–503.
Kitazawa T, Somlyo AP. Desensitization and muscarinic re-sensitization of force and myosin light chain phosphorylation to cytoplasmic Ca2+ in smooth muscle. Biochem Biophys Res Commun. 1990;172:1291–7.
Lee MR, Li L, Kitazawa T. Cyclic GMP causes Ca2+ desensitization in vascular smooth muscle by activating the myosin light chain phosphatase. J Biol Chem. 1997;272:5063–8.
Sausbier M, Schubert R, Voigt V, Hirneiss C, Pfeifer A, Korth M, Kleppisch T, Ruth P, Hofmann F. Mechanisms of NO/cGMP-dependent vasorelaxation. Circ Res. 2000;87:825–30.
Etter EF, Eto M, Wardle RL, Brautigan DL, Murphy RA. Activation of myosin light chain phosphatase in intact arterial smooth muscle during nitric oxide-induced relaxation. J Biol Chem. 2001;276:34681–5.
Bonnevier J, Arner A. Actions downstream of cyclic GMP/protein kinase G can reverse protein kinase C-mediated phosphorylation of CPI-17 and Ca(2+) sensitization in smooth muscle. J Biol Chem. 2004;279:28998–9003.
Wooldridge AA, MacDonald JA, Erdodi F, Ma C, Borman MA, Hartshorne DJ, Haystead TA. Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of Serine 695 in response to cyclic nucleotides. J Biol Chem. 2004;279:34496–504.
Nakamura K, Koga Y, Sakai H, Homma K, Ikebe M. cGMP-dependent relaxation of smooth muscle is coupled with the change in the phosphorylation of myosin phosphatase. Circ Res. 2007;101:712–22.
Neppl RL, Lubomirov LT, Momotani K, Pfitzer G, Eto M, Somlyo AV. Thromboxane A2-induced bi-directional regulation of cerebral arterial tone. J Biol Chem. 2009;284:6348–60.
Shirinsky VP, Vorotnikov AV, Birukov KG, Nanaev AK, Collinge M, Lukas TJ, Sellers JR, Watterson DM. A kinase-related protein stabilizes unphosphorylated smooth muscle myosin minifilaments in the presence of ATP. J Biol Chem. 1993;268:16578–83.
Khromov AS, Wang H, Choudhury N, McDuffie M, Herring BP, Nakamoto R, Owens GK, Somlyo AP, Somlyo AV. Smooth muscle of telokin-deficient mice exhibits increased sensitivity to Ca2+ and decreased cGMP-induced relaxation. Proc Natl Acad Sci U S A. 2006;103:2440–5.
Rattan S. Ca2+/calmodulin/MLCK pathway initiates, and RhoA/ROCK maintains, the internal anal sphincter smooth muscle tone. Am J Physiol Gastrointest Liver Physiol. 2017;312:G63–6.
Bashashati M, Moraveji S, Torabi A, Sarosiek I, Davis BR, Diaz J, McCallum RW. Pathological findings of the antral and pyloric smooth muscle in patients with gastroparesis-like syndrome compared to gastroparesis: similarities and differences. Dig Dis Sci. 2017;62:2828–33.
Chander RB, Mullin GE, Passi M, Zheng X, Salem A, Yolken R, Pasricha PJ. A prospective evaluation of ileocecal valve dysfunction and intestinal motility derangements in small intestinal bacterial overgrowth. Dig Dis Sci. 2017;62:3525–35.
Bharucha AE. Pelvic floor: anatomy and function. Neurogastroenterol Motil. 2006;18:507–19.
Doodnath R, Puri P. Internal anal sphincter achalasia. Semin Pediatr Surg. 2009;18:246–8.
Shafik A, Ahmed I, El SO, Shafik AA. Interstitial cells of Cajal in reflux esophagitis: role in the pathogenesis of the disease. Med Sci Monit. 2005;11:BR452–6.
Vanderwinden JM, Liu H, De Laet MH, Vanderhaeghen JJ. Study of the interstitial cells of Cajal in infantile hypertrophic pyloric stenosis (published erratum appears in Gastroenterology 1996 Nov;111(5):1403). Gastroenterology. 1996;111:279–88.
Acknowledgements
We are extremely grateful to the NIDDK for their support through Grant No. DK078736.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Keef, K., Cobine, C. (2019). Generation of Spontaneous Tone by Gastrointestinal Sphincters. In: Hashitani, H., Lang, R. (eds) Smooth Muscle Spontaneous Activity. Advances in Experimental Medicine and Biology, vol 1124. Springer, Singapore. https://doi.org/10.1007/978-981-13-5895-1_2
Download citation
DOI: https://doi.org/10.1007/978-981-13-5895-1_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-5894-4
Online ISBN: 978-981-13-5895-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)