Abstract
Sucrase-type glycosyltransferases that classified into non-Leloir glycosyltransferases, named glucansucrase and fructansucrase, catalyze in transfer of either a glucose or a fructose from sucrose to produce glucans or fructans. The reactions need only a renewable carbon resource, such as sucrose, and proceed very efficiently, with high yields, with regio- and stereoselectivity, and in one-pot water-based system. This chapter provides an overview of the glucansucrase and fructansucrase enzymes, their reaction, and product specificity. Finally, we discuss the potential applications of α-glucans produced by glucansucrase in new bio-based materials.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Polysaccharides, natural polymers composed of sugar units linked via glycosidic bonds, have been considered as interesting bio-based materials for utilization in many applications such as plastics and biomedical field with currently increasing amount of researches. The advantages are that they are made from renewable resources supporting the trend to reduce the consumption of plastics made from petrochemicals, and with the concept of carbon neutrality, they can be regarded as eco-friendly materials [1]. Plants typically produce polysaccharides such as well-known cellulose and hemicelluloses such as xyloglucan, xylan, and glucomannan. However, hemicelluloses are branched, and they are extracted from wood via alkali or acid process leading to chain degradation that lowers the molecular weight. Besides, microorganisms can synthesize many polysaccharides in the culture medium as well such as pullulan from Aureobasidium pullulans; curdlan from Agrobacterium, Rhizobium, and Cellulomonas; dextran from Leuconostoc and Streptococcus; and hyaluronic acid from Streptococcus and Pasteurella [2]. In any case, however, it is required complicated purification step in order to purify the target polysaccharides, and the limitation of the direct production of polysaccharides by microorganisms is the difficulty in structure and composition control due to the nature of each producer.
In vitro enzymatic polymerization of polysaccharides is convenient and environmentally friendly method for production of polysaccharides. Sucrase-type glycosyltransferases classified into non-Leloir glycosyltransferases have been employed as catalysts for the practical synthesis of polysaccharides by both polymerization and modification. It is to be mentioned that sucrases, e.g., glucansucrase and fructansucrase, belong to glycosidases (EC 3.2.1). These enzymes catalyze in transfer of either a glucose or a fructose moiety of sucrose to produce glucans or fructans of different types with respect to glycosidic linkages and side chains. The reactions proceed very efficiently, with high yields, with regio- and stereoselectivity, and in one-pot water-based system. The background of this convenient synthetic pathway is the high energy of the glycosidic bond of sucrose, which is similar to that of nucleotide-activated sugars. The simplified reaction manners are represented as follows.
-
Glucansucrase: n Sucrose → Glucan + n Fructose
-
Fructansucrase: n Sucrose → Fructan + n Glucose
In this chapter, biochemical characterizations of glucansucrases and fructansucrases are summarized, and their recent applications with a focus on in vitro-synthesized α-glucan by glucansucrase are described.
4.2 Glucansucrase
Glucansucrases are extracellular enzymes mainly produced by lactic bacteria Lactococcus, Leuconostoc, and oral Streptococcus [3]. Glucansucrases catalyze the synthesis of high molecular weight D-glucose polymers named glucans from sucrose (Fig. 4.1). Dextran, α(1 → 6)-glucan is the first reported and most common glucan synthesized by a kind of glucansucrase, was one of the first biopolymer to be produced on an industrial scale in 1948 [4]. The glucansucrase responsible for the synthesis of dextran was first reported in Leuconostoc [5] and was named as dextransucrase (EC 2.4.1.5). Amylosucrase (EC 2.4.1.4) is the most extensively studied glucansucrase [6,7,8]. Amylosucrase was found in the genus Neisseria and named because of its enzymatic conversion of sucrose to a glycogen- or amylopectin-like polymer [9]. Until now, amylosucrase gene from Neisseria polysaccharea was cloned and heterologously expressed in Escherichia coli [10], followed by reports of its three-dimensional structure [11, 12].
Glucansucrases of oral streptococci, like Streptococcus mutans, play a key role in the cariogenesis process, as the synthesized glucans enhance the attachment and colonization of cariogenic bacteria [13, 14]. Therefore, in order to develop vaccines against dental caries, studies for the isolation of the gene encoding for these glucansucrases of oral streptococci were initiated more than 40 years ago. The first genes encoded glucansucrases (named gtf) were cloned from Streptococcus downei, using γ phage as cloning vector and screening on sucrose-containing medium [15]. The term GTF (glucosyltransferases) has been the one preferred by researchers with oral bacteria; this term does carry to confusion with the nucleotide sugar-dependent intercellular glycosyltransferases [16]. In the sucrase-type enzymes, energy necessary to catalyze all the reactions comes only from the cleavage of the glycosidic bond of sucrose. The mediation of nucleotide-activated sugars or cofactors is not necessary. The term glucansucrase has been favorably used in recent years, the specificity of the enzyme being indicated by a name derived from the main glucan produced, e.g., α(1 → 6)-glucan (dextransucrase) and α(1 → 4)-glucan (amylosucrase).
A long-standing question about glucansucrase relates to the mechanisms of chain elongation and the determinants of type of glycosidic linkage introduced to the growing glucan chain. As nucleotide sequences of glucansucrases became available, multiple alignments of the deduced amino acid sequences revealed conserved unique features which may explain which amino acid residues or domains are responsible for these properties. Of the oral streptococci, S. mutans produces three distinct glucansucrases, while in S. sobrinus, the closely related S. downei and S. salivarius each produce four glucansucrase genes (Table 4.1) [17]. In contrast, S. gordonii, S. sanguinis, and S. oralis make only a single glucansucrase.
4.2.1 Catalytic Mechanism of Glucansucrase
The sequences of about 500 different glucansucrase genes including ORF fragment are listed on the CAZY database of Carbohydrate Active Enzymes (http://www.cazy.org/) [18,19,20]. And more than 60 glucansucrases have been biochemically characterized. In the CAZY classification system, all glucansucrases except amylosucrases are classified as family GH70. Amylosucrase is the only enzyme of family GH13 displaying polymerase activity and is clearly unique in the family GH13 that mainly contains starch-degrading enzymes. These enzymes from families GH13 and GH70 are also known as the part of the α-amylase superfamily and are classified in clan GHH [18,19,20]. In the α-amylase enzymes, the central catalytic core of these enzymes is predicted to have alternating α-helixes and β-sheets, in an arrangement the same as found in the (β/α)8 barrel found in amylase. Although these enzymes catalyze transglycosylation or hydrolysis reactions on differently linked α-glucan polymers, they use the same set of key amino acid residues to catalyze their reaction [21,22,23,24]. During the 2000s, the role of these residues and the mechanism of the reaction have been extensively studied in GH13 enzymes such as Neisseria polysaccharea amylosucrase [25], Aspergillus oryzae α-amylase [26], and Bacillus circulans cyclodextrin glucanotransferase [27]. A decade later, final evidence for the catalytic mechanism of GH70 glucansucrase came from the three-dimensional structure and its complexes with sucrose or maltose [23].
General reaction mechanism for glucansucrase is shown in Fig. 4.2. Based on the sequence homology within the α-amylase superfamily, the corresponding catalytic residues in glucansucrases had been identified, and the mechanism was proposed to be similar [24]. The most important catalytic residues are a nucleophile (aspartate), a general acid/base (glutamate), and a transition-state stabilizer (aspartate). This mechanism is based on a detailed structural analysis of B. circulans 251 CGTase in complex with intact substrate and on a covalent intermediate of the same enzyme [27]. In this mechanism, first the glycosidic linkage of the sucrose is cleaved, resulting in a covalent α-glucosyl-enzyme intermediate; in the second half-reaction, the glucosyl moiety is transferred to an acceptor with retention of the α-anomeric configuration. After formation of the covalent intermediate, fructose is released, and the glucosyl moiety is transferred to an accepting sugar (transglycosylation) or to a water molecule (hydrolysis). The excellent review articles have been also published to provide detailed information of structure and function of glucansucrase [28,29,30].
4.2.2 Synthesis of α-Glucans by Glucansucrases
Different kinds of α-glucans with different sizes and structures, depending on the glucansucrase-producing bacterium, are synthesized. Based on their main glycosidic linkage type, these α-glucans are divided into five categories: dextran, amylose, mutan, alternan, and reuteran (Fig. 4.1). This structural variability results in a wide range of physicochemical properties, which may be suitable for different applications.
Dextran
Dextran is a water-soluble α-glucan mainly composed of α(1 → 6) linkage connected by varying amounts and arrangements of α(1 → 2), α(1 → 3), and α(1 → 4) linkages (Fig. 4.1, Table 4.2). The glucansucrase responsible for the synthesis of dextran is designated as dextransucrase and was first reported in Leuconostoc [5]. Subsequently, dextransucrases from various species of the genera Lactobacillus, Streptococcus, and Weissella were also identified and characterized [31,32,33]. The dextran is widely used in separation technology, biotechnology, and several applications in medicine [34, 35]. Dextran for clinical and technical applications is marketed in most developed countries all over the world. Of industrial relevance is the dextran produced by Leuconostoc mesenteroides NRR B- 512F DSR-S; the dextransucrase of this strain converts sucrose into a high molar mass (up to 1 MDa) polymer with 95% α(1 → 6) linkages in the main chains and 5% α(1 → 3)-branching linkages [36,37,38]. This native or partially degraded dextran and its derivatives have found many industrial applications in medicine (e.g., blood plasma expander, anticoagulant, and antithrombotic agents), pharmacy (e.g., lubricant and carrier), food (e.g., thickening, stabilizing, and gelling agent), and biotechnology (e.g., chromatography matrix) [38,39,40].
Another intensively studied dextransucrase is the Lactobacillus reuteri 180 Gtf180 GS producing an α-glucan with a high molecular weight of 30 MDa containing 69% of α(1 → 6) linkages plus single α(1 → 3) linkages in linear (21%) and branched (13%) orientations [41]. Notably, different α-glucans with unique highly branched structures have been reported in Leuconostoc strains. The Leuconostoc citreum NRRL B-1299 was found to synthesize a dextran polymer with mostly α(1 → 6) linkages but also containing about 30% of α(1 → 2) linkages, as well as a limited amount of α(1 → 3)-branching linkages [42,43,44]. This strain encodes six different glucansucrases, namely, DSR-A, DSR-B, DSR-E, DSR-M, DSR-P, and BRS-A [43].
Amylose
Amylosucrase can catalyze three types of enzymatic reactions when sucrose is the only substrate: polymerization, isomerization, and hydrolysis [45]. The polymerization reaction, a unique characteristic of amylosucrase, synthesizes α-glucan with only α(1 → 4) linkages and has no need for any primer. Recombinant amylosucrase from Neisseria polysaccharea synthesizes amylose with DP of 35–58 in vitro. By changing only the initial sucrose concentration, it was possible to obtain amyloses with different morphology and structure [46]. Simultaneously, amylosucrase produces a certain number of sucrose isomers, turanose and trehalulose, through isomerization reactions, and catalyzes a hydrolysis reaction releasing glucose and fructose from sucrose [45]. In addition, in the presence of sucrose and extra glycosyl acceptors, amylosucrase has transglycosylation capacity to attach glucose molecules from sucrose to glycosyl acceptors, such as glycogen [47, 48], starch [49], and flavanone [50]. These unique reactions make it a vital transglucosylation tool in producing novel polysaccharides and carbohydrate-based bioactive compounds.
Using the self-assembly process of α(1 → 4)-glucans produced by amylosucrase, several amylose microbeads and their applications were reported. Amylose-single-walled carbon nanotube microbeads are the first exploration of enzymatic synthesis of microparticles by amylosucrase [51]. Amylose magnetic microbeads were also prepared by the similar enzymatic approach in the presence of iron oxide nanoparticles [52]. The produced microbeads had a well-defined spherical shape and exhibited excellent magnetic responses and dispersibility in aqueous solutions.
Mutan
Mutan polymers are water-insoluble α-glucan mainly composed of α(1 → 3) linkage in the linear backbone and minor amounts of α(1 → 6) linkages. Mutansucrase-synthesizing mutan polymers are mainly found in Streptococcus strains. The ability of Streptococcus mutans and Streptococcus sobrinus to convert sucrose into water-insoluble glucans was found to be important in the etiology of dental caries by facilitating the colonization of tooth surfaces [53]. Consequently, glucansucrases are regarded as potential targets for anticaries drugs [54]. Mutansucrases have also been found in Lactobacillus and Leuconostoc strains [55, 56]. Mutan is regarded as a potentially low-cost polymer, which may be used to develop new bio-based materials [57, 58]. In particular, chemical modification of mutans to ester derivatives has shown to improve the thermoplasticity of this polysaccharide (see below for further details) [57, 58]. Moreover, chemically sulfated mutan showed fibrinolytic, anti-inflammatory, and antimicrobial properties [59,60,61,62]. The use of mutan for a variety of applications as fibers, films, and resins has been patented. The in vitro-synthesized mutan by recombinant mutansucrase (GtfJ from S. salivarius ATCC 25975) showed only α(1 → 3) linkage without any branching structure, which is difficult to obtain from native sources.
A transmission electron micrograph of in vitro-synthesized mutan is shown in Fig. 4.3 (Kimura and Iwata unpublished data). The synthetic mutan are wavy fibril-like crystals, which aggregated into plates. By electron diffraction analysis, it was indicated that the glucan chain was not extended parallel to the fibril axis as with other fibrillar polysaccharides, such as cellulose and chitin, but folded in the fibrils [63]. Several structural studies of mutan-like glucans have been reported by X-ray diffraction analysis [64,65,66,67]. In either case, however, regenerated mutan derived from the precipitate of an alkaline extraction from fungi cell walls was used, and the detailed structures of mutan have not been elucidated. The in vitro-synthesized mutan was a pure and completely linear α(1 → 3)-glucan without branches; for the first time, it was confirmed the morphology of native mutan [63].
Alternan
Alternan, with alternating α(1 → 6) and α(1 → 3) linkages, was found in L. mesenteroides NRRL B-1355 [68, 69]. Due to its backbone structure of alternating α(1 → 6) and α(1 → 3) linkages, alternan is resistant to enzymatic digestion by most known mammalian and microbial hydrolytic enzymes [70]. These properties make alternan valuable as low-calorie food additive [71]. So far the only enzymes reported to hydrolyze alternan are isomaltodextranases and alternanase [70,71,72,73].
Reuteran
Reuteran is a water-soluble branched α-glucan mainly composed of α(1 → 4)-glucan segments interconnected by single α(1 → 6) linkage. Only two reuteransucrase have been characterized, both of them present in Lactobacillus reuteri strains and producing reuteran polymers differing in the amount of α(1 → 4) and α(1 → 6) linkages [74, 75]. Reuteran has been described as a potentially health-promoting food ingredient.
4.3 Fructansucrase
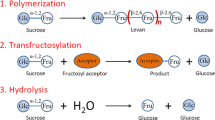
Fructansucrases transfer the fructose units of sucrose onto polysaccharides or appropriate acceptors with release of glucose. Fructans, thus produced, are either levan composed of β(2 → 6)-linked fructose residues by levansucrase or inulin composed of β(2 → 1)-linked fructose residues by inulosucrase. Figure 4.4 has shown the typical structure of levan and inulin. When sucrose is used as the acceptor in the initial priming reaction, synthesized fructans contain a nonreducing glucose unit at the end of the chain. In bacteria, fructansucrases are extracellular enzymes; levansucrases are widely distributed in both gram-positive and gram-negative bacteria, while inulosucrases are exclusively present in lactic acid bacteria. Most of the research of fructansucrases has been performed on levansucrases, in particular on enzymes from Bacillus spp. [76,77,78,79] and Zymomonas spp. [80,81,82,83].
Fructansucrases cleave the glycosidic bond of sucrose and use the released energy to couple a fructose unit (i) to a growing fructan chain (transfructosylation), (ii) to sucrose, (iii) to water (hydrolysis), or (iv) to another acceptor (such as raffinose) [84, 85]. Because sucrose is used as the acceptor in the initial priming reaction, bacterial fructans contain a nonreducing glucose unit at the end of the chain [86] (Fig. 4.4). In the initial reaction of fructansucrases, the fructose of a sucrose molecule is coupled by the enzyme to another nonreducing fructose with a free primary alcohol at position C2, acting as an acceptor substrate, e.g., sucrose, raffinose, or a fructan molecule [87, 88]. This is also referred to as the priming reaction. In subsequent steps, the enzyme elongates the primer. A clear difference between fructansucrase and glucansucrase enzymes is the fact that glucansucrase enzymes cannot use sucrose as an acceptor but rather the cleaved glucose residue. The molecular masses of the fructans produced show a large variation, from 2 × 104 to 50 × 106 Da [28]. There are some reports that the molecular mass of the fructan produced is dependent on incubation conditions, the temperature, salinity, and sucrose concentration [89,90,91]. Sucrose analogues with a similar glycosidic linkage to sucrose have been used for the synthesis of new poly- and oligosaccharides by fructansucrase. For example, a wide range of fructansucrases recognize most of the sucrose analogues, such as those which were composed of galactose, mannose, xylose, fucose, and rhamnose in place of glucose, giving rise to novel poly- and oligosaccharides [92,93,94,95,96,97].
4.4 In Vitro-Synthesized α-Glucan as New Bio-Based Materials
The usage of naturally obtained mutan in the material application had not attracted any attention until now as the mutan has a branched structure; they lack a uniform composition, and purification costs are relatively high. The GtfJ enzyme, a kind of glucansucrase from Streptococcus salivarius, can effectively catalyze the one-pot water-based enzymatic polymerization of linear α(1 → 3)-glucan without branches. The synthesis process is environmentally friendly with a reaction in water medium, without organic solvent, and also convenient: only mixing sucrose solution with enzyme and storing at designated temperature. In addition, the molecular weight of the mutan can be adjusted by reaction conditions. Thus, in vitro-synthesized mutan can be considered as a potentially low-cost polymer for future prospect. A disadvantage of mutan, like all other polysaccharides, is the insolubility against most organic solvents and its thermally unprocessability. Up until now, many researchers have been attempting to improve this drawback by various techniques. One interesting method is the introduction of acyl groups to the hydroxyl groups of sugar units, or esterification, which raised the thermoplastic properties of cellulose known as so-called cellulose acetate and also improved the thermal properties and solubility of other polysaccharides in reported researches recently.
The series of mutan ester derivatives with Mw of about 200 kDa having different acid chain length were synthesized, and their thermal and mechanical properties of mutan ester films were investigated [58]. Hopefully, this material could be a substitution product to come on board replacing petroleum-derived plastics so as to avoid the uncertainty and sensitivity from oil’s booms and busts, mitigate the environmental issues, and create the sustainability for future generations. Glass transition and melting behaviors of mutan ester series from C2 (acetate) to C8 (octanoate) are shown in Fig. 4.5. The thermal and mechanical properties of mutan esters and its degree of crystallinity can be controlled by changing the length of its ester chain. Figure 4.6 shows the superior melting and glass transition temperature of mutan acetate and propionate over commercially available petroleum-based thermoplastics and currently interesting bio-based polymers; thus these materials are regarded as promising candidates for future thermoplastic application [57]. It was showed that unbranched mutan can be conveniently produced by green method using sucrose as a low-cost material. Furthermore, fully substituted mutan esters which their thermal and mechanical properties can be adjusted are of interest for developing new thermoplastic materials.
Glass transition (Tg) and melting temperature (Tm) of mutan esters series from C2 (acetate) to C8 (octanoate). Redrawn on the basis of the reference [58]
Comparison of glass transition (Tg) and melting temperature (Tm) between those of mutan acetate and propionate, esters of other polysaccharides, and commercially available polymers. PE, PP, and PET are polyethylene, polypropylene, and polyethylene terephthalate, respectively. Redrawn on the basis of the reference [57]
4.5 Conclusions
Sucrase-type (non-Leloir-type) enzymes can catalyze to produce both polysaccharides and oligosaccharides using sucrose as a renewable cheap substrate via one-pot water-based reaction. The sucrase-type enzymes can be a very efficient tool to provide the synthesis of tailor-made glucan, fructan polysaccharides, and oligosaccharides for a wide range of applications. Several enzymatic processes with these enzymes already have been established for polysaccharide and oligosaccharide synthesis, some of which are applied industrially for a variety of applications. Further breakthroughs in this field are expected in the future, with enzyme engineering approaches increasingly allowing new construction of mutant enzymes and the discovery of new types of sucrase enzymes.
References
Iwata T (2015) Biodegradable and bio-based polymers: future prospects of eco-friendly plastics. Angew Chem Int Ed 54:3210–3215
Rehm BHA (2010) Bacterial polymers: biosynthesis, modifications and applications. Nat Rev Microbiol 8:578–592
Sedebotham RL (1974) Dextrans. Adv Carbohydr Chem 30:371–444
Groenwall AJ, Ingelman BJA (1948) Manufacture of infusion and injection fluids. US Patent 2:437–518
Hehre EJ, Sugg JY (1942) Serologically reactive polysaccharides produced through the action of bacterial enzymes: I. Dextran of Leuconostoc mesenteroides from sucrose. J Exp Med 75:339–353
Albenne C, Skov LK, Mirza O et al (2004) Molecular basis of the amylose-like polymer formation catalyzed by Neisseria polysaccharea amylosucrase. J Biol Chem 279:726734
van der Veen BA, Potocki-Véronese G, Albenne C et al (2004) Combinatorial engineering to enhance amylosucrase performance: construction, selection, and screening of variant libraries for increased activity. FEBS Lett 560:91–97
van Leeuwen SS, Kralj S, Eeuwema W et al (2009) Structural characterization of bioengineered α-D-glucans produced by mutant glucansucrase GTF180 enzymes of Lactobacillus reuteri strain 180. Biomacromolecules 10:580–588
Hehre EJ, Hamilton DM (1946) Bacterial synthesis of an amylopectin-like polysaccharide from sucrose. J Biol Chem 166:777–778
Büttcher V, Welsh T, Willmitzer L et al (1997) Cloning and characterization of the gene for amylosucrase from Neisseria polysaccharea: production of a linear α-1,4-glucan. J Bacteriol 179:3324–3330
Skov LK, Mirza O, Henriksen A et al (2001) Amylosucrase, a glucan-synthesizing enzyme from the α-amylase family. J Biol Chem 276:25273–25278
Skov LK, Mirza O, Sprogoe D et al (2002) Oligosaccharide and sucrose complexes of amylosucrase – structural implications for the polymerase activity. J Biol Chem 277:47741–47747
Hamada S, Slade HD (1980) Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev 44:331–384
Loesche WJ (1986) Role of Streptococcus mutans in human dental decay. Microbiol Rev 50:353–380
Gilpin ML, Ruccell RRB, Morrissey P (1985) Cloning and expression of two Streptococcus mutans glucosyltransferases in Escherichia coli K-12. Infect Immun 49:414–416
Lairson LL, Henrissat B, Davies GJ et al (2008) Glycosyltransferases: structures, functions, and mechanisms. Annu Rev Biochem 77:521–555
Simpson CL, Cheetham NWH, Giffard PM et al (1995) Four glucosyltransferases, GtfJ, GtfK, GtfL and GtfM, from Streptococcus salivarius ATCC 25975. Microbiology 141:1451–1460
Cantarel BL, Coutinho PM, Rancurel C et al (2009) The carbohydrate-active enzymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res 37:D233–D238
Henrissat B, Davies G (1997) Structural and sequence-based classification of glycoside hydrolases. Curr Opin Struct Biol 7:637–644
MacGregor EA, Janecek S, Svensson B (2001) Relationship of sequence and structure to specificity in the α-amylase family of enzymes. Biochim Biophys Acta 1546:1–20
Uitdehaag JCM, van der Veen BA, Dijkhuizen L et al (2002) Catalytic mechanism and product specificity of cyclodextrin glycosyltransferase, a prototypical transglycosylase from the α-amylase family. Enzyme Microbial Technol 30:295–304
Barends TR, Bultema JB, Kaper T et al (2007) Three-way stabilization of the covalent intermediate in amylomaltase, an α-amylase-like transglycosylase. J Biol Chem 282:17242–17249
Vujicic-Zagar A, Pijning T, Kralj S et al (2010) Crystal structure of a 117 kDa glucansucrase fragment provides insight into evolution and product specificity of GH70 enzymes. Proc Natl Acad Sci USA107:21406–21411
Devulapalle KS, Goodman SD, Gao Q et al (1997) Knowledge based model of a glucosyltransferase from the oral bacterial group of mutans streptococci. Protein Sci 6:2489–2493
Skov LK, Mirza O, Sprogoe D et al (2002) Oligosaccharide and sucrose complexes of amylosucrase. Structural implications for the polymerase activity. J Biol Chem 277:47741–47747
Brzozowski AM, Davies GJ (1997) Structure of the Aspergillus oryzae α-amylase complexed with the inhibitor acarbose at 2.0 A resolution. Biochemistry 36:10837–10845
Uitdehaag JCM, Mosi R, Kalk KH et al (1999) X-ray structures along the reaction pathway of cyclodextrin glycosyltransferase elucidate catalysis in the α-amylase family. Nat Struct Biol 6:432–436
van Hijum SAFT, Kralj S, Ozimek LK et al (2006) Structure-function relationships of glucansucrase and fructansucrase enzymes from lactic acid bacteria. Microbiol Mol Biol Rev 70:157–176
Leemhuisa H, Pijning T, Justyna M et al (2013) Glucansucrases: three-dimensional structures, reactions, mechanism, α-glucan analysis and their implications in biotechnology and food applications. J Biotechnol 163:250–272
Gangoitia J, Pijning T, Dijkhuizen L (2018) Biotechnological potential of novel glycoside hydrolase family 70 enzymes synthesizing α-glucans from starch and sucrose. Biotechnol Adv 36:196–207
Hanada N, Kuramitsu HK (1989) Isolation and characterization of the Streptococcus mutans gtfD gene, coding for primer-dependent soluble glucan synthesis. Infect Immun 57:2079–2085
van Leeuwen SS, Kralj S, van Geel-Schutten IH, Gerwig GJ et al (2008) Structural analysis of the α-D-glucan (EPS180) produced by the Lactobacillus reuteri strain 180 glucansucrase GTF180 enzyme. Carbohydr Res 343:1237–1250
Kang HK, Oh JS, Kim D (2009) Molecular characterization and expression analysis of the glucansucrase DSRWC from Weissella cibaria synthesizing a α(1→6) glucan. FEMS Microbiol Lett 292:33–41
Gavie EI (1984) Separation of species of the genus Leuconostoc and differentiation of the Leuconostoc from other lactic acid bacteria. In: Methods in microbiology (Bergan T Ed), vol 16. Academic Press, London, pp 147–178
Soetaert W, Sxhwengers D, Bucholz K, Vandamme EJ (1995) A wide range of carbohydrate modifications by a single microorganism: Leuconostoc mesenteroides. In: Pererson SB, Svensson B, Peterson S (eds) Carbohydrate Bioengineering, vol 10. Elsevier, Amsterdam, pp 351–358
Monchois V, Remaud-Simeon M, Russell RR et al (1997) Characterization of Leuconostoc mesenteroides NRRL B-512F dextransucrase (DSRS) and identification of amino-acid residues playing a key role in enzyme activity. Appl Microbiol Biotechnol 48:465–472
Passerini D, Vuillemin M, Ufarté L et al (2015) Inventory of the GH70 enzymes encoded by Leuconostoc citreum NRRL B-1299 -identification of three novel α-transglucosylases. FEBS J 282:2115–2130
Zannini E, Waters DM, Coffey A et al (2016) Production, properties, and industrial food application of lactic acid bacteria-derived exopolysaccharides. Appl Microbiol Biotechnol 100:1121–1135
Naessens M, Cerdobbel A, Soetaert W et al (2005) Leuconostoc dextransucrase and dextran: production, properties and applications. J Chem Technol Biotechnol 80:845–860
Badel S, Bernardi T, Michaud P (2011) New perspectives for Lactobacilli exopolysaccharides. Biotechnol Adv 29:54–66
van Leeuwen SS, Kralj S, van Geel-Schutten IH et al (2008) Structural analysis of the α-D-glucan (EPS35-5) produced by the Lactobacillus reuteri strain 35-5 glucansucrase GTFA enzyme. Carbohydr Res 343:1251–1265
Fabre E, Bozonnet S, Arcache A et al (2005) Role of the two catalytic domains of DSR-E dextransucrase and their involvement in the formation of highly α-1,2 branched dextran. J Bacteriol 187:296–303
Passerini D, Vuillemin M, Ufarté L et al (2015) Inventory of the GH70 enzymes encoded by Leuconostoc citreum NRRL B-1299 -identification of three novel α-transglucosylases. FEBS J 282:2115–2130
Vuillemin M, Claverie M, Brison Y (2016) Characterization of the first α-(1→3) branching sucrases of the GH70 family. J Biol Chem 291:7687–7702
Potocki de Montalk G, Remaud-Simeon M, Willemot RM et al (2000) Amylosucrase from Neisseria polysaccharea: novel catalytic properties. FEBS Lett 471:219–223
Potocki-Veronese G, Putaux JL, Dupeyre D et al (2005) Amylose synthesized in vitro by amylosucrase: morphology, structure, and properties. Biomacromolecules 6:1000–1011
Potocki de Montalk G, Remaud-Simeon M, Willemot RM et al (2000) Characterisation of the activator effect of glycogen on amylosucrase from Neisseria polysaccharea. FEMS Microbiol Lett 186:103108
Putaux JL, Potockivéronèse G, Remaudsimeon M et al (2006) α-D-Glucan-based dendritic nanoparticles prepared by in vitro enzymatic chain extension of glycogen. Biomacromolecules 7:1720–1728
Rolland-Sabaté A, Colonna P, Potocki-Véronèse G et al (2004) Elongation and insolubilisation of α-glucans by the action of Neisseria polysaccharea amylosucrase. J Cereal Sci 40:17–30
Overwin H, Wray V, Hofer B (2015) Flavonoid glucosylation by non-Leloir glycosyltransferases: formation of multiple derivatives of 3,5,7,3′,4′-pentahydroxyflavane stereoisomers. Appl Microbiol Biotechnol 99:9565–9576
Lim MC, Seo DH, Jung JH et al (2014) Enzymatic synthesis of amylose nanocomposite microbeads using amylosucrase from Deinococcus geothermalis. RSC Adv 4:26421–26424
Lim MC, Lee GH, Huynh DTN et al (2015) Amylosucrase-mediated synthesis and self-assembly of amylose magnetic microparticles. RSC Adv 5:36088–36091
Tsumori H, Kuramitsu H (1997) The role of the Streptococcus mutans glucosyltransferases in the sucrose-dependent attachment to smooth surfaces: essential role of the GtfC enzyme. Oral Microbiol Immunol 12:274–280
Zhang R, Zhang W, Hu T (2011) Dextran glucosidase: a potential target of iminosugars in caries prevention. Med Hypotheses 76:574–575
Waldherr FW, Doll VM, Meissner D et al (2010) Identification and characterization of a glucan-producing enzyme from Lactobacillus hilgardii TMW 1.828 involved in granule formation of water kefir. Food Microbiol 27:672–678
Côte GL, Skory CD (2012) Cloning, expression, and characterization of an insoluble glucan-producing glucansucrase from Leuconostoc mesenteroides NRRL B-1118. Appl Microbiol Biotechnol 93:2387–2394
Puanglek S, Kimura S, Enomoto-Rogers Y et al (2016) In vitro synthesis of linear α-1,3-glucan and chemical modification to ester derivatives exhibiting outstanding thermal properties. Sci Rep 6:30479
Puanglek S, Kimura S, Iwata T (2017) Thermal and mechanical properties of tailor-made unbranched α-1,3-glucan esters with various carboxylic acid chain length. Carbohydr Polym 169:245–254
Buddanaa SK, Varanasia YVN, Shettya PR (2015) Fibrinolytic, anti-inflammatory and anti-microbial properties of α-(1→3)-glucans produced from Streptococcus mutans (MTCC 497). Carbohydr Polym 115:152–1159
Huang Q, Zhang L (2011) Preparation, chain conformation and anti-tumor activities of water-soluble phosphated (1,3)-α-D-glucan from Poria cocos mycelia. Carbohydr Polym 83:1363–1369
Kiho T, Yoshida I, Nagai K et al (1989) (1→3)-α-D-glucan from an alkaline extract of Agrocybe cylindracea and antitumor activity of its O-carboxymethylated derivatives. Carbohydr Res 189:273–279
Wiater A, Paduch R, Choma A et al (2012) Biological study on carboxymethylated (1→3)-α-D-glucans from fruiting bodies of Ganoderma lucidum. Int J Biol Macromol 51:1014–1023
Kobayashi K, Hasegawa T, Kusumi R et al (2017) Characterization of crystalline linear (1→3)-α-D-glucan synthesized in vitro. Carbohydr Polym 177:341–346
Jelsma J, Kreger DR (1979) Polymorphism in crystalline (1→3)-α-D-glucan from fungal cell-walls. Carbohydr Res 71:51–64
Ogawa K, Misaki A, Oka S et al (1979) X-ray diffraction data for (1→3) -α-D-glucan. Carbohydr Res 75:C13–C16
Ogawa K, Okamura K, Sarko A (1981) Molecular and crystal structure of the regenerated form of (1→3)-α-D-glucan. Int J Biol Macromol 3:31–36
Ogawa K, Yui T, Okamura K et al (1994) Crystalline features of streptococcal (1→3)-α-D-glucans of human saliva. Biosci Biotechnol Biochem 58:1326–1327
Côté GL, Robyt JF (1982) Isolation and partial characterization of an extracellular glucansucrase from Leuconostoc mesenteroides NRRL B-1355 that synthesizes an alternating (1 →6), (1→3)- α-D-glucan. Carbohydr Res 101:57–74
Arguello-Morales MA, Remaud-Simeon M, Pizzut S et al (2000) Sequence analysis of the gene encoding alternansucrase, a sucrose glucosyltransferase from Leuconostoc mesenteroides NRRL B-1355. FEMS Microbiol Lett 182:81–85
Biely P, Côté GL, Burgess-Cassler A (1994) Purification and properties of alternanase, a novel endo-α-1,3-α-1,6-d-glucanase. J Biochem 226:633–639
Côté GL, Ahlgren JA (2001) The hydrolytic and transferase action of alternanase on oligosaccharides. Carbohydr Res 332:373–379
Sawai T, Tohyama T, Natsume T (1978) Hydrolysis of fourteen native dextrans by Arthrobacter isomaltodextranase and correlation with dextran structure. Carbohydr Res 66:195–205
Sawai T, Ohara S, Ichimi Y et al (1981) Purification and some properties of the isomaltodextranases Actinomadura strain R10 and comparison with that of Arthrobacter globiformis T6. Carbohydr Res 89:289–299
Kralj S, van Geel-Schutten GH, van der Maarel MJ et al (2004) Biochemical and molecular characterization of Lactobacillus reuteri 121 reuteransucrase. Microbiology 150:2099–2112
Kralj S, Stripling E, Sanders P et al (2005) Highly hydrolytic reuteransucrase from probiotic Lactobacillus reuteri strain ATCC 55730. Appl Environ Microbiol 71:3942–3950
Bezzate S, Steinmetz M, Aymerich S (1994) Cloning, sequencing, and disruption of a levanase gene of Bacillus polymyxa CF43. J Bacteriol 176:2177–2183
Li Y, Triccas JA, Ferenci T (1997) A novel levansucrase-levanase gene cluster in Bacillus stearothermophilus ATCC12980. Biochim Biophys Acta 1353:203–208
Steinmetz M, Le Coq D, Aymerich S et al (1985) The DNA sequence of the gene for the secreted Bacillus subtilis enzyme levansucrase and its genetic control sites. Mol Gen Genet 200:220–228
Tang LB, Lenstra R, Borchert TB et al (1990) Isolation and characterization of levansucrase encoding gene from Bacillus amyloliquefaciens. Gene 96:89–93
Hartmeier W, Reiss M, Heidel M et al (1994) Biochemical and economical aspects of levan synthesis by Zymomonas mobilis. Biocatalysis 10:131–136
Song KB, Joo HK, Rhee SK (1993) Nucleotide sequence of levansucrase gene (levU) of Zymomonas mobilis ZM1 (ATCC10988). Biochim Biophys Acta 1173:320–324
Yanase H, Iwata M, Nakahigashi R et al (1992) Purification, crystallization and properties of the extracellular levansucrase from Zymomonas mobilis. Biosci Biotechnol Biochem 56:1335–1337
Yanase H, Maeda M, Hagiwara E et al (2002) Identification of functionally important amino acid residues in Zymomonas mobilis levansucrase. J Biochem (Tokyo) 132:565–572
Seibel J, Beine R, Moraru R et al (2006) A new pathway for the synthesis of oligosaccharides by the use of non-Leloir glycosyltransferases. Biocatal Biotransformation 24:157–165
Seibel J, Jordening HJ, Buchholz K (2006) Glycosylation with activated sugars using glycosyltransferases and transglycosidases. Biocatal Biotransformation 24:311–342
French AD, Waterhouse AL (1993) Chemical structure and characteristics. In: Suzuki M, Chatterton NJ (eds) Science and technology of fructans. CRC Press Inc, Boca Raton, pp 41–82
Dedonder R (1966) Levansucrase from Bacillus subtilis. In: Neufeld EF, Ginsburg V (eds) Methods in enzymology. Academic Press, New York, pp 500–505
Robyt JF (1998) Essentials of carbohydrate chemistry. Springer-Verlag, New York
Ben Ammar Y, Matsubara T, Ito K et al (2002) Characterization of a thermostable levansucrase from Bacillus sp. TH4-2 capable of producing high molecular weight levan at high temperature. J Biotechnol 99:111–119
Tanaka T, Oi S, Yamamoto T (1980) The molecular structure of low and high molecular weight levans synthesized by levansucrase. J Biochem (Tokyo) 87:297–303
Tanaka T, Oi S, Yamamoto T (1979) Synthesis of levan by levansucrase some factors affecting the rate of synthesis and degree of polymerization of levan. J Biochem (Tokyo) 85:287293
Baciu IE, Jördening HJ, Seibel J et al (2005) Investigations of the transfructosylation reaction by fructosyltransferase from B. subtilis NCIMB 11871 for the synthesis of the sucrose analogue galactosyl-fructoside. J Biotechnol 116:347–357
Seibel J, Moraru R, Götze S (2005) Biocatalytic and chemical investigations in the synthesis of sucrose analogues. Tetrahedron 61:7081–7086
Seibel J, Moraru R, Götze S et al (2006) Synthesis of sucrose analogues and the mechanism of action of Bacillus subtilis fructosyltransferase (levansucrase). Carbohydr Res 341:2335–2349
Seibel J, Hellmuth H, Hofer B et al (2006) Identification of new acceptor specificities of glycosyltransferase R with the aid of substrate microarrays. Chembiochem 7:310–320
Beine R, Moraru R, Nimtz M et al (2008) Synthesis of novel fructooligosaccharides by substrate and enzyme engineering. J Biotechnol 138:33–41
Homann A, Seibel J (2009) Towards tailor-made oligosaccharides – chemo-enzymatic approaches by enzyme and substrate engineering. Appl Microbiol Biotechnol 83:209–216
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kimura, S., Iwata, T. (2019). Synthesis of Polysaccharides III: Sucrase as Catalyst. In: Kobayashi, S., Uyama, H., Kadokawa, Ji. (eds) Enzymatic Polymerization towards Green Polymer Chemistry. Green Chemistry and Sustainable Technology. Springer, Singapore. https://doi.org/10.1007/978-981-13-3813-7_4
Download citation
DOI: https://doi.org/10.1007/978-981-13-3813-7_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3812-0
Online ISBN: 978-981-13-3813-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)