Abstract
Total colonic aganglionosis (TCA) is a rare form of Hirschsprung disease and is defined as aganglionosis extending to the cecum or distal ileum. The mortality rate of this disease declined dramatically recently, but the morbidity rate is still high. Although several operative procedures have been adopted for TCA such as Martin, Duhamel, Soave, Swenson, and colon patch, with some modifications, the optimal procedure is not apparent, and we need to develop a novel treatment strategy to improve the high morbidity rate after pull-through operation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Total colonic aganglionosis (TCA) is a rare form of Hirschsprung disease and is defined as aganglionosis extending from the rectum to the cecum or distal ileum. Clinically, the length of the distal ileum that is affected by aganglionosis in patients with TCA is defined to be less than 50 cm in Western countries (or less than 30 cm in Japan). The more severe form is called extensive aganglionosis, and the most severe type is called total intestinal aganglionosis in which the entire or nearly entire intestine is affected. TCA and the more severe type of aganglionosis are different from short-segment aganglionosis in several respects as described below, and the management and treatment strategy of TCA and extensive aganglionosis should be considered separately.
The first successful management of TCA was reported in 1953 by Sandegard [1], and since then, many case series have been reported, but the enrolled number of cases was not large because of its rarity. A systematic review with meta-analysis that summarized cases published in the past 30 years was published in 2012 by Laughlin et al. [2], and comprehensive reviews were published in 2012 and 2015 by Moore [3, 4]. These reviews are valuable for obtaining an overall picture of TCA.
25.1 Clinical Characteristics of TCA
TCA is a relatively rare form of aganglionosis, and its incidence has been reported to be approximately 2–13% out of all aganglionosis cases [2, 5]. A recent meta-analysis reported its incidence as 9.1% which was obtained by analyzing published papers from 1980 to 2011 [2].
In contrast to the tendency of male dominance in short-segment aganglionosis, the male-to-female ratio is lower in TCA. The male-to-female ratio was 1.86:1 in a meta-analysis [2], and it was 2.2:1 in a survey of cases over a 5-year period from 1998 to 2002 in Japan [6]. Another important feature of TCA is its high tendency of familial recurrence [3, 4]. The genetic background is not fully understood, but abnormal RET gene signaling and dysregulation of the endothelin system are suspected of being some causes of the abnormal distribution of enteric nerve cells. Some reports showed a higher risk of familial recurrence in longer-segment aganglionosis, and a progression of severity through subsequent generations has been reported [7]. In a nationwide survey of TCA in Japan, the rate of positive family history of TCA was about 11.9% in a recent 5-year period from 1998 to 2002 [6].
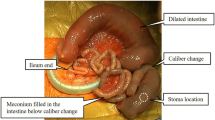
In short-segment aganglionosis, the typical first symptom in neonates is intestinal obstructive symptoms such as bilious vomiting and abdominal distention. However, in TCA, some cases do not show typical obstructive symptoms in the neonatal period, and more subtle and milder symptoms appear later in life. Ieiri et al. [6] reported that only 54.5% of TCA patients were diagnosed in the first month of life in Japan. This atypical presentation sometimes makes it difficult to diagnose TCA. Moreover, the radiological and histological characteristics of TCA are different from those of short-segment aganglionosis. Even in neonatal patients with intestinal obstructive symptoms, typical radiological features by contrast enema are not seen. For instance, a typical caliber change between normal and aganglionic bowel is not seen in patients with TCA. In a case series, two TCA patients did not show typical colonic contrast enema findings and were misdiagnosed by radiologists [8]. Another large case series reported that three types of colonic textures were seen by contrast enema among TCA patients [9]: (1) normal caliber (53%), (2) microcolon (29%), and (3) so-called question mark shape (18%). These facts strongly suggest that it is difficult to make a correct diagnosis of TCA from only the results of contrast enema even when we suspect that the patient suffers from TCA, and we should obtain a definitive diagnosis in such cases by the free use of the available diagnostic tools. It is also difficult to make a histological diagnosis of TCA because some cases show faint immunohistochemical staining of enteric nerve markers in transition and aganglionic segment and absence of the thick nerve bundle in aganglionic segment [10]. In addition to the atypical pathological findings, the transition zone (in other words, the hypoganglionic segment) may be longer than that in short-segment aganglionosis [3]. If a patient’s clinical signs and symptoms suggest TCA, we should be careful when interpreting the pathological findings in biopsy specimens, and it would be necessary to perform repeat biopsies in order to reach a definitive diagnosis when the results are obscure. During the pull-through operation, we should also be careful when distinguishing the sites with normal ganglionic cells and aganglionic sites.
TCA is clinically more severe than short-segment aganglionosis, the overall mortality rate of TCA in a meta-analysis of 969 patients was 19.9% [2], and the postoperative mortality rate after pull-through operation in 739 patients was 5.7% [2]. In a Japanese survey, the mortality rate of TCA was as high as 30.4% about 40 years ago, and it decreased to 7.1% in the recent 5-year period from 1998 to 2002 [6]. It is worth noting that extensive aganglionosis still showed a high mortality rate of 35.5% in the same 5-year period according to the same survey in Japan [6].
25.2 Operative Treatment
As described earlier, it is difficult to make an early and exact diagnosis of TCA. It is generally accepted that a definite diagnosis should be made after performing full-thickness biopsy of the intestine and after creating an ileostomy in a normal ganglionic segment by laparotomy. A laparoscopic procedure may replace open laparotomy, and successful one-stage laparoscopic pull-through operation has been reported by some authors [11, 12]. However, the laparoscopic procedure is still not widely accepted, and a long-term follow-up study is needed to determine its feasibility.
Several operative procedures for TCA have been reported (Table 25.1), and the most popular procedure in the past, which was Martin method, was replaced by the Duhamel method (Table 25.2). The Soave method and patch method are also often adopted. Since the first report of the new operative procedure by Martin [13] in 1968, which was a modified technique of the Duhamel operation and where the left side colon is used for water absorption, Martin’s technique was the most widely performed technique in the world for several years. A meta-analysis [2] reported that Martin’s technique was performed in 230 (31.9%) out of a total of 722 pull-through operations, which had been calculated from cases reported in papers published in the last 30 years. The second most widely performed procedure was the Duhamel method (229/722, 31.7%). The third was the Soave or Swenson method, which is straight ileoanal anastomosis, at a rate of 197/722 (27.3%). Although the Martin method was widely performed in the past, it has become less popular recently due to several reasons. Ieiri et al. [6] reported the frequencies of surgical procedures performed for the treatment of TCA in Japan between 1998 and 2002 as follows: (1) Martin method 28.9%, (2) Duhamel method 33.3%, (3) Soave method 15.7%, and (4) right colon patch method 18.1%. This Japanese survey also reported that the past frequencies of the Martin method were 83.6% in 1978 to 1982 and 53.5% in 1988 to 1992. These rates clearly demonstrate a transition in the preferred surgical technique for TCA by Japanese pediatric surgeons. The reason why the Martin method has been avoided in recent years is that it is a complex procedure with long side-to-side anastomosis. This technique might result in postoperative anastomotic leakage and kinking of the long anastomotic segment, causing periodic obstruction. Also, long anastomosis sometimes causes severe ulcer formation and anemia. Currently, the most popular pull-through method in Japan is the Duhamel method. However, the transanal endorectal pull-through (TAEPT) method with or without laparoscopic assistance has become very popular for the treatment of short-segment aganglionosis, and its rate has become as high as 49.6% during the 5-year period from 2008 to 2012 in Japan [5]. With this tendency, the Soave or Swenson type transanal pull-through method is expected to become the most popular procedure in the future. The right colon patch method was first reported by Kimura et al. [14] in 1981, and a long-term follow-up study of patients who underwent this procedure showed satisfactory results [15]. With this method, it is expected that water and electrolytes will be absorbed from the right hemi-colon, and some pediatric surgeons prefer this method as mentioned above (rate of 18.1% in the 5-year period from 1998 to 2002 in Japan) [6]. However, this procedure is not widely accepted by pediatric surgeons because the surgical procedure is complicated and a staged operation is needed. A similar technique was proposed by Sauer et al. in 1989 [16] in which the ascending colon was used as a patch and the ileocecal valve was preserved. The modified one-stage technique was also reported by the same author in 1993 [17]. Recently, in a long-term follow-up study of patients who underwent this procedure, the patients maintained a good quality of life, although the bowel function score was low in TCA patients treated by this technique [18].
Several modifications of the operative procedure for TCA have been proposed to reduce the postoperative complications. One of the modifications is J-pouch creation in ileoanal anastomosis to preserve the retaining ability of feces in the ileum and to prevent postoperative perineal excoriation [19]. However, pouchitis is an adverse effect. Another modification is to add internal anal sphincterectomy or sphincterotomy in ileoanal anastomosis to prevent postoperative obstruction syndrome and enteritis [20, 21], and its feasibility was reported by Li et al. [22] in 2016.
Creating a covering ileostomy at the time of pull-through operation is one option to prevent postoperative severe perineal excoriation. Bischoff et al. [23] insisted that the covered ileostomy should be kept open until the patient is completely toilet-trained for urine and can sit on a potty. This proposal remains controversial, and some pediatric surgeons do not agree with this because intimate perineal skin care can prevent severe perineal excoriation. In order to reach a definite conclusion concerning this issue, a prospective controlled study should be performed in the future.
Extensive aganglionosis is more difficult to treat than TCA, and a very specific treatment strategy may be needed. Patients with extensive aganglionosis suffer from short bowel syndrome without a colon, and intravenous hyperalimentation is often needed. One novel operation for the treatment of total intestinal aganglionosis was proposed by Ziegler et al. [24] in 1993. The operation is the extended myectomy-myotomy method with jejunostomy created at the 40 cm distal site from Treitz ligament. The authors reported 16 cases treated by this method, and the survival rate was 62.5%, and some patients could tolerate enteral feeding to various degrees. Another operative option for total intestinal aganglionosis is intestinal transplantation or multivisceral transplantation, and a recent meta-analysis by Nakamura et al. [25] showed promising results with an overall survival rate of 66%.
25.3 Postoperative Complications (Table 25.3)
As mentioned above, several complications may occur after pull-through operation despite various operative modifications. The most serious problem is postoperative enteritis. According to the meta-analysis, 42% of TCA patients experienced enteritis after pull-through operation [2], and in another report 55.4% of patients experienced enteritis [26]. Enteritis may be triggered by congestion of intestinal fluid and overgrowth of harmful bacteria. However, it has been hypothesized that some immunological defect might exist in the normo-ganglionic segment of the intestine in patients with aganglionosis, thereby causing severe inflammation and enteritis. These issues are still open for discussion and further studies are needed. Postoperative perineal excoriation is another complication. This complication may be prevented by intimate skin care provided by specialists such as wound, ostomy, and continence nurses. However, if refractory soiling and incontinence do exist for some reason, it is very difficult to avoid perineal dermatitis and deep skin injury. In some cases, permanent ileostomy was the final selection to resolve the problem. Bischoff et al. [23] emphasized that a proper and meticulous operative technique is needed to solve this problem. Obstructive syndrome sometimes occurs when the pull-through operation is done using a long aganglionic segment, and this symptom may result in severe ulcer formation and intestinal bleeding. Bischoff et al. proposed a radical opinion that we should abandon all complex techniques such as the patch procedure and J-pouch formation to prevent this complication [23].
25.4 Does an Optimal Operative Procedure for the Treatment of TCA Exist or Not?
The optimal operative technique for TCA is still uncertain even though several controlled studies have been performed in the past [26,27,28,29]. Such studies were not prospective controlled studies, and the number of patients enrolled was small. We do not have a definitive conclusion for the question of which operative technique is optimal for TCA. This is also supported by the fact that the postoperative complication rates are very similar among several different techniques. The rate of postoperative enteritis is still high after any surgical technique with any added option, and we must develop a new treatment strategy to prevent enteritis and to preserve and activate residual bowel function at the maximum level. It is strongly expected that a completely novel idea to reconstruct a normally nerved ileum and colon will be developed by adopting a stem cell technology or some other promising strategy.
References
Sandegard E. Hirschsprung’s disease with ganglion cell aplasia of the colon and terminal ileum: report of a case treated with total colectomy and ileo-anostomy. Acta Chir Scand. 1953;106:369–76.
Laughlin DM, Friedmacher F, Puri P. Total colonic aganglionosis: a systematic review and meta-analysis of long-term clinical outcome. Pediatr Surg Int. 2012;28:773–9.
Moore SW. Total colonic aganglionosis and Hirschsprung’s disease: a review. Pediatr Surg Int. 2015;31:1–9.
Moore SW. Total colonic aganglionosis in Hirschsprung disease. Semin Oediatr Surg. 2012;21:302–9.
Taguchi T, Obata S, Ieiri S. Current status of Hirschsprung’s disease: based on a nationwide survey of Japan. Pediatr Surg Int. 2017;33:497–504.
Ieiri S, Suita S, Nakatsuji T, Akiyoshi J, Taguchi T. Total colonic aganglionosis with or without small bowel involvement: a 30-year retrospective nationwide survey in Japan. J Pediatr Surg. 2008;43:2226–30.
Moore SW, Rode H, Miller AJ, Albertyn R, Cywes S. Familial aspects of Hirschsprung disease. Eur J Pediatr Surg. 1991;1:97–107.
Jamieson DH, Dundas SE, Belushi SA, Cooper M, Blair GK. Does the transition zone reliably delineate aganglionic bowel in Hirschsprung’s disease? Pediatr Radiol. 2004;34:811–5.
Stranzinger E, DiPietro MA, Teitelbaum DH, Strouse PJ. Imaging of total colonic Hirschsprung disease. Pediatr Radiol. 2008;38:1162–70.
Huang Y, Anupama B, Zheng S, Xiao X, Chen L. The expression of enteric nerve markers and nerve innervation in total colonic aganglionosis. Int J Surg Pathol. 2011;19:303–8.
Cheung ST, Tam YH, Chong HM, Chan KW, Mou WC, Shhoe DYJ, Lee KH. An 18-year experience in total colonic aganglionosis: from staged operation to primary laparoscopic endorectal pull-through. J Pediatr Surg. 2009;44:2352–4.
Yagi M, Okuyama H, Oue T, Kubota A, Ohyanagi H. One-stage laparoscopic pull-through operation without stoma for total colon aganglionosis. Pediatr Endosurg Innovative Tech. 2003;7:169–73.
Martin LW. Surgical management of Hirschsprung’s disease involving the small intestine. Arch Surg. 1968;97:183–9.
Kimura K, Nishijima E, Muraji T, Tsugawa C, Matsumoto Y. A new surgical approach to extensive aganglionosis. J Pediatr Surg. 1981;16:840–3.
Nishijima E, Kimura K, Tsugawa C, Muraji T. The colon patch graft procedure for extensive aganglionosis: long-term follow-up. J Pediatr Surg. 1998;33:215–9.
Sauer H, Klos I. A proposal to preserve the ileocecal valve and right colon in total colonic aganglionosis. J Pediatr Surg. 1989;24:457–61.
Sauer H, Fasching G. Preservation of ileocecal valve and right colon in total colonic aganglionosis. J Pediatr Surg. 1993;28:1640–3.
Amerstorfer EE, Fasching G, Till H, Huber-Zeyringer A, Hollwarth ME. Long-term results of total colonic aganglionosis patients treated by preservation of the aganglionic right hemicolon and the ileocecal valve. Pediatr Surg Int. 2015;31:773–80.
Hukkinen M, Koivusalo A, Rintala RJ, Pakarinen MP. Restorative proctocolectomy with j-pouch anastomosis for total colonic aganglionosis among neonates and infants. J Pediatr Surg. 2014;49:570–4.
Kimura K, Inimata Y, Soper RT. Posterior sagittal rectal myectomy for persistent rectal achalasia after Soave procedure for Hirschsprung disease. J Pediatr Surg. 1993;28:1200–1.
Zhang JS, Li L, Hou WY, Liu SL, Diao M, Zhang J, Ming AX, Cheng W. Transanal rectal mucosectomy and partial internal anal sphincterectomy for Hirschsprung disease. J Pediatr Surg. 2014;49:831–4.
Li Q, Li L, Jiang Q, Zhang Z, Xiao P. The mid-term outcomes of TRM-PIAS, proctocolectomy and ileoanal anastomosis for total colonic aganglionosis. Pediatr Surg Int. 2016;32:477–82.
Bischoff A, Levitt MA, Pena A. Total colonic aganglionosis: a surgical challenge. How to avoid complications? Pediatr Surg Int. 2011;27:1047–52.
Ziegler MM, Royal RE, Brandt J, Drasnin J, Martin LW. Extended myectomy-myotomy: a therapeutic alternative for total intestinal aganglionosis. Ann Surg. 1993;218:504–11.
Nakamura H, Henderson D, Puri P. A meta-analysis of clinical outcome of intestinal transplantation in patients with total intestinal aganglionosis. Pediatr Surg Int. 2017;33:837–41.
Menezes M, Prato AP, Jasonni V, Puri P. Long-term clinical outcome in patients with total colonic aganglionosis: a 31-year review. J Pediatr Surg. 2008;43:1696–9.
Blackburn S, Corbett P, Griffiths DM, Burge D, Beattie RM, Stanton M. Total colonic aganglionosis: a 15-year single center experience. Eur J Pediatr Surg. 2014;24:488–91.
Shen C, Song Z, Zheng S, Xiao X. A comparison of the effectiveness of the Soave and Martin procedure for the treatment of total colonic aganglionosis. J Pediatr Surg. 2009;44:2355–8.
Hukkinen M, Koivusalo A, Merras-Salmio L, Rintala RJ, Pakarinen MP. Postoperative outcome and survival in relation to small intestinal involvement of total colonic aganglionosis. J Pediatr Surg. 2015;50:1859–64.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kanamori, Y. (2019). Surgical Management of Total Colonic Aganglionosis and Extensive Aganglionosis. In: Taguchi, T., Matsufuji, H., Ieiri, S. (eds) Hirschsprung’s Disease and the Allied Disorders. Springer, Singapore. https://doi.org/10.1007/978-981-13-3606-5_25
Download citation
DOI: https://doi.org/10.1007/978-981-13-3606-5_25
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3605-8
Online ISBN: 978-981-13-3606-5
eBook Packages: MedicineMedicine (R0)