Abstract
Recently graphene has been drawing tremendous attention mainly due to its potential contributions to applications in biology, information technology and energy. Among these applications graphene-based biosensors have been particularly progressed caused in part by development of diverse derivatives of graphene such as graphene oxides (GOs) and graphene quantum dots (GQDs). In this chapter preparation and functionalization of the graphene and GOQs are described together with their optoelectronic properties. Recent progresses in graphene and GQD-based biosensors are also highlighted with emphasis on immunoassay which utilizes unique interaction between antigen and antibody, and oligonucleotide assay which utilizes hybridization process. Since electrical and optical features are the most prominent characteristics of graphene-based nanomaterials, biosensor systems will be focused on electrochemical and fluorescence-based detection scheme.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
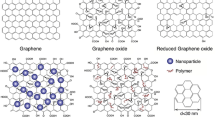
1 Graphene
1.1 Key Properties of Graphene
Graphene has been drawing tremendous attraction since the crystal graphene had been first observed by Novoselov in 2004 based on scotch-tape method (Novoselov et al. 2004). The name of the one-atom thick 2-D material, graphene is composed of two concepts, graphite and -ene. The thickness of graphene is the separation distance of the graphite, which is 0.335 nm recording the thinnest among all the nanomaterials developed until now. Graphene is 100–300 times stronger than steel with its Young’s modulus of 0.5–1.0 TPa and intrinsic strength of 130 GPa (Lee et al. 2008). The electron mobility at room temperature is 2.5 × 105 cm2 V−1 s−1 (Mayorov et al. 2011) with tis maximum current density reaching a few million times larger than copper (Liu et al. 2007). The single atom thick crystal material has high thermal conductivity of 3000 WmK−1 (Balandin et al. 2008) and high optical transmittance of 97.7% (Nair et al. 2008). Rightly these superb properties contributed Nobel Prize for Physics in 2010 and have been widely used for applications including sensors, electronics, energies and biology.
Two approaches are usually applied for the synthesis of graphene, bottom-up and top-down methods. In bottom-up method graphite is oxidized under very harsh condition to produce well-dispersed graphene oxide in aqueous medium, which in turn reduced to graphene. However, the reduction process does not produce completely -reduced graphene form. The chemical process generates graphene with some defects causing to be called reduced graphene oxide instead of graphene. In the top-down method chemical vapors are crystalized on a proper substrate form single-layer graphene. However, this is not suitable for mass production process. Many different types of exfoliation methods have been developed due to advantages of producing large amount of graphene in a relatively simple process.
1.2 Mechanical Exfoliation
The first discovery of graphene was processed based on mechanical exfoliation method using graphite, in which graphene is stacked together linked by van der Waals force. Although the van der Waals force itself is weak the force between graphenes in graphite is very strong since the van der Waals force works all over the surface of graphene. In order to produce graphene through exfoliation the van der Waals force is the first thing to overcome. Also another point to consider in exfoliation is lubricating effect in the lateral direction.
Sonication turned out to be a useful tool in exfoliation process due to advantage of producing large amount of graphene. When sonicated graphite powder is dispersed in solvents such as N, N-dimethylformamide (DMF) or N-methylpyrrolidone (NMP). However due to limit in dispersibility of graphene in those solvents, the concentration of graphene produced from sonication exfoliation is usually too low (~ 0.01 mg/mL) to be of any practical use. When the difference in surface energy between graphene and solvent is minimum, exfoliation tends to occur more easily. From the mechanistic point of view exfoliation is facilitated by liquid cavitation which in turn generates micro-bubbles. When the bubbles explode around the graphite the impulse helps to generate exfoliation process (Ciesielski and Samori 2014). However, sonication process has been reported to generate oxygen containing defects such as aldehyde, carboxylic acid, and ethers (Skaltsas et al. 2013).
Ball milling is another way for exfoliating graphite into graphene. In contrast to the sonication method where normal force is the major contributor, in the ball milling process shear force is the dominant factor. Another factor of force in the ball milling process is the balls which act on graphite through collisions. The ball milling can be processed either by wet or dry condition. In the wet process typically graphite is dispersed using solvents that are in good match with the surface energy of the graphite. Although mostly DMF and NMP are used as the matching solvents, combined use of 1-pyrenen carboxylic acid and methanol turned out to result in more efficient exfoliation than DMF alone (Aparna et al. 2013).
In spite of the scalability in production, the above-mentioned mechanical methods for exfoliation have to be developed to produce enhanced yield in monolayer graphene. Fragmentation effects also have to be reduced. In the case of sonication many interacting factors such as power, frequency, and time have to be optimized. Also detects of the graphene has to be minimized and uniformness of the product has to be enhanced.
1.3 Synthesis of Graphene
Although many different processes for exfoliation have been developed for large-scale production, the peeling-off method cannot avoid defects especially when it comes to producing large-scale graphene. Synthetic methods have been reported to be advantageous in this respect (Dreyer et al. 2010; Choi et al. 2010). Chemical vapor deposition (CVD) has been mostly widely used due to processibility of generating single-layer graphene over relatively large area (Li et al. 2009). For example, centimeter-scale graphene was able to be produced on a copper substrate using CVD method. Chemical synthetic method for graphene has also been widely used since the chemical wet process is suitable for large production. In the chemical process graphite is oxidized to produce graphene oxide (GO), which in turn is chemically reduced to reduced form of graphene oxide (rGO) (Li et al. 2008). Since the quality of rGO in the chemical synthetic method depends largely on the efficiency of reduction process, many different methods and reducing agents have been developed to obtain high quality rGO.
1.4 Characterization of Graphene
As described in the exfoliation and synthetic method for graphene, the product is usually a mixture of a single- and multi-layer of graphene. Many of the characterization methods for graphene is focused on the differentiating those species. Since, in the chemical synthetic method, GO is reduced to rGO, differentiation between these species and degree of reduction are critical aspects to be understood.
UV-visible spectroscopy can be used to identify GO and rGO. GO is characteristic of two absorption maxima (λMAX) at 234 nm and 299 nm. The shorter λMAX is attributed to π-π* transition in the aromatic C=C bonds while the longer λMAX is caused by n- π* transition in C=O bonds. In the case of rGO, λMAX appears at 269 nm which corresponds to π-π* transition in the aromatic C=C bonds. The red shift of π-π* transition in the aromatic C=C bonds for rGO indicates more delocalization of p-orbitals when GO is reduced to rGO (Paredes et al. 2008)
Raman spectroscopy is an efficient tool to identify pristine graphite, GO and rGO. Pristine graphite shows a very sharp G peak at 1581 cm−1, which is caused by the in-plane vibration in the sp2 carbons. When graphite is oxidized, the G peak shits to 1589 cm−1 in somewhat broadened mode and disordered structural pattern of GO, which has been caused by oxidation, produces a D band at 1352 cm−1 (Wang et al. 2009a, b, c). When GO is reduced to rGO, the G peak blue-shifts to 1582 cm−1 which is close to the G peak from the pristine graphite. Therefor the relative peak intensity between D and G can be used to understand the degree of defect in graphene.
XPS can be effectively used to understand the extent of reduction of GO. Since oxidized form of carbons in GO occurs as C-O (ethers and hydroxyls) and C=O (carbonyl) in addition to C-C (unoxidized carbons), C atoms in GO reveals at three different energies, 284.6 eV for C-C, 286.7 eV for C-O and 288.4 eV for C=O.
Atomic force microscopy (AFM) is a useful tool to identify single-layer graphene, of which thickness is reported to be 0.32 ~ 1.2 nm. More accurate way to characterize single-layer graphene than AFM is transmission electron microscopy (TEM), in which observation of transparency and edge of the graphene directly reveals whether the graphene is single-layered, double-layered or triple-layered, as well statistical analysis of the thickness (Hernandez et al. 2008).
1.5 Functionalization of Graphene
Even though graphene has great potential for applications in electronics, sensors, and various composites, in order to be of any practical utility, some barriers have to be overcome such as zero band gap, low dispersibility and inertness to chemical modifications. Many different methods for functionalizing graphene have been developed to enhance the real applicability of graphene. Functionalization on graphene can be processed either through covalent bonding or through non-covalent bonding. In this review functionalization of graphene will be focused mainly on method via covalent bonding. From the organic chemistry point of view, the chemical reactions can generally be processed by condensation reaction, addition reaction and substitution reaction, which can also be subdivided into nucleophilic and electrophilic substitution reactions.
In the condensation-type modification of graphene, thionyl chloride (SOCl2) chemistry is widely used to enable further functionalization on graphene. GO, which contains diverse oxygen-containing functional groups such as hydroxyl, epoxy and carboxylic groups is a good starting point to initiate functionalization process. The carboxylic acid on GO was reacted with SOCl2 to generate –COCl group which is labile to various types of nucleophiles. Indeed, alkylamine (RNH2) was reacted with –COCl to produce amide bond (-CONHR). Now the functional group R is linked with GO through amide bond, enabling the product with easy dispersibility in various types of solvents including THF, carbon tetrachloride (CCl4) and dichloroethane (Niyogi et al. 2006). Isocynate compound (RNCO) has been used to prepare amide bone (-CONHR) via reaction with carboxylic acid. Also the functionalization enables compatibility of GO with various polymers to produce graphene-polymer composite (Stankovich et al. 2006).
Different from condensation reaction which is mostly carried out via GO, addition reaction on graphene turned out to be a useful tool to enable direction chemical modification on graphene. Diazonium salt is an effective compound to carry out functionalization on graphene via free radicals which can be generated by heating the diazonium compound. Based on this chemistry, nitrophenyl diazonium compound (BF− 4 N+ 2-C4H4-NO2) was reacted on graphene to produce nirophenyl group. The addition reaction turned out to affect conductivity of graphene by transforming sp2 carbons to sp3 carbons. Based on this, conductivity of graphene was able to adjusted in a controlled manner, which in turn generated band gap on graphene making possible of applications as semiconducting materials (Sinitskii et al. 2010; Niyogi et al. 2010).
Substitution reactions can occur at epoxy groups on GO using diverse types of nucleophilic functionalities. Primary amine groups are good candidate for the purpose. Amines with varying length of alkyl groups (CnH2nNH2) were used to ad alkyl groups on GO (Bourlinos et al. 2003). When the alkyl chain was relatively short (n = 2, 4, 8, 12) the reaction was able to proceed at room temperature. However, when the alkyl chain was long (n = 18) the reaction had to be refluxed to complete the reaction.
2 Graphene Quantum Dots (GQDs)
Graphene quantum dot (GQD) is a graphene fragmented within around 20 nm. Although semiconductor quantum dots (SQDs) have been attracting much attention, more diverse and wider applications of GQDs are limited due to problems related with cost, toxicity, biocompatibility and chemical modification. In contrast, GQDs are understood be superior to SQDs with those problems. In this section synthetic methods and optical properties of GQDs are described.
2.1 Synthesis of GQDs
GQDs are generally prepared in two approaches, top-down and bottom-up. In the top-down method GQDs are prepared by exfoliating graphite followed by fragmentation step. The top-down method involves harsh reaction conditions and produces GQDs with irregular morphology and wide size distribution. Graphite is usually a starting material and is converted into GO using a modified version of the Hummers procedure (Hummers and Offeman 1958), in which sulfuric acid, sodium nitrate and potassium permanganate are involved. In the case of bottom-up method very uniform GQDs are produced. However, bottom-up preparation of GQDs involves complex synthetic steps.
Many different top-down methods have been developed and the major difference among them is how GO is converted into GQDs. Hydrothermal cutting is one of the widely used top-down method, in which relatively strong basic compound such NaOH is used as scissors for the carbon precursors. Pan et al. prepared GQD with diameter of 5–13 nm in aqueous media by thermally reducing GO to graphene and chemical oxidation of the graphene followed by hydrothermal reduction to GQD (Pan et al. 2010). From a mechanistic point of view, oxidation process generates epoxy groups in linear fashion along carbon lattice, which upon further oxidation are converted to carbonyl groups. Under hydrothermal condition the linear mode of oxidation acts as defect site for to be cut to produce GQDs.
Solvothermal method was used to produce GQDs. GO in DMF was sonicated and heated at 200 °C to produce GQDs with average diameter of 5.3 nm and thickness of 1.2 nm indicating most of the GQDs are in single layered or bi-layered state. The resulting GQOs displayed strong fluorescence with photoluminescence quantum yield of 11.4% (Zhu et al. 2011).
Microwave has been utilized for synthesizing GQDs (Li et al. 2012; Zhu et al. 2010; Chen et al. 2010, 2012). Use of microwave has advantage over the hydrothermal and oxidation procedure by providing high energy rapidly and uniformly throughout the reaction medium which results in short reaction time and enhanced uniformity of GQDs. One-pot microwave approach in the absence of stabilizer was reported to produce greenish yellow luminescent GQDs from GO (Li et al. 2012). Epoxy groups were oxidized to form a line of mixture of functional groups composed of minor amount of epoxy and majority of carbonyl groups. The greenish yellow-luminescent GQDs were mostly single and bi-layered graphene with average diameter of 4.5 nm with photoluminescence (PL) quantum yield of 11.7%. By treating the GQDs with NaBH4 blue-luminescent GQDs were obtained almost without change in dimension with PL quantum yield of 22.9%.
Electrochemical methods also have been used to prepare GQDs (Li et al. 2011; Zhang et al. 2012) based on anode oxidation and anion intercalation which in turn help exfoliate carbon anode. When graphite is used as a working electrode in the preparation of carbon dots, the high redox potential ranging from ±1.5 V to ±3 V can oxidize carbon-carbon single bond enabling oxidative cleavage (Lu et al. 2009). Also potential cycling allows the involving electrolytes to be intercalated into carbon anode. Zhang reported high yield process by exfoliating graphite based on electrochemical method. Dependency of the functionalization mode on temperature was reported. When the hydrazine reduction was processed at ambient temperature, reduction was exerted mostly on basal epoxy and hydroxyl groups. However, when the temperature was increased the reduction produced amidation on the edge carboxylic groups leading to hydrazide formation between two adjacent carboxylic groups.
In a bottom-up method, a polycyclic aromatic molecule, hexa-peri-hexabenzocoronene, was pyrolzed, oxidized and functionalized followed by reduction to produce GQDs (Liu et al. 2011). Yan et al. produced GQDs by oxidizing dendritic polyphenylene precursors. In order to provide solubility of the resulting GQDs phenyl rings were substituted with 3 long alkyl groups (Yan et al. 2010).
2.2 Luminescence Properties of GQDs
According to the calculation based on density function theory, the energy gap of GQDs of π –π* transition decrease as the size of GQDs increase (Eda et al. 2010). Depending on the way a graphene sheet is cut along the crystallographic direction, the edges can form zigzag or armchair mode. It is reported that types of edges affect the optoelectronic and magnetic properties of GQDs. Such size-dependent properties arise from quantum confinement effect (Chan and Nie 1998; Michalet et al. 2005; Smith and Nie 2010). In general, the smaller GQDs display luminescence at shorter wave length (Freeman and Willner 2012).
Also PL of GQDs tend to be affected by pH (Pan et al. 2010; Zhu et al. 2012). While hydrothermally prepared GQDs under basic condition display strong PL, GQDs prepared under acidic conditions show negligible PL. Under acidic conditions protonation of the zigzag edges disrupts the emissive triplet carbene state. In contrast under basic condition detachment of protons from the zigzag edges revive PL. Also PL patterns are affected by solvents and concentration of GQDs (Fan et al. 2012).
3 Applications of Graphene for Biosensors
3.1 Introduction
Many different types of sensing systems have been developed in order to maximize detection performance as well as to minimize handling procedures and the size of the sensors. Since electrical and fluorescent properties are the most prominent characteristics of graphene derivatives, sensing systems based on electrochemical and fluorescent properties of graphene have been much studied.
Use of graphene as an electrode in electrochemical applications has advantage in catalytic efficiency which is superior to that of carbon nanotube (CNT) with wide range of electrochemical potential of 2.5 V in 0.1 M PBS at pH 7.0 (Alwarappan et al. 2009). Graphene has lower charge-transfer resistance than graphite and glass carbon electrodes (GCEs) (Zhou et al. 2009). Electrical properties of were investigated for different redox systems, Ru(NH3)6 3+/2+, Fe(CN)6 3−/4-, Fe3+/2+ and dopamine (Tang et al. 2009). Two different electrodes were compared, bare glassy carbon (GC) and glassy carbon modified with rGO sheet film (rGSF). Much faster apparent electron-transfer rate constant (k° app) was observed on rGSF than on GC for both Ru(NH3)6 3+/2+, Fe(CN)6 3−/4-.
3.2 Graphene-Based Immunosensors
Immunoassay takes advantage of the interaction between antibody and antigen. Enzyme-linked immunoassay (ELISA) is a standard clinical diagnostic method, in which a capture antibody is in complexation with an antigen and a detection antibody in a sandwich-type fashion. One of the key factors for high sensitivity in electrochemical detection is how efficient the electron transfer is on the electrode surface. Highly conductive property of graphene is well suited for this purpose and much application has been developed to enhance the electrical conductivity on electrode surface by using graphene as surface modification medium. Many different types of protein-based cancer biomarkers have been detected using electrochemical immunoassay. Alpha-fetoprotein (AFP), a biomarker for hepatocellular cancer, was assayed on graphene-modified electrode in the hope of enhancing sensing performance by facilitating electron transfer rate of the electrode using graphene (Du et al. 2010).
Ultrasensitive immunoassay was processed by generating polyaniline (PAN) via catalysis with Horse-radish peroxidase (HRP)-conjugated AuNP (HRP-AuNP) (Fig. 4.1) (Lai et al. 2014). In the presence of poly(diallyldimethylammonium chloride) (PDDA) GO was reduced by hydrazine to produce composite of rGO/PDDA. AuNPs were mixed with the composite of rGO/PDDA to prepare rGO/AuNP. The rGO/AuNP was dispersed in water to be dropped on the screen-printed carbon electrode (SPCE), onto which anti-human IgG(anti-HIgG) was added. Due to high surface area of the rGO/AuNP nanocomposite provides large amount of anti-HIgG may be position, which would lead to highly sensitive assay system. In order to process immunoassay, HIgG was added followed by HRP-conjugated AuNP to form sandwich-typed complexation. Aniline monomer and H2O2 were added to form PAN. The aniline monomers are polymerized through catalysis by HRP oxidation of aniline by HRP-AuNP. Thus produced PAN is electrochemically determined for immunoassay. The nanocomposite system was reported to be able to detect 9.7 pg/mL of IgG with dynamic range covering 4 orders of magnitude. Such a high sensitivity is reported to have been contributed by the amplification effect of HRP-AuNP and the acceleration of electron transfer by rGO/AuNP.
Schematic view of the preparation for ultrasensitive detection of IgG based on rGO/AuNP system. (Lai et al. 2014. Copyright ⓒ Lai et al.)
Ultrasensitive electrochemical immunoassay system (limit of detection 100 fg/mL, 700aM) was reported using rGO (Fig. 4.2) (Monsur et al. 2012). Indium tin oxide (ITO) electrode was functionalized with amine group using amine-functionalized benzenediazonium. The amine functionalized ITO surface was coated with GO, in which the primary amine on the ITO surface was reacted with carboxylic acid positioned at the edge of GO. Then the GO on the ITO surface was electrochemically reduced to prepare rGO on ITO surface. Using π-π interaction of the aromatic rings positioned between ITO surface and polymer, the N-acryloxysuccinimide-functionalized poly(BMA-r-PEGMA-r-NAS) was coated on the rGO surface. IgG was tethered on the polymer coating through reaction of the primary amines in IgG with N-succinimide in the polymer. Then ELISA was processed via sandwich-type complexation with antigen and HRP-labeled secondary anti-IgG.
Schematic view of the preparation and working principle for the ultrasensitive electrochemical immunoassay. (Monsur et al. 2012 Copyright ⓒ Monsur et al.)
GQDs were used for immunoassay taking advantage of the unique luminescent and resonance energy transfer (RET) properties of GQDs to recognize 10 ng/mL of human IgG (Fig. 4.3) (Zhao et al. 2013). When mouse anti-human immunoglobulin G (mIgG) functionalized GQDs came in contact with graphene, the luminescent GQDs became quenched through π-π* stacking interaction. To this quenched system human IgG was added to restore some degree of luminescence caused by interruption of the π-π* stacking interaction due to the intervening IgG.
GQD as an immunoassay system via luminescence resonance energy transfer (LRET). (Zhao et al. 2013. Copyright ⓒ Zhao et al.)
3.3 Graphene-Based Detection of Oligonucleotides
DNA also can be recognized on graphene-based electrode via electrochemical analysis. Graphene-based electrode can catalytically oxidize the four DNA bases, A, G, C, and T, enabling direct detection of a single-nucleotide polymorphism (SNPP in a short oligonucleotide (Zhou et al. 2009). Immobilization of single-stand DNA can be processed either through adsorption or covalent bonding. Simple adsorption of single-strand DNA were processed on GO or rGO. Reduction of GO was processed electrochemically (Giovanni et al. 2012), thermally (Yang et al. 2013) or polyaniline-electrochemically (Wang et al. 2011). Covalent immobilization of DNA was processed by reacting amine-functionalized DNA on the oxygen containing rGO via carbodiimide (Bonanni et al. 2012).
Quain et al. reported fluorescence resonance energy transfer (FRET)-based DNA assay with limit of detection (LOD) of 75 pM and dynamic range of 6.7 ~ 46 nM (Fig. 4.4.) (Qian et al. 2014). GQDs were treated with NaBH4 to produce reduced GQDs (rGQDs), which can fluorescence prominently. Single-strand DNA (ssDNA) probes were tethered on rGQDs (ssDNA-rGQDs) through condensation reaction. The ssDNA-rGQDs came in contact with GO through π-π interaction leading to fluorescence quenching. When target DNA was added to form double-strand DNA (dsDNA), the dsDNA interrupts the π-π interaction to detach ssDNA-rGQDs from GO leading to restoring of fluorescence.
GQDs-based DNA detection system based on fluorescence resonance energy transfer (FRET). (Qian et al. 2014. Copyright ⓒ Qian Z S et al.)
Zhang et al. presented a molecular beacon (MB)-based miroRNA detection system utilizing GQDs (Fig. 4.5) (Zhang et al. 2015). Molecular beacons are oligonucleotides designed with stem and loop structures (Broude 2002; Wang et al. 2009a; Stobiecka and Chałupa 2015; Tang et al. 2009). The oligonucleotides are equipped with a fluorophore at one end and a quencher at the other. MicroRNAs were able to be analyzed with LOD of 100 pM and dynamic range of 0.1 ~ 200 nM. Pyrene-functionalized MB (py-MB) probes were prepared with 5′ modification on pyrene and 3′ modification of the fluorescence dyes, Cy3 and Cy5, while leaving the loop part of the sequence complementary to the target miRNA. The π-π interaction between pyrene part of the py-MBs and GQDs tethers the py-MBs on GQDs. When no target miRNAs came in contact with the probe py-MBs, FRET occurs between GQDs and the fluorescence dyes. However, when target miRNAs are added, double strands are formed preventing FRET from occurring.
Schematic view illustrating the detection mechanism of the py-MB detection system. (Zhang et al. 2015. Copyright ⓒ Zhang H et al.)
References
Alwarappan S, Erdem A, Liu C, Li C-Z (2009) Probing the electrochemical properties of graphene Nanosheets for biosensing applications. J Phys Chem C 113(20):8853–8857. https://doi.org/10.1021/jp9010313
Aparna R, Sivakumar N, Balakrishnan A, Nair AS, Nair SV, Subramanian KRV (2013) An effective route to produce few-layer graphene using combinatorial ball milling and strong aqueous exfoliants. J Renew Sustain Energy 5(3):033123–033123. https://doi.org/10.1063/1.4809794
Balandin AA, Ghosh S, Bao W, Calizo I, Teweldebrhan D, Miao F, Lau CN (2008) Superior thermal conductivity of single-layer graphene. Nano Lett 8(3):902–907. https://doi.org/10.1021/nl0731872
Bonanni A, Ambrosi A, Pumera M (2012) On oxygen-containing groups in chemically modified Graphenes. Chem Eur J 18(15):4541–4548. https://doi.org/10.1002/chem.201104003
Bourlinos AB, Gournis D, Petridis D, Szabó T, Szeri A, Dékány I (2003) Graphite oxide: chemical reduction to graphite and surface modification with primary aliphatic amines and amino acids. Langmuir 19(15):6050–6055. https://doi.org/10.1021/la026525h
Broude NE (2002) Stem-loop oligonucleotides: a robust tool for molecular biology and biotechnology. Trends Biotechnol 20(6):249–256. https://doi.org/10.1016/S0167-7799(02)01942-X
Chan WCW, Nie S (1998) Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 281(5385):2016–2018. https://doi.org/10.1126/science.281.5385.2016
Chen W, Yan L, Bangal PR (2010) Preparation of graphene by the rapid and mild thermal reduction of graphene oxide induced by microwaves. Carbon 48(4):1146–1152. https://doi.org/10.1016/j.carbon.2009.11.037
Chen S, Liu J-W, Chen M-L, Chen X-W, Wang J-H (2012) Unusual emission transformation of graphene quantum dots induced by self-assembled aggregation. Chem Commun 48(61):7637–7639. https://doi.org/10.1039/C2CC32984K
Choi W, Lahiri I, Seelaboyina R, Kang YS (2010) Synthesis of graphene and its applications: a review. Crit Rev Solid State Mater Sci 35(1):52–71. https://doi.org/10.1080/10408430903505036
Ciesielski A, Samori P (2014) Graphene via sonication assisted liquid-phase exfoliation. Chem Soc Rev 43(1):381–398. https://doi.org/10.1039/C3CS60217F
Dreyer DR, Park S, Bielawski CW, Ruoff RS (2010) The chemistry of graphene oxide. Chem Soc Rev 39(1):228–240. https://doi.org/10.1039/B917103G
Du D, Zou Z, Shin Y, Wang J, Wu H, Engelhard MH, Liu J, Aksay IA, Lin Y (2010) Sensitive Immunosensor for Cancer biomarker based on dual signal amplification strategy of graphene sheets and multienzyme functionalized carbon Nanospheres. Anal Chem 82(7):2989–2995. https://doi.org/10.1021/ac100036p
Eda G, Lin Y-Y, Mattevi C, Yamaguchi H, Chen H-A, Chen IS, Chen C-W, Chhowalla M (2010) Blue photoluminescence from chemically derived graphene oxide. Adv Mater 22(4):505–509. https://doi.org/10.1002/adma.200901996
Fan L, Hu Y, Wang X, Zhang L, Li F, Han D, Li Z, Zhang Q, Wang Z, Niu L (2012) Fluorescence resonance energy transfer quenching at the surface of graphene quantum dots for ultrasensitive detection of TNT. Talanta 101(Suppl C):192–197. https://doi.org/10.1016/j.talanta.2012.08.048
Freeman R, Willner I (2012) Optical molecular sensing with semiconductor quantum dots (QDs). Chem Soc Rev 41(10):4067–4085. https://doi.org/10.1039/C2CS15357B
Giovanni M, Bonanni A, Pumera M (2012) Detection of DNA hybridization on chemically modified graphene platforms. Analyst 137(3):580–583. https://doi.org/10.1039/C1AN15910K
Hernandez Y, Nicolosi V, Lotya M, Blighe FM, Sun Z, De S, McGovern IT, Holland B, Byrne M, Gun'Ko YK, Boland JJ, Niraj P, Duesberg G, Krishnamurthy S, Goodhue R, Hutchison J, Scardaci V, Ferrari AC, Coleman JN (2008) High-yield production of graphene by liquid-phase exfoliation of graphite. Nat Nanotechnol 3:563–568. https://doi.org/10.1038/nnano.2008.215
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80(6):1339–1339. https://doi.org/10.1021/ja01539a017
Lai G, Zhang H, Tamanna T, Yu A (2014) Ultrasensitive immunoassay based on electrochemical measurement of enzymatically produced polyaniline. Anal Chem 86(3):1789–1793. https://doi.org/10.1021/ac4037119
Lee C, Wei X, Kysar JW, Hone J (2008) Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321(5887):385–388. https://doi.org/10.1126/science.1157996
Li D, Müller MB, Gilje S, Kaner RB, Wallace GG (2008) Processable aqueous dispersions of graphene nanosheets. Nat Nanotechnol 3:101–105. https://doi.org/10.1038/nnano.2007.451
Li X, Cai W, An J, Kim S, Nah J, Yang D, Piner R, Velamakanni A, Jung I, Tutuc E, Banerjee SK, Colombo L, Ruoff RS (2009) Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 324(5932):1312–1314. https://doi.org/10.1126/science.1171245
Li Y, Hu Y, Zhao Y, Shi G, Deng L, Hou Y, Qu L (2011) An electrochemical avenue to green-luminescent graphene quantum dots as potential Electron-acceptors for photovoltaics. Adv Mater 23(6):776–780. https://doi.org/10.1002/adma.201003819
Li L-L, Ji J, Fei R, Wang C-Z, Lu Q, Zhang J-R, Jiang L-P, Zhu J-J (2012) A facile microwave avenue to Electrochemiluminescent two-color graphene quantum dots. Adv Funct Mater 22(14):2971–2979. https://doi.org/10.1002/adfm.201200166
Liu F, Ming P, Li J (2007) Ab initio. Phys Rev B 76(6):064120–064120
Liu R, Wu D, Feng X, Müllen K (2011) Bottom-up fabrication of Photoluminescent graphene quantum dots with uniform morphology. J Am Chem Soc 133(39):15221–15223. https://doi.org/10.1021/ja204953k
Lu J, J-x Y, Wang J, Lim A, Wang S, Loh KP (2009) One-pot synthesis of fluorescent carbon nanoribbons, nanoparticles, and graphene by the exfoliation of graphite in ionic liquids. ACS Nano 3(8):2367–2375. https://doi.org/10.1021/nn900546b
Mayorov AS, Gorbachev RV, Morozov SV, Britnell L, Jalil R, Ponomarenko LA, Blake P, Novoselov KS, Watanabe K, Taniguchi T, Geim AK (2011) Micrometer-scale ballistic transport in encapsulated graphene at room temperature. Nano Lett 11(6):2396–2399. https://doi.org/10.1021/nl200758b
Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S (2005) Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307(5709):538–544. https://doi.org/10.1126/science.1104274
Monsur A, Haque J, Park H, Sung D, Jon S, Choi S-Y, Kim K (2012) An electrochemically reduced graphene oxide-based electrochemical Immunosensing platform for ultrasensitive antigen detection. Anal Chem 84:1871−1878. https://doi.org/10.1021/ac202562v
Nair RR, Blake P, Grigorenko AN, Novoselov KS, Booth TJ, Stauber T, Peres NMR, Geim AK (2008) Fine structure constant defines visual transparency of graphene. Science 320(5881):1308–1308. https://doi.org/10.1126/science.1156965
Niyogi S, Bekyarova E, Itkis ME, McWilliams JL, Hamon MA, Haddon RC (2006) Solution properties of graphite and graphene. J Am Chem Soc 128(24):7720–7721. https://doi.org/10.1021/ja060680r
Niyogi S, Bekyarova E, Itkis ME, Zhang H, Shepperd K, Hicks J, Sprinkle M, Berger C, Lau CN, deHeer WA, Conrad EH, Haddon RC (2010) Spectroscopy of covalently functionalized graphene. Nano Lett 10(10):4061–4066. https://doi.org/10.1021/nl1021128
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306(5696):666–669. https://doi.org/10.1126/science.1102896
Pan D, Zhang J, Li Z, Wu M (2010) Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Adv Mater 22(6):734–738. https://doi.org/10.1002/adma.200902825
Paredes JI, Villar-Rodil S, Martínez-Alonso A, Tascón JMD (2008) Graphene oxide dispersions in organic solvents. Langmuir 24(19):10560–10564. https://doi.org/10.1021/la801744a
Qian ZS, Shan XY, Chai LJ, Ma JJ, Chen JR, Feng H (2014) A universal fluorescence sensing strategy based on biocompatible graphene quantum dots and graphene oxide for the detection of DNA. Nanoscale 6(11):5671–5674. https://doi.org/10.1039/C3NR06583A
Sinitskii A, Dimiev A, Corley DA, Fursina AA, Kosynkin DV, Tour JM (2010) Kinetics of Diazonium functionalization of chemically converted graphene nanoribbons. ACS Nano 4(4):1949–1954. https://doi.org/10.1021/nn901899j
Skaltsas T, Ke X, Bittencourt C, Tagmatarchis N (2013) Ultrasonication induces oxygenated species and defects onto exfoliated graphene. J Phys Chem C 117(44):23272–23278. https://doi.org/10.1021/jp4057048
Smith AM, Nie S (2010) Semiconductor nanocrystals: structure, properties, and band gap engineering. Acc Chem Res 43(2):190–200. https://doi.org/10.1021/ar9001069
Stankovich S, Dikin DA, Dommett GHB, Kohlhaas KM, Zimney EJ, Stach EA, Piner RD, Nguyen ST, Ruoff RS (2006) Graphene-based composite materials. Nature 442:282–286. https://doi.org/10.1038/nature04969
Stobiecka M, Chałupa A (2015) Biosensors based on molecular beacons. Chem Pap 69(1):62–76. https://doi.org/10.1515/chempap-2015-0026
Tang L, Wang Y, Li Y, Feng H, Lu J, Li J (2009) Preparation, structure, and electrochemical properties of reduced graphene sheet films. Adv Funct Mater 19(17):2782–2789. https://doi.org/10.1002/adfm.200900377
Wang J, Chen J, Chang P, LeBlanc A, Li D, Abbruzzesse JL, Frazier ML, Killary AM, Sen S (2009a) MicroRNAs in plasma of pancreatic ductal adenocarcinoma patients as novel blood-based biomarkers of disease. Cancer Prev Res 2(9):807–813. https://doi.org/10.1158/1940-6207.capr-09-0094
Wang H, Robinson JT, Li X, Dai H (2009b) Solvothermal reduction of chemically exfoliated graphene sheets. J Am Chem Soc 131(29):9910–9911. https://doi.org/10.1021/ja904251p
Wang K, Tang Z, Yang CJ, Kim Y, Fang X, Li W, Wu Y, Medley CD, Cao Z, Li J, Colon P, Lin H, Tan W (2009c) Molecular engineering of DNA: molecular beacons. Angew Chem Int Ed 48(5):856–870. https://doi.org/10.1002/anie.200800370
Wang Z, Zhang J, Chen P, Zhou X, Yang Y, Wu S, Niu L, Han Y, Wang L, Chen P, Boey F, Zhang Q, Liedberg B, Zhang H (2011) Label-free, electrochemical detection of methicillin-resistant staphylococcus aureus DNA with reduced graphene oxide-modified electrodes. Biosens Bioelectron 26(9):3881–3886. https://doi.org/10.1016/j.bios.2011.03.002
Yan X, Cui X, Li L-s (2010) Synthesis of large, stable colloidal graphene quantum dots with tunable size. J Am Chem Soc 132(17):5944–5945. https://doi.org/10.1021/ja1009376
Yang T, Li Q, Li X, Wang X, Du M, Jiao K (2013) Freely switchable impedimetric detection of target gene sequence based on synergistic effect of ERGNO/PANInanocomposites. Biosens Bioelectron 42(Suppl C):415–418. https://doi.org/10.1016/j.bios.2012.11.007
Zhang M, Bai L, Shang W, Xie W, Ma H, Fu Y, Fang D, Sun H, Fan L, Han M, Liu C, Yang S (2012) Facile synthesis of water-soluble, highly fluorescent graphene quantum dots as a robust biological label for stem cells. J Mater Chem 22(15):7461–7467. https://doi.org/10.1039/C2JM16835A
Zhang H, Wang Y, Zhao D, Zeng D, Xia J, Aldalbahi A, Wang C, San L, Fan C, Zuo X, Mi X (2015) Universal fluorescence biosensor platform based on graphene quantum dots and pyrene-functionalized molecular beacons for detection of MicroRNAs. ACS Appl Mater Interfaces 7(30):16152–16156. https://doi.org/10.1021/acsami.5b04773
Zhao H, Chang Y, Liu M, Gao S, Yu H, Quan X (2013) A universal immunosensing strategy based on regulation of the interaction between graphene and graphene quantum dots. Chem Commun 49(3):234–236. https://doi.org/10.1039/C2CC35503E
Zhou M, Zhai Y, Dong S (2009) Electrochemical sensing and biosensing platform based on chemically reduced graphene oxide. Anal Chem 81(14):5603–5613. https://doi.org/10.1021/ac900136z
Zhu Y, Murali S, Stoller MD, Velamakanni A, Piner RD, Ruoff RS (2010) Microwave assisted exfoliation and reduction of graphite oxide for ultracapacitors. Carbon 48(7):2118–2122. https://doi.org/10.1016/j.carbon.2010.02.001
Zhu S, Zhang J, Qiao C, Tang S, Li Y, Yuan W, Li B, Tian L, Liu F, Hu R, Gao H, Wei H, Zhang H, Sun H, Yang B (2011) Strongly green-photoluminescent graphene quantum dots for bioimaging applications. Chem Commun 47(24):6858–6860. https://doi.org/10.1039/C1CC11122A
Zhu S, Zhang J, Liu X, Li B, Wang X, Tang S, Meng Q, Li Y, Shi C, Hu R, Yang B (2012) Graphene quantum dots with controllable surface oxidation, tunable fluorescence and up-conversion emission. RSC Adv 2(7):2717–2720. https://doi.org/10.1039/C2RA20182H
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kim, Y.J., Jeong, B. (2018). Graphene-Based Nanomaterials and Their Applications in Biosensors. In: Noh, I. (eds) Biomimetic Medical Materials. Advances in Experimental Medicine and Biology, vol 1064. Springer, Singapore. https://doi.org/10.1007/978-981-13-0445-3_4
Download citation
DOI: https://doi.org/10.1007/978-981-13-0445-3_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-0444-6
Online ISBN: 978-981-13-0445-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)