Abstract
Protein cage nanoparticles are made of biomaterials, proteins, and have well-defined cage-like architectures designed and built by nature. They are composed of multiple copies of one or a small number of chemically identical subunits having a highly uniform nano-size and symmetric structure. Protein cage nanoparticles have genetic and chemical plasticity amenable to simultaneously introducing multiple cell-specific targeting ligands, diagnostic agents, and their corresponding therapeutic agents at desired sites depending on its purpose. A wide range of protein cage nanoparticles, such as ferritin, lumazine synthase, encapsulin, and virus-like particles, has been extensively explored and utilized in biomedical fields as effective delivery nanoplatforms of diagnostics and/or therapeutics. Highly biocompatible and plastic protein cage nanoparticles may provide a new paradigm for developing simple, but versatile in vivo delivery systems.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Along with the innovative development of nanotechnology, a wide range of nanoscale delivery vehicles, including liposomes, micelles, inorganic and polymeric nanoparticles, and protein cage nanoparticles, has been developed to effectively deliver therapeutic and/or diagnostic reagents to the target sites (Allen and Cullis 2013; Brigger et al. 2002; Lee et al. 2016; Rösler et al. 2001; Wang et al. 2012). The nanoscale and modifiable surface of delivery nanoplatforms generally result in efficient passive delivery of cargo molecules mainly relying on enhanced permeability and retention (EPR) effects of nanoparticles in tumor tissues (Brigger et al. 2002). EPR effects of delivery nanoplatforms frequently allow a long circulation time in the bloodstream and deep penetration of delivered cargoes, such as therapeutic and/or diagnostic reagents. For the localized treatment of diseases, minimizing side-effects, and target-specific diagnosis of symptoms in early stage, the active targeted delivery of diagnostic or/and therapeutic reagents to desired sites using nanoparticles has been widely attempted.
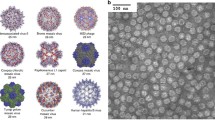
Among various delivery nanoplatforms, protein cage nanoparticles are considered to be excellent candidates for multifunctional delivery nanoplatforms due to their well-defined architectures and high biocompatibility (Lee et al. 2016; Maham et al. 2009). A variety of protein cage nanoparticles, such as ferritin, lumazine synthase, encapsulin, and virus-like particles, have been extensively studied and their atomic resolution crystal structures have been solved allowing us to easily manipulate them genetically and chemically (Fig. 2.1). Protein cage nanoparticles have three distinct interfaces: interior and exterior surfaces as well as the interfaces between subunits. These versatile interfaces allow them to be utilized as delivery nanoplatforms for diverse applications (Douglas and Young 2006; Uchida et al. 2010). The defined interior spaces and/or surfaces of protein cage nanoparticles are used as rooms for synthesizing size-constraint biomimetic nanomaterials or for encapsulating diagnostic and/or therapeutic reagents (Bode et al. 2011; Flenniken et al. 2009; Kang and Douglas 2010; Lee et al. 2016). The exterior surfaces of protein cage nanoparticles provide the sites for presenting various types of molecules including affinity tags, antibodies, fluorophores, carbohydrates, nucleic acids, and targeting peptides (Kang et al. 2012, 2014; Kim et al. 2016; Min et al. 2014a, b; Moon et al. 2013, 2014a, b). Chimeric protein cage nanoparticles having multifunctions can also be generated by modulating assembly of pre-functionalized subunits either in cells or in vitro (Kang et al. 2008a, b, 2009; Suci et al. 2010). The highly symmetric and uniform, but multivalent nature of protein cage nanoparticle makes them attractive as multifunctional delivery nanoplatforms. In this chapter, we will briefly discuss about recent development of protein cage nanoparticles as delivery nanoplatforms and their broad usages in biomedical fields.
Surface diagram representations of various types of protein cage nanoparticles. (a) Ferritin (PDB:2JD6) (b) Lumazine synthase (PDB:1HQK) (c) Encapsuline (PDB:3DKT) (d) CCMV (PDB:1CWP) (e) bacteriophage Qβ (PDB: 1qbe) (f) bacteriophage P22 procapsid (PDB:3IYI). One of subunits is represented as ribbon diagram in red. All the images are generated by using UCSF chimera
2 Therapeutic and/or Diagnostic Agent Delivery Nanoplatforms
Nature provides a wide range of protein cage nanoparticles which have their own unique biochemical and biophysical properties, such as size, composition, stability and biological activity. The various types of protein cage nanoparticles having different origins and compositions have been used depending on their applications.
2.1 Small-Sized Protein Cage Nanoparticles: Ferritin, Lumazine Synthase, and Encapsulin
Ferritins are iron storage proteins found in almost all living organisms from bacteria to animals (Theil et al. 2013). Ferritins are composed of 24 subunits and self-assemble into highly symmetric 12 nm closed shells having 8 nm inner diameter cavity. Recently, RGD-modified ferritin was used to encapsulate doxorubicin (Dox) up to 73.49wt% by pre-complexing with Cu(II) and, similarly, cisplatin which are Pt-based drugs up to 50 molecules via metal-ferritin interaction and selectively delivered them to the target sites (Zhen et al. 2013). Non-covalent loading and unloading of hydrophobic drug-like molecules to ferritin was also demonstrated by chemically conjugating β-cyclodextrins (β-CDs) on the surface of ferritin which spontaneously capture hydrophobic drug molecules and reversibly release them (Kwon et al. 2012). For the targeted delivery of ferritin, monosaccharides, mannoses or galactoses, were chemically attached to the surface of ferritin (Kang et al. 2014). Mannose- or galactose-displaying ferritins recognized and tightly bound to DC-SIGN or ASGP-R lectins on the surface of the mammalian cells, DCEK or HepG2 cells. Antibodies are ideal ligands for targeted delivery of various therapeutics and/or diagnostics because they have extremely high binding affinity and specificity for their target molecules and a variety of antibodies against virtually any desired targets can be readily obtained on demand. Thirteen residue Fc-binding peptides (FcBP) were genetically inserted onto the surface of ferritin to couple antibodies and ferritin without altering the targeting capability of displayed antibodies (Kang et al. 2012). FcBP-presenting ferritin formed stable non-covalent complexes with both IgGs derived from human and rabbit. Using a human anti-HER2 antibody and a rabbit anti-folate receptor antibody along with fluorescently labeled FcBP-ferritin, the specific binding of these complexes to breast cancer cells and folate receptor over-expressing cells were respectively demonstrated by fluorescent cell imaging (Kang et al. 2012).
Similar antibody-mediated targeted delivery nanoplatforms were also established with lumazine synthase. The lumazine synthase, isolated from hyperthermophile Aquifex aeolicus (AaLS), consists of 60 identical subunits assembled into icosahedral capsid architecture with an exterior diameter of 15 nm and an 8 nm interior cavity (Zhang et al. 2001). While AaLS is an enzyme that catalyzes the penultimate step in riboflavin biosynthesis inside the cell (Zhang et al. 2003), its hollow spherical architecture has been used as a template for the encapsulation of cargo proteins (Azuma et al. 2017; Beck et al. 2015; Frey et al. 2016; Seebeck et al. 2006; Wörsdörfer et al. 2011, 2012). Instead of Fc-binding peptides, antibody Fc-binding domain (ABD) from protein A was genetically fused to the C-termini of AaLS subunit and ABD-displaying AaLS (ABD-AaLS) were successfully produced without altering cage architecture and stability (Kim et al. 2016). It was demonstrated that ABD-AaLS effectively capture various types of antibodies derived from diverse species, such as human, rabbit, and mouse, on demand and the resulting complexes have the capability of selective recognition and binding to their target cells guided by antibodies displayed on the surface of ABD-AaLS (Kim et al. 2016). AaLS exhibits an unusual heat stability and genetic and chemical versatility. The AaLS templates acquired two different types of cell-specific targeting peptides, RGD4C and SP94 peptides, in two different positions individually and corresponding cargo molecules, either detecting molecules, NHS-fluorescein and fluorescein-5-maleimide, or therapeutic molecules, aldoxorubicin and bortezomib (BTZ), were chemically attached in combination without disrupting the overall cage architecture. RGD4C- and SP94-AaLS individually exhibited specific binding capability toward their target cells, KB and HepG2 cells respectively, and the enhanced cytotoxicity of delivered Dox and BTZ. (Min et al. 2014a, b)
Encapsulin, another heat stable protein cage nanoparticle isolated from thermophile Thermotoga maritima, is assembled from 60 copies of identical 31 kDa monomers having a thin and icosahedral symmetric cage structure with interior and exterior diameters of 20 and 24 nm, respectively (Giessen 2016; Sutter et al. 2008). Encapsulin has a large enough central cavity and tendency to encapsulate a large amount of therapeutic and/or diagnostic reagents. SP94-peptides were presented on the exterior surface of engineered encapsulin through either chemical conjugation or genetic insertion and SP94-encapsulin exhibited specific binding capability to hepatocellular carcinoma cells, HepG2, and an ability to carry imaging probes or prodrug molecules (Moon et al. 2014a, b). In a similar approach, FcBP was introduced onto the surface loop region of encapsulin and FcBP-displaying encapsulin was demonstrated to selectively recognize and specifically bind to squamous cell carcinoma 7 (SCC-7) cells, which overexpress a cell surface glycoprotein CD44 involved in cell-cell interactions, cell adhesion and migration, over Hela, HepG2, MDA-MB-231 and KB cells (Moon et al. 2014a, b).
2.2 Large-Sized Protein Cage Nanoparticles: Virus-Like Particles (VLPs)
Virus-like particle (VLP) is one of the most widely used protein cage nanoparticles for biomedical applications (Ma et al. 2012). VLPs are generally derived from viral capsids, especially bacterial and plant viruses. Similar to the other protein cage nanoparticles, VLPs have a uniform size distribution and a symmetric and well-defined multivalent structure. The cowpea mosaic virus (CPMV) and the cowpea chlorotic mottle virus (CCMV) are plant viruses and self-assemble into an icosahedral symmetric cage structure having an overall outer diameter of 28 nm (Brumfield et al. 2004; Ochoa et al. 2006; Sutter et al. 2008). CPMV exhibits a natural affinity to bind to and penetrate mammalian cells and fluorescent dye-labeled CPMVs were used for intravital imaging of vascular development (Leong et al. 2010; Lewis et al. 2006). Covalent conjugation of anticancer drugs, Dox, to the CPMV was achieved and Dox-CPMV conjugates exhibited superior cytotoxic effect in Hela cells to that of free Dox (Aljabali et al. 2013). The light-absorbing molecules, zinc phthalocyanines (ZnPC), were loaded into CCMV VLPs using pH- and ionic-strength mediated structural changes and the ZnPC-loaded CCMV VLPs were used for photodynamic therapy. RAW 264.7 macrophages efficiently took up ZnPC-loaded CCMV VLPs and were effectively killed upon red light irradiation (Brasch et al. 2011).
In addition to plant viruses, bacterial viruses, bacteriophage MS2, Qβ, and P22, have been used widely in biomedical applications (Lee et al. 2016; Ma et al. 2012; Shukla and Steinmetz 2015). Bacteriophage MS2 (Peabody 2003) and Qβ (Brown et al. 2009) contain RNA molecules as their genomes and they are composed of 180 subunits to form closed icosahedral shells with an outer diameter of 28 nm similar to that of CPMV and CCMV. MS2 has been used for the delivery of nucleic acids, such as siRNA, miRNA, and antisense ssDNA, anticancer drugs including Dox and 5-fluorouracil, and ricin toxin (Ashley et al. 2011; Galaway and Stockley 2013; Pan et al. 2012a, b; Wu et al. 2005). For the photodynamic therapy, the interior surface of MS2 VLPs was chemically conjugated with 180 photodynamic agents, porphyrins, and the exterior was decorated with approximately 20 copies of a Jurkat-specific aptamer using an oxidative coupling reaction targeting an unnatural amino acid. The doubly modified MS2 VLPs selectively targeted the Jurkat cells and killed more than 76% of them upon 20 min illumination (Stephanopoulos et al. 2010). Similar approach using Qβ VLPs as alternative photodynamic agent carriers was reported (Rhee et al. 2012). Alkyne-derivatized Qβ VLPs were prepared by acylation of the wild-type Qβ VLPs with N-hydroxysuccinimide ester and subsequently the zinc tetraaryl porphyrins and glycan, Siaα2-6Galβ1-4GlcNAc, were attached by the copper-catalyzed azide-alkyne cycloaddition (CuAAC) reaction as photodynamic agents and a specific ligand for the B-cell CD22 receptor, respectively. It was shown that the doubly modified Qβ VLPs selectively bind to CD22 receptor bearing Chinese hamster ovary (CHO) cells and efficiently generate singlet oxygen upon full-spectrum xenon lamp irradiation showing dose-dependent phototoxicity (Rhee et al. 2012). Fullerenes (C60) were also used as an alternate photosensitizing moiety and their successful cellular uptake into HeLa cells was reported (Steinmetz et al. 2009).
P22 VLPs have approximately twice the outer diameter (~60 nm) of other VLPs that are commonly used (~28 nm) (Kang et al. 2008a, b). With the aid of approximately 300 copies of internal scaffolding proteins, four hundred and twenty copies of identical 46 kDa capsid subunits initially assemble into a 58-nm icosahedral procapsid structure which transforms into 64-nm mature capsid upon DNA packaging (Prevelige et al. 1988). Recently, P22 VLPs have been popularly used for encapsulation of a wide variety of proteins, including fluorescent proteins, influenza nucleoproteins, alcohol dehydrogenase D, and hydrogenase complexes by truncating scaffolding proteins and genetically fusing a cargo protein of interest to the N-terminus (Jordan et al. 2016; O’Neil et al. 2012; Patterson et al. 2012, 2013, 2014; Qazi et al. 2016; Schwarz and Douglas 2015; Sharma et al. 2017). P22 VLPs have genome-free hollow architectures, with sufficient space for accommodating small chemotherapeutic agents and/or diagnostic probes within their cavity. While catechol ligands were attached to the interior surface of the P22 WB VLPs through thiol-maleimide Michael-type addition with N-(3,4-dihydroxyphenethyl)-3-maleimido-propanamide, hepatocellular carcinoma (HCC) cell targeting SP94 peptides were chemically conjugated to the exterior surface of them (Min et al. 2014a, b). Anticancer drug, BTZ, formed a stable complex with catechol ligand within P22 VLPs at neutral and alkaline pH through the boric acid-diol complexation and became dissociated under cancerous acidic conditions to kill them. The doubly modified P22 VLPs encapsulated up to 280 molecules of BTZ per particle at pH 9.0 and release them completely within 12 h with a half-life of approximately 5 h at pH 5.5. They efficiently bound to and killed HepG2 hepatocellular carcinoma cells in a dose-dependent manner (Min et al. 2014a, b).
The blood brain barrier (BBB) is often an insurmountable obstacle for a large number of candidate drugs, including peptides, antibiotics, and chemotherapeutic agents. P22 VLPs were tailored to deliver analgesic ziconotide across a BBB model by genetically incorporating ziconotide into scaffolding protein in the interior cavity and chemically attaching cell penetrating HIV-Tat peptide on the exterior of the capsid (Anand et al. 2015). P22 VLPs containing ziconotide were successfully transported in several BBB models of rat and human brain microvascular endothelial cells (BMVEC) using a recyclable noncytotoxic endocytic pathway (Anand et al. 2015).
3 Vaccine Delivery Nanoplatforms
To date, vaccination is considered as the most effective way for control and prevention of infectious diseases. Most vaccines currently available are based on live attenuated or killed pathogens against their own original disease-causing pathogens (Berzofsky et al. 2001). However, they often cause severe side-effects at some frequency in population and there are limitations for developing vaccines for non-pathogen derived diseases, such as cancer, in these approaches. Although subunit vaccines that are derived from specific components of disease-causing pathogens or tissues have been developed to circumvent these drawbacks, they generally exhibited limited immunogenicity and longevity (Bachmann and Jennings 2010). In contrast, protein cage nanoparticles self-assemble and form highly symmetric morphology mimicking disease-causing viruses without infectious genetic materials. They are efficiently taken up by professional antigen presenting cells probably due to their nanometer-range size and surface patterns and lead to the efficient induction of strong humoral and cellular immune responses (Bachmann and Jennings 2010; Chackerian 2007; Grgacic and Anderson 2006; Kushnir et al. 2012; Plummer and Manchester 2011; Schwarz and Douglas 2015). Protein cage nanoparticles have been genetically, chemically, and/or post-translationally modified to be used as delivery nanoplatforms for exogenous antigenic molecules.
3.1 Chemical Conjugation of Antigenic Molecules to Protein Cage Nanoparticles
Qβ VLPs were investigated as potential delivery nanoplatforms for chemically conjugating self-antigens that induce neutralizing autoantibody responses (Jennings and Bachmann 2009; Maurer et al. 2005; Tissot et al. 2008). Fourteen different self-molecules were individually attached on the surface of Qβ VLPs and four out of them were selected and clinically tested (Jennings and Bachmann 2009). Clinical studies with AngQβ, which target angiotensin II, reported that three immunizations with 300 μg of AngQβ reduced blood pressure in patients with mild to moderate hypertension during the daytime and especially in the early morning (Tissot et al. 2008). Similarly, approximately 585 nicotine molecules were chemically attached to a Qβ VLP to form NicQβ and NicQβ induced strong antibody responses in preclinical studies (Maurer et al. 2005). Vaccinated mice with NicQβ significantly reduced nicotine levels in the brain compared with control group upon intravenous nicotine challenge. In a phase I study, 32 healthy non-smokers were immunized with NicQβ and all volunteers who received NicQβ showed nicotine-specific IgM antibodies at day 7 and nicotine-specific IgG antibodies at day 14 (Maurer et al. 2005).
Similarly, a model antigen, ovalbumin (OVA), was chemically conjugated to the exterior of a small heat shock protein (sHsp), which consists of 24 identical protein subunits forming a near spherical shell of 12 nm exterior and 6.5 nm interior diameter, and a single intranasal vaccination of mice with OVA-sHsp resulted in accelerated and intensified OVA-specific IgG1 responses within 5 days (Richert et al. 2012). It was also shown that pretreatment of mice with P22 VLPs further accelerated the onset of the antibody response to OVA-sHsp, demonstrating the utility of conjugating antigens to VLPs for pre-, or possibly post-exposure prophylaxis of lung, all without the need for adjuvant (Richert et al. 2012).
The effective generation of robust cytotoxic CD8+ T cell immune responses is considered a primary goal in cancer immunotherapy because functional cytotoxic CD8+ T cells not only kill their target cells directly but also secrete the cytokine IFN-γ. E2 protein cage nanoparticles were used as nanoplatforms for simultaneous delivery of CD8+ T cell-specific OT-1 peptide (SIINFEKL) and adjuvant, CpG molecules, to dendritic cells (DCs). E2 is a non-viral protein cage nanoparticle composed of 60 identical subunits forming a hollow dodecahedral shell with 25 nm outer diameter. OT-1 peptides and CpGs were chemically conjugated to E2 and they were effectively delivered to DCs being displayed on MHC I threefold greater than the control. Co-delivery of OT-1 peptides and CpGs by E2 to DCs showed increased and prolonged cytotoxic CD8+ T cell activation (Molino et al. 2013).
3.2 Genetic Insertion of Antigenic Molecules to Protein Cage Nanoparticles
In addition to chemical conjugation of antigenic molecules, genetic modifications have been widely used for VLP-based vaccine development. Bacteriophage MS2 VLP was used for displaying viral epitope and binding motif on its surface. Peptides from the V3 loop of HIV gp120 and the ECL2 loop of the HIV coreceptor, CCR5, were genetically inserted into the surface of MS2 VLPs and these genetically modified MS2 VLPs showed the potent immunogenicity (Peabody et al. 2008). The RNA bacteriophage AP205 was also investigated as a nanoplatform for heterologous display of many antigens. The AP205 VLP is composed of 180 copies of the capsid protein and both its N-terminus and C-terminus are tolerant to the fusion of long and complex epitopes. A fusion of a gonadotropin releasing hormone (GnRH) epitope to AP205 VLPs successfully induced antibodies and vaccination of mice with AP205 VLPs genetically fused with an extracellular domain of the Influenza A M2 protein resulted in 100% protection from lethal infection with influenza virus (Tissot et al. 2010). The insect virus flock house virus (FHV) has been also widely used for antigen display and delivery in animals (Chen et al. 2006; Manayani et al. 2007; Scodeller et al. 1995). FHV also forms icosahedral capsid consisting of 180 copies of the capsid protein and has several surface exposed loops which are popular sites for inserting antigenic epitopes. Chimeric FHV VLPs that carry both Hepatitis C virus (HCV) and hepatitis B virus (HBV) epitopes simultaneously was constructed and they elicited anti-HCV and anti-HBV responses in guinea pig (Chen et al. 2006). The principal neutralizing domain, IGPGRAF sequence, from the V3 loop of HIV-1 was genetically inserted into the surface of FHV VLPs and these hybrid VLPs induced strong and broad specific immune response in guinea pigs against different V3 loop sequences (Scodeller et al. 1995). In addition to peptide epitopes, large antigens were displayed on the surface of FHV VLPs through genetic insertions. The 181 amino acid ANTXR2 VWA domain was inserted into a loop of capsid protein and displayed on the surface of modified FHV VLPs (Manayani et al. 2007). Vaccination with engineered FHV VLPs induced a potent immune response against lethal toxin and protected rats against lethal toxin challenge after a single administration without adjuvant (Manayani et al. 2007).
VLP is not the only one type of protein cage nanoparticles used for antigen display and delivery. The ectodomain of A/New Caledonia/20/1999 (1999 NC) haemagglutinin (HA) was genetically fused to the N-terminus of ferritin subunit to form HA-ferritin. HA-ferritin self-assembled and spontaneously generated eight trimeric viral spikes on its surface (Kanekiyo et al. 2013). Immunization with HA-ferritin elicited haemagglutination inhibition antibody titers more than tenfold higher than those from the licensed inactivated vaccine (Kanekiyo et al. 2013). Antibodies elicited by HA-ferritin neutralized H1N1 viruses from 1934 to 2007 protected ferrets from an unmatched 2007 H1N1 virus challenge (Kanekiyo et al. 2013). Further structure-based development of an H1 HA stem-only immunogen was carried out. H1 HA stabilized stem (H1-SS) without the immunodominant head domain was generated and genetically fused to ferritin to form H1-SS-ferritin. Vaccination with H1-SS-ferritin in mice and ferrets elicited broadly cross-reactive antibodies that completely protected mice and partially protected ferrets against lethal heterosubtypic H5N1 influenza virus challenge (Kanekiyo et al. 2013). AaLS and encapsulin were also used as delivery nanoplatforms to polyvalently display germline-targeting HIV-1 gp120 outer domain immunogens (eOD-GT6) and the receptor-binding portion of Epstein-Barr virus (EBV) gp350, respectively. eOD-GT6-AaLS successfully activated germline and mature VRC01-class B cells that produce broadly neutralizing antibodies (bNAbs) against HIV-1 (Jardine et al. 2013) and EBV gp350-encapsulin induced neutralizing antibody responses in mice and non-human primates that significantly exceeded the level obtained with soluble EBV gp350 protein (Kanekiyo et al. 2015).
Exterior surface is not the only place where protein cage nanoparticles can carry antigenic epitopes. A variety of antigenic peptides and proteins can be encapsulated into spacious interior cavity of protein cage nanoparticles and/or inserted into the protein sequences. The conserved nucleoprotein (NP) from influenza was genetically fused to SP and NP-encapsulated P22 VLPs were successfully generated (Patterson et al. 2013). Vaccination of mice with NP-encapsulated P22 VLPs resulted in multi-strain protection against 100 times lethal doses of influenza in an NP specific cytotoxic CD8+ T cell-dependent manner (Patterson et al. 2013). Ferritin and AaLS were evaluated as efficient vaccine platforms for systematic studies of epitope-specific immune responses (Han et al. 2014; Ra et al. 2014). Antigenic peptides, OT-1 (SIINFEKL) or OT-2 (ISQAVHAAHAEINEAGR) which are derived from ovalbumin, were genetically introduced to various sites of ferritin and AaLS, effectively delivered to DCs, and processed within endosomes. Vaccination of naïve mice with antigenic peptide bearing ferritin and AaLS induced an efficient differentiation of OT-1 specific CD8+ T cells into functional effector cytotoxic T cells and an effective differentiation of proliferated OT-2 specific CD4+ T cells into functional CD4+ Th1 and Th2 cells which produces IFN-γ/IL-2 and IL-10/IL-13 cytokines, respectively (Han et al. 2014; Ra et al. 2014). As an extension of these studies for cancer vaccine development, antigenic OT-1 peptide was genetically incorporated into three different positions of the encapsulin subunit and their efficacies of inducing DC-mediated antigen-specific T cell cytotoxicity followed by B16-OVA tumor rejection were evaluated (Choi et al. 2016). Vaccination of mice with OT-1-Encap effectively activated OT-1 peptide specific cytotoxic CD8+ T cells before or even after B16-OVA melanoma tumor generation and led to subsequent infiltration of OT-1-specific cytotoxic CD8+ T cells into the tumor sites upon tumor challenges, providing tumor suppression (Choi et al. 2016).
3.3 Post-translational Addition of Antigenic Molecules to Protein Cage Nanoparticles
Genetic fusion of antigenic proteins to the viral capsid proteins may be the most commonly used approach to display antigenic proteins on VLPs. However, genetic fusion of two different proteins, antigenic proteins and viral capsid proteins, often leads to misfolding of antigenic proteins and/or impairing VLP assembly. To circumvent these issues, antigenic proteins and VLPs were individually expressed with extra glue domains and then covalently combined together post-translationally using recently developed SpyTag/SpyCatcher (ST/SC) protein ligation system (Moon et al. 2016; Zakeri et al. 2012). In the ST/SC protein ligation system, the 15 kDa SC protein recognizes the 13-amino acid ST (AHIVMVDAYKPTK) and they spontaneously form an irreversible isopeptide covalent bond. ST and SC can be genetically fused to antigenic proteins and VLPs, respectively or reciprocally, and they maintain their individual functions as well as stability of the fused proteins (Moon et al. 2016; Zakeri et al. 2012).
AP205 VLPs were genetically fused to SC (SC-AP205 VLPs) and subsequently ligased with ST-fused malaria antigens, including cysteine-rich Inter-Domain Region (CIDR) and P. falciparum sexual-stage antigen (Pfs25) (Brune et al. 2016). Covalent couplings between SC-AP205 VLPs and ST-fused malaria antigens were quantitatively achieved (Brune et al. 2016). Vaccination with SC-AP205 VLPs decorated with malarial antigens efficiently induced antibody responses after only a single immunization (Brune et al. 2016). ST-AP205 VLPs were also generated and used for ligating full-length 3d7 circumsporozoite protein (CSP) fused with SC or Pfs48/45 protein fused with SC (Janitzek et al. 2016). The CSP is an attractive target for malaria vaccine and the immunogenicity of CSP-AP205 VLPs was evaluated in mice (Janitzek et al. 2016). 112 CSP molecules were presented on the surface of an AP205 VLP (180 subunits) on average and mice vaccinated with CSP-AP205 VLPs generated 2.6 fold higher antibody titers over a course of 7 months than those of the control group (Janitzek et al. 2016). CSP-AP205 VLPs also induced production of IgG2a antibodies which are linked with a more efficient clearing of intracellular parasite infection (Janitzek et al. 2016). Genetic fusion of ST or SC to the N-terminus and/or C-terminus of AP205 VLPs produced stable, nonaggregated VLPs expressing one SC, one ST or two ST per capsid protein (Thrane et al. 2016). Eleven different vaccine antigens fused to SC or ST were attempted to be ligased to ST- or SC-AP205 VLPs and antigen-AP205 VLP conjugates were obtained with coupling efficiencies of ranging from 22% to 88% (Thrane et al. 2016). AP205 VLPs displaying Pfs25 or VAR2CSA drastically increased antibody titer, affinity, longevity and functional efficacy compared to corresponding monomeric protein vaccines. AP205 VLPs displaying cancer or allergy-associated self-antigens, including PD-L1, CTLA-4 and IL-5, also effectively broke B cell self-tolerance eliciting potent and durable antibody responses upon vaccination (Thrane et al. 2016). As extension of these studies, the amount and efficacy of antibodies induced by three different nanoplatforms were evaluated side-by-side (Leneghan et al. 2017). Plasmodium falciparum malaria transmission blocking antigen Pfs25 was selected as a transmission blocking malaria vaccine (TBV) candidate and it was genetically fused to IMX313, which is a multimerization domain derived from the chicken complement inhibitor C4b-binding protein, chemically crosslinked onto the surface of Qβ VLPs, or conjugated through ST/SC ligation to SC-AP205 VLPs. While chemically-crosslinked Pfs25-Qβ VLPs elicited the highest quantity of anti-Pfs25 antibodies, Pfs25-AP205 VLPs elicited the highest quality anti-Pfs25 antibodies for transmission blocking upon mosquito feeding (Leneghan et al. 2017). It is anticipated that Pfs25 displayed on AP205 VLPs maintains its native conformation better than that of Qβ VLPs producing more functionally relevant monoclonal antibodies (Leneghan et al. 2017).
4 MRI Contrasting Agent (CA) Delivery Nanoplatform
Magnetic resonance imaging (MRI) is one of most powerful in vivo imaging techniques that provide highly resolved anatomical and functional information without using harmful ionizing radiation. However, it is difficult to distinguish selected tissues of interest, such as diseased area, from background tissues because they generally produce similar signal intensities. To overcome this issue, contrast agents (CAs) are frequently used to increase the sensitivity of MR to tissues of interest (Caravan 2006). Both positive (T1-weighted, brightening) and negative (T2-weighted, darkening) contrast agents are being actively explored for in vivo applications. Paramagnetic gadolinium ion (Gd(III)) complexed with poly(aminocarboxylate) compound chelating agents, such as tetraazacyclododecane tetraacetic acid (DOTA) and diethylenetriamine pentaacetic acid (DTPA), is the most frequently used positive contrast agent for contrast enhancement by reducing spin-lattice relaxation times (Caravan 2006; Lauffer 1987) and ferromagnetic iron oxide nanoparticles is the most popularly used negative contrast agents for contrast enhancement by promoting T2 shortening (Shukla and Steinmetz 2015). A variety of protein cage nanoparticles have been used as templating nanoplatforms for both positive and negative contrast agents.
4.1 Positive (T1) Contrast Agents: Gd(III)-Chelating Agent/Protein Cage Nanoparticle Conjugates
Paramagnetic gadolinium ion (Gd(III)) enhances the image contrast with increased signal intensity from T1-weighted image acquisition due to the greatly reduced spin-lattice relaxation times produced by the interaction between the proton and unpaired electron spins of Gd(III) (Caravan 2006; Lauffer 1987). However, the free form of Gd(III) is toxic and, therefore, should be complexed with chelating agents or sequestered by composites (Caravan 2006). Furthermore, covalent conjugation of Gd(III)-chelating agent complexes to macromolecules generally improves both the blood circulation time and relaxivity value for high resolution/contrast MR image acquisition (Anderson et al. 2006; Datta et al. 2008; Ferreira et al. 2012; Liepold et al. 2009). Our discussion will focus on covalent protein cage nanoparticle conjugates with Gd(III)-chelating agent complexes.
CCMV VLPs were used as a templating macromolecules to attach Gd(III)-DOTA and each particle contained 60 Gd(III)-DOTAs on average. The resulting Gd(III)-DOTA-CCMV conjugates exhibited ionic and particle T1 relaxivities of 46 and 2806 mM−1s−1, respectively, at 60 MHz (Liepold et al. 2007). To increase the number of Gd(III) ions per particle and conjugate size, various VLPs and chemical methods were applied (Anderson et al. 2006; Datta et al. 2008; Garimella et al. 2011; Hooker et al. 2007; Min et al. 2013; Pokorski et al. 2011; Prasuhn et al. 2007; Qazi et al. 2013). 360 and more than 500 Gd(III)-DTPAs were attached onto the P22 and MS2 VLPs and they generated enhanced T1 relaxivities up to 20503 and 7200 mM−1s−1 per particle at 60 MHz, respectively (Anderson et al. 2006; Min et al. 2013). The potential use of Gd(III)-DTPA-P22 conjugates as in vivo MRI contrast agents was also demonstrated by imaging the blood vessels of a mouse including the carotid, mammary arteries, the jugular vein and, the superficial vessels of the head (Min et al. 2013). Another Gd(III)-chelating agent complex, Gd(III) hydroxypyridonate (Gd(III)-HOPO), was also polyvalently attached to MS2 VLPs obtaining 180 Gd(III) ions per nanoparticle and the resulting Gd(III)-HOPO-MS2 exhibited maximum ionic and particle T1 relaxivities of 41 and 7416 mM−1s−1, respectively, at 60 MHz (Datta et al. 2008; Garimella et al. 2011; Hooker et al. 2007).
Polymerization chemistry along with VLPs allowed conjugation of remarkable amounts of Gd(III) ions to VLPs. The polymerization of oligo(ethylene glycol)-methacrylate (OEGMA) and its azido-functionalized analogue (OEGMA-N3) was directly grafted from the outer surface of Qβ VLPs by atom transfer radical polymerization (ATRP) and the resulting surface-grafted Qβ VLPs held 610 Gd(III) ions exhibiting maximum ionic and particle T1 relaxivities of 11.6 and 7092 mM−1s−1, respectively, at 60 MHz (Pokorski et al. 2011). Approximately 1900 Gd(III) ions were loaded into P22 VLP cavity by using the branched polymerization of p-SCN-Bn-DTPA-Gd(III) and 2-azido-1-azidomethyl-ethylamine (DAA) via stepwise click reactions inside of P22 VLPs and they exhibited maximum ionic and particle T1 relaxivities of 21.7 and 41300 mM−1s−1, respectively, at 28 MHz (Qazi et al. 2013). Similar polymerization approach was applied to non-VLP protein cage nanoparticle, sHsp. Gd(III)-DTPA containing branched polymers were grown inside of sHsp via stepwise click reactions and the resulting Gd(III)-DTPA-sHsp exhibited maximum ionic and particle T1 relaxivities of 25 and 4200 mM−1s−1, respectively, at 31 MHz (Liepold et al. 2009).
In both preclinical and clinical settings, a demand for MRI contrast agents with improved relaxivity at higher magnetic fields ( >300 MHz or 7 T) is being hugely increased. The T1 enhancement ability tends to decrease significantly (more than tenfold) as the magnetic field is increased and often causes a major problem in in vivo MRI at high field. AaLS was polyvalently decorated with Gd(III)-DOTA to evaluate its potential as an in vivo MR CA at the high magnetic field strength of 7 T. Each AaLS was conjugated with 60 Gd(III)-DOTAs on its surface and the T1 relaxivities of Gd(III)-DOTA-AaLS were 30.2 and 16.5 mM−1s−1 at 60 and 300 MHz, respectively, making it attractive as a T1 contrast agent at high field (7 T) (Song et al. 2015). 3D MR angiography of mice demonstrated the feasibility of vasculature imaging within 2 h of intravenous injection of Gd(III)-DOTA-AaLS and a significant reduction of T1 values in the tumor region at 7 h post-injection in the SCC-7 flank tumor model implied potential use of Gd(III)-DOTA-AaLS as an tumor-targeting MR CA at high magnetic field (Song et al. 2015).
4.2 Negative (T2) Contrast Agents: Iron-Oxide Nanoparticle/Protein Cage Nanoparticle Core-Shells
Ferritin is probably the best protein cage nanoparticle for preparation of ferrimagnetic iron oxide nanoparticles because it inherently sequestrates irons in vivo and converts and stores them as forms of iron oxide (Fe2O3) (Uchida et al. 2006). Recombinant human H chain ferritin (rHFn) was used as size-constrained nanoplatforms for ferromagnetic iron oxide nanoparticle synthesis and it generated a series of iron oxide nanoparticles with diameters ranging from 3.6 to 5.9 nm with increasing iron loading amounts from 1000 to 5000 iron ions per rHFn (Uchida et al. 2008). The iron oxide-mineralized rHFn exhibited comparable MR signals to known iron oxide-based MRI CAs, such as ferumoxtran-10, and they were readily taken up by macrophages in vitro and provided strong T2-weighted MR contrast (Uchida et al. 2008). The iron oxide-mineralized rHFn were also used to image vascular macrophages in vivo in murine carotid arteries through MRI (Terashima et al. 2011). The iron oxide-mineralized rHFn accumulated in vascular macrophages in mice atherosclerotic lesions without any additional macrophage targeting moieties allowing in vivo MR imaging of atherosclerosis (Terashima et al. 2011). Recently, the iron oxide-mineralized rHFn were demonstrated to be targeted to numerous types of cancer cell lines that express high transferrin receptor 1 (TfR1) levels (Fan et al. 2012). As a following study, the iron oxide-mineralized rHFn with the core size of 5.3 nm were prepared and exhibited extremely high relaxivity (T2) of up to 224 mM−1s−1 (Cao et al. 2014). TfR1-positive MDA-MB-231 or U87 tumor-bearing mice were treated with the iron oxide-mineralized rHFn and tumor sites either in thigh or brain were successfully visualized with MRI (Cao et al. 2014). This study indicated that the iron oxide-mineralized rHFn can cross the endothelium, epithelium, and BBB layers (Cao et al. 2014). In vivo MRI of vascular inflammation and angiogenesis in experimental carotid disease and abdominal aortic aneurysm (AAA) were also performed with RGD peptide displaying rHFn which mineralized iron oxide nanoparticles within its cavity (RGD-HFn-Fe3O4) (Kitagawa et al. 2017). RGD-HFn-Fe3O4 was taken up more than HFn-Fe3O4 in both the ligated left carotid arteries and AAAs probably due to active targeting of cells and thus exhibited significantly enhanced MRI signals (Kitagawa et al. 2017).
VLPs have been also popularly used as templating nanoplaforms for negative MRI CAs. BMV VLPs derived from plant virus, brome mosaic virus, were disassembled and reassembled with pre-formed ferromagnetic iron oxide nanoparticles to generate core-shell hybrid composites comprising an iron oxide core and a BMV capsid protein shell (Huang et al. 2011). The resulting hybrid composites showed T2 relaxivity of 376 mM−1s−1, which is 4- to 6-fold higher than commercially available contrast agents, and penetrated into tissue and transferred long-distance through the vasculature in Nicotiana benthamiana leaves (Huang et al. 2011). Similar core-shell formation approach using Rotavirus or Simian virus 40 (SV40) VLPs derived from mammalian viruses along with the ferromagnetic iron oxide nanoparticles was carried out and it was demonstrated that the resulting core-shell hybrid composites (Chen et al. 2012; Enomoto et al. 2013) were efficiently internalized by their target cells significantly improving cellular MRI sensitivity compared with commercially available surface passivated iron oxide nanoparticles (Chen et al. 2012).
5 Conclusion
Macromolecular composites, including synthetic polymers, dendrimers, liposomes, carbohydrates, and inorganic nanoparticles, have been extensively studied for development of versatile in vivo delivery nanoplatforms. Although protein cage nanoparticles are in the very early stages of development as in vivo delivery nanoplatforms for diagnostics and/or therapeutics, they are a promising class of macromolecular composites for development of in vivo delivery nanoplatforms because they have a high biocompatibility and well-defined monodisperse structure which are hardly achieved by other types of macromolecular composites. Protein cage nanoparticles also have the genetic and chemical plasticity that can be used to acquire diverse functions, such as cargo encapsulation, targeting ligand presentation, and functional molecule conjugation, by design depending on their purposes. Numerous studies discussed in this chapter present that various encapsulation strategies of cargo molecules in combination with diverse presentation strategies of targeting ligand molecules are applicable to many protein cage nanoparticles and protein cage nanoparticles are promising in vivo delivery nanoplatforms for diagnosis, prevention, and therapy of diseases. Although there are some clinical trials using protein cage nanoparticle-based delivery nanoplatforms undergone and planned, further through studies related to their fate within target cells, in vivo immune alteration caused by them, and their bio-distribution and pharmacodynamics upon in vivo administration should be carried out before clinical applications can be considered.
References
Aljabali AAA, Shukla S, Lomonossoff GP, Steinmetz NF, Evans DJ (2013) CPMV-DOX delivers. Mol Pharm 10(1):3–10. https://doi.org/10.1021/mp3002057
Allen TM, Cullis PR (2013) Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev 65(1):36–48. https://doi.org/10.1016/j.addr.2012.09.037
Anand P, O’Neil A, Lin E, Douglas T, Holford M (2015) Tailored delivery of analgesic ziconotide across a blood brain barrier model using viral nanocontainers. Sci Rep 5:12497. https://doi.org/10.1038/srep12497
Anderson EA, Isaacman S, Peabody DS, Wang EY, Canary JW, Kirshenbaum K (2006) Viral nanoparticles Donning a paramagnetic coat: conjugation of MRI contrast agents to the MS2 capsid. Nano Lett 6(6):1160–1164. https://doi.org/10.1021/nl060378g
Ashley CE, Carnes EC, Phillips GK, Durfee PN, Buley MD, Lino CA, Padilla DP, Phillips B, Carter MB, Willman CL, Brinker CJ, Caldeira JC, Chackerian B, Wharton W, Peabody DS (2011) Cell-specific delivery of diverse cargos by bacteriophage MS2 virus-like particles. ACS Nano 5(7):5729–5745. https://doi.org/10.1021/nn201397z
Azuma Y, Zschoche R, Hilvert D (2017) The C-terminal peptide of Aquifex aeolicus riboflavin synthase directs encapsulation of native and foreign guests by a cage-forming lumazine synthase. J Biol Chem 292(25):10321–10327. https://doi.org/10.1074/jbc.C117.790311
Bachmann MF, Jennings GT (2010) Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol 10(11):787–796. https://doi.org/10.1038/nri2868
Beck T, Tetter S, Künzle M, Hilvert D (2015) Construction of Matryoshka-type structures from supercharged protein nanocages. Angew Chem Int Ed 54(3):937–940. https://doi.org/10.1002/anie.201408677
Berzofsky JA, Ahlers JD, Belyakov IM (2001) Strategies for designing and optimizing new generation vaccines. Nat Rev Immunol 1(3):209–219. https://doi.org/10.1038/35105075
Bode SA, Minten IJ, Nolte RJM, Cornelissen JJLM (2011) Reactions inside nanoscale protein cages. Nanoscale 3(6):2376–2389. https://doi.org/10.1039/C0NR01013H
Brasch M, de la Escosura A, Ma Y, Uetrecht C, Heck AJR, Torres T, Cornelissen JJLM (2011) Encapsulation of Phthalocyanine supramolecular stacks into virus-like particles. J Am Chem Soc 133(18):6878–6881. https://doi.org/10.1021/ja110752u
Brigger I, Dubernet C, Couvreur P (2002) Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev 54(5):631–651. https://doi.org/10.1016/S0169-409X(02)00044-3
Brown SD, Fiedler JD, Finn MG (2009) Assembly of hybrid bacteriophage Qβ virus-like particles. Biochemistry 48(47):11155–11157. https://doi.org/10.1021/bi901306p
Brumfield S, Willits D, Tang L, Johnson JE, Douglas T, Young M (2004) Heterologous expression of the modified coat protein of Cowpea chlorotic mottle bromovirus results in the assembly of protein cages with altered architectures and function. J Gen Virol 85(4):1049–1053. https://doi.org/10.1099/vir.0.19688-0
Brune KD, Leneghan DB, Brian IJ, Ishizuka AS, Bachmann MF, Draper SJ, Biswas S, Howarth M (2016) Plug-and-display: decoration of virus-like particles via isopeptide bonds for modular immunization. Sci Rep 6:19234. https://doi.org/10.1038/srep19234
Cao C, Wang X, Cai Y, Sun L, Tian L, Wu H, He X, Lei H, Liu W, Chen G, Zhu R, Pan Y (2014) Targeted in vivo imaging of microscopic tumors with Ferritin-based nanoprobes across biological barriers. Adv Mater 26(16):2566–2571. https://doi.org/10.1002/adma.201304544
Caravan P (2006) Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem Soc Rev 35(6):512–523. https://doi.org/10.1039/B510982P
Chackerian B (2007) Virus-like particles: flexible platforms for vaccine development. Expert Review of Vaccines 6(3):381–390. https://doi.org/10.1586/14760584.6.3.381
Chen Y, Xiong X, Liu X, Li J, Wen Y, Chen Y, Dai Q, Cao Z, Yu W (2006) Immunoreactivity of HCV/HBV epitopes displayed in an epitope-presenting system. Mol Immunol 43(5):436–442. https://doi.org/10.1016/j.molimm.2005.03.002
Chen W, Cao Y, Liu M, Zhao Q, Huang J, Zhang H, Deng Z, Dai J, Williams DF, Zhang Z (2012) Rotavirus capsid surface protein VP4-coated Fe3O4 nanoparticles as a theranostic platform for cellular imaging and drug delivery. Biomaterials 33(31):7895–7902. https://doi.org/10.1016/j.biomaterials.2012.07.016
Choi B, Moon H, Hong SJ, Shin C, Do Y, Ryu S, Kang S (2016) Effective delivery of antigen–encapsulin nanoparticle fusions to dendritic cells leads to antigen-specific cytotoxic T cell activation and tumor rejection. ACS Nano 10(8):7339–7350. https://doi.org/10.1021/acsnano.5b08084
Datta A, Hooker JM, Botta M, Francis MB, Aime S, Raymond KN (2008) High relaxivity gadolinium hydroxypyridonate-viral capsid conjugates: nanosized MRI contrast agents. J Am Chem Soc 130(8):2546–2552. https://doi.org/10.1021/Ja0765363
Douglas T, Young M (2006) Viruses: making friends with Old Foes. Science 312(5775):873. https://doi.org/10.1126/science.1123223
Enomoto T, Kawano M, Fukuda H, Sawada W, Inoue T, Haw KC, Kita Y, Sakamoto S, Yamaguchi Y, Imai T, Hatakeyama M, Saito S, Sandhu A, Matsui M, Aoki I, Handa H (2013) Viral protein-coating of magnetic nanoparticles using simian virus 40 VP1. J Biotechnol 167(1):8–15. https://doi.org/10.1016/j.jbiotec.2013.06.005
Fan K, Cao C, Pan Y, Lu D, Yang D, Feng J, Song L, Liang M, Yan X (2012) Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat Nano 7(7):459–464. https://doi.org/10.1038/nnano.2012.90
Ferreira MF, Mousavi B, Ferreira PM, Martins CIO, Helm L, Martins JA, Geraldes CFGC (2012) Gold nanoparticles functionalised with stable, fast water exchanging Gd3+ chelates as high relaxivity contrast agents for MRI. Dalton Trans 41(18):5472–5475. https://doi.org/10.1039/c2dt30388d
Flenniken ML, Uchida M, Liepold LO, Kang S, Young MJ, Douglas T (2009) A library of protein cage architectures as nanomaterials. Curr Top Microbiol Immunol 327:71–93
Frey R, Hayashi T, Hilvert D (2016) Enzyme-mediated polymerization inside engineered protein cages. Chem Commun 52(68):10423–10426. https://doi.org/10.1039/C6CC05301G
Galaway FA, Stockley PG (2013) MS2 viruslike particles: a robust, semisynthetic targeted drug delivery platform. Mol Pharm 10(1):59–68. https://doi.org/10.1021/mp3003368
Garimella PD, Datta A, Romanini DW, Raymond KN, Francis MB (2011) Multivalent, high-relaxivity MRI contrast agents using rigid Cysteine-reactive Gadolinium complexes. J Am Chem Soc 133(37):14704–14709. https://doi.org/10.1021/ja204516p
Giessen TW (2016) Encapsulins: microbial nanocompartments with applications in biomedicine, nanobiotechnology and materials science. Curr Opin Chem Biol 34:1–10. https://doi.org/10.1016/j.cbpa.2016.05.013
Grgacic EVL, Anderson DA (2006) Virus-like particles: passport to immune recognition. Methods 40(1):60–65. https://doi.org/10.1016/j.ymeth.2006.07.018
Han J-A, Kang YJ, Shin C, Ra J-S, Shin H-H, Hong SY, Do Y, Kang S (2014) Ferritin protein cage nanoparticles as versatile antigen delivery nanoplatforms for dendritic cell (DC)-based vaccine development. Nanomedicine 10(3):561–569. https://doi.org/10.1016/j.nano.2013.11.003
Hooker JM, Datta A, Botta M, Raymond KN, Francis MB (2007) Magnetic resonance contrast agents from viral capsid shells: a comparison of exterior and Interior Cargo strategies. Nano Lett 7(8):2207–2210. https://doi.org/10.1021/nl070512c
Huang X, Stein BD, Cheng H, Malyutin A, Tsvetkova IB, Baxter DV, Remmes NB, Verchot J, Kao C, Bronstein LM, Dragnea B (2011) Magnetic virus-like nanoparticles in N. benthamiana Plants: a new paradigm for environmental and agronomic biotechnological research. ACS Nano 5(5):4037–4045. https://doi.org/10.1021/nn200629g
Janitzek CM, Matondo S, Thrane S, Nielsen MA, Kavishe R, Mwakalinga SB, Theander TG, Salanti A, Sander AF (2016) Bacterial superglue generates a full-length circumsporozoite protein virus-like particle vaccine capable of inducing high and durable antibody responses. Malar J 15:545. https://doi.org/10.1186/s12936-016-1574-1
Jardine J, Julien J-P, Menis S, Ota T, Kalyuzhniy O, McGuire A, Sok D, Huang P-S, MacPherson S, Jones M, Nieusma T, Mathison J, Baker D, Ward AB, Burton DR, Stamatatos L, Nemazee D, Wilson IA, Schief WR (2013) Rational HIV immunogen design to target specific germline B cell receptors. Science (New York, NY) 340(6133):711–716. https://doi.org/10.1126/science.1234150
Jennings GT, Bachmann MF (2009) Immunodrugs: therapeutic VLP-based vaccines for chronic diseases. Annu Rev Pharmacol Toxicol 49(1):303–326. https://doi.org/10.1146/annurev-pharmtox-061008-103129
Jordan PC, Patterson DP, Saboda KN, Edwards EJ, Miettinen HM, Basu G, Thielges MC, Douglas T (2016) Self-assembling biomolecular catalysts for hydrogen production. Nat Chem 8(2):179–185. https://doi.org/10.1038/nchem.2416
Kanekiyo M, Wei C-J, Yassine HM, McTamney PM, Boyington JC, Whittle JRR, Rao SS, Kong W-P, Wang L, Nabel GJ (2013) Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 499(7456):102–106. https://doi.org/10.1038/nature12202
Kanekiyo M, Bu W, Joyce MG, Meng G, Whittle JRR, Baxa U, Yamamoto T, Narpala S, Todd J-P, Rao SS, McDermott AB, Koup RA, Rossmann MG, Mascola JR, Graham BS, Cohen JI, Nabel GJ (2015) Rational design of an Epstein-Barr Virus vaccine targeting the receptor-binding site. Cell 162(5):1090–1100. https://doi.org/10.1016/j.cell.2015.07.043
Kang S, Douglas T (2010) Some enzymes just need a space of their own. Science 327(5961):42–43. https://doi.org/10.1126/science.1184318
Kang S, Lander GC, Johnson JE, Prevelige PE (2008a) Development of bacteriophage P22 as a platform for molecular display: genetic and chemical modifications of the procapsid exterior surface. Chembiochem 9(4):514–518. https://doi.org/10.1002/cbic.200700555
Kang S, Oltrogge LM, Broomell CC, Liepold LO, Prevelige PE, Young M, Douglas T (2008b) Controlled assembly of bifunctional chimeric protein cages and composition analysis using noncovalent Mass spectrometry. J Am Chem Soc 130(49):16527–16529. https://doi.org/10.1021/ja807655t
Kang S, Suci PA, Broomell CC, Iwahori K, Kobayashi M, Yamashita I, Young M, Douglas T (2009) janus-like protein cages. Spatially controlled dual-functional surface modifications of protein cages. Nano Lett 9(6):2360–2366. https://doi.org/10.1021/nl9009028
Kang HJ, Kang YJ, Lee Y-M, Shin H-H, Chung SJ, Kang S (2012) Developing an antibody-binding protein cage as a molecular recognition drug modular nanoplatform. Biomaterials 33:5423–5430. https://doi.org/10.1016/j.biomaterials.2012.03.055
Kang YJ, Yang HJ, Jeon S, Kang Y-S, Do Y, Hong SY, Kang S (2014) Polyvalent display of monosaccharides on Ferritin protein cage nanoparticles for the recognition and binding of cell-surface lectins. Macromol Biosci 14(5):619–625. https://doi.org/10.1002/mabi.201300528
Kim H, Kang YJ, Min J, Choi H, Kang S (2016) Development of an antibody-binding modular nanoplatform for antibody-guided targeted cell imaging and delivery. RSC Adv 6(23):19208–19213. https://doi.org/10.1039/C6RA00233A
Kitagawa T, Kosuge H, Uchida M, Iida Y, Dalman RL, Douglas T, McConnell MV (2017) RGD targeting of human ferritin iron oxide nanoparticles enhances in vivo MRI of vascular inflammation and angiogenesis in experimental carotid disease and abdominal aortic aneurysm. J Magn Reson Imaging 45(4):1144–1153. https://doi.org/10.1002/jmri.25459
Kushnir N, Streatfield SJ, Yusibov V (2012) Virus-like particles as a highly efficient vaccine platform: diversity of targets and production systems and advances in clinical development. Vaccine 31(1):58–83. https://doi.org/10.1016/j.vaccine.2012.10.083
Kwon C, Kang YJ, Jeon S, Jung S, Hong SY, Kang S (2012) Development of protein-cage-based delivery Nanoplatforms by Polyvalently displaying β-Cyclodextrins on the surface of Ferritins through Copper(I)-catalyzed Azide/Alkyne cycloaddition. Macromol Biosci 12(11):1452–1458. https://doi.org/10.1002/mabi.201200178
Lauffer RB (1987) Paramagnetic metal complexes as water proton relaxation agents for NMR imaging: theory and design. Chem Rev 87(5):901–927. https://doi.org/10.1021/cr00081a003
Lee EJ, Lee NK, Kim I-S (2016) Bioengineered protein-based nanocage for drug delivery. Adv Drug Deliv Rev 106:157–171. https://doi.org/10.1016/j.addr.2016.03.002
Leneghan DB, Miura K, Taylor IJ, Li Y, Jin J, Brune KD, Bachmann MF, Howarth M, Long CA, Biswas S (2017) Nanoassembly routes stimulate conflicting antibody quantity and quality for transmission-blocking malaria vaccines. Sci Rep 7:3811. https://doi.org/10.1038/s41598-017-03798-3
Leong HS, Steinmetz NF, Ablack A, Destito G, Zijlstra A, Stuhlmann H, Manchester M, Lewis JD (2010) Intravital imaging of embryonic and tumor neovasculature using viral nanoparticles. Nat Protoc 5(8):1406–1417. https://doi.org/10.1038/nprot.2010.103
Lewis JD, Destito G, Zijlstra A, Gonzalez MJ, Quigley JP, Manchester M, Stuhlmann H (2006) Viral nanoparticles as tools for intravital vascular imaging. Nat Med 12(3):354–360. https://doi.org/10.1038/nm1368
Liepold L, Anderson S, Willits D, Oltrogge L, Frank JA, Douglas T, Young M (2007) Viral capsids as MRI contrast agents. Magn Reson Med 58(5):871–879. https://doi.org/10.1002/mrm.21307
Liepold LO, Abedin MJ, Buckhouse ED, Frank JA, Young MJ, Douglas T (2009) supramolecular protein cage composite MR contrast agents with extremely efficient relaxivity properties. Nano Lett 9(12):4520–4526. https://doi.org/10.1021/Nl902884p
Ma Y, Nolte RJM, Cornelissen JJLM (2012) Virus-based nanocarriers for drug delivery. Adv Drug Deliv Rev 64(9):811–825. https://doi.org/10.1016/j.addr.2012.01.005
Maham A, Tang Z, Wu H, Wang J, Lin Y (2009) Protein-based nanomedicine platforms for drug delivery. Small 5(15):1706–1721. https://doi.org/10.1002/smll.200801602
Manayani DJ, Thomas D, Dryden KA, Reddy V, Siladi ME, Marlett JM, Rainey GJA, Pique ME, Scobie HM, Yeager M, Young JAT, Manchester M, Schneemann A (2007) A viral nanoparticle with dual function as an Anthrax Antitoxin and vaccine. PLoS Pathog 3(10):e142. https://doi.org/10.1371/journal.ppat.0030142
Maurer P, Jennings GT, Willers J, Rohner F, Lindman Y, Roubicek K, Renner WA, Müller P, Bachmann MF (2005) A therapeutic vaccine for nicotine dependence: preclinical efficacy, and phase I safety and immunogenicity. Eur J Immunol 35(7):2031–2040. https://doi.org/10.1002/eji.200526285
Min J, Jung H, Shin H-H, Cho G, Cho H, Kang S (2013) Implementation of P22 viral capsids as intravascular magnetic resonance T1 contrast Conjugates via site-selective attachment of Gd(III)-chelating agents. Biomacromolecules 14(7):2332–2339. https://doi.org/10.1021/bm400461j
Min J, Kim S, Lee J, Kang S (2014a) Lumazine synthase protein cage nanoparticles as modular delivery platforms for targeted drug delivery. RSC Adv 4(89):48596–48600. https://doi.org/10.1039/C4RA10187A
Min J, Moon H, Yang HJ, Shin H-H, Hong SY, Kang S (2014b) Development of P22 viral capsid nanocomposites as anti-cancer drug, Bortezomib (BTZ), delivery nanoplatforms. Macromol Biosci 14(4):557–564. https://doi.org/10.1002/mabi.201300401
Molino NM, Anderson AKL, Nelson EL, Wang S-W (2013) biomimetic protein nanoparticles facilitate enhanced dendritic cell activation and cross-presentation. ACS Nano 7(11):9743–9752. https://doi.org/10.1021/nn403085w
Moon H, Kim WG, Lim S, Kang YJ, Shin H-H, Ko H, Hong SY, Kang S (2013) Fabrication of uniform layer-by-layer assemblies with complementary protein cage nanobuilding blocks via simple His-tag/metal recognition. J Mater Chem B 1(35):4504–4510. https://doi.org/10.1039/C3TB20554A
Moon H, Lee J, Kim H, Heo S, Min J, Kang S (2014a) Genetically engineering encapsulin protein cage nanoparticle as a SCC-7 Cell targeting optical nanoprobe. Biomaterials research 18:21. https://doi.org/10.1186/2055-7124-18-21
Moon H, Lee J, Min J, Kang S (2014b) Developing genetically engineered encapsulin protein cage nanoparticles as a targeted delivery nanoplatform. Biomacromolecules 15:3794–3801. https://doi.org/10.1021/bm501066m
Moon H, Bae Y, Kim H, Kang S (2016) Plug-and-playable fluorescent cell imaging modular toolkits using the bacterial superglue, SpyTag/SpyCatcher. Chem Commun 52(97):14051–14054. https://doi.org/10.1039/C6CC07363H
O’Neil A, Prevelige PE, Basu G, Douglas T (2012) coconfinement of fluorescent proteins: spatially enforced communication of GFP and mCherry encapsulated within the P22 capsid. Biomacromolecules 13(12):3902–3907. https://doi.org/10.1021/bm301347x
Ochoa WF, Chatterji A, Lin T, Johnson JE (2006) Generation and structural analysis of reactive empty particles derived from an icosahedral virus. Chem Biol 13(7):771–778. https://doi.org/10.1016/j.chembiol.2006.05.014
Pan Y, Jia T, Zhang Y, Zhang K, Zhang R, Li J, Wang L (2012a) MS2 VLP-based delivery of microRNA-146a inhibits autoantibody production in lupus-prone mice. Int J Nanomedicine 7:5957–5967. https://doi.org/10.2147/IJN.S37990
Pan Y, Zhang Y, Jia T, Zhang K, Li J, Wang L (2012b) Development of a microRNA delivery system based on bacteriophage MS2 virus-like particles. FEBS J 279(7):1198–1208. https://doi.org/10.1111/j.1742-4658.2012.08512.x
Patterson DP, Prevelige PE, Douglas T (2012) Nanoreactors by programmed enzyme encapsulation inside the capsid of the bacteriophage P22. ACS Nano 6(6):5000–5009. https://doi.org/10.1021/nn300545z
Patterson DP, Rynda-Apple A, Harmsen AL, Harmsen AG, Douglas T (2013) Biomimetic antigenic nanoparticles elicit controlled protective immune response to influenza. ACS Nano 7(4):3036–3044. https://doi.org/10.1021/nn4006544
Patterson DP, Schwarz B, Waters RS, Gedeon T, Douglas T (2014) Encapsulation of an enzyme cascade within the bacteriophage P22 virus-like particle. ACS Chem Biol 9(2):359–365. https://doi.org/10.1021/cb4006529
Peabody DS (2003) A viral platform for chemical modification and multivalent display. Journal of Nanobiotechnology 1(1):5. https://doi.org/10.1186/1477-3155-1-5
Peabody DS, Manifold-Wheeler B, Medford A, Jordan SK, Caldeira JC, Chackerian B (2008) Immunogenic display of diverse peptides on virus-like particles of RNA phage MS2. J Mol Biol 380(1):252–263. https://doi.org/10.1016/j.jmb.2008.04.049
Plummer EM, Manchester M (2011) Viral nanoparticles and virus-like particles: platforms for contemporary vaccine design. Wiley Interdiscip Rev Nanomed Nanobiotechnol 3(2):174–196. https://doi.org/10.1002/wnan.119
Pokorski JK, Breitenkamp K, Finn MG (2011) Functional virus-based polymer-protein nanoparticles by atom transfer radical polymerization. J Am Chem Soc 133(24):9242–9245. https://doi.org/10.1021/ja203286n
Prasuhn JDE, Yeh RM, Obenaus A, Manchester M, Finn MG (2007) Viral MRI contrast agents: coordination of Gd by native virions and attachment of Gd complexes by azide-alkyne cycloaddition. Chem Commun 12:1269–1271. https://doi.org/10.1039/B615084E
Prevelige PE, Thomas D, King J (1988) Scaffolding protein regulates the polymerization of P22 coat subunits into icosahedral shells in vitro. J Mol Biol 202(4):743–757. https://doi.org/10.1016/0022-2836(88)90555-4
Qazi S, Liepold LO, Abedin MJ, Johnson B, Prevelige P, Frank JA, Douglas T (2013) P22 viral capsids as nanocomposite high-relaxivity MRI contrast agents. Mol Pharm 10(1):11–17. https://doi.org/10.1021/mp300208g
Qazi S, Miettinen HM, Wilkinson RA, McCoy K, Douglas T, Wiedenheft B (2016) Programmed self-assembly of an active P22-Cas9 nanocarrier system. Mol Pharm 13(3):1191–1196. https://doi.org/10.1021/acs.molpharmaceut.5b00822
Ra J-S, Shin H-H, Kang S, Do Y (2014) Lumazine synthase protein cage nanoparticles as antigen delivery nanoplatforms for dendritic cell-based vaccine development. Clin Exp Vaccine Res 3(2):227–234. https://doi.org/10.7774/cevr.2014.3.2.227
Rhee J-K, Baksh M, Nycholat C, Paulson JC, Kitagishi H, Finn MG (2012) Glycan-targeted virus-like nanoparticles for photodynamic therapy. Biomacromolecules 13(8):2333–2338. https://doi.org/10.1021/bm300578p
Richert LE, Servid AE, Harmsen AL, Rynda-Apple A, Han S, Wiley JA, Douglas T, Harmsen AG (2012) A virus-like particle vaccine platform elicits heightened and hastened local lung mucosal antibody production after a single dose. Vaccine 30(24):3653–3665. https://doi.org/10.1016/j.vaccine.2012.03.035
Rösler A, Vandermeulen GWM, Klok H-A (2001) Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv Drug Deliv Rev 53(1):95–108. https://doi.org/10.1016/S0169-409X(01)00222-8
Schwarz B, Douglas T (2015) Development of virus-like particles for diagnostic and prophylactic biomedical applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol 7(5):722–735. https://doi.org/10.1002/wnan.1336
Scodeller EA, Tisminetzky SG, Porro F, Schiappacassi M, De Rossi A, Chiecco-Bianchi L, Baralle FE (1995) A new epitope presenting system displays a HIV-1 V3 loop sequence and induces neutralizing antibodies. Vaccine 13(13):1233–1239. https://doi.org/10.1016/0264-410X(95)00058-9
Seebeck FP, Woycechowsky KJ, Zhuang W, Rabe JP, Hilvert D (2006) A simple tagging system for protein encapsulation. J Am Chem Soc 128(14):4516–4517. https://doi.org/10.1021/ja058363s
Sharma J, Uchida M, Miettinen HM, Douglas T (2017) Modular interior loading and exterior decoration of a virus-like particle. Nano 9(29):10420–10430. https://doi.org/10.1039/C7NR03018E
Shukla S, Steinmetz NF (2015) Virus-based nanomaterials as PET and MR contrast agents: from technology development to translational medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol 7(5):708–721. https://doi.org/10.1002/wnan.1335
Song Y, Kang YJ, Jung H, Kim H, Kang S, Cho H (2015) Lumazine synthase protein nanoparticle-Gd(III)-DOTA conjugate as a T1 contrast agent for high-field MRI. Sci Rep 5:15656. https://doi.org/10.1038/srep15656
Steinmetz NF, Hong V, Spoerke ED, Lu P, Breitenkamp K, Finn MG, Manchester M (2009) Buckyballs meet viral nanoparticles: candidates for biomedicine. J Am Chem Soc 131(47):17093–17095. https://doi.org/10.1021/ja902293w
Stephanopoulos N, Tong GJ, Hsiao SC, Francis MB (2010) Dual-surface modified virus capsids for targeted delivery of photodynamic agents to cancer cells. ACS Nano 4(10):6014–6020. https://doi.org/10.1021/nn1014769
Suci P, Kang S, Gmur R, Douglas T, Young M (2010) Targeted delivery of a photosensitizer to aggregatibacter actinomycetemcomitans biofilm. Antimicrob Agents Chemother 54(6):2489–2496. https://doi.org/10.1128/aac.00059-10
Sutter M, Boehringer D, Gutmann S, Gunther S, Prangishvili D, Loessner MJ, Stetter KO, Weber-Ban E, Ban N (2008) Structural basis of enzyme encapsulation into a bacterial nanocompartment. Nat Struct Mol Biol 15(9):939–947. https://doi.org/10.1038/nsmb.1473
Terashima M, Uchida M, Kosuge H, Tsao PS, Young MJ, Conolly SM, Douglas T, McConnell MV (2011) Human Ferritin cages for imaging vascular macrophages. Biomaterials 32(5):1430–1437. https://doi.org/10.1016/j.biomaterials.2010.09.029
Theil EC, Behera RK, Tosha T (2013) Ferritins for Chemistry and for life. Coord Chem Rev 257(2):579–586. https://doi.org/10.1016/j.ccr.2012.05.013
Thrane S, Janitzek CM, Matondo S, Resende M, Gustavsson T, de Jongh WA, Clemmensen S, Roeffen W, van de Vegte-Bolmer M, van Gemert GJ, Sauerwein R, Schiller JT, Nielsen MA, Theander TG, Salanti A, Sander AF (2016) Bacterial superglue enables easy development of efficient virus-like particle based vaccines. J Nanobiotechnol 14:30. https://doi.org/10.1186/s12951-016-0181-1
Tissot AC, Maurer P, Nussberger J, Sabat R, Pfister T, Ignatenko S, Volk H-D, Stocker H, Müller P, Jennings GT, Wagner F, Bachmann MF (2008) Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: a double-blind, randomised, placebo-controlled phase IIa study. Lancet 371(9615):821–827. https://doi.org/10.1016/S0140-6736(08)60381-5
Tissot AC, Renhofa R, Schmitz N, Cielens I, Meijerink E, Ose V, Jennings GT, Saudan P, Pumpens P, Bachmann MF (2010) Versatile virus-like particle carrier for epitope based vaccines. PLoS One 5(3):e9809. https://doi.org/10.1371/journal.pone.0009809
Uchida M, Flenniken ML, Allen M, Willits DA, Crowley BE, Brumfield S, Willis AF, Jackiw L, Jutila M, Young MJ, Douglas T (2006) Targeting of cancer cells with ferrimagnetic ferritin cage nanoparticles. J Am Chem Soc 128(51):16626–16633. https://doi.org/10.1021/ja0655690
Uchida M, Terashima M, Cunningham CH, Suzuki Y, Willits DA, Willis AF, Yang PC, Tsao PS, McConnell MV, Young MJ, Douglas T (2008) A human ferritin iron oxide nano-composite magnetic resonance contrast agent. Magn Reson Med 60(5):1073–1081. https://doi.org/10.1002/mrm.21761
Uchida M, Kang S, Reichhardt C, Harlen K, Douglas T (2010) The ferritin superfamily: supramolecular templates for materials synthesis. Biochim Biophys Acta Gen Subj 1800:834–845. https://doi.org/10.1016/j.bbagen.2009.12.005
Wang AZ, Langer R, Farokhzad OC (2012) Nanoparticle delivery of cancer drugs. Annu Rev Med 63(1):185–198. https://doi.org/10.1146/annurev-med-040210-162544
Wörsdörfer B, Woycechowsky KJ, Hilvert D (2011) Directed evolution of a protein container. Science 331(6017):589–592. https://doi.org/10.1126/science.1199081
Wörsdörfer B, Pianowski Z, Hilvert D (2012) Efficient in vitro encapsulation of protein cargo by an engineered protein container. J Am Chem Soc 134(2):909–911. https://doi.org/10.1021/ja211011k
Wu M, Sherwin T, Brown WL, Stockley PG (2005) Delivery of antisense oligonucleotides to leukemia cells by RNA bacteriophage capsids. Nanomedicine 1(1):67–76. https://doi.org/10.1016/j.nano.2004.11.011
Zakeri B, Fierer JO, Celik E, Chittock EC, Schwarz-Linek U, Moy VT, Howarth M (2012) Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc Natl Acad Sci U S A 109(12):E690–E697. https://doi.org/10.1073/pnas.1115485109
Zhang X, Meining W, Fischer M, Bacher A, Ladenstein R (2001) X-ray structure analysis and crystallographic refinement of lumazine synthase from the hyperthermophile Aquifex aeolicus at 1.6 Å resolution: determinants of thermostability revealed from structural comparisons. J Mol Biol 306(5):1099–1114. https://doi.org/10.1006/jmbi.2000.4435
Zhang X, Meining W, Cushman M, Haase I, Fischer M, Bacher A, Ladenstein R (2003) A structure-based model of the reaction catalyzed by Lumazine synthase from Aquifex aeolicus. J Mol Biol 328(1):167–182. https://doi.org/10.1016/S0022-2836(03)00186-4
Zhen Z, Tang W, Chen H, Lin X, Todd T, Wang G, Cowger T, Chen X, Xie J (2013) RGD-modified Apoferritin nanoparticles for efficient drug delivery to tumors. ACS Nano 7(6):4830–4837. https://doi.org/10.1021/nn305791q
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Choi, B., Kim, H., Choi, H., Kang, S. (2018). Protein Cage Nanoparticles as Delivery Nanoplatforms. In: Noh, I. (eds) Biomimetic Medical Materials. Advances in Experimental Medicine and Biology, vol 1064. Springer, Singapore. https://doi.org/10.1007/978-981-13-0445-3_2
Download citation
DOI: https://doi.org/10.1007/978-981-13-0445-3_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-0444-6
Online ISBN: 978-981-13-0445-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)