Abstract
Rheology as the science of flow properties of materials is one of the most vital concepts that have greatly attracted the attention of biomedical engineers and bioengineers over the past few decades. It has been less than a century since the science of rheology was formally introduced to the scientific society. Over the past few decades rheological properties of living things including cells and tissues have been investigated and studied thoroughly. As a result various measurement techniques have been developed and used ever since such as Atomic Force Microscopy (AFM), optical measurement techniques, etc. Recent advancements of technology regarding submicron scale measurement enabled scientists to evaluate cellular behavior towards mechanical stimuli with ultra-precision. Data obtained from these studies have revealed vital information regarding effects of mechanical properties of cells on cellular functions such as adhesion, proliferation and migration, and differentiation. Additionally rheological properties of most tissues in human body were measured and some results have been configured into engineered scaffolds along with being used as diagnostic tools. Despite the fact that there are hundreds of studies regarding rheological properties of cells and tissues, there are still so many unsolved problems and unanswered questions concerning fabrication of ideal devices and implants that need to be solved and answered. Hence the hot topic of principles and influence of rheology on living materials has become an appealing and amusing research subject resulting in attainment of fascinating results, which so far have proven to be greatly helpful in unlocking wide range of unknowns of life itself. Of the uncountable number of researches conducted to this day regarding rheological properties of biomaterials a small portion of is discussed in this chapter as an introduction to the concept of rheology in living things and its significance.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 What Is Rheology?
The term rheology is derived from the Greek words rheo, “to flow,” and logos, “science.” Rheology, therefore, is “the scientific study of the deformation and flow properties of matter” [ 79].
The discipline of rheology was first introduced formally on April 29, 1929 by Professor E. C. Bingham of Lafayette College and G. W. Scott Blair [82]. The importance of this concept was so rapidly understood that in the same year at the Third Plasticity Symposium in 1929 a permanent organization with the aim of study and development of the new discipline of rheology under the name of “The Society of Rheology” was founded.
To this day, the role of rheology in materials both of natural origin and synthetic along with the extent of its efficacy has being investigated and during past century with the advancement of measurement techniques, studying rheology in small scale has become possible. Hence the hot topic of principles and influence of rheology on living materials has become an appealing and amusing research subject resulting in attainment of fascinating results which so far have proven to be greatly helpful in unlocking wide range of mysteries of life itself.
2 Role of Rheology in Life
Biological materials including cells and tissue, are complex structures made of diverse unites scaling from nano-scale to micron. Cells, as building blocks of living things, have various types of structural units and subunits namely nucleus, mitochondria and bilayer cell membrane each with their specific physicochemical and mechanical properties. Combination and accumulation of numerous of these sub-unites inside cytosol, the gel-like aqueous component of cytoplasm which constitutes about 70% of the total volume of a normal cell, gives rise to a nonhomogeneous entity [22]. Despite the fact that cells are made of solid and fluidic materials, the nonhomogeneous nature of this complex leads to unique characteristics unlike solid and liquid such as flow properties.
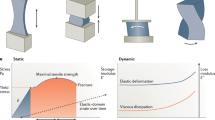
In material science mechanical properties of materials are usually classified in three main categories of elastic, plastic and viscous. Elasticity is a constant value calculated by scaling between stress and strain of a solid material also called the Young’s modulus which determines the ability of solid materials to sustain their original shape when mechanical stress is applied. Plasticity on the other hand, refers to very large deformation of a material as it is subjected to stress levels beyond its certain yield limits [79]. In contrast to solids, if stress is applied to fluids, elastic energy cannot be stored and the flow pattern of the fluid changes. The concept of changes of the rate of flow under applied load is known as viscosity [51]. Deformation of a material can be measured while a force is applied to it. Figure 1 is an illustration of various ways a force that can be applied to a material.
Cells, being constructed of mostly fluidic and partially solid materials show neither elastic nor viscous characteristics. Hence cells can be categorized as viscoelastic [79]. Unlike soft materials, biological materials do not just react to a certain mechanical stimuli; they behave via generating an active response by the means of mechanotransduction.
Mechanotransduction refers to cell’s ability of generating biochemical signals in response to external mechanical cues with the aim of transporting information from nucleus to other organelles inside the cell and vice versa in order to trigger series of particular responses. These responses usually results in functional behaviors like adhesion, migration, proliferation and differentiation as well as apoptosis or necrosis [17, 34, 78].
Activation of mechanosensitive signaling pathways is one of the most basic and vital physiological mechanisms through which cells are able to react to environmental conditions and physical cues. These reactions occur by reorganization and force generation of cytoskeletal structure. It has also been found that any disruption in cytoskeletal architecture caused by genetic mutations or pathogens can lead to changes in cell mechanical properties such as elasticity, adhesiveness, and viscosity [34, 78]. Hence studying the mechanical behavior of cells and therefore tissues has attracted considerable interest over the past century.
Since majority of cell volume contains fluidic substance, among mechanical properties, study of flow properties of cells has attracted significant attention resulting in the massive amount of research regarding the concept of cellular rheology [34, 49].
In material science, during the past century, data obtained from rheological measurements of nonliving complex materials has provided vast range of information regarding conformational changes or rearrangements of material’s constituents in micro and nano-scale when they undergo mechanical force [49]. These findings shed light on the potential effects and importance of rheological properties of cells on their function. Thus, over the past decade there have been many studies concerning the role of rheological properties on cellular functions namely cell crawling, wound healing, protein regulation, cell malignancy and how they may cause deficiency in cell’s life [73].
As a result, today there exist a myriad of experimental techniques and devices for measuring flow properties of biological materials such as Atomic Force Microscopy (AFM) that can provide massive amount of information (in the form of interdisciplinary concepts of combined molecular biology and advanced cell mechanics) which ease our understanding of cellular mechanisms and effects of mechanical forces on their physiology, metabolism, development, function and disease [17].
3 Techniques and Methods of Measurement
When it comes to measuring quantitative values of a property, the scale of measurement plays the most important role of all. In case of cells, almost all sorts of measurements need to be done at micron scale [34]. Thus devices and instruments of choice must be in submicron scale and precision. Since over the past decades, techniques of micron and nano-scale measurements have been developed extensively and thoroughly, it has become quite easy and simple to measure flow properties of cells of different origin in diverse conditions, namely Atomic force Microscopy (AFM), Optical Tweezers, Magnetic Tweezers or Magnetic Twisting Cytometry, Particle Tracking Microrheology, Microplate Rheometry or Whole Cell Stretching, Micropipette Aspiration, Traction Force Microrheology and Optical Cell Stretcher [34, 51, 68]. Each of these methods is briefly mentioned in Table 1 ‚ and Fig. 2 represents a schematic of each technique.
Schematic representation of microrheology measurement techniques: a AFM, b optical tweezers, c magnetic tweezers, d particle tracking microrheology, e micropipette rheometry, f microplate aspiration, g traction force microrheology (embedded microbeads), h traction force microrheology (micropillars), i optical cell stretcher
Unfortunately all of the measurements techniques developed so far show some disadvantages. For instance AMF provides high resolution cell images, quantitative information about the cell surface properties, the Young modulus, the viscosity or the relaxation times but the measurements are too slow. In optical tweezers, local heating and prototoxicity occurs during measurements but it is a highly sensitive technique. Magnetic tweezers can only probe microenvironment inside the cell whereas Traction force microscopy can only measure relation between cell and ECM. Micropipette Aspiration technique as a quantitative method, relies heavily on theoretical models, thus results may not be much reliable. Overall it can be said that in order to characterize rheological properties of cells, a combination of these methods must be used so as to obtain realistic and reliable results [3, 5, 41, 43, 56, 60, 75].
4 Rheological Properties of Cells: Principles, Discoveries and Applications
4.1 Principles of Cell Mobility: Mechanotransduction
In human body there is a wide and diverse range of mechanical stimuli in form of mechanical stress being applied to almost all cells with both external and internal origins [1]. In case of occurrence of a mechanical stimulus, the cells are equipped with sensing mechanisms which are called mechanoreceptors. These receptors help cells feel their surrounding environment in terms of mechanical stress. Since the main goal of dynamic nature of cells is to promote homeostasis, including maintenance of internal structural integrity of cell while interacting with its ECM, which is vital for cell’s survival, the fundamental importance of motion regulators, mechanoreceptors and mechanotransduction process are well recognized and appreciated [35].
Hence mechanotransduction was defined as sequential processes of understanding how mechanical forces are sensed by cells, how sensed forces are transduced into biochemical signals and finally how they affect cellular metabolism and function [50]. Mechanotransduction involves two main procedures: mechanosensing and mechanoregulation. In the former, mechanical stresses applied to the cell are detected and identified via mechanoreceptors and in the latter cells regulate series of functions so as to respond to the sensed force in the most appropriate way [35].
Over the years many studies have been conducted on revealing unknown facts of mechanosensing and so far several molecules and structures have been identified as to be involved in this process namely ion channels, cell adhesion receptors, cytoskeletal and extracellular matrix molecules [30]. Some of the cytoskeletal molecules that are immensely involved in cellular motion are actin and myosin. Actin is a protein that can shape microfilaments and can be found in almost all eukaryotic cells. It is found in two forms of actin stress fibers and actin microfilaments. Actin structures can also be found in the form of thin filaments in contractile apparatus of muscle tissue in Z disc [19].
Just like actin, myosin is one of the major components of cell cytoskeleton as well and it is a motor protein that can be found in most eukaryotic cells specially muscle cells [78]. Myosin proteins are categorized in two groups: myosin I and myosin II. Myosin I is in close coordination with actin proteins and since it has contractile properties by itself, plays a vital role in contractility of muscle cells. On the other hand myosin II, having a massive amino acidic side, is a molecule which is divided in two main parts: head domain which merges with actin and initiates force and tail domain that acts as a coordination tool between other myosin subunits and communication facilitator with cargo muscle [54, 78].
Actin and myosin together coordinate in a systematic manner to control cellular mobility and deformation along with many other functions that require structural deformations of cell. This systematic regulation is called actomyosin machinery [30, 35, 54].
As mentioned previously, cell movement mainly starts with cell adhesion which in turn starts with formation of binding sites between cell membrane and underlying substrate called focal adhesion sites. Integrins are one of the most important adhesive membrane receptors that bind the cell to the surrounding environment mechanically. Integrins are structures inside the cells that are in direct contact with focal adhesion sites [1, 25].
Many mechanical stresses that cells sense, are inflicted by ECM. There are also many forces that are generated within the contractile cytoskeleton of cell itself [50]. Convergence of both of these forces occurs on membrane integrin receptors that assemble together within the focal adhesion sites. Focal adhesions are the linking point of cytoskeleton to ECM [50]. The forces that are sensed by the cell enforces to generate a stress-induces strengthening reaction as the result of adaptation to cell generated applied forces to integrin or rigidity changes of the ECM. Therefore the mechanical stiffness of the cell increases as the overall applied force rises. The ability of cells to change their stiffness is an indication of a level of tolerance towards membrane tearing [83].
Microbead microrheology and optical tweezers method have been used in order to understand the mechanism by which cells respond to mechanical cues and it was revealed that cells need to create focal adhesion at the site of applied force as a vital step on stiffening the cell. In short, stiffening (in response to external stimulus) starts by changes in focal adhesion size, composition, and position which simultaneously occur within the first few seconds to minutes after external stresses are sensed by mechanoreceptors [65].
When the cell is subjected to internal mechanical stress which is applied to integrins, focal adhesions are again assembled and the cell stiffness changes due to activation of actin and myosin. In this process myosin light chain is phosphorylated causing increase in cell contractility along with polymerization of actin. These procedures occur in a very complex and precise manner in the matter of seconds to minutes based on the intensity of applied stress [65, 80].
In addition to activation of actin-myosin network, mechanosensitive calcium ion channels can also be activated due to the stress imposed on integrins resulting in calcium influx into the cell which can cause contractility and therefore changing cell stiffness [53].
In addition to internal mechanical stimuli, there are external mechanical forces which apply sheer stress on the cells. For instance upon some cells‚ these forces are applied continuously such as sheer and stretch forces applied to Endothelial Cells (ECs) in blood vessels via blood flow which are called hemodynamic forces. These forces have the ability to change the physical shape and function of the ECs. These changes occur due to the ability of ECs to sense and adapt to the pressure fluctuations in order to maintain in an optimal stage of homeostasis and therefore function [13]. In general it can be said that mechanosensing is a vital regulatory mechanism of the cells with the main purpose of keeping the cell alive and at highest functional performance.
4.2 Rheological Properties of Eukaryotic Cells
Cells are composed of mostly fluid trapped inside a lipid membrane with various types of channels embedded inside this membrane allowing transportation of all sorts of molecules. The fluid inside the cell, cytoplasm, contains solid-like tiny structures called organelles. Existence of these organelles inside the cell along with constant chemical composition changes of the cytoplasm result in a unique flow characteristic which is referred to as viscoelastic. This viscoelastic fluid trapped in a lipid sack has extraordinary mobility abilities such as proliferation, migration, differentiation, along with contract, stiffen, stretch, fluidize, reinforce, crawl, intravasate, extravasate, invade, engulf, divide, swell, shrink or remodel [6].
Over the past century many studies have been conducted so as to measure flow properties of different eukaryotic cells in various stages of cell’s life. As a result many techniques have been developed and extremely valuable insights have been provided in this regard. It has been found that in eukaryotic cells, for approximately 1% of applied strain, cell-generated contractile stress which result in cell deformation, is described by weak power law worldwide and is strongly related to cell contents in terms of composition and number of organelles [72, 84]. Hence cellular rheological behaviors cannot be measured and defined in time-dependent manners.
So far most rheological studies of cells have focused on red blood cells, lung alveolar epithelium, and brain cells along with human mesenchymal-derived stem cells, primary osteoblasts, chondrocytes, and adipocytes. In addition some studies focused on mechanical properties of cell nucleus [27]. AFM, micropipette aspiration, tweezers and optical measurement methods are the most preferred methods of microrheometry [62].
Starting from stem cells in 2007 Pajerowski et al. conducted a study on physical plasticity of nucleus of primary human embryonic stem cells (ESCs) also known as naive stem cells, proving the fact that structural reorganizations due to fundamental and massive amount of changes in gene expression necessary for differentiation, affect the flexibility of cell nucleus noticeably [62]. These internal structural deformations occur by condensation of chromatin and immobilization of nucleoprotein. The results of this study indicate that after sixfold through terminal differentiation, the nuclei becomes so stiffen that it loses the ability of going back to the original shape. Lamin A/C was identified to be the nucleo-skeletal component behind this phenomenon. By knocking down lamin A/C in human epithelial cells and bearing in mind that this component does not express in stem cells, deformability of epithelial cells were measured at close values to that of adult hematopoietic stem cells [62, 72, 84].
One of the major outcomes of this study was proving the flow, distend, and reorganization of chromatin whilst the lamina stretches, meaning that despite the fact that rheological behavior of nucleus is mainly regulated by nucleoplasm/chromatin interactions, lamina is the modulator of extent of nucleus deformations [58].
Blood as a tissue contains many cell types such as red blood cell (erythrocytes), white blood cells (leucocytes) and platelets. Each of these go through extensive deformations through out their life, erythrocytes change their physical form in order to be able to move in and out of narrow capillaries. This function requires the cells to have the capability to regulate cellular stiffness and elasticity imperative for desired actions. Hence studying mechanical and rheological properties of red blood cells are of great importance [63].
Like other eukaryotic cells, blood cells have a lipid bilayer attached to the two dimensional elastic spectrin networks. This network is the main regulator of morphological and mechanical changes of the cell [59]. Studying mechanical deformations and therefore rheological properties of red blood cells are mostly done in two aspects: deformability and aggregability [63].
Aggregability occurs when erythrocytes attached to one another which can impair blood circulation at the areas where shear rate is low (Matthias Brust 2013) [15, 63]. Investigating rheological properties of red blood cells via micropipette aspiration has revealed that shape deformations of erythrocytes occur due to changes in volume of the cell rather than membrane disfigurement and structural changes of cell membrane. By measuring surface area and cell volume of aspirated cells verses relaxed cells, it was discovered that while the total surface area of the cell remains constant, as the cell is being aspirated, the volume of the cell changes in order to fit in the micropipette. This result proved the fact that, cell membrane of red blood cells has resistance properties against mechanical forces [38]. Erythrocyte stiffness and relaxation time are the fundamental factors that affect blood flow and viscosity to a great extent by controlling deformation and aggregation. These factors have been investigated thoroughly over that past century and plenty of methods have been developed ever since namely Microscopic Photometric Monolayer Technique. In this method erythrocytes’ resistance to deformation (elongation) is measured along with relaxation time when cells are subjected to various physicochemical conditions [3].
Despite the fact that majority of the cells found in blood are erythrocytes and white blood cells, also known as leucocytes, can only negligibly affect blood flow and viscosity in main blood vessels, they contribute significantly to the hemodynamic forces at the tissue level. In fact in microcirculation leucocytes have major effects on flow rate and viscosity since the diameter of capillaries is close to diameter of a single cell or even smaller. There are many types of white blood cells such as Granulocytes which have the ability to alter its morphology when an external mechanical force is sensed such as the resistance for passing applied by the internal wall of narrow capillaries. The capability of leucocytes for shape alterations (mainly dependent on type) is considered to be greater than erythrocytes but the transient time necessary for passing a leucocyte of any kind is much longer than that of erythrocytes. In cases of severe infection, due to increase in the rigidity of leucocytes, this phenomenon can cause blockages in the microcirculation network and lead to serious problems [7].
One type of cell in human body that is always subjected to considerable amount of physical tension is alveolar epithelial lung cells. These cells constantly undergo stretching during inhale and exhale. This high amount of mechanical force changes cell viscoelastic properties. In all eukaryotic cells, stabilized cytoskeletal structure occurs mainly due to overall tensile stresses endured by filamentous structures. Consequently, cell stiffness increases relatively with the level of the tensile stress, which is called the prestress [83].
Alveolar cells like other cells are under pre-stress. In a detailed study regarding viscoelastic properties of these cells under stretch, it was found that cytoskeletal prestress is the main regulator of the frictional and elastic properties of lung alveolar cells. This result was obtained via experiments on human epithelial lung cells under equibiaxial stretch forces. It was observed by Trepat et al. that the applied force in form of stretch, increased cellular viscoelasticity but inhibited by latrunculin A via sequestering G-actin and preventing F-actin assembly [83].
This process once again proves the vital role of actin network in cell stiffness. Another important outcome of this study was the idea which suggested the cell detachment from the substrate and increase in cell stiffness coincide in high stretch forces revealing the highest limit to which alveolar cells can withstand the mechanical strain forces. This discovery shed some light on the disruption of alveolar barrier in injured lung by explaining the unbalanced forces both in cell-cell and cell-ECM interaction caused by cell stiffening induced by stretching forces [76].
Another organ in the body under constant shear stress is brain. Human brain tissue is a quite elastic and plastic tissue consisting of diverse types of cells called neurons. This flexible organ floats in a liquid called cerebrospinal fluid. Cerebrospinal fluid is the recycling system of the central nervous system since it circulates nutritious and chemicals which were filtered from the blood along with removing waste products of cells. Additionally it acts as a shield for brain. Constant circulation of this fluid causes a slight shear force on the brain tissue [18].
Apart from this, some neurological activities also change the physical form of brain tissue at specific regions namely, playing a musical instrument for a long time. Physical deformation of brain tissue starts with shape change of neurons. In human brain there are diverse types of nerve cells categorized in two groups of primary signaling cells or neurons and supporting cells or glial cell. Glial cells were discovered nearly 150 years ago as supporting cells for neurons. The name glial comes from a Greek word meaning glue. In the early days of discovery of glial cells, they were thought to be either supporting cells that glued primary signaling neurons together or act as scaffolds for them [76]. Over the past century it has been discovered that these cells have individual vital functions apart from protecting primary neuron. Glial cells can only be found in central nervous system.
The shape changing characteristics of human brain has made it an appealing research subject in terms of mechanical properties [47]. Most preferred method in studying viscoelastic characteristics of human brain cells are bulk rheology, AFM, Scanning Force Microscopy (SFM), and optical measurement methods. Primary findings of these experiments indicated that all cells of central nervous system behave more like elastic materials rather than viscous materials [47].
Secondly, unlike most eukaryotic cells, both primary signaling neurons and glial cells are very soft biological structures, but when comparing primary neurons with glial cells, neurons are considerably stiffer than glial cells. Thirdly, and most interestingly, different part of nerve cells showed different mechanical properties. This noticeable local diversity of viscoelastic values was assumed to be due to distribution of organelles. These findings revealed that initial roles assigned to glial cells cannot be true and they serve neither as support cells nor as scaffolds for primary neurons [47].
Taking another step forward towards discerning mysteries of mechanical properties of eukaryotic cells, mesenchymal-derived stem cells, primary osteoblasts, chondrocytes, and adipocytes attracted particular attention among mechanobiologists. The aim of investigating viscoelastic properties of these cells simultaneously by Darling et al. [17] was to understand the differences in mechanical behavior between stem cells and primary stem cells of mesenchymal lineage. They chose primary osteoblasts, chondrocytes, and adipocytes cells for this purpose. Their findings demonstrated that viscoelastic characteristics of cells not only affect cell-cell and cell-ECM interactions, but also greatly influences the production of biomarkers on the cells. Biomarkers are biological markers that demonstrate phenotypical changes that cells experienced during cellular transformation, differentiation and changes which were caused by diseases. A group of these biomarkers can provide precise and detailed information regarding phenotypic characteristics of different types of stem cells. Over the past years, focus of mechanobiology has been on the identification of these biomarkers via cells’ mechanical characteristics with the aim of using the results for identification of different stem cells from mesenchymal stem cells. According to results of this study, when comparing adipocytes, osteoblast and chondrocyte with mesenchymal stem cells, the three cell types were all found to be stiffer than mesenchymal stem cells. A comparison among adipocytes, osteoblast and chondrocyte showed that adipocytes are the softest of them all followed by osteoblast and then chondrocyte as the stiffest cell type of this group. Interestingly, adipose-derived stem cells showed similar viscoelastic values to that of mesenchymal stem cells, but the two were mechanically distinct from primary differentiated stem cells [17].
5 Rheological Properties of Tissues
After detailed investigation on rheological properties of cells, it is also necessary to study rheological properties of tissues as well. Since tissue are biological structures made of considerable number of cells bound together by ECM, and almost all types of cells in human body demonstrate viscoelastic behaviors, it would be rational to assume that tissues would show similar mechanical characteristics as well. Hence a noticeable number of scientists in the fields of biology, mechanics and biomedical science have focused on studying the mechanical properties of tissues of human body, in particular their rheological behaviors [79].
Among all tissues in the body some have been more investigated than others including blood and brain tissue (white and grey matters). There are indeed many studied conducted over the years to investigate rheological properties of tissues but here some major examples are given and discussed with the aim of introducing the concepts and demonstrating their importance.
Blood as discussed before consists of three major cell types. Despite the common belief that blood is a viscous fluid, accumulation of these cells each with their unique mechanical characterizations, result in blood’s behavior to be far from viscous material. Instead, it can be considered as a two phase liquid or in more simple term as a solid-liquid suspension.
In case of blood as a tissue, the mechanical behavior of blood in terms of flow mainly depends on plasma viscosity, red blood cell aggregation and mechanical properties of erythrocytes. As it was discussed previously, erythrocytes have reversible deformation capabilities during which cells’ stiffness and rheology changes constantly depending on the amount of shear stress imposed on cells during circulation. It is also important to mention that aggregation of erythrocytes is an outcome of high shear stress especially during microcirculation. When there is high amounts of shear force like during microcirculation, the aggregates dismantle and individual erythrocytes deform in order to enter capillaries. In case of low shear stress, red blood cells attached to one another in the matter of seconds and form aggregates that have unique and size dependent mechanical characteristics. Hence it can be concluded that formation and size of aggregates has an inverse proportional relation to the amount of applied forces [7].
Apart from effects of aggregates on blood flow patterns, there are other factors that alter flow patterns of blood such as temperature, rheological properties of plasma and leucocytes and of course aging. For instance plasma as the suspension fluid for dispersed elements (erythrocytes, leukocytes, etc.), is a liquid with temperature dependent rheology. At 37 °C the viscosity of plasma varies between 1.10 and 1.35 cp and in case of an injury or disease these values are much higher [37].
Brain just like blood is another organ in human body that has been under extensive investigations so as to understand the rheological properties if its tissues. There are numerous studies regarding the biomechanical characteristics of tissues of central nervous system and brain, in particular [70]. The results of these studies revealed many inconsistencies for biomechanical properties of same tissues that are apparently tested under similar conditions. These inconsistencies may result from operator errors, age and gender of the subject tissue, tissue pretreatment protocols, etc. Unfortunately there are no accurate values for rheological properties of brain tissue which makes it difficult for scientists and engineers to apply obtained results in design and fabrication of biological scaffolds and material for diseases of CNS, but so far the database of biomechanical properties of brain and spinal cord of humans includes very enlightening information [12]. For instance three different regions of brain were investigated in term of biomechanical properties, in particular rheology. The selected sections of both white and grey matter were Corpus callosum (CC), Thalamus (Th) and Corona radiata (CR). The experiments were done by subjecting tissue specimens to repeated creep-recovery shear force. According to the results, all three tissues have both reversible and irreversible deformations happening on different sections. The former is due to the elastic nature of specific sections of the tissue and the latter occurs because of the viscous characteristic of those sections. Results also demonstrated that all three tissues have distinct biomechanical properties. Also it was observed that the highest stiffness value belonged to thalamus tissue and lowest stiffness belonged to corpus callosum [9].
Apart from these findings, biomechanical properties of brain tissue under the classification of white and grey matter were investigated. It is well known that human brain consists of mainly two layers named grey and white matter. The white matter which is the inner layer of the brain, consist of myelinated axons whereas the gray matter that is the outer layer, houses cell bodies, dendrites, and unmyelinated axons.
Studying biomechanical differences between grey and white matter can be useful in understanding many neurological advancement such as neurodevelopment and the environmental factors involved in morphological abnormalities of brain tissue such as brachycephaly and plagiocephaly, flat or asymmetric heads, and hydrocephalus, abnormal accumulation of cerebrospinal fluid in the brain [10].
By studying biomechanical characteristics of grey and white matter via indentation, it was revealed that white matter is approximately 39% stiffer than gray matter based on the data obtained for average modulus of both tissues to be at 1.895 and 1.389 kPa respectively. Viscosity test results also showed that white matter is more viscous than grey matter; hence, the response time of white matter to mechanical loading was much slower than grey matter [10].
Measuring the differences between mechanical properties of gray and white matter and understanding the reasons behind them as well as their impact on health of the brain can be of great importance in better understanding of neurological networks and developments and diagnosis of neurological disorders.
In 2016 Kofahl et al. modified MRI imaging machine in order to detect tumorous brain tissue based on the biomechanical differences between healthy and tumorous tissue. This combination of rheological characterization and novel imaging techniques is an excellent illustration of how results of extensive studies regarding rheological properties of different tissue types can be applied to modern detection and diagnostic techniques [40].
6 Rheological Properties of Scaffolds and Their Biomedical Applications
After addressing the concept, followed by clarifying the influence and importance of these rheological properties in cells and tissues, almost all of the discoveries made so far can be utilized in synthesis and fabrication of biomaterials and related structures such as 2D and 3D scaffolds as hydrogels or sponges. Just like cells and tissues, there has been extensive research focusing on creation of perfect artificial structures suitable for biomedical applications on both in vitro and in vivo uses, namely hydrogels, bone fillers and constructed heart valves.
Cardiovascular diseases are one of the main reasons of death in 21th century. Among the heart diseases, heart valve defects are one of the most common ones and unfortunately its rate seems to be increasing every year which arises the urgent need for artificial tissue engineered heart valves. Despite all efforts spent so far on fabrication and modification of functional tissue engineered heart valves that match characteristics of native human heart valve in terms of biocompatibility and mechanical function and in general implantability, engineered valves are far from perfect [29, 40]. One of the main biomechanical characteristics necessary for a fully functional heart valve is its rheological properties of the soft tissue. Heart valves are constantly under mechanical force. Hence for the valves to have an acceptable performance, it is necessary to respond to wide range of mechanical stimuli instantaneously and precisely. Dynamic and static mechanical properties of tissue including tensile strength, viscoelastic and rheological properties are the main regulators of valve’s response. A tissue engineered heart valve must be as similar to native tissue as possible in terms of fatigue, flexural, rheological and viscoelastic properties [32].
Tissue engineered heart valves are fabricated via cell seeding or cell encapsulation into 3D scaffolds made of biodegradable and biocompatible materials. The seeded scaffolds are then placed in bioreactors for culturing. When the cells reach a certain level of viability, the scaffolds can be implanted. The aim of using bioreactor is to induce tissue formation rather than 2D cell layer formation [31]. As the tissue engineered scaffolds are implanted, they can obtain mechanical and structural properties of the native tissue as they adapt to in vivo conditions by remodeling, repair and tissue growth [32]. Since any implanted heart valve is subjected to a great amount of physical force upon implantation, it is imperative for the tissue to provide adequate mechanical strength and flawless function. This can be achieved provided that all mechanical properties of native tissue are known and precisely calculated and integrated into the fabricated artificial valve in a stable manner [20].
Despite the fact that data obtained for biomechanical properties of human heart valve are not very thorough due to the limited availability of fresh human heart valves and ideal measurement methods, a useful set of data are available regarding biomechanical properties of human heart valves [32, 39]. For instance transvalvular pressure of human mitral valve, aortic valve, tricuspid valve and pulmonary valve are reported at 120.0, 80.0, 25.0 and 10.0 mmHg respectively whereas values of the same pressure for bovine heart valves are found at 144.0, 92.4, 27.2 and 119.0 mmHg for bovine mitral valve, aortic valve, tricuspid valve and pulmonary valve respectively [32].
Although by knowing these and similar other values of mechanical and geometric characteristics of the native human and animal valves along with methods of manipulation and adjustment of mechanical properties of xenografts and polymeric scaffolds to desired values, fabrication of suitable tissue engineered heart valves have become possible, extensive and thorough investigation are still required to fabricate an exact replica of native human heart valve [32].
Bone diseases are another major problem of modern medicine and hot topic of interest for biomedical researchers. In human body bones have many responsibilities, namely providing a structural stability and a means for flawless motion along with acting as a physiological reservoir of hemocytoblasts and mesenchymal cells.
There are many of reasons for bone diseases such as trauma, tumors, degenerative diseases, osteoporosis, etc., that cause bone defects. Bone filler also known as bone cement is one of the popular methods for treating these bone defects by repairing damaged tissue via imparting desired biomechanical and physiological healing factors for the bones. Bone filler is so far the best alternative for allografts and xenografts which have problems such as availability, infection hematoma and rejection in case of allografts and reduced osteogenic capacity, reduced revascularization post implantation, etc. in case of xenografts [52].
Synthetic materials used in treatment of bone defects have problematic issues as well such as functional defects, difficulties in handling during implantation, unbalanced or insufficient local density, low adhesion problem, and mechanical instability with time. Despite these issues, among all treatment methods, bone fillers seem to be more efficient if and only if they are properly designed in terms of chemical compositions, biodegradability, biocompatibility, stiffness, flow properties, tensile strength and many other mechanical, chemical and biological properties [66].
Hydroxyapatite, tricalcium phosphate, hydroxyapatite cement, bioglass, methylmethacrylate polymer and porous polyethylene polymers are the most commonly used materials in the novel medicine as bone substitutes. There are other materials in form of composites which are used as bone cements such as zinc phosphate cement, zinc polycarboxylate cement and glass polyalkenoate cement which are less used nowadays. Bone cements are usually viscoelastic materials which look like toothpastes which turn into solid once injected or manually placed [23, 61]. Solidification occurs by an external cross linker. Each material has its own unique cross linker. For instance, PMMA, widely used in orthopedic surgery, is composed of two main components: PMMA powder and methyl methacrylate liquid. Once these two are mixed, a homogenous dough-like white material is obtained. Solidification of this dough after placement into the intended site happens via polymerization of methylmetacrylate. There is benzoyl peroxide present in the powder which starts producing free radicals as it reacts with N,N-dimethyl-p-toluidine present in the liquid [23].
There are many aspects that must be carefully studied and calculated in designing new bone filler materials such as chemical composition, biocompatibility and of course mechanical properties. Depending on the site where the bone cement needs to be injected, the flow properties of the filler is of vital importance along with all factors that can affect the quality and duration of polymerization such as pH, temperature, chemical compositions of plasma, etc. On the other hand the implanted cement must be completely biocompatible with similar mechanical strength as similar to desired value for the intended function as possible [23, 61].
7 Conclusion
Rheology is one of the characteristics of materials, which is categorized under mechanical properties, is a representative of flow properties of materials. Human body both in large scale (organs) and in small scale (cells) consists of mostly fluid. In material science fluids mostly show viscous behaviors. However, in case of fluids of human body, viscous behavioral patterns are useless due to the solid small components within cell and tissues, ECM, phenotype differences of cells and tissues along with compositional differences between different body fluids which lead to diverse structural and metabolic differences. Fortunately combination of concepts of elasticity and viscosity is able to define biomechanical nature of cells, tissues and related structures.
By advancement of science and technology over that past century a great number of methods have been developed for measuring mechanical properties of almost all types of materials in nano-scale and micron. In particular, investigating rheological properties of cells in micron and nano-scale has revealed incredibly comprehensive information. These invaluable data can help understand biomechanical and therefore physiological processes which take place in cells and their relation with cellular functions, diseases, mutations and in general, life.
There are many other studies regarding rheological studies of cells, tissues, organs, biomaterials, artificially engineered structure that have great applications in biomedical field. Despite the fact that almost all cell types have been biomechanically characterized along with almost all tissue types, and biomaterials such as polymers, ceramics, metals and composites fabricated in form of membranes, 3D scaffolds, hydrogels, fibrous structures, etc. there are still so many unsolved problems and unanswered questions concerning fabrication of ideal devices and implants that need to be solved and answered.
The number of researches conducted to this day regarding rheological properties of biomaterials is uncountable but unfortunately only a small portion of it was discussed in this chapter as in introductory to the world of rheology in living things. Wish of solving the riddle of life can only come true if all is revealed in subatomic level and that is the ultimate goal of today and the inevitable certainty of the future.
References
Alenghat, F. J., & Ingber, D. E. (2002). Mechanotransduction: All signals point to cytoskeleton, matrix, and integrins. Science’s STKE : Signal Transduction Knowledge Environment, 119, pe6.
Amblard, F., Maggs, A. C., Yurke, B., Pargellis, A. N., & Leibler, S. (1996). Subdiffusion and anomalous local viscoelasticity in actin networks. Physical Review Letters, 77(21), 4470–4473.
Artmann, G. M. (1995 Sep–Oct). Microscopic stiffness and relaxation time of red blood in a flow chamber of stiffness and relaxation time of red blood cells in a flow chamber. Biorheology, 32(5), 553–570.
Artmann, G. M., Sung, K. L., Horn, T., Whittemore, D., Norwich, G., & Chien, S. (1997). Micropipette aspiration of human erythrocytes induces echinocytes via membrane phospholipid translocation. Biophysics Journal, 72, 1434.
Ayala, Y. A., Pontes, B., Ether, D. S., Pires, L. B., Araujo, G. R., Frases, S., et al. (2016). Rheological properties of cells measured by optical tweezers. BMC Biophysics, 9, 5.
Badalà, Federico, Nouri-mahdavi, Kouros, & Raoof, Duna A. (2008). NIH public access. Computer, 144(5), 724–732.
Baskurt, Oguz K., & Meiselman, Herbert J. (2003). Blood rheology and hemodynamics. Seminars in Thrombosis and Hemostasis, 29(5), 435–450.
Bausch, A. R., Ziemann, F., Boulbitch, A. A., Jacobson, K., & Sackmann, E. (1998). Local measurements of viscoelastic parameters of adherent cell surfaces by magnetic bead microrheometry. Biophysical Journal, 75(4), 2038–49. http://dx.doi.org/10.1016/S0006-3495(98)77646-5.
Boudjema, F., Lounis, M., Khelidj, B., & Bessai, N. (2015). Rheological regional properties of brain tissue studied under cyclic creep/recovery shear stresses. Journal of Physics: Conference Series, 602(1), 6.
Budday, S., Nay, R., de Rooij, R., Steinmann, P., Wyrobek, T., Ovaert, T. C., & Kuhl, E. (2015). Mechanical properties of gray and white matter brain tissue by indentation. Journal of the Mechanical Behavior of Biomedical Materials, 46, 318–330. http://dx.doi.org/10.1016/j.jmbbm.2015.02.024.
Chan, C. J., Whyte, G., Boyde, L., Salbreux, G., & Guck, J. (2014). Impact of heating on passive and active biomechanics of suspended cells. Interface Focus, 4(2), 20130069.
Cheng, S., Clarke, E. C., & Bilston, L. E. (2008). Rheological properties of the tissues of the central nervous system: A review. Medical Engineering & Physics, 30(10), 1318–1337.
Chien, S. (2007). Mechanotransduction and endothelial cell homeostasis: The wisdom of the cell. American Journal of Physiology-Heart and Circulatory Physiology, 292(3), H1209–H1224.
Chien, S., Sung, K. L., Skalak, R., Usami, S., Tözeren, A. (1978, November 24). Theoretical and experimental studies on viscoelastic properties of erythrocyte membrane. Biophysical Journal, 24, 463–487.
Chmiel, B., Karkoszka, H., Cierpka, L., & Wiecek, A. (2005). Rheological properties of red blood cells in kidney transplant recipients: The role of lipid profile and type of immunosuppresion. Transplantation Proceedings, 37(4), 1885–1888.
Crocker, J. C., & Hoffman, B. D. (2007). Multiple-particle tracking and two-point microrheology in cells. Methods in Cell Biology, 83(7), 141–178.
Darling, E. M., Topel, M., Zauscher, S., Vail, T. P., & Guilak, F. (2008). Viscoelastic properties of human mesenchymally-derived stem cells and primary osteoblasts, chondrocytes, and adipocytes. Journal of Biomechanics, 41(2), 454–464.
Di Terlizzi, R., & Platt, S. (2006). The function, composition and analysis of cerebrospinal fluid in companion animals: Part I—Function and composition. Veterinary Journal, 172(3), 422–431.
Doherty, G. J., & McMahon, H. T. (2008). Mediation, modulation, and consequences of membrane-cytoskeleton interactions. Annual Review of Biophysics, 37(1), 65–95.
Driessen, N. J. B., Mol, A., Bouten, C. V. C., & Baaijens, F. P. T. (2007). Modeling the mechanics of tissue-engineered human heart valve leaflets. Journal of Biomechanics, 40(2), 325–334.
Drury, J. L., & Dembo, M. (1999). Hydrodynamics of micropipette aspiration. Biophysical Journal, 76(1 Pt 1), 110–128.
Engelking, L. R. (2011). Chemical composition of living cells. Textbook of veterinary physiological chemistry (pp. 2–6). Academic Press. eBook ISBN: 9780123919106.
Farrar, D. F., & Rose, J. (2001). Rheological properties of PMMA bone cements during curing. Biomaterials, 22(22), 3005–3013.
Franck, C., Maskarinec, S. A., Tirrell, D. A., & Ravichandran, G. (2011). Three-dimensional traction force microscopy: A new tool for quantifying cell-matrix interactions. PLoS ONE, 6(3).
Goldmann, W. H. (2012). Mechanotransduction in cells. Cell Biology International, 36(6), 567–570.
Guck, J., Ananthakrishnan, R., Mahmood, H., Moon, T. J., Cunningham, C. C., & Käs, J. (2001). The optical stretcher: A novel laser tool to micromanipulate cells. Biophysical Journal, 81(2), 767–784.
Guilak, F., Tedrow, J. R., & Burgkart, R. (2000). Viscoelastic properties of the cell nucleus. Biochemical and Biophysical Research Communications, 269(3), 781–786.
Haase, K., & Pelling, A. E. (2015). Investigating cell mechanics with atomic force microscopy. Journal of the Royal Society, Interface/the Royal Society, 12(104), 20140970.
Hale, C. M., Sun, S. X., & Wirtz, D. (2009). Resolving the role of actoymyosin contractility in cell microrheology. PLoS ONE, 4(9).
Han, M. K. L., & de Rooij, J. (2016). Converging and unique mechanisms of mechanotransduction at adhesion sites. Trends in Cell Biology, 26(8), 612–623. https://doi.org/10.1016/j.tcb.2016.03.005.
Hasan, A., Memic, A., Annabi, N., Hossain, M., Paul, A., Dokmeci, M. R., et al. (2014). Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomaterialia, 10(1), 11–25.
Hasan, A., Ragaert, K., Swieszkowski, W., Selimović, Š., Paul, A., Camci-Unal, G., et al. (2014). Biomechanical properties of native and tissue engineered heart valve constructs. Journal of Biomechanics, 47(9), 1949–1963.
Hochmuth, R. M. (2000). Micropipette aspiration of living cells. Journal of Biomechanics, 33(1), 15–22.
Hoffman, B. D., & Crocker, J. C. (2009). Cell mechanics: Dissecting the physical responses of cells to force. Annual Review of Biomedical Engineering, 11, 259–288.
Humphrey, J. D., Dufresne, E. R., & Schwartz, M. A. (2014). Mechanotransduction and extracellular matrix homeostasis. Nature Reviews Molecular Cell Biology, 15(12), 802–812. http://dx.doi.org/10.1038/nrm3896.
Icard-Arcizet, Delphine, Cardoso, Olivier, Richert, Alain, & Hénon, Sylvie. (2008). Cell stiffening in response to external stress is correlated to actin recruitment. Biophysical Journal, 94(7), 2906–2913.
Irace, C., Carallo, C., Scavelli, F., Esposito, T., De Franceschi, M. S., Tripolino, C., & Gnasso, A. (2014). Influence of blood lipids on plasma and blood viscosity. Clinical Hemorheology and Microcirculation, 57(3), 283–290.
Jay, A. W., & Canham, P. B. (1977). Viscoelastic properties of the human red blood cell membrane. II. Area and volume of individual red cells entering a micropipette. Biophysical Journal, 17(2), 169–178. http://dx.doi.org/10.1016/S0006-3495(77)85634-8.
Kalejs, M., Stradins, P., Lacis, R., Ozolanta, I., Pavars, J., & Kasyanov, V. (2009). St Jude Epic Heart Valve bioprostheses versus native human and porcine aortic valves—Comparison of mechanical properties. Interactive Cardiovascular and Thoracic Surgery, 8(5), 553–556.
Kofahl, A. L., Theilenberg, S., Bindl, J., Ulucay, D., Wild, J., Napiletzki, S., et al. (2016). Combining rheology and MRI: Imaging healthy and tumorous brains based on mechanical properties. Magnetic Resonance in Medicine, 0, 1–11.
Kollmannsberger, P., & Fabry, B. (2007). High-force magnetic tweezers with force feedback for biological applications. Review of Scientific Instruments, 78(11), 1–6.
Kraning-Rush, C. M., Califano, J. P., & Reinhart-King, C. A. (2012). Cellular traction stresses increase with increasing metastatic potential. PLoS ONE, 7(2).
Laperrousaz, B., Drillon, G., Berguiga, L., Nicolini, F., Audit, B., Satta, V. M., et al. (2016). From elasticity to inelasticity in cancer cell mechanics: A loss of scale-invariance. AIP Conference Proceedings (p. 1760).
Last, J. A., Liliensiek, S. J., Nealey, P. F., & Murphy, C. J. (2009). Determining the mechanical properties of human corneal basement membranes with atomic force microscopy. Journal of Structural Biology, 167(1), 19–24.
Lomakina, E. B., Spillmann, C. M., King, M. R., & Waugh, R. E. (2004). Rheological analysis and measurement of neutrophil indentation. Biophysical Journal, 87(6), 4246–4258.
Lombardo, M., Lombardo, G., Carbone, G., De Santo, M. P., Barberi, R., & Serrao, S. (2012). Biomechanics of the anterior human corneal tissue investigated with atomic force microscopy. Investigative Ophthalmology and Visual Science, 53(2), 1050–1057.
Lu, Y.-B., Franze, K., Seifert, G., Steinhäuser, C., Kirchhoff, F., Wolburg, H., et al. (2006). Viscoelastic properties of individual glial cells and neurons in the CNS. Proceedings of the National Academy of Sciences, 103(47), 17759–17764.
Lulevich, V., Zink, T., Chen, H. Y., Liu, F. T., & Liu, G. Y. (2006). Cell mechanics using atomic force microscopy-based single-cell compression. Langmuir, 22(19), 8151–8155.
Massiera, G., Van Citters, K. M., Biancaniello, P. L., & Crocker, J. C. (2007). Mechanics of single cells: Rheology, time dependence, and fluctuations. Biophysical Journal, 93(10), 3703–3713. https://doi.org/10.1529/biophysj.107.111641.
Matthews, B. D., Overby, D. R., Mannix, R., & Ingber, D. E. (2006). Cellular adaptation to mechanical stress: Role of integrins, rho, cytoskeletal tension and mechanosensitive ion channels. Journal of Cell Science, 119(Pt 3), 508–518.
Moeendarbary, E., & Harris, A. R. (2014). Cell mechanics: Principles, practices, and prospects. Wiley Interdisciplinary Reviews: Systems Biology and Medicine, 6(5), 371–388.
Morra, M., Giavaresi, G., Sartori, M., Ferrari, A., Parrilli, A., Bollati, D., et al. (2015). Surface chemistry and effects on bone regeneration of a novel biomimetic synthetic bone filler. Journal of Materials Science: Materials in Medicine, 26(4). http://dx.doi.org/10.1007/s10856-015-5483-6.
Munevar, S. (2004). Regulation of mechanical interactions between fibroblasts and the substratum by stretch-activated Ca2+ entry. Journal of Cell Science, 117(1), 85–92.
Müller, U. (2004). Integrins and extracellular matrix in animal models. Handbook of Experimental Pharmacology, 165, 217–241.
Nagayama, K., Adachi, A., & Matsumoto, T. (2011). Heterogeneous response of traction force at focal adhesions of vascular smooth muscle cells subjected to macroscopic stretch on a micropillar substrate. Journal of Biomechanics, 44(15), 2699–2705.
Nawaz, S., Sánchez, P., Bodensiek, K., Li, S., Simons, M., & Schaap, I. A. (2012). Cell visco-elasticity measured with AFM and optical trapping at sub-micrometer deformations. PLoS ONE, 7(9).
Oh, M.-J., Kuhr, F., Byfield, F., & Levitan, I. (2012). Micropipette aspiration of substrate-attached cells to estimate cell stiffness. Journal of Visualized Experiments, 67, e3886.
Pajerowski, J. D., Dahl, K. N., Zhong, F. L., Sammak, P. J., & Discher, D. E. (2007). Physical plasticity of the nucleus in stem cell differentiation. Proceedings of the National Academy of Sciences of the United States of America, 104(40), 15619–15624.
Park, Y., Best, C. A., Badizadegan, K., Dasari, R. R., Feld, M. S., Kuriabova, T., et al. (2010). Measurement of red blood cell mechanics during morphological changes. Proceedings of the National Academy of Sciences of the United States of America, 107(15), 6731–6736.
Pierini, F., Zembrzycki, K., Nakielski, P., Pawłowska, S., & Kowalewski, T. A. (2016). Atomic force microscopy combined with optical tweezers (AFM/OT). Measurement Science and Technology, 27(2), 25904.
Pryor, L. S., Gage, E., Langevin, C. J., Herrera, F., Breithaupt, A. D., Gordon, C. R., et al. (2009). Review of bone substitutes. Craniomaxillofacial Trauma & Reconstruction, 2(3), 151–160.
Pullarkat, P. A., Fernández, P. A., & Ott, Albrecht. (2007). Rheological properties of the eukaryotic cell cytoskeleton. Physics Reports, 449(1–3), 29–53.
Quinto-Su, P. A., Kuss, C., Preiser, P. R., & Ohl, C.-D. (2011). Red blood cell rheology using single controlled laser-induced cavitation bubbles. Lab on a Chip, 11(4), 672–678.
Raucher, D., Stauffer, T., Chen, W., Shen, K., Guo, S., York, J. D., et al. (2000). Phosphatidylinositol 4,5-Bisphosphate functions as a second messenger that regulates cytoskeleton–plasma membrane adhesion. Cell, 100(2), 221–228.
Riveline, D., Zamir, E., Balaban, N. Q., Schwarz, U. S., Ishizaki, T., Narumiya, S., et al. (2001). Focal contacts as mechanosensors. The Journal of Cell Biology, 153(6), 1175–1186.
Roohani-Esfahani, S.-I., Newman, P., & Zreiqat, H. (2016). Design and fabrication of 3D printed scaffolds with a mechanical strength comparable to cortical bone to repair large bone defects. Scientific Reports, 6(February 2015), 19468.
Rother, J., Nöding, H., Mey, I., & Janshoff, A. (2014). Atomic force microscopy-based microrheology reveals significant differences in the viscoelastic response between malign and benign cell lines. Open Biology, 4(5), 140046.
Sasaki, N. (2012). Viscoelastic properties of biological materials. Annals of the New York Academy of Sciences, 99–122. http://www.ncbi.nlm.nih.gov/pubmed/7076685.
Schulze, C., Wetzel, F., Kueper, T., Malsen, A., Muhr, G., Jaspers, S., et al. (2012). Stiffening of human skin fibroblasts with age. Clinics in Plastic Surgery, 39(1), 9–20.
Shuck, L. Z., & Advani, S. H. (1972). Rheological response of human brain tissue in shear. Journal of Basic Engineering Trans ASME, 94 Ser D(4), 905–911.
Sleep, J., Wilson, D., Simmons, R., & Gratzer, W. (1999). Elasticity of the red cell membrane and its relation to hemolytic disorders: An optical tweezers study. Biophysical Journal, 77(6), 3085–3095.
Stamenović, D. (2008). Rheological behavior of mammalian cells. Cellular and Molecular Life Sciences, 65(22), 3592–3605.
Supriya, B., Jun, D., Paul, B. C., & Dahms, T. E. S. (2012). Viscoelasticity in biological systems: A special focus on microbes. ISBN 978-953-51-0841-2, Publisher: InTech.
Thoumine, O., & Ott, A. (1997). Time scale dependent viscoelastic and contractile regimes in fibroblasts probed by microplate manipulation. Journal of Cell Science, 110(Pt 1), 2109–2116.
Tozeren, A., Skalak, R., Sung, K. P., & Chien, S. H. U. (1982). Viscoelastic behavior of erythrocyte membrane. Biophysical Society, 39(1), 23–32.
Trepat, X., Grabulosa, M., Puig, F., Maksym, G. N., Navajas, D., & Farré, R. (2004). Viscoelasticity of human alveolar epithelial cells subjected to stretch. American Journal of Physiology, 287(5), L1025–L1034.
Tseng, Y., Kole, T. P., & Wirtz, D. (2002). Micromechanical mapping of live cells by multiple-particle-tracking microrheology. Biophysical Journal, 83(6), 3162–3176.
Van Citters, K. M., Hoffman, B. D., Massiera, G., & Crocker, J. C. (2006). The role of F-actin and myosin in epithelial cell rheology. Biophysical Journal, 91(10), 3946–3956.
Verdier, C., Etienne, J., Duperray, A., Preziosi, L., et al. (2009). Review: Rheological properties of biological materials. Comptes Rendus Physique, 10(8), 790–811. http://dx.doi.org/10.1016/j.crhy.2009.10.003.
Von Wichert, G., Jiang, G., Kostic, A., De Vos, K., Sap, J., & Sheetz, M. P. (2003). RPTP-α acts as a transducer of mechanical force on αv/β3-integrin–cytoskeleton linkages. The Journal of Cell Biology, 161(1), 143–153. http://doi.org/10.1083/jcb.200211061.
Vonna, Laurent, Wiedemann, Agnès, Aepfelbacher, Martin, & Sackmann, Erich. (2003). Local Force Induced Conical Protrusions of Phagocytic Cells. Journal of Cell Science, 116(Pt 5), 785–790.
Walters, K. (1980). History of rheology. Rheology I.
Wang, N., Tolic-Nørrelykke, I. M., Chen, J., Mijailovich, S. M., Butler, J. P., Fredberg, J. J., & Stamenovic, D. (2002). Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. American Journal of Physiology. Cell Physiology, 282(3), C606–C616.
Zhou, S., Sokolov, A., Lavrentovich, O. D., & Aranson, I. S. (2014). Living liquid crystals. Proceedings of the National Academy of Sciences of the United States of America, 111(4), 1265–1270. http://www.ncbi.nlm.nih.gov/pubmed/24474746.
Acknowledgements
The authors would like to thank Prof. Dr. Herbert J. Meiselman, Prof. Dr. Aysegül Temiz Artmann and Prof. Dr. Oguz K. Baskurt for their invaluable research in this field as the result of their lifetime of intelligence and hard work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Rostami, S., Garipcan, B. (2018). Rheological Properties of Biological Structures, Scaffolds and Their Biomedical Applications. In: Artmann, G., Artmann, A., Zhubanova, A., Digel, I. (eds) Biological, Physical and Technical Basics of Cell Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-10-7904-7_5
Download citation
DOI: https://doi.org/10.1007/978-981-10-7904-7_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7903-0
Online ISBN: 978-981-10-7904-7
eBook Packages: EngineeringEngineering (R0)