Abstract
The problem of creation and use of sorption materials are of current interest for the practice of the modern medicine and agriculture. The knowledge of physical and chemical rules of carbonization, activation as well as sorption and desorption processes is of particular importance in the case of application of the nanostructured carbon sorbent agent for high purification of water contaminated with pesticides, as well as for reducing the concentration of cytokines in the blood of sepsis patients. Practical importance is production of a biostimulant using a carbon sorbent for a significant increase in productivity, which is very relevant for the regions of Kazakhstan. It is now known that a plant phytohormone—fusicoccin in nanogram concentrations transforms cancer cells to the state of apoptosis. In this regard, there is a scientific practical interest in the development of a highly efficient method for producing fusicoccsin from extract of germinated wheat seeds. This method is based on selective sorption of fusicoccin by a nanostructured carbon sorbent. Thus, it becomes possible to create a high-performance domestic anticancer drug.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
1.1 Carbon Sorption Materials
The useful properties of activated carbon have been known since ancient times. This traces back to 1500 BC when Egyptians used charcoal as an adsorbent for medicinal purposes and a purifying agent. Around 420 BC it was observed that Hippocrates dusted wounds with powdered charcoal to remove their odor. Ancient Hindu societies purified their water by filtration through charcoal. In 1773, the Swedish chemist Karl Wilhelm Scheele was the first to observe adsorption of gases on charcoal. A few years later activated carbons began being used in the sugar industry as a decolorizing agent for syrup.
In the early 20th century the first plant to produce activated carbon industrially was built for use in sugar refining industry in Germany. Many other plants emerged in the early 1900s to produce activated carbons primarily for decolorization. During World War I activated carbon was used in gas masks for protection against hazardous gases and vapors.
Today, activated carbons are used to remove color from pharmaceutical and food products, as air pollution control devices for industrial and automobile exhaust, for chemical purification, and as electrodes in batteries. 500,000 tons per year of activated carbon are produced globally [1,2,3,4,5,6,7]. 80% of this is used for liquid phase applications, and 20% is used for solid phase applications.
Activated carbon (AC) is a unique material with its immense capacity for adsorption from gas and liquid phases. It occupies a special place in terms of producing a clean environment involving water purification as well as separations and purification in the chemical and associated industries. In these roles, it exhibits a remarkable efficiency as the international production is a little more than a million tons per year, with perhaps 2 million tones being in continuous use. Broadly AC has been used for three main purposes:
-
1.
AC is predominantly used for purposes of adsorption in liquid-phase. The wide-ranging scenarios for AC are:
-
drinking water availability, to improve taste, smell and color including removal of chlorinated compounds and other volatile organic carbons (VOCs);
-
purification of ground water purity from contaminants coming from disused sites of heavy industries;
-
treatment of both industrial and municipal wastewater;
-
mining operations require feed water treatment, metallic ion adsorption (gold and other metals), adsorption of excess flotation reagents and adsorption of natural organic matter (NOM);
-
pharmaceutical processes, including purification of process water, use with fermentation broths and purification of many products;
-
the food, beverage and oil industries for removal of color and unacceptable tastes;
-
the dry-cleaning industries require purification of solvents;
-
the electroplating industries require purification of wastewaters containing Pb, Cr, etc.;
-
household water purification, cleaning of aquaria and use in oven-extract hoods;
-
the sugar and sweetener industries need decolorization agents for production of white sugar, etc.
-
-
2.
AC in different forms (granular, extruded, fiber or cloth) is used for production of pure gases in the chemical industry, to reduce pollutant gases to very low concentrations in a single stage, in protection against poisonous gases, in air conditioning, for removal of oil from compressed air, to separate gases in mixtures by sieving, etc.
-
3.
Carbon materials have been used for some time in heterogeneous catalysis, acting as direct catalysts or as a catalyst support. Catalytic particles can be supported within the porosity to promote required catalytic conversions [8,9,10].
Activated carbon is a microcrystalline form of carbon with very high porosity and surface area. It may be visualized as foam solid that has a large surface within a rigid granule or particle structure of relatively small volume. Its chemical structure allows it to preferentially adsorb organic materials and other nonpolar compounds from gas or liquid streams.
Activated carbon has become one of the most technically important and most widely used adsorbents because of its high adsorptive capacity. Present technology demands a very large production of activated carbons with appropriate characteristics for each particular application. In general, activated carbon which is used in any of the most common applications must have adequate adsorptive capacity, chemical purity, mechanical strength, etc.
Furthermore, all these specifications should coexist with a low production cost. Activated carbon is obtained from a carefully controlled process of dehydration, carbonization and oxidation of organic substances. It can be prepared for research in the laboratory from a large number of materials. However, the most commonly used ones in commercial practice are peat, coal, lignite, wood and agricultural by-products such as coconut shell, almond shell, rice husk, etc.
Pyrolysis of the starting material with exclusion of air and without addition of chemical agent usually results in an inactive material with a specific surface area of the order of several m2/g and low adsorption capacity. One can prepare carbon with a large adsorption capacity by activating the carbonized products with a reactive gas. The majority of activated carbon used throughout the world is produced by steam activation (physical activation). In this process, the carbonized product is reacted with steam over 900 ℃.
Another procedure used in the production of activated carbon involves the use of chemical activating agents before the carbonization step. The most commonly used activating agents are phosphoric acid, zinc chloride and salts of sodium and magnesium etc. Many chemical substances act as dehydration agents and therefore may restrict the formation of tar during carbonization. Chemical activation is usually carried out at lower temperatures than the simple pyrolysis and the activation process with steam or carbon dioxide. The production at lower temperatures promotes the development of a porous structure because under these conditions elementary crystallites of smaller dimensions are formed [11, 12].
Most of the available surface area of activated carbon is nonpolar in nature. However, during production the interaction of surface with oxygen produces specific active sites giving the surface of slightly polar nature. As activated carbon is one of the most commonly used adsorbents in many industrial applications for its adsorptive capacity [13,14,15].
Most activated carbons contain pores of different sizes: micropores, transitional mesopores and macropores. According to the IUPAC definition, pores can be distinguished in three groups with respect to their dimensions.
-
Macropores—Pores with larger than 50 nm;
-
Mesopores—Pores with diameters between 2 and 50 nm;
-
Micropores—Pores with diameters less than 2 nm.
Microporous carbons have a unique structure which essentially is a three dimensional network, or labyrinth of carbon atoms some in hexagonal arrangements and some as individual carbon atoms bonded together extremely close but not close packed. This bonding arrangement results in space existing between the atoms to create an interconnecting three dimensional passage way in which every space unit has a connection to all others within the carbon. The dimensions of the passage way (width) are those of molecules, about 0.5 nm, such that any molecule which enters into this space is subjected to intense dispersion forces from the carbon atoms (about eight per space) which make up the “surface” of the space.
These forces can “trap” the molecule for periods of time much longer than on an open surface and so the phenomenon of physical adsorption of gases is generated.
The site, place or space where a molecule can be trapped (adsorbed) is called an adsorption site. These adsorption sites can be modified in terms of their size (widened or narrowed) and in terms of their chemical composition [16]. The surface can be bonded to hydrogen, oxygen, chlorine, nitrogen, etc. to alter the polarity of the surface. Changing this polarity can enhance the adsorption process for polar molecules. The porosity can be widened by gasification with water vapor, oxygen or carbon dioxide. The parent materials may also be impregnated with zinc chloride, potassium hydroxide or phosphoric acid, these treatments improving adsorption characteristics, a process known as activation. The variables, within an adsorption system (dominantly an aqueous solution) controlling extents of adsorption include [17, 18]:
-
1.
Volume of narrow micropores, <0.7 nm (VNM—cm3/g), including the pore-size distributions.
-
2.
Volume of wider micropores, 0.7–2.0 nm (VWM—cm3/g), including the pore-size distributions.
-
3.
Volume in mesopores, >2.0 and <50 nm (VME—cm3/g), including the pore-size distributions.
-
4.
The parameter “surface area” is a crude parameter and fails to quantify, adequately, the capacity of the all-important micropores and their size distributions.
-
5.
The controlled additions of surface oxygen complexes from known sources, e.g. nitric acid, H2O2, etc., with controlled removal of the complexes, thermally or by reaction with hydrogen at temperature >750 ℃.
-
6.
The effects of additions of surface oxygen complexes upon pore volumes and pore size distributions (pore dimensions can be reduced).
-
7.
The characterization of surface oxygen complexes.
-
8.
The pH of the solution.
-
9.
Ionic strength.
-
10.
Temperature.
Nowadays in Kazakhstan the necessity of creation of carbon materials with high sorption properties has come. A pioneer in this field was “Laboratory of carbon nanomaterials” at the Institute of Combustion problems and at Al-Farabi KazNU created by Professor R. M. Mansurova. In this laboratory from waste rice grinding production by carbonation the first carbonized carbon biomaterial was obtained which has the ability to adsorb not only molecules, but even whole microbial cells. In the work [19,20,21] on the carbonized carbon material from rice husk cells of the following microorganisms were sorbed. The choice of these microorganisms was due to the fact that they are widely used for detoxification of sewage. In this work we have obtained very interesting results. It is shown that microorganisms adsorbed on carbon materials remained viable in the presence of highly toxic ions of cadmium, lead and copper [22,23,24]. Sorption of microorganisms in the carbon sorbent from rice husk four times increased the viability of the microorganisms in the presence of toxic ions, in this case the total sorption capacity of these toxic ions increased several times. All these results suggest that unusual carbon material with sorbed living cells of microorganisms was created and it has the ability to high sorption of highly toxic ions. This new material can be widely used for industrial detoxification of polluted water contaminated with heavy metal ions.
In this laboratory headed by Professor R. M. Mansurova, carbon materials have been created by carbonization of the waste from local raw materials. Interesting work has been done on the sorption of toxic ions by obtained carbonized carbon materials derived from rice husk and wheat bran [25, 26]. It was found that activation of the obtained carbon materials with hydrogen peroxide and ammonia significantly increases the sorption capacity for heavy metal ions and radionuclides.
Thus, developed on the basis of vegetative raw materials of Kazakhstan carbonized carbon materials have a great potential for detoxification of water contaminated with ions of heavy metals and radionuclides. Moreover, they offer the prospect of using these materials for the enrichment of rare and expensive metals. Owing to excellent sorption quality of such materials it is necessary to expand and deepen the study on the creation of properties of new carbon materials derived from the vegetative raw materials of Kazakhstan [27].
The main trends of development in science and technology is currently focused on the study of nanoscale structural components of matter and functional materials. Progress in this area is of great theoretical and practical importance because such materials can be widely used in ecology, biology and medicine and just because in these areas of science the greatest social and economic effect from the introduction nanomaterials is predicted.
It should be noted that prospects for practical use of nanostructured sorbents are obtained by high temperature carbonization of cheap secondary vegetative raw materials. Carbon obtained by the carbonization of plant materials, keeps its original finely organized structure. By varying the conditions of carbonization, it is possible to obtain a complex of compositions of carbon from other materials. Some of these materials can find practical application. Availability and annual renewability of raw materials, low content of mineral impurities, developed porous structure, ecological production allow to obtain cheap, fast regenerable carbon sorbents [28,29,30].
1.2 The Properties of Fusicoccin and Its Application as an Anticancer Preparation
Fusicoccin was discovered in 1964 by the Italian scientist Alessandro Ballio as the phytotoxin of the phytopathogenic fungus “Fusicoccum amygdali Del”. This phytotoxin was able to kill young almond trees as follows: this toxin opened stomata of leaves which did not close after. In consequence of the excessive transpiration and because of the weakness of the root system young trees were dying rapidly from drought, i.e. fusicoccin is a natural desiccant. In the laboratory of Professor A. Ballio the structure of this phytotoxin was determined as is shown in Fig. 1. Considering the fact that fusicoccin refers to natural terpenoids as well as the gibberellin Gronevald et al. developed a hypothesis about the kinship of gibberellin and fusicoccin. Indeed, they both are diterpenoids, metabolites of phytopathogenic fungi. Over time, the number of known gibberellins increased rapidly from few in 1960 to more than eighty by the end of the 80s. The same for fusicoccins: now more than 15 related compounds of this group are known (denoted by literal characters—A, B, C, etc.).
Formula of fusicocin—phytotoxin of the phytopatogenic fungus Fusicoccum amygdali Del. [31]
Preliminary data on the presence of substances of the fusicoccin family in higher plants were published first by Muromtsev and his co-wokers in 1980. Later for the detection of fusicoccin a gas chromatography, mass-spectrometry was used (GC/MS) with a preliminary fractionation of the material by the high performance liquid chromatography (HPLC). Initially, the authors have developed an identification method for fusicoccinal metabolites in the culture fluid of the fungus F. amygdali by the combined method of the gas chromatography and mass spectrometry of trisilil derivatives, when the detection is performed through several chosen characteristic ions. To obtain fusicoccin, the authors used corn cobs and cabbage leaves, which were homogenized in ethanol. The alcohol extracts were concentrated. The concentrate was extracted by chloroform. The chloroform extract was subjected to the liquid chromatography. The obtained peaks were subjected to the gas-liquid chromatography followed by mass spectrometry. The content of the endogenous fusicocin A in plants was 10−11–10−12 M, which is 2–3 orders lower than that of the endogenous gibberellins. Hence it becomes clear that the problem of preparative quantities extraction of natural fusicoccin from higher plants is a very difficult experimental problem.
Then, the same authors tried another shot to extract natural fusicoccin from higher plants. At this time, as the object for the fusicoccin extraction the authors used a sterile culture of transformed horseradish roots distinguished by the intensive growth. This object was chosen for the following reasons. Firstly, it eliminates the possibility of contamination by microorganisms and fungi that may be sources of fusicoccin. Second, the culture of roots cells can be obtained in very large quantities due to their intensive growth.
From cultures of roots cells the authors obtained alcohol extracts. The extracts were evaporated in vacuo, the aqueous residues were extracted by chloroform, followed by determination of the presence of fusicoccin in aqueous and chloroform phases by immune methods. Fusicoccin was in chloroform, whereas in the aqueous phase there were found only trace amounts, that eliminates the possibility of effective interaction of such compounds as proteins and sugars with receptors and antibodies. Substances similar to fusicoccin were discovered at all stages of roots cultivation (since 14 days). It was found that the maximum content of fusicoccin in grown sterile roots is up to 150 nM per kilogram of roots.
Mass-spectrometric analysis of the obtained fractions showed mainly the presence of fusicoccin type A, also these analyses indicated the possible presence of another unidentified fusicoccin. As a result of undertaken studies Muromtsev and his co-workers made a conclusion that identification of endogenous fusicoccin is a very difficult task [32].
The first and main of the identified fusicoccins, fusicoccin A, is a glycoside of the carbotricyclic diterpene with a molecular weight of 680 Da gross and the formula C36H56O12 (Fig. 1). The aglycon part of fusicoccin molecules is represented by the tricyclic system including one eight-membered and two five-membered rings. Besides the basic compound (fusicoccin A) the fungus produces some similar compounds (more than 15), which differ in the degree of acetylation, positions of acetyl groups, the degree of oxidation of the aglycone part of the molecule. Thus, ten fusicoccins represent monoacetates, diacetates and triacetates. Another series of fusicoccins has a more simple structure: their C20-atom is not oxidized. The analysis of literature data shows that the fusicoccin molecule is not a unique structure and connections with the similar “skeleton” which are derivatives of the dicyclopentane and cyclooctane (the ring system 5-8-5), are widely distributed in the nature. They were found in fungi and algae, higher plants—liverworts, flowering plants, and even among animals (insects). If and when discovered and identified various representatives of this class of terpenoids the trivial names of organisms-producers are assigned to them. Thus, for example, the fusicoccin extracted from the liverwort Plagiochila acanthophylla is called fusicoplagin C, the fusicoccin extracted from the liverwort Anastrepta orcadensin is anadensin, the fusicoccin extracted from the giant kelp Dictyota dichtoma is epoxydictimen. It was proposed to combine compounds having dicyclopentane and cyclooctane “skeleton” into groups of terpenoids of the fusicoccane range. The basis of the range of such compounds was formed by the hypothetical trans-sin-trans C20 hydrocarbon that was named fusicoccine.
-
1.
The further study of fusicoccin showed that they demonstrate various physiological and biochemical properties. This made it possible to class it among natural regulators of the plant growth.
-
2.
The cells of many organs and tissues of higher plants respond to the treatment by fusicoccin with the increase of the volume, with which such effects can be associated as the opening of the stomata, cells growth and elongation. There are data on the possible role of fusicoccin as an endogenous regulator of seed germination and root formation.
-
3.
Fusicoccin effectively activates seeds from the deep rest. The laboratory of Muromtsev first discovered that fusicoccin actively stimulates the rhizogenesis for number of cultures. Fusicoccin had a very interesting effect on germination of pea seeds. So it stimulated the growth of cotyledon cells and at the same time inhibited the growth of the embryonic axis, which indicates the polarity of the fusicoccin action. It was found that fusicoccin plays an important activating role at early stages of legumes contamination with microorganisms of the genus Rhizobium spp, i.e. fusicoccin activates the nodulation in legumes.
-
4.
Fusicoccin activates absorption of carbon dioxide by columnar cells of the leaf. Also fusicoccin regulates the concentration of ascorbate in the apoplast of coleoptile cells Vigna angularis.
-
5.
One of the most striking hormonal properties of fusicoccin is its anti-stress activity. Muromtsev and his co-workers showed that fusicoccin can increase the seed germination in conditions unfavorable for germination, for example: at elevated and low temperatures, excessive moisture, salination. The co-workers of the Institute of Plant Physiology showed that the seeds soaking (0.68 mg/l of fusicoccin) and also spraying of plants of winter wheat, rye, barley (0.34 mg/l of fusicoccin) leads to the increase of the frost hardiness of plants at booting and tillering phases. Moreover, the increase of the frost hardiness correlated well with the degree of development of the photosynthetic apparatus and sugar accumulation, as well as with the development strengthening of the endoplasmic reticulum in cells. It is shown that fusicoccin protects rice plants under salinity, increases the resistance of potato tubers to some diseases. Fusicoccin is undoubtedly one of the most powerful anti-stress compounds for plants. The positive role of fusicoccin in adaptation to osmotic stress is shown in the work [33].
The available data on the study of the biochemical activity of fusicoccin are of particular interest for us.
For the first time [33] Ballio and his co-workers discovered fusicoccinal receptors on the plasma membrane isolated from the roots of maize seedlings. They also created proteoliposomes with the inclusion of isolated fusicoccinal receptors and studied properties of this artificial system. De Boer and his co-wokers developed a method for purification of FC-binding proteins on a special affine sorbent to which the biotinylated fusicoccin is ligated as the active group. A number of authors have also performed some studies on the extraction of fusicoccin-binding proteins [34].
For fusicoccin the alleged receptors are described—fusicoccin-binding proteins (FCBP) of two types. The first type has a high affinity for fusicoccin with a dissociation constant (Kd) about 10−10 M (high-affinity HA), and the second receptors type with a lower affinity, Kd about 10−7 M (low-affinity LA). They were first detected in the fraction containing plasma membranes of maize coleoptiles. It was shown in the publication that the ratio of the quantity of high-affinity receptors sites to low-affinity receptor sites on isolated membranes [HA]/[LA] is approximately one to two. The authors made a conclusion that only the high-affinity binding site is involved in action of fusicoccin. This point of view in the following years became dominant. Thus, the authors suggested that participation of the high-affinity site as a fusicoccin receptor is according to the reconstruction of the system in vitro consisting of fusicoccin-binding protein and H+-ATPase A [35,36,37].
A small quantity or absence of low-affinity sites on most isolated membranes may be due to their inactivation during the isolation process. It is explained by a much greater lability of low-affinity binding sites in comparison with high-affinity ones. Currently, the issue of the functional role of the high- and low-affinity sites is the subject of research.
Membrane effects of fusicoccin belong to rapid answers: usually stimulation of protons emission begins immediately after adding fusicoccin and has no lag phase. In addition to the rapid membrane effects FC may have a prolonged, generalized action on plants that speaks in favor of its hormonal properties. Conspicuous is the fact that the current fusicoccin doses (10–20 mg/ha) are by two-three orders lower than that of other plant phytohormones and growth regulators (including those for gibberellin). In the publication of Babakov [38] the role of FC in the activation of protein kinase is discussed.
Claudio Olivari and his co-wokers [39] first stated that the activation process of H+-ATPase by fusicoccin requires the presence of another protein. In his work Claudio Olivari found that the phenylarsine oxide was a specific inhibitor of activation process of H+-ATPase by fusicoccin.
Very interesting is the action of fusicoccin on cells. First of all it concerns the acidification of the content of the cell under the influence of fusicoccin. The data that fusicoccin stimulates the protons emission in oat coleoptile cells were first specified in the work. The listed processes can be activated upon condition of the work stimulation of H+-ATPase or reduction of the ions leakage through ion channels of a plasmalemma. Actually, according to the latest data, fusicoccin affects both the activity of H+-ATPase, and the conductivity of plasmalemma potassium channels [40].
1.3 Natural Small Molecules and Their Use as an Anticancer Drug
Some small molecules that are used in preclinical as well as clinical studies influence PPIs of the Bcl-2 protein family of anti-apoptotic proteins [41]. Several compounds have been identified that target the hydrophobic binding pocket, which forms the BH3 binding site in Bcl-2, and prevent thereby the hetero-dimerization of Bcl-2 with pro-apoptotic members and thereby the induction of apoptosis. Another well known example is the nutlins (cis-imidazoline analogs) that target the p53/MDM2 interaction. These compounds bind selectively to MDM2 and prevent thereby the interaction with p53 and enhance the activity of the p53 pathway. It is interesting that healthy wild-type p53 cells retained their viability when treated with these nutlins, while wild-type p53 expressing cancer cells are killed. Besides these examples many other small molecules are known that prevent PPIs that are beneficial in the treatment of cancer. However, so far not many small molecules are known that affect cancer cells by stabilizing PPIs.
In recent years several reviews have been published about the isolation of (novel) natural compounds and active small molecules with an anticancer activity from various sources. Plants supplied several of these compounds while other anticancer drugs have been isolated from marine organisms and microorganisms like fungi. Although the working mechanism of many active small molecules is not yet (completely) clarified, most these compounds have been shown to belong to diverse structural classes, including polyketides, terpenoids, terpenes, alkaloids, and steroids and to influence PPIs. Furthermore, many drugs (>60%) that are currently used in cancer treatment are derived from natural occurring small molecules, either directly or indirectly. Paclitaxel (taxol) is, for instance, isolated from the bark of the Pacific yew tree Taxus brevifolia [42] and the vinca alkaloids vinblastine and vincristine are isolated from the Madagascar periwinkle Catharanthus roseus.
Fusicoccanes are a large group of active small molecules that refer to the terpenoids. They have a typical dicyclopenta[a,d] cyclooctane skeleton (5-8-5 core ring structure), to which various side chains are linked [43]. They are found throughout nature and are amongst others produced by various fungi, higher plants, liverworts, and even insects. A number of biological effects have been described for fusicoccanes on both plants and other organisms, and several of them are caused by the selective targeting of a particular protein or PPI. Some well-studied fusicoccanes are Fusicoccin-A (FC), Cotylenin-A (Cot-A), and Ophiobolin-A (OPH-A) (Fig. 2).
Chemical structure of Fusicoccin-A, Cotylenin-A and Ophiobolin-A [44]
The fungus infects peach and almond trees, in which the secreted FC stabilizes the complex formed between the C-terminal tip of the plasma membrane H+-ATPase and a 14-3-3 protein. This stabilization irreversibly activates the H + -ATPase, that drives the opening of stomata resulting in uncontrolled water loss and wilting of the tree. Although the only known FC target in plants is the H+-ATPase, at the cell and organ level FC affects a range of different processes, like opening the stomata, breaking seed dormancy, inducing an apoptotic like cell death in sycamore cells, and reducing hydrogen peroxide and nitric oxide production in guard cells [45].
Many of these processes can be (in)directly explained by the activation of the H+-ATPase while others suggest the presence of another FC target. FC is not only active on all higher plants, but on other organisms as well.
It randomizes, for instance, the left-right asymmetry early in the Xenopus laevis embryonic development [46] and induces apoptosis in cancer cells derived from different origins, for which the efficacy of FC can be enhanced by combining it with the cytokine interferon (IFNα).
Cotylenin-A was first described by Sassa (REF) who discovered it in a fungal culture filtrate of Cladosporium sp and described its growth enhancing effect on plant cotyledons. The structure of Cot-A is rather similar to that of FC, and comparisons made on their effects in plants show that both molecules have similar physiological effects. In some cases FC is more potent (e.g. induction of seed germination and wilting of tomato cuttings), while Cot-A is more effective in others (e.g. stomatal opening and cotyledon elongation). Besides this, Cot-A has been shown to induce differentiation in human myeloid leukemia cells and inhibits the growth of various tumor types both in vitro and in vivo, when combined with IFNα or rapamycin.
Ophiobolins (OPHs) are produced by plant pathogenic fungi belonging to the Bipolaris species. Currently 23 analogs have been identified, to which various biological activities are ascribed that are all damaging plants either directly or indirectly. Besides being harmful for plants, OPHs also possess anti-microbial activities as well as toxicity towards animals and even cancer cells. The working mechanism of most OPHs is not completely clear and probably differs per analog. OPH-A, however, has been shown to mainly function through the specific inhibition of calmodulin, an important signalling molecule.
Anticancer effects have been described for several fusicoccanes, including FC, Cot-A and OPH-A. All show a clear growth reduction at relatively low concentrations (IC50-value mostly in the μM range, depending on the cell line). Although the tumor selectivity differs per molecule, with some being relatively cancer selective while others are indiscriminate and kill healthy cells as well as cancer cells, fusicoccanes form an interesting group of potentially new anticancer compounds. The working mechanism of most fusicoccanes in inhibiting the cancer cell growth is not clear yet. OPH-A most likely acts through the selective inhibition of calmodulin by binding covalently to calmodulin with its C7-aldehyde group [47]. FC and Cot-A both lack this aldehyde group, suggesting that these compounds function through a different cellular target. When combined with IFN, FC and Cot-A both induce apoptosis by the induction of the TRAIL pathway, however, this is most likely the final effect of the combined treatment and the primary target of both compounds is therefore still unknown.
2 Experimental Methods
2.1 Preparation of Activated Carbons Based on Vegetable Raw Materials
In this work, a series of experiments on the physical and chemical activation patterns of raw materials was performed. Physical activation of raw materials samples was performed in a rotating stainless steel reactor of 0.5 dm3 at 19 rpm. Carbon dioxide gas, was chosen as an activating agent which is supplied from the bubbler into the reaction zone at a rate of 50 cm3/min. Carbonization was carried out in a horizontal pyrolysis installation with adjustable electric heating (Fig. 8) in the temperature range from 400 to 900 ℃, duration 1 h after carbonization yield at a predetermined temperature. For the experiment, crushed walnut shells, highlighting the sieving of the product of crushing the working fraction with a diameter of 2–4 mm [48].
2.2 Chromatographic Separation of Fusicoccin
For the experiment, 1 kg of seeds of spring wheat cultivar “Steklovidnaya-24” was taken. The seeds which were soaked for one day in a sterile cool tap water supplemented with 1 mM 6-BAP (6-benzyl aminopurine). 6-BAP was added as an inducer of the fusicoccin compounds. Then germinated seeds were homogenized in 3 l of 70% ethanol on the blade type homogenizer MPW-302 (Poland). The homogenate was centrifuged for 10 min at 10,000 × g. The resultant extract was purified by column nanostructured carbon sorbent.
In the presented work was used a column manufactured as follows (Fig. 3).
For a glass tube a rubber tube of the desired size was selected that it enters the tube only half or less. Then, with the narrow end of the plug blade we chose gentle concave surface, deepening the center plug, and then polishing the surface with sandpaper and the exact center of the plug was inserted into the plastic nozzle or syringe with a diameter of 1.5–2.5 mm. The nozzle must be inserted so that one end accounted for exactly the bottom surface of the funnel, and the other free exit from the wider end of the plug. If possible, it is desirable to have a plastic plug screw thread, which can be tightly screwed to various connecting plastic tubing or plug. After making the bottom plug with a hose, the narrow end of its snug nylon fabric (preferably from the children’s bow) was inserted into the cork with a cloth in the lower end of the column. After the plug firmly, but not much comes to a column sticking out of the column pieces of nylon fabric carefully cut with a razor cap more tightly planted on the column and the column was checked for leaks. It was very convenient to use the plug with the union in the upper part of the column. In this case, the cut surface of the funnel is not necessary, and the nozzle or needle is located on both sides of the plug. The top tube provides complete sealing of the column and continuity in the elution solution from a supply vessel with a minimum hydrostatic pressure [49].
2.3 Study of Biological Activity
2.3.1 Cytotoxic Activity
Cytotoxicity was assessed with the help of the survival test of brine shrimp larvae of Artemia salina (Leach). Experiments were carried out in 2-day-old larvae in cultivation conditions in vitro. The larvae were grown up by dipping of brine shrimp eggs A. salina (Leach) into the artificial sea water and by the 48 h incubation at a temperature of 37 ℃. A weighed portion of the test sample was dissolved in 2 ml of methanol, and then it was taken from that solution by 500 μl (3 parallels), 50 μl (3 parallels), 5 μl (3 parallels). After evaporation of methanol 5 ml of artificial seawater were added to each vial. Thus, if the initial sample weight was 2 mg, the final sample concentrations were 100 µg/ml, 10 µg/ml and 1 µg/ml, respectively of each concentration in 3 replications. 10 2-day-old brine shrimp larvae A. salina were put into each vial containing the sample using the Pasteur pipette. Thereafter, all the vials were left at room temperature exposed to light for 24 h. After 24 h survived and dead larvae were counted. Then, using the obtained data of the upper and lower toxic limits a half-toxic sample dose was calculated.
2.3.2 Antimicrobial Activity
The study of antimicrobial activity of the samples mentioned above was performed pertaining to gram-positive bacteria strains Staphylococcus aureus, Bacillus subtilis, gram-negative strains Escherishia coli and Candida albicans yeast fungus by the method of diffusion into the agar wells. Comparator agents were gentamicin for bacteria and nystatin for C. albicans yeast fungus.
Cultures were grown in a fluid medium with pH 7.3 ± 0.2 at a temperature of 30–35 ℃ during 18–20 h. The cultures were diluted 1:1000 in a sterile 0.9% sodium chloride isotonic solution; then they were poured into cups with 1 ml of solution with relevant elective nutrient media clarify for the studied test-strains and inoculated according to the method of “continuous lawn”. After drying on the surface of the agar wells of 6.0 mm size were formed, which were filled with the solution of the test sample, gentamicin and nystatin. Ethanol in equivoluminar quantities was used as control. Thus, the sample was tested in quantity of 1 µg and the comparator agent in quantity of 1 mg. The inoculations were incubated at 37 ℃, the accounting of growing colonies was performed after 24 h.
The antimicrobial activity of the samples was estimated by the diameter of test-strains growth inhibition zones (mm). The diameter of inhibition zones lower than 10 mm and the continuous increase in the Petri dish were estimated as the absence of the antibacterial activity, 10–15 mm—as a weak activity, 15–20 mm—as a moderate activity, over 20 mm—as an expressed one (REF). Each sample was tested in three parallel experiments. Statistical reporting was performed by methods of the parametric statistics with the calculation of arithmetic mean and standard error.
2.3.3 Analgesic Activity
The experimental objects were studied in the dose range from 25 to 100 mg/kg when administered intragastrically. The comparator agent diclofenac sodium was tested in the dose range from 25 to 100 mg/kg. The experimental objects and comparator agent were administered 30 min before administration of the 0.75% acetic acid solution.
2.3.4 Phagocytosis-Stimulating Activity
The comparator agent “Immunorm” (juice of Echinacea purpurea in alcohol, “Merkle”, Germany) which was tested at a dilution of 0.9% sodium chloride solution in the final concentration of 8%.
Blood sampling was taken from a healthy donor in the fasting state from the ulnar vein into heparinized test tubes. As an object of the phagocytosis the daily culture S. aureus (strain 209) was used. At the microscopic examination (the magnification 15 × 90, oil immersion) the number of phagocytic cells (the phagocytic index, PI) out of 100 neutrophils (quantitative indicator) and the number of staphylococci, absorbed by one neutrophil—the phagocytic number (PN, qualitative indicator of the phagocytosis) were counted after 1 h of study, Table 5.
75 mg of the dry sample with account of its solubility were diluted in 0.2 ml of 96% alcohol until fully dissolved and made up to 2 ml of 0.9% sodium chloride solution. The substances were tested at a concentration of 1 mg/ml in three parallel experiments. The smears of the blood incubated with the dilution medium (96% alcohol and saline 1:9) were served as controls.
Statistical results reporting was performed by methods of the non-parametric statistics with the calculation of the arithmetic mean (M) and its standard error (m) [50].
3 Results
3.1 Computer Modeling of Activated Carbon for Fusicoccin
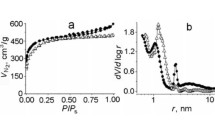
We have retrieved an X-ray structure of fusicoccin from the Brookhaven Protein Database (entry 3IQV) and optimised its geometry using molecular mechanics (MMFF) followed by semiempirical calculations (PM3). The size of the molecule (to its van der Waals limit) is about 1.9 × 1.5 × 1.1 nm. As it can rotate freely in solution it is unlikely to be bound within pores that are smaller than the compound’s largest dimension. Overlaying the molecule on a model of a graphene sheet and removing carbon atoms until fusicoccin just fits leaves a pore just over 2.1 nm in diameter. To allow for any solvent and rapid molecular rotation, we would suggest a pore size of 2.5 nm as the minimum that could be expected to bind the molecule.
According to the results of computer modeling, cleaning composite components of fusicoccin using microporous carbon adsorbents not suitable as the size of the molecule of fusicoccin more than micropores.
And in the following figure (Fig. 4) the optimum pore size for purification of constituents of fusicoccin was determined by computer simulation.
As the results of computer simulation in this work study show that the most effective recognized mesoporous activated carbons.
3.2 Extract of Phytohormone of Fusicoccin Containing Components
Fusicoccin was obtained by a technique developed in the ICP, as part of the bouquet of organic compounds. In this connection, there arose the task of separation of biologically active substances. To solve this task, the technique has been used with liquid chromatography sorbent made of Greek walnut (GW). A distinguishing property of the selected sorbent is that it contains carbon and silica in its structure, this leading to the presence of both hydrophobic and hydrophilic properties. To control the chromatographic separation, a UV monitor type Uvicord S II manufactured by LKB (Sweden) was used.
To release columns from unadsorbed substances, the column was washed with 10% ethanol to completely remove them, and then bounded with sorbent phytohormone was eluted with 50% ethanol.
The spectroscopic study of purified GW was conducted on spectrophotometer Ultrospec +1100 pro company of Amersham Biosciences (UK) in the ultraviolet and visible regions of the spectrum.
Fot the test, GW samples obtained at temperature of 750 ℃ were chosen. To compare the specific characteristics, nanomaterial organic gel Octyl Sepharose CL-4B (Sweden) used in the world today was taken.
The liquid containing FC was passed through a separation column with the experimental material. The results of the chromatographic separation are shown in Fig. 5.
The first peak (Fig. 5) contained substances that do not bind with sorbent. For release the column from unadsorbed substances, the column was washed with 10% ethanol prior to complete removal, and then the phitohormone bound with the sorbent was eluted with 50% ethanol (2nd peak). Analysis of the figures of separation suggests that the synthesized sorbent has separation characteristics which are not inferior to the world analogues. However, the greatest advantage of this material is that it has a high resistance with respect to the microbiological media and the lack of parasitic sorption, unlike organic gel Octyl Sepharose CL-4B (b), having agarose in its structure [51].
3.3 Mass Spectrometric Analysis. Fragmentation of Fusicoccin Using MS/MS
Figures 6 and 7 show the spectra obtained when the polarity of the voltage applied in the electrospray (ESI) (source coupling the LC and MS) was positive and negative, respectively.
The full scan spectra in the preferential formation of a sodium adduct of fusicoccin [fusicoccin + Na]+. Other species without such adduct were not detected. Multiple low intense peaks appeared, when infusing fusicoccin with ESI (−).
ESI (+) was chosen as the optimal conditions to monitor fusicoccin given that ESI (−) caused low intense peaks that we could not relate to fusicoccin.
The full scan spectra in F the preferential formation of a sodium adduct of fusicoccin [fusicoccin + Na]+. Other species without such adduct were not detected. The most abundant product ions are 633 and 431. Fragmentation (MS/MS) of the ion m/z 703.3 with the amplitude applied in the trap shown in Fig. 8.
The optimal fragmentation amplitude (0.7 V) was chosen as the one causing the highest intensity of product ions keeping at least 5% of precursor ion (Fig. 9).
Fragmentation MS3 of m/z 703 [fusicoccin + Na]+ and analysis of the resultant ions by product ion scan mode shown in Fig. 10.
Respectively, in Fig. 11 shown fragmentation MS3 of m/z 703 [fusicoccin + Na]+ (fragmentation route 2) and analysis of the resultant ions by product ion scan mode.
3.4 Analysis of Samples on Chromatogram
Ethanol extracts obtained from the elution of the retained fraction of wheat samples from a carbon column. In addition, there was a solid extract that was extracted at Brighton University by mixing it with 5 ml methanol and sonication (30 min), Fig. 12.
In Fig. 12 shown the full scan analisys from the solid extract sample.
Two intense peaks can be detected, the first one with m/z 701 as most abundant ion (see spectra below), which also contains m/z 703 as isotopic ion, and in the second one, m/z 703 (rt 30–35 min) can also be detected (fusicoccin m/z).
3.5 Determination of the Chemical Composition of the Samples by Liquid Chromatography
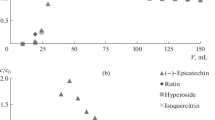
Objects of the study: extract obtained by elution with 50% ethanol in the separation of 70% ethanol extract of milk-ripe wheat and extract obtained by elution with 10% ethanol in the separation of 70% ethanol extract of milk-ripe wheat, Figs. 13, 14 and 15.
The fraction extract obtained by elution with 10% ethanol in the separation of 70% ethanol extract of milk-ripe wheat shown in Fig. 14.
The fraction obtained by elution with a 50% ethanol in the separation of 70% ethanol extract of milk-ripe wheat shown in Fig. 15.
The results of the quantitative content of fusicoccin in fractions after preparative separation are shown in Table 1.
The fraction extract obtained by elution with 10% ethanol in the separation of 70% ethanol extract of milk-ripe wheat don’t have fusicoccin.
3.6 The Examination of the Cytotoxic Activity of the Obtained Fractions
Objects of the study were 8 sample substances for the presence of cytotoxic activity pertaining to brine shrimp larvae Artemia salina (Leach) under cultivation conditions in vitro.
Samples designation (name):
-
1.
A0—crude extract;
-
2.
A1—fraction after the preparative separation;
-
3.
A2—fraction after the preparative separation;
-
4.
A3—fraction after the preparative separation;
-
5.
A4—fraction after the preparative separation;
-
6.
A5—fraction after the preparative separation;
-
7.
A6—fraction after the preparative separation;
-
8.
A7—fraction after the preparative separation.
Statistical results reporting was conducted using FNI computer program.
Findings of investigations.
The results of the testing of the cytotoxic activity of the samples A0, A1, A2, A3, A4, A5, A6, A7 pertaining to brine shrimp larvae A. salina (Leach) under cultivation conditions in vitro are listed in the Table 2.
As the table shows the crude extract A0 and the following fractions after the preparative separation (A1, A4, A5, A6, A7) exhibit the cytotoxic activity pertaining to brine shrimp larvae A. salina (Leach).
3.7 Antimicrobial Activity Tests
Materials and methods: Objects of the study were 8 samples of fusicoccin compounds for the presence of antimicrobial activity.
Samples designation (name):
Findings of investigations. The results of the samples’ antimicrobial activity testing are listed in Table 3.
It was stated that all presented samples exhibit an antibacterial activity pertaining to the gram-positive microorganisms S. aureus and B. subtilis, and the gram-negative test-strain E. coli.
3.8 The Study of the Analgesic Activity of Naturally Occurring Substances and Their Derivatives
The screening results of the analgesic activity of the crude extract and its fractions after preparative separation are listed in the Table 4. The analgesic effect of the submitted samples was determined by the ability to reduce the number of “writhes” (in %) calculated for 10, 15, 20 and 30 min and compared to the corresponding figures in animals in the control group, Table 4.
The study revealed that the fraction after preparative separation (7) has an expressed analgesic effect at a dose of 100 mg/kg, the remaining samples showed a weak analgesic activity.
3.9 Testing for the Phagocytosis-Stimulating Activity
The results of the screening study of the blood cells phagocytic activity are listed in the table (Table 5).
Based on data from the table the comparator agent “Immunorm” has an expressed phagocytosis-stimulating action pertaining to both quantitative and qualitative indicators of the blood neutrophils phagocytosis.
4 Conclusion
For the first time a method of determining the optimum pore size of sorbents for the selection of fusicoccin was developed using the program Spartan Graphical interface and found that as optimal sorbent with mesoporous structure for cleaning fusicoccin was used walnut shell. A sensitive and selective method is developed in samples of milky ripe wheat by analysis of fusicoccin by liquid chromatography and mass spectrometry. As a result of the investigation of the cytotoxic activity and biological activities of the extracts fusicoccin.
References
Dubinin, M. M. (1978). Adsorbents, their preparation, properties and applications. -M, 4–22.
Aygun, A., Yenisoy-Karakas, S., & Duman, I. (2003). Production of granular activated carbon from fruit stones and nutshells and evaluation of their physical, chemical and adsorption properties. Microporous and Mesoporous Materials, 66, 189–195.
Guo, Y., Yang, S., Yu, K., Zhao, J., Wang, Z., & Xu, H. (2002). Carbon materials for sorption. Materials Chemistry and Physics, 74, 320.
Daud, W. M., & Ali, W. S. (2004). Comparison on pore development of activated carbon produced from palm shell and coconut shell. Bioresource Technology, 93, 63–69.
Benaddi, H., Bandosz, T. J., Jagiello, J., Schwarz, J. A., Rouzaud, J. N., Legras, D., et al. (2000). Surface functionality and porosity of activated carbons obtained from chemical activation of wood. Carbon, 38, 669–674.
Asma, B. M. (2000). Apricot production. Malatya Evin Ofset, Turkey, 240 p.
Smisek, M., & Cerny, S. (1970). Active carbon manufacture, properties and aplications (370 p). New York: Elsevier Publishing Company.
Khezami, L., Chetouan, A., Taou, B., & Capar, R. (2005). Production and characterisation of activated carbon from wood components in powder: Cellulose, lignin, xylan. Powder Technology, 157, 48–56.
Hayashi, J., Kazehaya, A., Muroyama, K., & Watkinson, A. P. (2000). Preparation of activated carbons from lignin by chemical activation. Carbon, 38, 1873–1878.
Jandosov, J. M., Shabanova, T. A., Shamalov, M., Biysenbaev, M. A., & Mansurov, Z. A. (2010). Preparation of carbon materials with high specific surface area. Combustion and Plasma Chemistry, 8(3), 257–261.
Klijanienko, A., Grabowska, E. L., & Gryglewicz, G. Z. (2008). Development of mesoporosity during phosphoric acid activation of wood in steam atmosphere. Bioresource Technology, 99, 7208–7214.
Jibril, B., Houache, O., Maamari, R. A., & Rashidi, B. A. (2008). Effects of H3PO4 and KOH in carbonization of lignocellulosic material. Journal of Analytical and Applied Pyrolysis, 83, 151–158.
Azat, S. (2013). Synthesis of carbonized nano mesoporous sorbents based on vegetable raw materials. Nanoscience and Nanoengineering International Journal, 1(1), 41–44.
Azat, S., Pavlenko, V. V., Kerimkulova, A. R., & Mansurov, Z. A. (2012). Synthesis and structure determination of carbonized nano mesoporous materials based on vegetable raw materials. Advanced Materials Research, 535–537. Online available since Jun 14, 2012 at www.scientific.net.
Fierro, V., Fernandez, V. T., Montane, D., & Celzard, A. (2008). Adsorption of phenol onto activated carbons having different textural and surface properties. Microporous and Mesoporous Materials, 111, 276–284.
Cookson, J. T. (1980). Carbon adsorption handbook. In P. N. Cheremisinoff & F. Ellerbusch (Eds.) (pp. 241–279). Michigan: Ann Arbor Sci.
Keltsev, N. V. (1976). Fundamentals of adsorption technology. Moscow Chemistry, 512.
Greg, S., & Singh, K. (1970). Adsorption, surface area, porosity. M.: Mir, 259.
Mansurov, Z. A., Zhylybaeva, N. K., Ualieva, P. S., & Mansurova, R. M. (2002). Obtaining procedure and properties of the sorbents from plant raw material. Chemistry for Sustainable Development, 3, 321–328.
Mansurov, Z. A., Digel, I., Biisenbaev, M. A., Savitskaya, I., Kistaubaeva, A., Akimbekov, N., et al. (2012). Composites and their applications. INTECH 2012 Chap. 11, 271–295.
Mansurov, Z. A., Shabanova, T. A., & Mansurova, R. M (2004). The morphology of micro nano particles of carbonized plant materials. Bulletin of KazNU. Series of Chemical, 2(34), 129–135.
Basso, M. C., Cerrella, E. G., & Cukierman, A. L. (2002). Activated carbons from a rapidly renewable biosource for removal of cadmium(II) and nickel(II) ions from dilute aqueous solutions. Industrial & Engineering Chemistry Research, 41, 180–189.
Jia, Y. F., & Thomas, K. M. (2000). Adsorption of cadmium ions on oxygen surface sites in activated carbon. Langmuir, 16, 1114.
Azat, S., Kerimkulova, A. R., & Mansurov, Z. A. (2012). Synthesis and structure determination carbonated nanomaterials based on vegetable raw materials. In VII International Symposium “Physics and Chemistry of Carbon Materials/Nanoengineering”, Almaty (pp. 124–126). On Sept 19–21.
Mansurov, Z. A. (2008). Synthesis of carbon nanomaterials and their applied aspects. Herald A Series of Chemical, 2(50), 16–31.
Azat, S. Mansurov, Z. A. (2011). Wastewater treatment using for carbonized nanosorbents. Vestnik KazNU Chemical Series, 1(61), 166–169.
Emuranov, M., Yu, S., Zhylybaeva, N. K., Biysenbaev, M. A., Shabanova, T. A., Ryabikin, Yu., et al. (2006). Multifunctional nanostructured carbonized sorbents. Bulletin of National Academy of Sciences of Kazakhstan A Series of Chemical, 4, 35–41.
Bevla, F. R., Rico, D. P., & Gomis, A. F. (1984). Activated carbon from almond shells. Chemical activation. Activating reagent selection and variables influence. Industrial Engineering Chemistry Product Research and Development, 23, 266–269.
Aygun, A., Yenisoy-Karakas, S., & Duman, I. (2003). Production of granular activated carbon from fruit stones and nutshells and evaluation of their physical, chemical and adsorption properties. Microporous and Mesoporous Materials, 66, 189–195.
Razvigorova, M., Budinova, T., Petrov, N., & Minkova, V. (1998). Purification of water by activated carbons from apricot stones, lignites and anthracite. Water Research, 32, 2135–2139.
Ballio, A., Chain, E. B., DeLeo, P., Erlanger, B. F., Mauri, M., & Tonolo, A. (1964). Fusicoccin: A new wilting toxin produced by Fusicoccum Amygdali Del. Nature, 203(4642), 297.
Babakov, A. V., Abramycheva, N. Y., Bilushi, S. T., & Shevchenko, V. P. (1990). Research fusicoccin interaction with the plasma membrane of higher plants. Biology Membrane, 7.2. M, 107–112.
Tajima, N., Nukina, M., Kato, N., & Sassa, T. (2004). Novel fusicoccins R and S, and the fusicoccin S aglycon (phomopsiol) from Phomopsis amygdali Niigata 2-A, and their seed germination-stimulating activity in the presence of abscisic acid. Bioscience, Biotechnology, and Biochemistry, 68(5), 1125–1130.
Aducci, P., Ballio, A., Fogliano, V., Fullone, M. R., Marra, M., & Proietti, N. (2003). Purification and photoaffinity labeling of fusicoccin receptors from maize. Development, 130(20), 4847–4858.
De Boer, A. H., Watson, B. A., & Cleland, R. E. (1989). Purification and identification of the fusicoccin binding protein from oat root plasma membrane. Plant Physiology, 89(1), 250–259.
Trofimova, M. S., Smolenskaya, I. N., Drabkin, A. V., Galkin, A. V., & Babakov, A. V. (1997). Does plasma membrane H+ATPase activation by fusicoccin involve protein kinase. Physiologia Plantarum, 99(2), 221–226.
Olivari, C., Meanti, C., De Michelis, M. I., & Rasi-Caldogno, F. (1998). Fusicoccin binding to its plasma membrane receptor and the activation of the plasma membrane H + -ATPase IV. Fusicoccin induces the association between the plasma membrane H + -ATPase and the fusicoccin receptor. Plant Physiology, 116(2), 529–537.
Oecking, C., Eckerskorn, C., & Weiler, E. W. (1994). The fusicoccin receptor of plants is a member of the 14-3-3 superfamily of eukaryotic regulatory proteins. FEBS Letters, 352, 163–166.
Olivari, C., Albumi, C., Pugliarello, M. C., & De Michelis, M. I. (2000). Phenylarsine oxide inhibits the fusicoccin-induced activation of plasma membrane H + -ATPase. Plant Physiology, 122(2), 463–470.
Baunsgaard, L., Fuglsang, A. T., Jahn, T., Korthout, H. A. A. J., De Boer, A. H., & Palmgren, M. G. (1998). The 14-3-3 proteins associate with the plant plasma membrane H + -ATPase to generate a fusicoccin binding complex and a fusicoccin responsive system. The Plant Journal 13(5), 661–671.
De Vries-van Leeuwen, I. J., Kortekaas-Thijssen, C., Nzigou Mandouckou, J. A., Kas, S., Evidente, A., & De Boer, A. H. (2010). Fusicoccin-A selectively induces apoptosis in tumor cells after interferon-a priming. Cancer Letters, 293(2), 198–206.
Sassa, T., Tajima, N., Sato, M., Takahashi, A., & Kato, N. (2002). Fusicoccins P and Q, and 3-epifusicoccins H and Q, new polar fusicoccins from isolate Niigata 2-A of a peach fusicoccum canker fungus. Bioscience, Biotechnology, and Biochemistry, 66(11), 2356–2361.
Come, C., Laine, A., Chanrion, M., Edgren, H., Mattila, E., Liu, X., et al. (2009). CIP2A is associated with human breast cancer aggressivity. Clinical Cancer Research, 15(16), 5092–5100.
Wurtele, M., Jelich-Ottmann, C., Wittinghofer, A., & Oecking, C. (2003). Structural view of a fungal toxin acting on a 14-3-3 regulatory complex. EMBO Journal, Chemical structure of Fusicoccin-A, Cotylenin-A and Ophiobolin-A, 22(5)(C), 987–994.
Korthout, H. A. A. J., & De Boer, A. H. A. (1994). fusicoccin binding protein belongs to the family of 14-3-3 brain protein homologs. The Plant Cell, 6(11), 1681–1692.
Sassa, T., Tajima, N., Sato, M., Takahashi, A., & Kato, N. (2002). Fusicoccins P and Q, and 3-epifusicoccins H and Q, new polar fusicoccins from isolate Niigata 2-A of a peach fusicoccum canker fungus. Bioscience, Biotechnology, and Biochemistry, 66(11), 2356–2361.
Bunney, T. D., De Boer, A. H., & Levin, M. (2003). Fusicoccin signaling reveals 14-3-3 protein function as a novel step in left-right patterning during amphibian embryogenesis. Development, 130(20), 4847–4858.
Pavlenko, V. V., Anurov, S. A., Mansurov, Z. A., Biysenbaev, M. A., Konkova, T. V., Azat, S., et al. (2014). Preparation of microporous activated carbons based on carbonized shell apricot. Vestnik KazNU, Chemical Series, 3(75), 103–113.
Mansurov, Z. A., Jandosov, J. M., Kerimkulova, A. R., Azat, S., Zhubanova, A. A., Digel, I. E., et al. (2013). Nanostructured carbon materials for biomedical Use. Eurasian Chemico-Tecnological Journal, 15(3), 209–217.
Mansurov, Z. A., Azat, S., Adekenova, A. S., Kerimkulova, A. R., Ivasenko, S. A., Shulgau, Z. T., et al. (2013). Extraction fusicoccin from wheat seeds using nanocarbon sorbents. Advanced Materials Research, 647, 67–70.
Azat, S., Adekenova, A. S., Ivasenko, S. A., Seydahmetova, R. B., Kerimkulova, A. R., & Mansurov, Z. A. (2012). Development of technology for drug fusicoccin on nanocarbon sorbents and the study of the biological activity. Pharmaceutical Bulletin Scientific Journal, 2–3(164), 57–60.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mansurov, Z.A., Azat, S., Kerimkulova, A.R. (2018). Extraction of Fusicoccin-like Compounds Using Nanocarbon Sorbents and Study of Their Biological and Cytotoxic Activity. In: Artmann, G., Artmann, A., Zhubanova, A., Digel, I. (eds) Biological, Physical and Technical Basics of Cell Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-10-7904-7_12
Download citation
DOI: https://doi.org/10.1007/978-981-10-7904-7_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7903-0
Online ISBN: 978-981-10-7904-7
eBook Packages: EngineeringEngineering (R0)