Abstract
Biomimetic artificial membranes are convenient, versatile models that borrow from the principles of biological systems and mimic the physiological characteristics of natural cell membranes by exploiting simple nanostructured materials. To construct an artificial membrane, it is important to first understand the biology of natural membranes and to recognize the primary differences between the cellular membranes of different organisms. The creation of biomimetic membranes can be achieved with a minimal number of living or non-living components while sufficiently retaining the basic properties of cellular life. The successful development of biomimetic membranes promotes an understanding of basic cellular functions and assists in the generation of semi-natural systems with new functions, the fabrication of selective and sensitive biosensing platforms, and the development of new biotechnology in different fields ranging from medicine to the environment. This chapter presents the most common model biomimetic membranes that are currently available and their applications, as well as their preparation methods, general investigation techniques, properties, and limitations.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Biological membranes
- Biomimetic model membranes
- Supported lipid membranes
- Lipid vesicles
- Membrane proteins
- Biosensing platforms
- Nanostructured materials
1 Introduction
Synthetic biomimetic membranes that designed to mimic the fundamental architecture of biological membranes are discussed in chapter “Can We Rebuild the Cell Membrane?” written by Dr. Samar Damiati. This chapter describes the most common biomimetic membrane models that are fabricated to simplify the complexity of biological membranes by exploiting simple nanostructured materials and the natural molecular mechanisms. Development of biomimetic membranes is a good example for synthetic biology which aims not only to advance our understanding of the biological systems by mimicking existing cellular structure and function, but also to design and engineer semi-natural systems with new functions which do not occur naturally. This chapter can be presented as a synthetic biology toolbox for biomimetic membranes assembly.
Nature is the richest source of inspiration to design and develop man-made molecular machines. Copying nature is a highly demanded skill that can be used to synthesize new biocomponents or to modify existing biocomponents, respectively, to perform novel functions that are not found in nature or to enhance existing systems. The complexity observed in nature arises from the highly organized structures of biomolecules and their complicated interactions. Hence, bioinspired mimics can help build an understanding of how biological systems work. Rapid advances in molecular engineering and high-resolution analytical techniques have allowed scientists to create biomimetic structures with high molecular precision. Understanding and engineering biological systems through mimicry can be defined as synthetic biology. This science is a rapidly growing multidisciplinary field that involves biology, chemistry, physics, mathematics and engineering, and it aims to redesign or construct a semi-synthetic system with the basic properties of cellular life by minimizing the chemical components required and controlling the physical parameters of the system. Although this strategy sounds simple, building an artificial system with the desired characteristics is still difficult and requires many trials to ensure that the system is effective. Although constructing a simple biological system depends on the assembly of basic biological components to perform specific functions, the generated system usually does not behave as expected due to the lack of some biological information. Hence, synthetic biology involves two approaches to building semi-synthetic systems: bottom-up and top-down [53]. In a bottom-up strategy, the basic elements (non-living components) of biological origins, such as nucleic acids, amino acids, lipids, and sugars, are exploited to create artificial life de novo. By contrast, the top-down approach starts with a complex living organism and attempts to make it as simple as possible while considering the biological elements as parts of the system.
Synthetic biology has generated interest in biomimetic membranes due to the ubiquitous presence of membranes in biological systems. Moreover, the complex nature of the structure and function of biological membranes is a challenge that can be overcome by constructing biomimetic models. However, membrane technology can be easily scaled up, requires mild conditions, no or low energy consumption, and that presently has a broad range of applications in small- and industrial-scale separations. Biomimetic or bioinspired membranes can be generated through the self-assembly of carefully chosen building materials in a near-natural environment in which several parameters, such as the temperature, pressure, and aqueous surroundings, are controlled. The elementary materials that are used to build the membrane are usually common biocomponents with excellent wetting, adhesive, hydrodynamic, and mechanical properties.
So, can we rebuild a cell membrane? The simple answer is yes, we can. This chapter provides insight into the basic characteristics of biological membranes in terms of membrane composition. The answer to the question above is described by a brief overview of the different biomimetic model membranes and their preparation methods. Furthermore, the advantages and disadvantages of each model are summarized.
2 Biological Membranes
The biological membrane has a critical role in cellular life. It has important functions in cells, such as protection, compartmentalization, and maintaining two-dimensional fluidity, and furthermore, it acts as an effective diffusion barrier that controls membrane permeability. Natural membranes possess unique features such as self-healing, anti-fouling, and selective permeability. These membranes are only a few nanometers thick, they are perfectly organized at the molecular level, and they are composed of three essential components: lipids, proteins, and carbohydrates. Lipids and proteins present a highly complex composition in biological membranes, whereas carbohydrates are located outside the membrane and are attached to lipids or proteins to form glycolipids or glycoproteins, respectively. Hence, the structure and function of the cell membrane are based on the fluid lipid bilayer and the incorporated proteins.
2.1 Lipid Compositions of Biological Membranes
The lipid compositions of biological membranes are primarily glycerophospholipids (also known as phospholipids), sphingolipids, and, in eukaryotic cells, sterols. Lipids are small, amphiphilic molecules consisting of a hydrophilic head group and a hydrophobic tail. These two components, and the interface formed between them, define the physical characteristics of any lipid molecule. The cell membrane is formed by a spontaneous self-assembly process driven by the amphipathic nature of phospholipid molecules; in this assembly process, a planar bilayer is formed with the non-polar groups pointed toward the hydrophobic interior of the bilayer and the polar head groups oriented toward the external aqueous phase [24]. Lipid head groups have been classified into several categories, each of which has unique chemical properties. For example, some head groups have a negative charge and exhibit charge-charge repulsion, which forms a larger effective cross-sectional area [26]. Moreover, the changes in the pH values that affect the lipid charge depend on the pKa values of the head group either by imparting or eliminating the charges [12]. However, there are significant differences between bacterial and mammalian membranes (Fig. 1).
Bacteria are classified into two broad groups as follows based on the cell wall structure: Gram-positive bacteria, which have a single cytoplasmic membrane surrounded by a thick layer of peptidoglycan, and Gram-negative bacteria, which have two membranes consisting of cytoplasmic and outer membranes surrounded by a thin layer of peptidoglycan. Both classes of bacteria have different lipopolysaccharides in their membranes. Gram-positive bacteria contain lipoteichoic acids (LTAs) embedded within the cytoplasmic membrane, whereas Gram-negative bacteria contain lipopolysaccharides that form the primary lipid component of the outer leaflet of the outer membrane. Indeed, there is a difference in the lipid compositions of the bacterial cytoplasmic membranes. The predominant zwitterionic phospholipids in most bacteria are phosphatidylethanolamines (PEs), whereas the predominant anionic phospholipids are phosphatidylglycerol (PG) and cardiolipin (CL). Gram-positive bacteria have lower PE contents than Gram-negative bacteria, and there are at least 15% anionic lipids in both bacterial types [25]. Prokaryotic cells lack cholesterol in their membranes; they instead have sterol-like molecules called hopanoids [59]. The exposure of these anionic lipids provides for the selectivity of cationic antimicrobial agents that ensure toxicity against bacterial cells but not against mammalian cells.
Mammalian membranes are primarily composed of different structural lipids, such as phosphatidylcholine (PC), phosphatidylserine (PS), sphingomyelin (SM), phosphatidylinositol (PI), PE and cholesterol. These lipid species are present at different ratios depending on the cell type and function. In general, in most eukaryotic membranes, PC accounts for more than 50% of the phospholipids [75]. Lipids are asymmetrically distributed in the bilayer structure; PE and PS are primarily found in the inner leaflet of the plasma membrane, whereas PC and sphingomyelins are found in the outer leaflet. Cholesterol, the primary steroid in eukaryotic cells, has a significant role in determining membrane fluidity. The polar hydroxyl groups of cholesterol are inserted into the lipid bilayer close to the hydrophilic head groups of phospholipids, whereas the rigid hydrocarbon rings interact with the fatty acid chains. This interaction increases membrane rigidity and decreases its mobility. However, membrane lipids can be present in cylindrical (e.g., PC, and PS), conical (e.g., PE) or inverted conical (e.g., lysophosphatidylcholine) shapes (Fig. 2) [37, 47].
This diversity in shapes (polymorphism) influences the localization of lipids within the biological membranes and is responsible for phase separation within single monolayer leaflets. The shape of a lipid molecule in a membrane depends on the relative sizes of its polar head group and polar tails [69]. Biological membranes contain more lipids than proteins because lipid molecules are smaller than protein molecules and can act as a scaffold for membrane proteins.
2.2 Membrane Proteins
Although lipid molecules provide the basic structure of biological membranes, peptides and proteins are the dominant building blocks in biological systems because they perform most of the specific functions of organic tissues. Hence, peptides and proteins provide the cellular membrane with its functional characteristics. The basic building blocks of any protein are 20 amino acids that are encoded by DNA and are composed of two parts: an identical backbone (a carboxyl group and an amino group) and a side chain that varies in size and polarity. The amino acids in any polypeptide chain occur in a specific order (the primary structure) and are folded into a three-dimensional (3D) structure to establish the function of the protein and enhance its stability. Therefore, proteins are proposed to fit into a specific environment, such as an aqueous environment or in a membrane, where they interact with lipids or exist in a complex with other proteins. Protein folding occurs in a complex and crowded molecular environment within the cell and usually requires the assistance of molecular chaperones to prevent protein misfolding. Indeed, these chaperone molecules help the protein to engage in localization, assembly and disassembly without becoming permanent parts of the structure [3, 23].
There are two types of membrane-associated proteins: peripheral and integral proteins. Peripheral proteins are water-soluble molecules that are not inserted into the lipid bilayer but rather are attached to the inner or outer side of the membrane via non-covalent interactions, such as hydrophobic and electrostatic interactions. These proteins can also be attached covalently to the membrane via hydrophobic amino acids. By contrast, integral proteins are water-insoluble molecules that are embedded into a single lipid monolayer or bilayer. Most integral proteins are transmembrane proteins that function as gateways that control the movement of ions and small molecule into or out of the cell. Membrane proteins usually tend to denature once they are isolated from the lipid environment, losing their functionality due to misfolding. Furthermore, the low abundance of these proteins makes their investigation a challenging task due to the difficulty of the isolation and purification processes.
3 Biomimetic Model Membranes
The sophisticated structures and functions of biological membrane components (lipids and proteins) have inspired scientists to design artificial membranes through different chemically and physically controlled mechanisms. Moreover, in biological membranes, the lipid-lipid, protein-lipid, and protein-protein interactions are highly complex, which complicates the investigation of the distinct membrane components that are responsible for specific effects. Hence, biomimetic membranes offer a promising platform to overcome these limitations.
Bioinspired or biomimetic membranes are fabricated with natural or natural-like components by exploiting natural molecular mechanisms and the molecular self-assembly process to generate membranes that possess biologically accurate properties. The most common biomimetic model membranes are described below.
3.1 Black Lipid Membranes (BLMs)
The BLM was the earliest artificial membrane derived from extracted brain lipids, and it was introduced by Mueller and colleagues in 1962 [45]. The name of this membrane is attributed to its appearance under optical microscopy, which results from the optical behavior of the membrane when formed. Under reflected light, this very thin lipid film (20–25 Å) reflects little to no light relative to its surroundings and appears “black”; hence, the term “black lipid membrane”. The thickness of this membrane fluctuates because it is compressible [52]. There are several methods of forming BLMs, but all of the procedures involve the formation of a free-standing lipid bilayer over a small aperture, which is usually less than 1 mm in diameter and is made in a hydrophobic substrate material, such as Teflon or polyethylene. The aperture is usually part of a wall separating two chambers filled with aqueous solutions containing reference electrodes. The two most popular methods of forming BLMs are described below.
3.1.1 Painting Technique
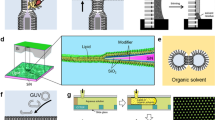
This method involves the use of a brush to paint a lipid dissolved in an organic solvent, such as n-decane or squalene, directly over an aperture under aqueous conditions (Fig. 3). The deposited lipid mass becomes thinner as it spreads, resulting in the spontaneous formation of a BLM. The primary drawback of the painting BLM technique is the use of hydrocarbon solvents, which are not present in natural biological membranes. Moreover, the resulting membrane retains the solvent, which can change its characteristics and reduce its stability [14].
3.1.2 Monolayer Folding Painting
This method is a modified form of the previous technique that allows for organic solvent evaporation [44]. Here, it is important to have an experimental cell that controls the solution level independently in each chamber (Fig. 4). The lipid solution is applied on top of one chamber above the aqueous solution and the researcher waits until the volatile solvent evaporates, which results in the formation of a lipid monolayer. The solution level containing the resultant monolayer is slowly lowered below the aperture and then raised again. This step deposits a monolayer on each pass, resulting in the formation of a solvent-free lipid bilayer film.
Both the painting and monolayer folding painting techniques generate a free-standing planar lipid bilayer that allows the transmembrane protein to be fused. Therefore, BLMs are widely used as a model system to study membrane proteins. The suspension of BLMs in solution in the absence of an underlying support allows the membrane proteins to remain fully mobile and active, which makes the BLM similar to a living membrane system. BLMs have been used to examine different biophysical processes, such as the formation of ion channels in the lipid bilayer by pore/channel-forming biomolecules, which include peptides, proteins, and antibiotics [14, 35]. BLMs can be investigated electrochemically or by simple light microscopy. For example, the thickness of a generated BLM may be calculated using the measurements of reflected light intensity [56]. Importantly, BLMs suffer from a limited lifetime (usually less than one hour), poor mechanical stability, and limited detection methods. This poor stability can be addressed by using a supportive substrate. Developing and improving BLM architectures through the use of, for example, chip-based platforms improves the stability, reproducibility, and generation of membranes with different geometries [15]. Indeed, simple imaging and electrochemical techniques are usually used with BLMs, and more advanced optical/fluorescence microscopy or advanced techniques are in high demand.
3.2 Langmuir Monolayers
Another simple model that mimics the biological membrane is the Langmuir monolayer. This two-dimensional system provides a unique model for studying the interactions of small peptides with phospholipids at the air-water interface due to its homogeneity, stability and planar geometry. The Langmuir monolayer is useful in characterizing lipid-lipid interactions and evaluating the insertion of amphipathic molecules, such as drugs or antimicrobial peptides, into the membrane [4, 41]. These monolayers are easily prepared by continuously adding a lipid solution to the air-water interface of a constant area, which subsequently forms an insoluble film via the self-assembly of lipid molecules in a specific orientation. In this self-assembly process, the head groups point toward the aqueous phase, whereas the acyl chains point toward the air; thus, the resulting structure is considered a half-membrane (Fig. 5). This model is suitable for mimicking processes at the membrane surface but is not suitable for investigating transmembrane processes. Active compounds (e.g., peptides, drugs, and biosurfactants) can be injected into the water sub-phase after stabilizing the lipid monolayer at a defined initial surface pressure. The interaction between the active compound and the lipid film can be recorded by monitoring the increases in the surface pressure [22]. However, monolayers present a rich polymorphism that can easily be characterized by several techniques, such as optical microscopy and X-ray diffraction [13]. Even so, Langmuir monolayers have some limitations, such as the absence of bilayer structure similar to that of the biological membrane, the high surface tension of water, and its unsuitableness for membrane protein reconstitution [14, 16].
3.3 Hybrid Bilayer Lipid Membranes (hBLMs)
This biomimetic model is a modified lipid monolayer that has been introduced by using alkanethiols (e.g., octadecanethiol) to form self-assembling monolayers (SAMs) on several metals, such as gold (the most widely used), silver, and mercury. Indeed, several polymer films with hydrophobic side chains can also be used to form SAMs (Fig. 6). These SAMs can be formed by incubating alkanethiol in an ethanol solution on an electrode surface, for 12–24 h on gold or silver, and for 5–20 min on mercury. An alternative preparation method involves a Langmuir-Blodgett (LB) transfer (this technique will be described later in this chapter). Both methods produce a tightly packed and well-ordered hydrophobic monolayer. However, the self-assembly of a SAM includes the anchoring of the thiol via the sulfhydryl groups to the metal surface, which leads the hydrocarbon chains to point toward the aqueous solution. There are two general methods for introducing a lipid monolayer (which serves as the upper leaflet) to the SAM that covers the hydrophobic surface. The first method involves the spontaneous fusion of lipid vesicles onto the SAM, whereas the second involves the horizontal transfer of a lipid monolayer that is supported in a Langmuir trough from an air-water interface to a SAM. Both methods result in the generation of a hybrid bilayer membrane (hBLM) that consists of a single lipid layer stabilized onto an alkanethiol SAM [14]. Due to the strong interactions between the SAM layer and the underlying surface, hBLMs are very robust and stable over long time periods. Moreover, some of the properties of a alkanethiol/lipid hybrid bilayer generated at the air-water interface can be preserved without change after the bilayer has been dried and rehydrated.
Different alkanethiols and lipids can alter the physical properties of hBLMs. For example, the membrane capacitance decreases when a thicker membrane is generated due to the increased chain length of the alkanethiol or phospholipids. However, hBLMs can be characterized by a wide range of techniques, such as surface plasmon resonance (SPR), quartz crystal microbalance with dissipation monitoring (QCM-D), and electrochemical measurements that include cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). In fact, hBLMs allow for the detection of non-labeled analytes due to the direct coupling of the membrane to the metallic surface. Despite the close packing and rigidity of alkanethiol, this SAM still has some limitations. This supported lipid monolayer model presents a less flexible and fluid environment because its structure (a half-membrane) is more crystalline than a normal lipid bilayer, which affects the protein accommodation and functionality [28].
3.4 Supported Lipid Bilayer Membranes (sLBMs)
Since 1958, when Tamm and McConnell were the first to report the formation of a planar supported lipid bilayer membrane (sLBM) in direct contact with a solid surface, this approach has become one of the most popular biomimetic models for developing a surface-confined membrane system (Fig. 7). The simplest technique for obtaining this model is by fusing small unilamellar vesicles onto a smooth, clean solid substrate, such as borosilicate glass, mica, or silicon oxide wafers. Several attempts have been made to deposit a supported lipid bilayer onto single crystals of SrTiO2 and TiO2 or onto thin films of SiO2 or LiNbO3 crystals [2, 55]. This one-step fusion procedure results in a flat lipid membrane that can be obtained using different lipid mixtures. When the lipid vesicles (usually liposomes) touch a solid surface, they may adsorb, rupture, or spread to form a lipid bilayer on a hydrophilic surface. Lipid molecules are attached to the substrate surface via electrostatic, hydration, van der Waals and steric forces [51]. In the bilayer model, the polar head groups of one lipid monolayer face toward the solid surface while the hydrocarbon chains are in contact with the other lipid monolayer.
Alternatively, the Langmuir-Blodgett (LB) and Langmuir-Schaefer (LS) techniques can be used to prepare asymmetric bilayers (Fig. 8). In the LB (vertical) technique, dipping the lipid molecules spreads them at the air-water interface to form a lipid monolayer. A solid wafer is then raised or lowered vertically through the generated layer to transfer the lower leaflet of lipids to the solid support. Next, the same support is immersed again through the air-water interface to generate the upper leaflet, resulting in the formation of a lipid bilayer. In the LS (horizontal) technique, dipping the solid wafer, upon which the first lipid monolayer is adhered, pushes the monolayer horizontally through a second lipid monolayer [27]. A combination of the previous methods can be employed to form the lower and upper leaflets of a lipid membrane; namely, the LB technique is used to form the lower leaflet, and vesicle fusion is subsequently used to form the upper leaflet. The successful generation of the lipid bilayer is dependent on several factors, such as the temperature, vesicle size and composition, and membrane density [38]. In contrast to BLM, sLBM is more stable and robust, and it can be investigated using several surface-specific analytical techniques that include SPR, QCM-D, and atomic force microscopy (AFM).
The fluidity of the sLBM is maintained by a layer of trapped water that separates the lipid membrane from the solid surface at an estimated thickness of 10–20 Å. This hydration layer near the hydrophilic surface is most likely highly ordered, more viscous and has a lower dielectric constant than bulk water. The transmembrane space measures approximately 0.5–2 nm in width, which provides stability and robustness to the generated lipid membrane but is not fully decoupled from the underlying solid surface. Moreover, the space limitation of the membrane-substrate restricts the incorporation and mobility of large integral membrane proteins and allows for direct contact between the surface and the protein, which may lead to protein denaturation and a loss of functionality. It is difficult to control the orientation of the incorporated membrane protein into the sLBM, which also suffers from the frequent presence of a defective area in the lipid membrane [58].
To circumvent these limitations, an auxiliary strategy has been developed in which the lipid membrane is decoupled from the solid substrate by the introduction of an intermediate spacer layer, which is typically less than 100 nm thick and which extends the membrane-substrate distance. A water reservoir increases the thickness of the water layer, which reduces the interaction between the lipid bilayer or incorporated protein and the substrate and minimizes the non-specific adsorption of aqueous protein from the solution. To regulate the lateral diffusivity and function of accommodated membrane proteins into this biomimetic model, it is important to quantitatively control the length and lateral density of the polymer layer by controlling both the membrane-substrate distance and the viscosity of the polymer layer [49, 73].
3.5 Polymer-Cushioned Lipid Bilayer Membranes
This model is the modified form of hBLM and sLBM and was pioneered by Sackmann and colleagues [57, 58]. In this biomimetic model, the lipid bilayer membrane is decoupled from the solid surface using soft polymeric molecules, such as chitosan, cellulose, dextran, lipopolymer tethers, and polyelectrolytes, and it presents a model membrane that has greater stability while maintaining the dynamic and structural integrity of a lipid bilayer membrane (Fig. 9). Another alternative to using polymers is to use quasi-crystal bacterial cell surface-layer (S-layer) proteins that can be recrystallized either in suspension or on several solid substrates, including silica, gold, and silicon dioxide. On a solid support, S-layer proteins can form a 5–10-nm-thick, highly porous lattice that effectively organizes the lipid-protein membrane [61, 68]. In general, a cushion layer provides several advantages, such as acting as a supporter that rests on the substrate to support the lipid bilayer without any direct linkage with the substrate. The cushion layer also acts as a lubricant that reduces the interactions between membrane-incorporated proteins and the solid surface (thereby minimizing the risk of protein denaturation), assists in the self-healing of local defects in the membrane, and provides uniform coverage over macroscopically large surfaces (cm2), which is important because a large number of defect sites contribute to a low signal-to-noise ratio and a high background response. In fact, the polymeric spacer provides a thick (larger than 3 nm) hydrated layer that allows for the incorporation of large transmembrane proteins and prevents the dewetting of the membrane [31, 36, 43, 73, 76]. In general, it is better to choose a polymer support that is soft, hydrophilic, not extensively cross-linked, not too highly charged, and has excellent wetting properties to prevent surface dehydration. All these features lead to the generation of a thermodynamically and mechanically stable supported membrane [58, 72]. To generate polymer-supported membranes, polymer molecules bind to the solid substrate first, and the lipid membrane is subsequently deposited onto the cushion layer. Several techniques have been reported to generate a lipid bilayer on the polymer cushion, such as vesicle fusion and LB/LS transfers.
The fabrication and design of a cushioned membrane can be inspired by nature. For example, the cytoskeleton, which is a protein matrix, supports the lipid bilayer of the cellular membrane in erythrocytes, providing the cell with its distinct shape and support. Thus, a well-designed cushioned membrane should behave similarly to a cytoskeleton and significantly address the balancing of surface forces [57]. The deposition of a lipid membrane on a cushion layer has no chemical link between the lipid molecules and the polymer cushion, but the membrane has attractive and repulsive physical forces that limit potential interactions between the membrane and the solid substrate. However, in a physiological system, an unstable biomimetic system can result from weak interactions between the lipid bilayer and the cushion layer. Hence, to tether the lipid membrane to the cushion layer effectively, the polymer cushions first need to be covalently attached to the underlying substrate, and subsequently, the insertion of anchor lipids or alkyl side chains into the lipid membrane can be employed [72, 73]. Polymer-cushioned lipid membranes are rigid, stable, and allow for the incorporation of large transmembrane proteins in a good orientation and under non-denaturing conditions. This biomimetic model has been reported in many studies for the successful reconstitution of several proteins in their functional forms. It is important to note that the nature and density of the polymer cushion can affect the diffusion of embedded lipids and accommodated proteins. In fact, the polydispersity and swelling behavior of the polymer may affect membrane properties, such as thickness, by producing bilayers containing holes and defects [21, 70, 76].
3.6 Tethered Bilayer Lipid Membranes (tBLMs)
As a polymer-cushioned lipid bilayer membrane, the tBLM is an alternative form of a sLBM that includes a reservoir layer to separate the lipid bilayer and solid support; this structure allows the membrane to behave similarly to the free-standing membrane (Fig. 9). The tBLM minimizes the direct contact between the incorporated membrane protein and the solid surface. Several types of molecules can be used as tethers, such as peptides (e.g., P19), lipopolymers [e.g., poly(2-ethyl-2-oxazoline)] or any self-assembling molecules that contain amino thiols or aminosilane groups [50, 66, 67]. To build a tBLM, tether molecules are self-assembled via thiol chemistry on a mercury substrate, via silane chemistry on a silicon substrate, or via phosphoric acid chemistry on an aluminum substrate [6, 11, 54]. The most commonly used solid substrate is gold, which can easily be functionalized by thiol- or sulfur-based anchor moieties [60]. The final lipid bilayer can be formed by vesicle fusion or via the LB technique. It is important to achieve the correct balance between the chemistry, density, and length of the tether molecules. Having large number of tethers attached to the solid substrate (high density) may reduce the mobility of the lipid bilayer and may change the phase transition temperature. The tether length may affect the membrane integrity; long tethers allow for the homogeneous incorporation of large membrane proteins into the membrane structure, whereas short tethers help in the generation of distinct protein patches in the membrane. However, significant increases in the tether length may lead to a greater number of defective areas in the membrane due to the storing of water molecules in the membrane (high membrane hydration), which reduces the electrical sealing properties [5, 40]. Many studies have used tBLM as a good platform for investigating the incorporation of pore- and channel-forming peptides and proteins [32, 42]. The tBLM, which offers a highly adaptable biosensor that is functionalized by the incorporation of ion channels, has been used to investigate the effects of some nanoparticles on cellular responses [29].
3.7 Lipid Vesicles
In [7], Bangham and colleagues observed the ability of phospholipids to form closed bilayer structures that were hollow and spherical in aqueous solution. These vesicles are versatile biomimetic model membranes that are widely used in biophysical and molecular biology research and, importantly, as a pharmaceutical carrier [34, 74]. Establishing a stable delivery system requires an understanding of lipid polymorphisms. Depending on the type of lipids used and the temperature, many types of assembled vesicles can be generated. However, lipid vesicles are produced by the dispersion of lipid molecules (a single lipid or a mixture of different types of lipids) in aqueous solution, which spontaneously assemble to enclose a small, aqueous compartment [22]. The generated vesicles can be a monolayer (micelles), which presents a simple form of biological membrane structure, or a bilayer (liposomes and nanodiscs), which more closely mimics biological membranes (Fig. 10).
3.7.1 Micelles
Typical micelles are spherical in shape, water soluble, and form spontaneously when surfactants or detergent/lipid mixtures are dispersed in a liquid solution. It is also possible to generate micelles in different shapes, such as cylinders, cones, ellipsoids, and bilayers. Several factors, such as the pH, temperature, ionic strength, surfactant concentration, and molecular geometry of the surfactants, affect the size and shape of the resulting micelles. Two types of micelles can be formed by the micellization process: in a normal-phase micelle (an oil-in-water micelle), the hydrophilic head faces the aqueous exterior, whereas the hydrophobic tail faces the inner core; by contrast, in an inverse micelle (water-in-oil micelle), the head groups face toward the interior side whereas the tail groups face toward the exterior aqueous solution [33, 64]. Therefore, micelles are able to encapsulate non-polar molecules, such as vitamins, anti-oxidants, and antimicrobials.
3.7.2 Liposomes
Liposomes are spherical, self-assembled structures with internal aqueous compartments inside and between the lipid bilayer. These structures vary in size from 10 nm to several hundred micrometers. Liposomes are thermodynamically unstable, and their preparation methods can influence their diameter, size distribution, lamellarity, and encapsulation efficiency. Thus, liposomes are classified by either their structural properties or the method of preparation [30]. Some examples of developed methods presented by Bangham include organic solvent injection and reverse phase evaporation.
The different types of liposomes are as follows:
-
Multilamellar Lipid Vesicles (MLVs): These vesicles are characterized by 5–20 concentric lipid bilayers that are separated by water molecules and a bilayer ranging from 0.5–10 µm. MLVs can be formed by the vigorous hydration of a dried lipid film at a temperature above that of the lipid phase transition, and then the size of the generated particles can be reduced by sonication or by performing several freeze-thaw cycles. To form unilamellar vesicles, MLVs can be extruded through a porous membrane, resulting in the generation of either SUVs or LUVs [8, 46].
-
Small Unilamellar Vesicles (SUVs): These vesicles are single lipid bilayers varying in size from 20–100 nm, and they can be formed by the hydration or sonication of a lipid film [8].
-
Large Unilamellar Vesicles (LUVs): These vesicles are single lipid bilayers varying in size from 100–500 nm and can be formed by extrusion or by reverse-phase evaporation [71].
-
Giant Unilamellar Vesicles (GUVs): These vesicles vary in size from 5–100 µm and can be formed using many preparation protocols, which include the hydration of a lipid film at a temperature above the lipid phase transition for a long period of time (up to 36 h), gentle spontaneous swelling, the application of an eternal electrical field (electroformation method) at low or biologically relevant salt concentrations, or electroformation using native membrane extracts purified from biological cells [30, 77].
Similar to micelles, liposomes can encapsulate many functional ingredients. A major advantage is that due to their ability to encapsulate both water- and fat-soluble ingredients, liposomes can encapsulate hydrophilic agents in their internal core and hydrophobic agents into the membrane. Moreover, membrane proteins can be incorporated into the liposome membrane, thereby retaining their activities.
3.7.3 Nanodiscs
The nanodisc is a novel technology that was developed by Sligar and colleagues in the early 2000s. This synthetic model membrane system consists of self-assembled lipid-protein discoid nanoparticles (8–16 nm in diameter) composed of a phospholipid bilayer that is surrounded by a belt of an encircling helical protein called the membrane scaffolding protein (MSP). Each nanodisc is solubilized by two MSP molecules. However, MSPs are amphipathic peptides that are derived from apolipoprotein A-I, an important protein found in the human bloodstream that is fundamental to forming high-density lipoprotein (HDL). The diameter of a nanodisc is determined by the stoichiometry of the given lipid molecules and the length of the MSP. Nanodiscs are formed spontaneously by removing the detergent from a mixture of detergent solubilized-lipids and MSP, and the generated discoid bilayer particles are monodisperse, homogeneous, and can be obtained at high yields. These particles have a hydrophobic core of lipids that is shielded from the solvent by apolipoprotein molecules [9, 20].
Although micelles and liposomes can be used to solubilize and investigate insoluble membrane proteins, nanodiscs are a promising model that exhibit several advantages over other membrane mimetics. Such advantages include the following aspects: (i) their simplicity, which allows for the in vitro investigation of specific interactions between membrane receptors and their native ligands; (ii) their similarity to those of naturally occurring human lipoprotein, thus they can mimic the physiological environment of the plasma membrane, and allow for the incorporated protein to retain its stability in solution; (iii) their small size allows for the incorporation of a limited number of proteins of interest; and (iv) the MSP has the potential to be genetically modified with different tags [10, 19].
4 Applications of Biomimetic Membranes
Synthetic biomimetic membranes can be exploited for many practical applications, including the fabrication of biosensors, the development of nano-devices, and drug delivery systems. Indeed, these membranes help in understanding the molecular functions of biological membranes. Although more than 60% of drugs on the market target membrane proteins, most of their functional and structural properties are still ambiguous because it is difficult to study these proteins in their native environment. Moreover, these proteins tend to denature when isolated from the surrounded lipid membrane. Research on membrane proteins depends on the availability of the protein in its functional form for physical investigations, and it requires a lipid membrane for correct folding. Hence, artificial membranes provide promising strategies that allow for the investigation of membrane proteins in semi-natural environments that mimic biological environments. To create a successful lipid membrane model for embedding large transmembrane proteins, it is important to consider the protein rather than the lipid molecules as the basic building block of the platform. Subsequently, the effective generation of a membrane-incorporated protein can be used for physiological studies or as a sensing platform for diagnostic purposes and drug screening. Many attempts have been made to develop a suitable platform to study membrane proteins in bio-sensing applications. For example, the voltage-dependent anion channel (VDAC) protein, the major protein in the outer mitochondrial membrane, and the pore-forming toxin α-hemolysin, have been chosen for incorporation into the cushion-supported lipid membrane [S-layer-supported lipid bilayer membrane (SsLBM)]. The spontaneous incorporation of the VDAC protein into the SsLBM, which leads to the formation of an open channel in the shape of cylindrical channels that are permeable to many ions, has been successfully observed. The functionality of the formed channels can be detected by monitoring the flow of ions across the membrane, which is recorded as a significant decrease in the membrane resistance. The SsLBM model showed the ability to accommodate the VDAC protein but not α-hemolysin. Perhaps the mushroom-like shape of α-hemolysin could affect the functional incorporation of the protein into the synthetic membrane [17, 18]. Interestingly, α-hemolysin molecules could be functionally incorporated into a free-standing lipid bilayer onto which the S-protein has previously been recrystallized [62, 63].
Artificial membrane technology has attracted more attention by the medical field as an effective green method for the diagnosis, treatment, and prevention of diseases. A major research goal is the development of sensitive biosensors to detect large molecules (such as proteins, DNA, drugs, and toxins), viruses, bacteria, cancer cells, and low molecular weight molecules in samples while directly excluding any preliminary preparation steps. The application of biomimetic membranes in health care centers helps to improve diagnostic tests by producing sensitive (to picomolar concentrations), selective, rapid, easy-to-use, and inexpensive detection systems. Membrane biosensors are versatile and convenient models that can be employed to accurately detect pathogens. For example, a tBLM was developed to detect the influenza virus. This membrane is composed of a lipid bilayer, gramicidin (a peptide antibiotic), and antibody fragments (derived from monoclonal antibodies) that are embedded in the tethered lipid membrane. The functionality of the fabricated sensor is dependent on the recognition of the influenza nucleocapsid protein by the antibody fragments. In brief, chicken cells are infected with influenza virus. The cells are then lysed to extract the protein contents. The resulting lysate contains chicken and viral proteins, including the nucleocapsid protein. In the absence of an analyte, the gramicidin molecules in their monomeric forms are reconstituted into the upper and lower lipid leaflets, and they tend to assemble transiently and spontaneously into the dimeric form. After the analyte is exposed to the tethered lipid membrane, the nucleocapsid protein is captured by the antibody fragments, which prevents the transient formation of gramicidin dimers. These changes are recorded as a drop in admittance, and the signal response changes rapidly (on the order of minutes). This approach is known as the ion channel switch (ICS) biosensor [39].
Another influential use for biomimetic membranes is as a drug carrier. Nanocontainers, such as micelles, liposomes, and nanodiscs, are often used not only for biosensor applications but also for drug delivery. Using recombinant proteins as therapeutic agents has many drawbacks, such as low bioavailability, high toxicity, and rapid clearance [48, 78, 79]. The development of a drug delivery system (DDS) has become an increasingly attractive option for delivering hydrophilic and lipophilic drugs. These drug vehicles are able to increase the drug bioavailability, deliver a drug to a specific target site, control the drug release, and reduce toxicity by using a minimum dose to achieve good therapeutic effects. Due to the high stability, tunability and longevity of these carriers, they offer appropriate environmental conditions for storing drugs while maintaining their activities. In addition to these advantages, lipid nanoparticles have introduced new routes of delivery that were not commonly used in protein drug administration. For example, insulin, a small protein that is composed of two cross-linked peptide chains, is conventionally administered via the subcutaneous injection. As alternatives to this route, oral and nasal modes of administration have been exploited to deliver insulin. Encapsulating insulin in liposomes results in enhanced insulin bioavailability and protects the drug from enzymatic degradation while maintaining the pharmacological effect [1, 65].
5 Conclusion
Biomimetic models are commonly used to explore the biological membrane and, at present, show strong promise in this field. These bioinspired models offer excellent platforms for investigating natural membranes by simplifying their complex structure while maintaining their functionality and quality. This strategy, although simple in concept, is challenging to accomplish, but it can be achieved due to the rapid growth in synthetic biology, particularly via the bottom-up approach. To fabricate a semi-synthetic system, it is important to know what we want to create, which biological materials can be used, which ideas or concepts can be inspired by natural systems, how reorganized biological components can be controlled to perform a desired action, and what alternative solutions are available.
Biomimetic membranes can be built using a well-designed, easily generated system composed of a single lipid or a mixture of lipids to form mono- or bilayer membranes that are free-standing or supported by a solid substrate. As a result, this approach provides a large lipid membrane area that permits the reconstitution of transmembrane proteins. These membrane-based sensors allow the investigation of cellular principles, the functional properties of incorporated proteins, and the effects of proteins on lipid phase behavior. Moreover, biomimetic membrane may provide insight into the pathogenicity of some diseases to which lipids contribute during disease development, such as atherosclerosis. This novel approach will assist in disease diagnosis, treatment, and prevention. Currently, biomimetic membranes are in the phase of case-to-case development, and continuous improvement is revealing a more detailed understanding of cellular life.
Abbreviations
- 3D:
-
Three-dimensional
- AFM:
-
Atomic Force Microscopy
- BLM:
-
Black Lipid Membrane
- CL:
-
Cardiolipin
- EIS:
-
Electrochemical Impedance Spectroscopy
- GUVs:
-
Giant Unilamellar Vesicles
- hBLM:
-
Hybrid Bilayer Lipid Membrane
- HDL:
-
High-Density Lipoprotein
- LB:
-
Langmuir-Blodgett
- LS:
-
Langmuir-Schaefer
- LTA:
-
Lipoteichoic Acids
- LUVs:
-
Large Unilamellar Vesicles
- MLVs:
-
Multilamellar Lipid Vesicles
- MSP:
-
Membrane Scaffolding Protein
- PC:
-
Phosphatidylcholine
- PE:
-
Phosphatidylethanolamine
- PG:
-
Phosphatidylglycerol
- PI:
-
Phosphatidylinositol
- PS:
-
Phosphatidylserine
- QCM-D:
-
Quartz Crystal Microbalance with Dissipation monitoring
- S-layer:
-
Surface-layer
- SAM:
-
Self-Assembling Monolayer
- sLBM:
-
Supported Lipid Bilayer Membrane
- SM:
-
Sphingomyelin
- SsLBM:
-
S-layer-supported Lipid Bilayer Membrane
- SUVs:
-
Small Unilamellar Vesicles
- tBLM:
-
Tethered Bilayer Lipid Membrane
References
Agrawal, A., Harde, H., Thanki, K., & Jain, S. (2014). Improved stability and antidiabetic potential of insulin containing folic acid functionalized polymer stabilized multilayered liposomes following oral administration. Biomacromolecules, 5(1), 350–360.
Ajo-Franklin, C., Kam, L., & Boxer, S. (2001). High refractive index substrates for fluorescence microscopy of biological interfaces with high z contrast. Proceedings of National Academy of Sciences, 98(24), 13643–13648.
Albert, B., Johnson, A., Lewis, J., Morgan, D., Raff, M., Roberts, K., et al. (2014). Molecular biology of the cell (6th ed.). New York: Garland Science.
Alhakamy, N., Kaviratna, A., Berkland, C., & Dhar, P. (2013). Dynamic measurements of membrane insertion potential of synthetic cell penetrating peptides. Langmuir, 29(49), 15336–15349.
Andersson, J., & Köper, I. (2016). Tethered and polymer supported bilayer lipid membranes: Structure and function. Membranes, 6(2), 30.
Atanasov, V., Knorr, N., Duran, R. S., Ingebrandt, S., Offenhäusser, A., Knoll, W., et al. (2005). Membrane on a chip: A functional tethered lipid bilayer membrane on silicon oxide surfaces. Biophysical Journal, 89(3), 1780–1788.
Bangham, A., Standish, M., & Watkins, J. (1965). Diffusion of univalent ions across the lamellae of swollen phospholipids. Journal of Molecular Biology, 13, 238–252.
Batzri, S., & Korn, E. (1973). Single bilayer liposomes prepared without sonication. Biochimica et Biophysica Acta, 298, 1015–1019.
Bayburt, T., & Sligar, S. (2003). Self-assembly of single integral membrane proteins into soluble nanoscale phospholipid bilayers. Protein Science, 12, 2476–2481.
Bayburt, T. H., & Sligar, S. G. (2010). Membrane protein assembly into nanodiscs. FEBS Letters, 584, 1721–1727.
Becucci, L., & Guidelli, R. (2014). Mercury-supported biomimetic membranes for the investigation of antimicrobial peptides. Pharmaceuticals (Basel), 7(2), 136–168.
Bogdanov, M., Dowhan, W., & Vitrac, H. (2014). Lipids and topological rules governing membrane protein assembly. Biochimica et Biophysica Acta, 1843(8), 1475–1488.
Brezesinski, G., & Möhwald, H. (2003). Langmuir monolayers to study interactions at model membrane surfaces. Advances in Colloid and Interface Science, 100–102, 563–584.
Castellana, E., & Cremer, P. (2006). Solid supported lipid bilayers: From biophysical studies to sensor design. Surface Science Reports, 61(10), 429–444.
Chen, T., & Reinhard, B. M. (2013). Characterizing the lateral friction of nanoparticles on on-chip integrated black lipid membranes. Small (Weinheim an der Bergstrasse, Germany), 9, 876–884.
Costa, A., & Burgess, X. (2012). Langmuir balance investigation of speroxide dimutase interactions with mixed-lipid monolayers. Langmuir, 28, 10050–10056.
Damiati, S., Zayni, S., Schrems, A., Kiene, E., Sleytr, U.B., Chopineau, J., et al. (2015a). Inspired and stabilized by nature: Ribosomal synthesis of the human voltage gated Ion channel (VDAC) into 2D-protein-tethered lipid interfaces. Biomaterials Science, 3, 1406–1413.
Damiati, S., Schrems, A., Sinner, E., Sleytr, U.B. & Schuster, B. (2015b). Probing peptide and protein insertion in a biomimetic S-layer supported lipid membranes platform. International Journal of Molecular Sciences, 16, 2824–2838.
Denisov, I., & Sligar, S. (2016). Nanodiscs for structural and functional studies of membrane proteins. Nature Structural and Molecular Biology, 23, 481–486.
Denisov, I. G., Grinkova, Y. V., Lazarides, A. A., & Sligar, S. G. (2004). Directed self-assembly of monodisperse phospholipid bilayer nanodiscs with controlled size. Journal of the American Chemical Society, 126, 3477–3487.
Deverall, M. A., Gindl, E., Sinner, E. K., Besir, H., Ruehe, J., Saxton, M. J., et al. (2005). Membrane lateral mobility obstructed by polymer-tethered lipids studied at the single molecule level. Biophysical Journal, 88, 1875–1886.
Eeman, M., & Deleu, M. (2010). From biological membranes to biomimetic model membranes. Biotechnologie, Agronomie, Societe et Environnement, 14(4), 719–736.
Ellis, R. (2005). Chaperone function: The orthodox view. In B. Henderson & A. G. Pockley (Eds.), Molecular chaperones and cell signalling (pp. 3–21). Cambridge: Cambridge University Press.
Engelmann, D. (2005). Membranes are more mosaic than fluid. Nature, 438, 578–580.
Epand, R., & Epand, R. (2009). Lipid domains in bacterial membranes and the action of antimicrobial agents. Biochimica et Biophysica Acta, 1788, 289–294.
Fedyukina, D. V., Jennaro, T. S., & Cavagnero, S. (2014). Charge segregation and low hydrophobicity are key features of ribosomal proteins from different organisms. Journal of Biological Chemistry, 289(10), 6740–6750.
Girard-Ergot, A. P., & Blum, L. C. (2007). Langmuir-Blodgett technique for synthesis of biomimetic lipid membranes. In D. K. Martin (Ed.), Nanobiotechnology of biomimetic membranes (pp. 23–74). New York: Springer.
Glazier, S., Vanderah, D., Plant, A., Bayley, H., Valincius, G., & Kasianowicz, J. (2000). Reconstitution of the pore-forming toxin α-hemolysin in phospholipid/18-octadecyl-1-thiahexa (ethylene oxide) and phospholipid/n-octadecanethiol supported bilayer membranes. Langmuir, 16(26), 10428–10435.
Goreham, R. V., Thompson, V. C., Samura, Y., Gibson, C. T., Shapter, J. G., & Köper, I. (2015). Interaction of silver nanoparticles with tethered bilayer lipid membranes. Langmuir, 31(21), 5868–5874.
Imura, T., Gotoh, T., Otaka, K., Yoda, S., Takebayashi, Y., Yokoyama, S., et al. (2003). Control of physicochemical properties of liposomes using a supercritical reverse phase evaporation method. Langmuir, 19(6), 2021–2025.
Ingo, K. (2007). Insulating tethered bilayer lipid membranes to study membrane proteins. Molecular BioSystems, 3, 651–657.
Jadhav, S. R., Sui, D., Garavito, R. M., & Worden, R. M. (2008). Fabrication of highly insulating tethered bilayer lipid membrane using yeast cell membrane fractions for measuring ion channel activity. Journal of Colloid and Interface Science, 322(2), 465–472.
Jelinek, R., & Silbert, L. (2009). Biomimetic approaches for studying membrane processes. Molecular BioSystems, 5, 811–818.
Kamps, J. A. A. M., Scherphof, G. L., Sullivan, S., Gong, Y., & Hughes, J. (2003). In V. P. Torchilin & V. Weissig (Eds.), Liposomes, a practical approach (2nd ed., pp. 267–301). New York: Oxford University Press.
Khan, M. S., Dosoky, N. S., Berdiev, B. K., & Williams, J. (2016). Electrochemical impedance spectroscopy for black lipid membranes fused with channel protein supported on solid-state nanopore. European Biophysics Journal, 45, 843.
Kiessling, V., & Tamm, L. (2003). Measuring distances in supported bilayers by fluorescence interference-contrast microscopy: Polymer supports and snare proteins. Biophysical Journal, 84, 408–418.
Kroon, A., Rijken, P., & De Smet, C. (2013). Checks and balances in membrane phospholipid class and acyl chain homeostasis, the yeast perspective. Progress in Lipid Research, 52(4), 374–394.
Le Brun, A., Clifton, L., Holt, S., Holden, P., & Lakey, J. (2016). Deuterium labeling strategies for creating contrast in structure-function studies of model bacterial outer membranes using neutron reflectometry. Methods in Enzymology, 566, 231–252.
Lee, S. K., Cascão-Pereira, L. G., Sala, R. F., Holmes, S. P., Ryan, K. J., & Becker, T. (2005). Ion channel switch array: A biosensor for detecting multiple pathogens. Industrial Biotechnology, 1, 26–31.
Liu, C., & Faller, R. (2012). Conformational, dynamical and tensional study of tethered bilayer lipid membranes in coarse-grained molecular simulations. Langmuir, 28(45), 15907–15915.
Maget-Dana, R. (1999). The monolayer technique: A potent tool for studying the interfacial properties of antimicrobial and membrane-lytic peptides and their interactions with lipid membranes. Biochimica et Biophysica Acta, 1462, 109–140.
McGillivray, D. J., Valincius, G., Heinrich, F., Robertson, J. W., Vanderah, D. J., Febo-Ayala, W., et al. (2009). Structure of functional staphylococcus aureus alpha-hemolysin channels in tethered bilayer lipid membranes. Biophysical Journal, 96(4), 1547–1553.
Merzlyakov, M., Li, E., Gitsov, I., & Hristova, K. (2006). Surface-supported bilayers with transmembrane proteins: Role of the polymer cushion revisited. Langmuir, 22(24), 10145–10151.
Montal, M., & Mueller, P. (1972). Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proceedings of the National Academy of Sciences of the United States of America, 69(12), 3561–3566.
Mueller, P., Rudin, D., Tien, H., & Wscott, W. (1962). Reconstitution of excitable cell membrane structure in vitro. Circulation, 26, 1167–1170.
Nguyen, T., Tang, Q., Doan, D., & Dang, D. (2016). Micro and nano liposome vesicles containing curcumin for a drug delivery system. Advances in Natural Sciences: Nanoscience and Nanotechnology, 7(3), 035003.
Osman, C., Voelker, D. R., & Langer, T. (2011). Making heads or tails of phospholipids in mitochondria. Journal of Cell Biology, 192(1), 7–16.
Petrache, A., Machin, D., Williamson, D., Webb, M., & Beales, P. (2016). Sortase-mediated labelling of lipid nanodiscs for cellular tracing. Molecular BioSystems, 12, 1760–1763.
Purrucker, P., Förtig, A., Jordan, A., & Tanaka, M. (2004). Supported membranes with well-defined polymer tethers—Incorporation of cell receptors. Chem PhysChem, 5, 327–335.
Rebaud, S., Maniti, O., & Girard-Egrot, A. P. (2014). Tethered bilayer lipid membranes (tBLMs): Interest and applications for biological membrane investigations. Biochimie, 107, 135–142.
Reimhult, E., Zach, A., Höök, F., & Kasemo, B. (2006). A multitechnique study of liposome adsorption on Au and lipid bilayer formation on SiO2. Langmuir, 22, 3313–3319.
Ries, R., Choi, H., Blunck, R., Bezanilla, F., & Heath, J. (2004). Black lipid membranes: Visualizing the structure, dynamics, and substrate dependence of membranes. The Journal of Physical Chemistry B, 108, 16040–16049.
Roberts, M. A., Cranenburgh, R. M., Stevens, M. P., & Oyston, P. C. F. (2013). Synthetic biology: Biology by design. Microbiology, 159(7), 1219–1220.
Roskamp, R. F., Vockenroth, I. K., Eisenmenger, N., Braunagel, J., & Köper, I. (2008). Functional tethered bilayer lipid membranes on aluminum oxide. ChemPhysChem, 9(13), 1920–1924.
Rossetti, F. F., Bally, M., Michel, R., Textor, M., & Reviakine, I. (2005). Interactions between titanium dioxide and phosphatidyl serine-containing liposomes: Formation and patterning of supported phospholipid bilayers on the surface of a medically relevant material. Langmuir, 21, 6443.
Ryan, S. R., Hyeon, C., Rikard, B., Francisco, B., & James, R. H. (2004). Black lipid membranes: Visualizing the structure, dynamics, and substrate dependence of membranes. The Journal of Physical Chemistry B, 108, 16040–16049.
Sackmann, E. (1996). Supported membranes: Scientific and practical applications. Science, 271, 43–48.
Sackmann, E., & Tanaka, M. (2000). Supported membranes on soft polymer cushions: Fabrication, characterization and applications. Trends in Biotechnology, 18, 58–64.
Sáenz, J., Grosser, D., Bradley, A., Lagny, T., Lavrynenko, O., Broda, M., et al. (2015). Hopanoids as functional analogues of cholesterol in bacterial membranes. Proceedings of the National Academy of Sciences, 112(38), 11971–11976.
Schiller, S. M., Naumann, R., Lovejoy, K., Kunz, H., & Knoll, W. (2003). Archaea analogue thiolipids for tethered bilayer lipid membranes on ultrasmooth gold surfaces. Angewandte Chemie International Edition in English, 42(2), 208–211.
Schrems, A., Larisch, V. D., Stanetty, C., Dutter, K., Damiati, S., Sleytr, U. B., et al. (2011). Liposome fusion on proteinaceous S-layer lattices triggered via β-diketone ligand–europium (III) complex formation. Soft Matter, 7, 5514–5518.
Schuster, B., & Sleytr, U. B. (2002). Single channel recordings of α-hemolysin reconstituted in S-layer stabilized lipid bilayers. Bioelectrochemistry, 55, 5–7.
Schuster, B., Pum, D., Sara, M., Braha, O., Bayley, H., & Sleytr, U. B. (2001). S-layer ultrafiltration membranes: A new support for stabilizing functionalized lipid membranes. Langmuir, 17, 499–503.
Seddon, A., Curnow, P., & Booth, P. (2004). Membrane proteins, lipids and detergents: Not just a soap opera. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1666(1–2), 105–117.
Sharma, G., Sharma, A. R., Nam, J. S., Doss, G. P. C., Lee, S. S., & Chakraborty, C. (2015). Nanoparticle based insulin delivery system: The next generation efficient therapy for Type 1 diabetes. Journal of Nanobiotechnology, 13, 74.
Siegel, A. P., Hussain, N. F., Johnson, M., & Naumann, C. A. (2012). Metric between buckling structures and elastic properties in physisorbed polymertethered lipid monolayers. Soft Matter, 8, 5873–5880.
Sinner, E. K., Reuning, U., Kok, F. N., Sacca, B., Moroder, L., Knoll, W., et al. (2004). Incorporation of integrins into artificial planar lipid membranes: Characterization by plasmon-enhanced fluorescence spectroscopy. Analytical Biochemistry, 333(2), 216–224.
Sleytr, U. B., Schuster, B., Egelseer, E. M., & Pum, D. (2014). S-layers: Principles and applications. FEMS Microbiology Reviews, 38(5), 823–864.
Sprong, H., Sluijs, P., & Meer, G. (2001). How proteins move lipids and lipids move proteins. Nature Reviews Molecular Cell Biology, 2, 504–513.
Su, Z., Jiang, Y., Velázquez-Manzanares, M., Leitch, J. J., Kycia, A., & Lipkowski, J. (2013). Electrochemical and PM-IRRAS studies of floating lipid bilayers assembled at the Au (111) electrode pre-modified with a hydrophilic monolayer. Journal of Electroanalytical Chemistry, 688, 76–85.
Szoka, F., & Papahadjopoulos, D. (1978). Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proceedings of the National Academy of Sciences of the United States of America, 75, 4194–4198.
Tanaka, M. (2006). Polymer-supported membranes: Physical models of cell surfaces. MRS Bulletin, 31, 513–520.
Tanaka, M., & Sackmann, E. (2005). Polymer-supported membranes as models of the cell surface. Nature, 437, 656–663.
Torchilin, V. (2005). Recent advances with liposomes as pharmaceutical carriers. Nature Reviews Drug Discovery, 4, 145–160.
Van Meer, G., Voelker, D., & Feigenson, G. (2008). Membrane lipids: Where they are and how they behave. Nature Reviews Drug Discovery, 9(2), 112–124.
Wagner, M. L., & Tamm, L. K. (2000). Tethered polymer-supported planar lipid bilayers for reconstitution of integral membrane proteins: Silane-polyethyleneglycol-lipid as a cushion and covalent linker. Biophysical Journal, 79, 1400–1414.
Walde, P., Cosentino, K., Engel, H., & Stano, P. (2010). Giant vesicles: Preparations and applications. ChemBioChem, 11, 848–865.
Wu, W., & Jiang, X. (2016). Polymeric micelles for drug delivery. In Y. Zhao & Y. Shen (Eds.), Biomedical nanomaterials. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA.
Yu, X., Trase, I., Ren, M., Duval, K., Guo, X., & Chen, Z. (2016). Design of nanoparticle-based carriers for targeted drug delivery. Journal of Nanomaterials, 2016, 1–15.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Damiati, S. (2018). Can We Rebuild the Cell Membrane?. In: Artmann, G., Artmann, A., Zhubanova, A., Digel, I. (eds) Biological, Physical and Technical Basics of Cell Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-10-7904-7_1
Download citation
DOI: https://doi.org/10.1007/978-981-10-7904-7_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7903-0
Online ISBN: 978-981-10-7904-7
eBook Packages: EngineeringEngineering (R0)