Abstract
Pylorus-resecting pancreaticoduodenectomy (PrPD) is one of the procedures that may be recommended for treatment of periampullary neoplasms including pancreatic cancer. Two meta-analyses regarding comparison between PrPD and pylorus-preserving pancreaticoduodenectomy (PpPD) reported that PrPD resulted in a significant reduction of the incidence of delayed gastric emptying compared to PpPD. However, further studies are required to clarify the long-term QOL and/or nutritional status resulting after the use of these techniques.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Pylorus-resecting pancreaticoduodenectomy
- Pylorus-preserving pancreaticoduodenectomy
- Delayed gastric emptying
8.1 Introduction
Pancreaticoduodenectomy (PD) has evolved since Kausch performed the first successful procedure as a two-stage operation in Germany in 1912 [1] and later developed by Dr. Allen Oldfather Whipple, the American surgeon, for the treatment of carcinoma of the ampulla of Vater in 1941[2]. Afterward, pylorus-preserving pancreaticoduodenectomy (PpPD), in which the whole stomach and 2.5 cm of duodenum were preserved, was described by Watson in 1944 [3] in an effort to decrease postgastrectomy syndromes in post-Whipple patients. Moreover, PpPD was popularized for the treatment of chronic pancreatitis as a modification of conventional PD reported by the American surgeons, Traverso and Longmire, in the late 1970s [4].
PpPD has been reported to reduce postgastrectomy syndromes such as dumping, diarrhea, and bile reflux gastritis or to have a better nutritional status than PD [5,6,7,8,9]. Therefore, PpPD has been generally accepted for surgical procedure of periampullary neoplasms such as pancreatic head cancer, cancer of ampulla of Vater, and bile duct cancer. Delayed gastric emptying (DGE) after PpPD is a frustrating and persistent complication. Moreover, it results in a prolonged hospital stay that induces to increase hospital costs and to decrease quality of life. To preserve pylorus ring with denervation or devascularization in PpPD may cause DGE. In 2007, subtotal stomach-preserving pancreaticoduodenectomy (SSPPD), in which duodenum and the stomach 2–3 cm proximal to the pylorus ring were removed, has been reported for periampullary and pancreatic head tumors of malignancy by the Japanese surgeon Hayashibe [10]. However, the definition of SSPPD in resection site of stomach remains unclear. It has reported in 2011 that the new surgical procedure resecting just pylorus ring in pancreaticoduodenectomy was designed as pylorus-resecting pancreaticoduodenectomy (PrPD) [11]. We will focus on the technical aspects and perioperative impacts of PrPD.
8.2 Procedure of PrPD
The following shows procedure of PrPD for pancreatic cancer. Mesenteric approach is performed for pancreatic cancer located in the pancreatic head.
8.2.1 Mesenteric Approach
-
Mesenteric approach is an efficient and safe approach to pancreaticoduodenectomy when SMA involvement is suspected and makes it easy to determine resectability at the beginning of the operation.
-
Early ligation of IPDA minimizes bleeding by better exposure and dissection of the posterior connective tissues of SMA-SMV.
-
Useful approach in PV invasion with difficulty tunneling above PV.
First, the mesentery of the jejunum is resected at the line between the Treitz ligament and the third portion of the duodenum in order to identify the SMV and SMA at the line. SMV and SMA should be obtained using vessel loops. As the next step, the J1 and J2 branch are approached by exposing SMA (Fig. 8.1), and the inferior pancreaticoduodenal artery (IPDA) is also identified. After that, IPDA can be more readily ligated and divided. The connective tissues around the SMA and SMV are dissected completely (Fig. 8.2). If tumor is not invaded to the nerve plexus of the SMA, just lymph node dissection around the SMA is done. In this case, nerve plexus of SMA is preserved. In cases with abutment to SMA, the nerve plexus of the SMA should be resected in addition to this procedure in order to obtain negative surgical margins. The connective tissues along the SMV and SMA are dissected along its longitudinal axis toward the inferior border of the pancreatic body. On the way, the gastrocolic trunk root is ligated and divided. And then, tunneling is created between the anterior surface of the portal vein (PV) and the pancreas neck. In a case of invasion of the front side of the PV, tunneling between the anterior surface of the PV and the pancreas neck is impossible. However, in a case with invasion only of the right or left side of the PV, tunneling from the other side is possible.
8.2.2 Resection of Pylorus Ring
-
The stomach is divided adjacent to the pylorus ring, and whole stomach is mostly preserved.
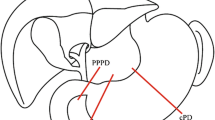
After mesenteric approach as artery-first approach, omentectomy is performed. The right gastric artery is dissected by the root, and the first pyloric branch is dissected around the pylorus ring. The first pyloric branch of the right gastroepiploic artery is also dissected along the greater curvature of the stomach. The pyloric branch of the vagal nerve is dissected along with lymph nodes around the pylorus ring (Fig. 8.1). In PrPD, the stomach is divided just adjacent the pylorus ring, and the nearly total stomach is preserved including antrum (Fig. 8.2).
8.2.3 Lymph Node Dissection Around Hepatoduodenal Ligament
-
Precise identification and taping of the right hepatic artery to avoid injury of the right hepatic artery during exposure of the bile duct
Next, the adipose tissue around hepatoduodenal ligament is cleaned followed by the adipose tissue around the common hepatic artery. During this manipulation, gastroduodenal artery is identified, followed by common hepatic artery taping. Continuously, lymph node dissection around the proper hepatic artery from hepatoduodenal ligament is done while identifying portal vein front wall. Generally, the right hepatic artery runs behind bile duct. Therefore, it is important to identify the right hepatic artery to avoid injury of the right hepatic artery during exposure of the bile duct. After cholecystectomy, the bile duct is cut at the level of the common hepatic duct, and the margin of the bile duct is pathologically diagnosed to determine whether cancer cells are present. The bile duct margin of liver side is clamped with blood vessel forceps to prevent pollution of operative field by bile juice. After that, the origin of gastroduodenal artery is ligated and divided, and portal vein trunk is exposed.
8.2.4 Transection of the Pancreas
-
The pancreas parenchyma is sharply transected with a cautery.
Before pancreatic resection, distal pancreas is gently fastened with a vessel loop to control bleeding from the remnant pancreatic stump. Caution must be used not to crush the pancreatic parenchyma during fastening by a vessel loop. The pancreas parenchyma is sharply transected with a cautery on the left side of the portal vein. Hemorrhage from the pancreatic stump of the remnant pancreas was ligated by 5-0 prolene. Preserving the blood stream of the surgical stump of the remnant pancreas is important to prevent pancreatic fistula. After complete hemostasis, 5-0 Fr pancreatic duct tube is inserted to confirm the patency and direction of the pancreatic duct.
8.2.5 Reconstruction
As the first step in reconstruction during PrPD, the proximal jejunum is brought through the transverse mesocolon by the retrocolic route. Duct-to-mucosa pancreaticojejunostomy during PrPD is done by a single layer of interrupted absorbable stitches. In seromuscular-parenchymal anastomosis, nonabsorbable interrupted stiches are placed in end to side. And then, a single layer choledochojejunostomy is constructed using interrupted stitches without a stent. Gastrojejunostomy in PrPD is performed by a two layer anastomosis via an antecolic route (Fig. 8.3). The final step is construction of the gastrojejunostomy using a two-layer anastomosis. The inner layer was 4–0 PDS-II and the outer layer used 3–0 silk for seromuscular anastomosis.
8.3 The Impact of Pylorus-Resecting Pancreaticoduodenectomy (PrPD)
DGE is a persistent complication after pancreaticoduodenectomy and results in significant prolongation of hospital stay. DGE after PpPD occurs due to several factors, such as (1) antroduodenal ischemia [12, 13], (2) gastric atony caused by vagotomy [14], (3) pylorospasm [15,16,17], (4) the absence of gastrointestinal hormones [18], (5) gastric dysrhythmia secondary to other complications such as a pancreatic fistula [19,20,21], and (6) antroduodenal congestion [22]. In particular, DGE after PpPD has been attributed to denervation and devascularization of the pyloric ring due to pylorospasms caused by injuries of the vagus nerves innervating the pyloric ring. In PrPD, the only pylorus ring is resected. The stomach is divided adjacent to the pylorus ring, and almost whole stomach is preserved. PrPD was designed with expectation in maintaining the favorable stomach pooling ability and reducing the incidence of DGE compared to PpPD [11]. The technical modification of resecting pylorus ring may provide a simple and effective method to prevent the incidence of DGE (Fig. 8.4).
Table 8.1 shows summary for comparative study between PpPD and PrPD (SSPPD) [11, 23,24,25,26,27]. There are two RCTs and five retrospective studies which compared PpPD to PrPD (SSPPD) based on DGE defined by the International Study Group of Pancreatic Surgery (ISGPS) [28]. RCT which compared PpPD with PrPD demonstrated that PrPD (4.5%) resulted in a significant reduction in the incidence of DGE compared with PpPD (17.2%) (P = 0.0244) [11]. On the other hand, another RCT by Matsumoto et al. reported that the incidence of DGE was 20% with PpPD and 12% with SSPPD (P = 0.414) [27]. The RCT demonstrated that no significant difference in the incidence of DGE was observed between PpPD and SSPPD. Matsumoto et al. discussed that this discrepancy between two RCTS was due to differences in the study subjects. So, in their study, pancreatic cancer was excluded because patients with pancreatic cancer underwent a more invasive surgery including portal vein resection and regional lymph node dissection than other benign or low-grade malignant lesions. However, Fujii et al. reported that SSPPD offer better perioperative and long-term outcomes for pancreatic cancer compared PpPD [24]. Two meta-analysis comparing PrPD with PpPD reported that PrPD resulted in a significant reduction of the incidence of DGE compared to PpPD [29, 30]. As a modified anastomosis to prevent occurrence of DGE in SSPPD, Nakamura et al. demonstrated the greater curvature side-to-side anastomosis of gastrojejunostomy [31]. In the side-to-side anastomosis, the jejunal loop is anastomosed to the greater curvature 5–10 cm proximal to the closed gastric stump, and the anastomosis is just the greater curvature, not the anterior nor the posterior wall of the stomach. The study reported that the incidence of DGE in side-to-side anastomosis was in 2.5 % in side-to-side anastomosis and 21.3% in end-to-side anastomosis (P = 0.0002). It was concluded that the greater curvature side-to-side anastomosis of gastrojejunostomy significantly reduced incidence of DGE compared to the gastric stump-to-jejunal end-to-side anastomosis in SSPPD. Now, PROPP study which compares PrPD to PpPD by RCT with sample size for 89 patients per group has been proceeding by Hackert et al. in Germany [32] (Fig. 8.5).
8.4 Long-Term Outcomes in PrPD
Advances in surgical techniques and perioperative management have led to a low mortality rate and long post-PD survival. Therefore, long-term outcomes after PD have been becoming a great matter of concern. In particular, nutritional status, body weight loss, dumping syndrome, or diarrhea after PD affects quality of life (QOL). The superiority of PrPD regarding long-term outcomes compared to PpPD remains still controversial. PrPD may have as an equally favorable pooling ability in the stomach as PpPD. However, PrPD with resection of the pylorus ring may result in the more frequent occurrence of dumping syndrome than PpPD. The study for 2-year follow-up period between PpPD and PrPD has shown that dumping syndrome occurred in only 1 of 66 patients (1.6%) with PrPD. The patients with dumping syndrome could be treated with dietary management alone. The study concluded that PrPD offer similar long-term outcomes with PpPD regarding QOL, nutritional status, and late complications [11]. The RCT by Matsumoto et al. also reported that SSPPD is equally effective in long-term nutritional status comparing to PpPD [27]. The study demonstrated that no significant differences were observed between PpPD and SSPPD regarding postoperative serum albumin levels, serum cholesterol levels, and body mass index during the 3-year follow-up period. On the other hand, Fujii et al. reported that serum albumin concentration and total lymphocyte count at 1 year postoperatively were significantly higher in SSPPD than in PpPD for patients with pancreatic cancer (P = 0.0303 and P = 0.0203, respectively) [24]. As the reason, they discussed that the gastric outlet diameter was larger after SSPPD than after PPPD, and this may have contributed to improved oral intake followed by more favorable nutritional status in their study.
Conclusion
PrPD is one of the procedures that may be recommended for treatment of periampullary neoplasms including pancreatic cancer. Two meta-analysis comparing PrPD with PpPD reported that PrPD resulted in a significant reduction of the incidence of DGE compared to PpPD. Further studies are required to clarify the long-term QOL and/or nutritional status resulting after the use of these techniques.
References
Das Carnimom KW. der Papilla duodeni und seine radikale Entfernung. Beitr Klin Chir. 1912;78:439–86.
Whipple AO, Parsons W, Mullins CR. Treatment of carcinoma of the ampulla of Vater. Ann Surg. 1935;102:763–79.
Watson K. Carcinoma of the ampulla of Vater. Successful radical resection. Br J Surg. 1944;31:368–73.
Traverso LW, Longmire WJ. Preservation of the pylorus in pancreaticoduodenectomy. Surg Gynecol Obstet. 1978;146:959–62.
Traverso LW, Longmire WJ. Preservation of the pylorus in pancreaticoduodenectomy: a follow-up evaluation. Ann Surg. 1980;192:306–10.
Jimenez RE, Femandez-del Castillo C, Rattner DW, et al. Outcome of pancreaticoduodenectomy with pylorus preservation or with antrectomy in the treatment of chronic pancreatitis. Ann Surg. 2000;231:293–300.
Brasssch JW, Deziel DJ, Rossi RL, et al. Pyloric and gastric preserving pancreatic resection. Experience with 87 patients. Ann Surg. 1986;204:411–8.
Hunt DR, McLean R. Pylorus-preserving pancreatectomy: functional results. Br J Surg. 1989;76:173–6.
van Berge Henegouwewn MI, van Gulik TM, De Wit LT, et al. Delayed gastric emptying after standard pancreaticoduodenectomy versus pylorus-preserving pancreaticoduodenectomy: an analysis of 200 consecutive patients. J Am Coll Surg. 1997;185:373–9.
Hayashibe A, Kameyama M, Shinbo M, et al. The surgical procedure and clinical results of subtotal stomach preserving pancreaticoduodenectomy (SSPPD) in comparison with pylorus preserving pancreaticoduodenectomy (PPPD). J Surg Oncol. 2007;95:106–9.
Kawai M, Tani M, Hirono S, Miyazawa M, Shimizu A, Uchiyama K, et al. Pylorus ring resection reduces delayed gastric emptying in patients undergoing pancreatoduodenectomy: a prospective randomized controlled trial of pylorus-resecting versus pylorus-preserving pancreatoduodenectomy. Ann Surg. 2011;253:495–501.
Ohwada S, Satoh Y, Kawate S, Yamada T, Kawamura O, Koyama T, et al. Low-dose erythromycin reduces delayed gastric emptying and improves gastric motility after Billroth I pylorus-preserving pancreaticoduodenectomy. Ann Surg. 2001;234:668–74.
Park JS, Hwang HK, Kim JK, Cho SI, Yoon DS, Lee WJ, et al. Clinical validation and risk factors for delayed gastric emptying based on the International Study Group of Pancreatic Surgery (ISGPS) Classification. Surgery. 2009;146:882–7.
Itani KM, Coleman RE, Meyers WC, Akwari OE. Pylorus-preserving pancreaticoduodenectomy. A clinical and physiologic appraisal. Ann Surg. 1986;204:655–64.
Gauvin JM, Sarmiento JM, Sarr MG. Pylorus-preserving pancreaticoduodenectomy with complete preservation of the pyloroduodenal blood supply and innervation. Arch Surg. 2003;138:1261–3.
Fischer CP, Hong JC. Method of pyloric reconstruction and impact upon delayed gastric emptying and hospital stay after pylorus-preserving pancreaticoduodenectomy. J Gastrointest Surg. 2006;10:215–9.
Kim DK, Hindenburg AA, Sharma SK, Suk CH, Gress FG, Staszewski H, et al. Is pylorospasm a cause of delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy? Ann Surg Oncol. 2005;12:222–7.
Kobayashi I, Miyachi M, Kanai M, Nagino M, Kondo S, Kamiya J, et al. Different gastric emptying of solid and liquid meals after pylorus-preserving pancreaticoduodenectomy. Br J Surg. 1998;85:927–30.
Räty S, Sand J, Lantto E, Nordback I. Postoperative acute pancreatitis as a major determinant of postoperative delayed gastric emptying after pancreaticoduodenectomy. J Gastrointest Surg. 2006;10:1131–9.
Riediger H, Makowiec F, Schareck WD, Hopt UT, Adam U. Delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy is strongly related to other postoperative complications. J Gastrointest Surg. 2003;7:758–65.
Yeo CJ, Barry MK, Sauter PK, Sostre S, Lillemoe KD, Pitt HA, et al. Erythromycin accelerates gastric emptying following pancreaticoduodenectomy: a prospective, randomized placebo-controlled trial. Ann Surg. 1993;218:229–38.
Kurosaki I, Hatakeyama K. Preservation of the left gastric vein in delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy. J Gastrointest Surg. 2005;9:846–52.
Kurahara H, Takao S, Shinchi H, et al. Subtotal stomach-preserving pancreaticoduodenectomy (SSPPD) prevents postoperative delayed gastric emptying. J Surg Oncol. 2010;102:615–9.
Fujii T, Kanda M, Kodera Y, et al. Preservation of the pyloric ring has little value in surgery for pancreatic head cancer: a comparative study comparing three surgical procedures. Ann Surg Oncol. 2012;19:176–83.
Nanashima A, Abo T, Sumida Y, et al. Comparison of results between pylorus-preserving pancreaticoduodenectomy and subtotal stomach-preserving pancreaticoduodenectomy: report at a single cancer institute. Hepatogastroenterology. 2013;60:1182–8.
Hackert T, Hinz U, Hartwig W, et al. Pylorus resection in partial pancreaticoduodenectomy: impact on delayed gastric emptying. Am J Surg. 2013a;206:296–9.
Matsumoto I, Shinzeki M, Asari S, et al. A prospective randomized comparison between pylorus- and subtotal stomach-preserving pancreatoduodenectomy on postoperative delayed gastric emptying occurrence and long-term nutritional status. J Surg Oncol. 2014;109:690–6.
Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142:761–8.
Hanna MM, Gadde R, Tamariz L, et al. Delayed Gastric Emptying After Pancreaticoduodenectomy: Is Subtotal Stomach Preserving Better or Pylorus Preserving? J Gastrointest Surg. 2015;19:1542–52.
Huang W, Xiong JJ, Wan MH, et al. Meta-analysis of subtotal stomach-preserving pancreaticoduodenectomy vs pylorus preserving pancreaticoduodenectomy. World J Gastroenterol. 2015;21:6361–73.
Nakamura T, Ambo Y, Noji T, et al. Reduction of the Incidence of Delayed Gastric Emptying in Side-to-Side Gastrojejunostomy in Subtotal Stomach-Preserving Pancreaticoduodenectomy. J Gastrointest Surg. 2015;19:1425–32.
Hackert T, Bruckner T, Dörr-Harim C, et al. Pylorus resection or pylorus preservation in partial pancreatico-duodenectomy (PROPP study): study protocol for a randomized controlled trial. Trials. 2013;14:44.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kawai, M., Yamaue, H. (2018). Pylorus-Resecting Pancreaticoduodenectomy: How I Do It. In: Tewari, M. (eds) Surgery for Pancreatic and Periampullary Cancer. Springer, Singapore. https://doi.org/10.1007/978-981-10-7464-6_8
Download citation
DOI: https://doi.org/10.1007/978-981-10-7464-6_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7463-9
Online ISBN: 978-981-10-7464-6
eBook Packages: MedicineMedicine (R0)