Abstract
Widespread application of non-renewable energy resources such as fossil fuels is limited mainly due to their adverse environmental impacts by increasing the amount of greenhouse gas (GHG) emissions. A solution to limit fossil-fuel pollution is the use of renewable energy resources. In the recent years, microalgae have received considerable attention as a suitable feedstock for biofuel production. Microalgae can grow in various aquatic wastewater media and are able to produce biomass, lipids, and hydrocarbons. Using different types of wastewaters as media for algae cultivation could not only reduce their freshwater footprint but also the costs associated with algae cultivation and biofuel production. This chapter presents an overview on various algal cultivation systems as well as on optimization of algal cultivations, while downstream processes including harvesting and drying of microalgae and lipid extraction systems are also reviewed and discussed. Subsequently, different microalgae biofuel production pathways are presented. Finally, the applications of microalgae in integrated systems, i.e., in wastewater treatment and biodiesel production systems and biofixation of carbon, are scrutinized.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Introduction
Energy resources are divided into non-renewables and renewables, with the latter having a minor contribution to the global energy market at the present time. However, due to the limitations on non-renewable energy resources and increasing greenhouse gas (GHG) emissions as a result of using these fuels, the share of renewable energy resources such as biofuels, hydro, wind, solar, and geothermal energies is bound to increase (Bwatanglang et al. 2015). Among the above-mentioned renewables, only biofuels are being used globally in the transportation sector, the main GHG emitter. Based on their feedstocks, biofuels are classified into first-generation biofuels (FGBs) produced from sugar, starch, animal fats, and vegetable oils; second-generation biofuels (SGBs) produced from non-food crops, agro-forest residues, and wastes; and third-generation biofuels (TGBs) produced from microalgae (Demirbas and Demirbas 2011; Laghari et al. 2015).
Microalgae, photosynthetic microorganisms, could be grown on non-arable land and have an acceptable growth rate (20–30 times faster than other conventional energy crops) and high photosynthetic conversion efficiency (Ullah et al. 2014). Cultivation of microalgae consumes less water than land crops, and unlike corn, soybean, and palm as main sources of biofuel production, algae is not used as a primary food source for human being, affirming that they can be used distinctively as fuel while having less impact on food security. Moreover, due to their ability to withstand high CO2 contents in gas stream, microalgae have high efficiency for CO2 mitigation as well (Demirbas and Demirbas 2010; Mata et al. 2010; Wang et al. 2008; Zhang 2015).
Microalgae are classified into four main taxonomic groups: diatoms (Bacillariophyceae), green algae (Chlorophyceae), cyanobacteria or blue-green algae (Cyanophyceae), and golden algae (Chrysophyceae). These microorganisms contain high lipid, high protein, and low carbohydrate content. Nowadays, the main interest is in cultivating microalgae to produce lipid as feedstock for biodiesel (Markou and Nerantzis 2013), while other types of biofuels are also of some interest. The lipid content, lipid productivity, and different types of biofuel reportedly produced from different microalgae are shown in Table 13.1. Moreover, a comparison among different biodiesel feedstocks in terms of their oil properties is also presented in Table 13.2.
Microalgae can grow in various aquatic environments, such as freshwater or marine water (Zhou et al. 2011a, b), industrial wastewaters (Wang et al. 2010, Zhou et al. 2013), municipal wastewaters (Kong et al. 2010), animal wastewaters (Wang et al. 2010; Zhou et al. 2012; Hu et al. 2012), and agricultural wastewaters. Accordingly, many studies have strived to promote biofuel production using wastewater resources as a means of improving the economic aspects of algal fuels production (Pittman et al. 2011; Wu et al. 2012). However, in a rather recent critical review, Chisti (2013) pointed out the constraints to microalgal biofuel commercialization. Among those was the calculations concerning the inadequacy of wastewater as a source of nitrogen and phosphorus for microalgal cultivation and that the algal biofuels produced using the wastewater generated in a metropolitan area such as New York city could only be sufficient to replace 1–3% of the petroleum demands of the city. As shown in Table 13.3, presenting the potential of algal fuel production from wastewater in major cities in the world, this is absolutely true and such a scenario would be totally inefficient if one places the main focus on biofuel production using wastewater.
To the contrary and by highlighting algal-based wastewater treatment instead of biofuels production, i.e., by looking at this scenario other way around, different conclusions could be made. Accordingly, biofuel production using wastewater would come second as a strategy to further justify, or economize, the algal-based treatment process of various types of wastewater. Table 13.4 tabulates the pros and cons of microalgal-based wastewater treatment systems and compares them with the conventional wastewater treatment procedures.
The present chapter aims to review the developments made and success stories reported in different aspects of algal cultivation and harvesting/extraction within the framework of integrated biofuel production/wastewater treatment systems. Furthermore, the application of microalgae in integrated systems, i.e., microalgae-driven wastewater treatment and algal-based carbon biofixation with simultaneous biofuels production, has been brought into attention.
13.2 Algae Cultivation Systems
13.2.1 Suspended Culture
The most common large-scale algae production systems are based on suspended culture. In these cultures, including open ponds and closed reactors, single cells and small groups of cells are maintained in liquid medium. This medium requires agitation and gas exchange.
13.2.1.1 Open Ponds
Open ponds can be categorized into natural waters (lakes, lagoons, and ponds) and artificial ponds. The most commonly used systems for algae cultivation include circular ponds and raceway ponds. Algae cultivation in open system has some disadvantages, such as the difficulties in controlling contamination, culture environment conditions, poor light utilization by the cells, and requirement for large areas of land, while biomass harvesting is costly as well (Carvalho et al. 2006). Circular ponds are generally round, simple, and mixed with a rotating circular arm fixed in the pond center (Lee and Lee 2001). Raceway ponds are shallow ponds and are used for commercial microalgal production, usually lined with plastic, with a 15–20 cm depth in which water and nutrients circulate around a racetrack with a rotating paddle wheel (Brennan and Owende 2010). High-rae algal ponds (HRAP)s are raceway-type ponds and have 0.2–1 m depth, paddle wheel-mixed, and provide improved wastewater treatment. They are efficient and cost-effective upgrades for treating municipal, industrial, and agricultural wastewater (Park et al. 2011; Craggs et al. 2012). These ponds are in fact a combination of algal reactor and amplified oxidation ponds.
13.2.1.2 Closed Reactors
Closed reactors are expensive to build. However, compared with open ponds, they are much easier to control contamination and environmental conditions. Closed reactors require chemical sterilizers to effectively sterilize. In such reactors, cost of harvesting is less than open ponds and the obtained biomass concentration is higher than open ponds (Lee and Lee 2001; Scott et al. 2010). There are four key requirements for algal growth in reactors. The photosynthetic activity of microalgae depends on light; therefore, light is one of the restrictive factors in the algae culture; if light is too low, growth of microalgae will be slow and their photosynthesis will decline. Conversely, if it is too high, photoinhibition and oxidative damage would occur (Kumar et al. 2010a, b, c). Another key parameter is temperature, and too low and too high values would result in slow growth and cell death, respectively. Fluctuations in temperature can lead to significant decreases in productivity, while the optimal growth temperature for microalgae is often in the range of 20–30 °C (Chisti 2008). Mixing is also an important parameter in microalgal cultivation that improves gas exchange, keeps cells in suspension, distributes the nutrients, and decreases photoinhibition on the surface (Ugwu et al. 2008). Mixing in photobioreactors (PBR) is provided by pumping or aeration through a variety of gas transferring systems. Finally, nutrients are also instrumental in achieving am efficient cultivation system. Low nutrient availability leads to growth inhibition, while high concentration may exert toxic effects. Essential elements for algal growth include nitrogen (N), phosphorus (P), and, in some cases, silicon (Chisti 2007).
Closed reactors can be categorized into flat-plate reactors and tubular reactors. Flat-plate reactors are vertical reactors made up of narrow panels with 10-mm glass plates that are pasted together. Tubular reactors are another type of closed reactors that can be categorized into: horizontal tubular, vertical airlift, and helical tubular. The only type of closed systems used on large scales is tubular reactors (Chisti 2007). The control of temperature and pH in tubular photobioreactors is better than that in open ponds. In comparison with open ponds, tubular photobioreactors can generally provide a better protection against culture contamination, less evaporative loss, better mixing, and higher cell densities (Mata et al. 2010). Horizontal tubular systems are composed of thin-diameter tubing lying or stacked horizontally, while vertical tubular systems are composed of vertical tubes that can be easily erased and kept sterile. This type of reactor is suitably set and manufactured at low cost (Ugwu et al. 2008). Helical tubular systems are constructed of tubing coiled around a circular framework, and hence, angle to sunlight is reduced; subsequently, the required land area is reduced (Morita et al. 2001). A comparison of open and closed culture systems for microalgae is shown in Table 13.5 (Pluz 2001; Brennan and Owende 2010; Ugwu et al. 2008). An efficient hybrid system of photobioreactor and open ponds was also suggested by Narala and co-authors (2016). Schematic diagrams for closed reactors and open pond are shown in Fig. 13.1.
13.2.2 Immobilized Algal Culture
Harvesting microalgae is a major challenge in suspended culture at large-scale algae production system. Using immobilized cultures (attached algal processes) could play a major role in overcoming this major challenge to production, i.e., harvesting of microalgae (Hoffmann 1998). In addition to that, immobilization offers a number of other advantages over free-cell systems as well, including less space requirements, easier handling, higher resistance to unfavorable environmental conditions, and the possibility of using higher cell densities in the process as well as reusing the biomass for product generation (Mallick 2002; de-Bashan and Bashan 2010; Christenson and Sims 2011; Eroglu et al. 2015). It should also be mentioned that immobilized cells have been reported to possess higher biosorption capacity and bioactivity (Mallick 2002). These collectively mark immobilized algal cultivation systems as cost-effective processes for scale-up processing. Among the various immobilization processes, the most common ones are discussed herein.
13.2.2.1 Matrix-Immobilized Microalgae
In this method, microalgal cells are immobilized or entrapped in a 3D matrix made of natural (such as agar, cellulose, alginate) or synthetic (such as polyacrylamide, polyurethane, polyvinyl) polymers. de-Bashan and Bashan (2010) argued that the latter is comparatively more stable in wastewater samples, while natural polymers such as alginate are advantageous in terms of their higher nutrient/product diffusion rates and their eco-friendly features (de-Bashan and Bashan 2010). In spite of the promising aspects of matrix immobilization of algal cells, this method is still limited to laboratory scale for the cost of the immobilization matrix is yet to be further decreased in order to be economically justified (Chevalier et al. 2000).
13.2.2.2 Algal Biofilms
The main advantage of algal cultivation systems designed based on algal biofilm is the facilitated harvesting of algal cells by scraping. As mentioned earlier, expensive harvesting systems used in suspended cultures, e.g., flocculation and centrifugation, generally jeopardize the economic viability of these systems, and therefore, algal biofilms when become economically available could assist with overcoming this shortcoming.
13.3 Algal Cultivation Optimization
In order to achieve an economically viable biodiesel production system from microalgal biomass, optimization of algal cultivation in terms of algal biomass and lipid content is of prominent importance. Numerous attempts of different nature have been made in order to achieve the above-mentioned goals. For instance, Bohutskyi et al. (2014) introduced an innovative, mixed trophic state process based on Auxenochlorella protothecoides grown phototrophically, to obtain high lipid content for generating algal biodiesel. They argued that simultaneous nitrogen deprivation and glucose supplementation during the heterotrophic stage could boost total lipid content by over threefolds. They also proposed to couple biodiesel production with anaerobic digestion in order to produce biogas from the remaining biomass after oil extraction and stated that the overall energy output of the coupled process could be increased by up to 40% (Bohutskyi et al. 2014).
In a different study, various nutritional modes, including glucose supplementation, were investigated with an aim to enhance biomass and lipid productivity in different microalgal strains. They reported that lipid productivity ranged from 2 to 13% under photoautotrophic conditions, 1.7–32% under mixotrophic conditions, and 0.9–20% under heterotrophic conditions. While under heterotrophic conditions where glucose supplementation was practiced, polyunsaturated fatty acids (PUFA) fraction of the oil was decreased by around 2–4-folds depending on the microalgae strain under investigation. On the other hand, saturated fatty acid (SFA) fraction was also negatively impacted by glucose supplementation. Oils rich in SFA containing low PUFA are ideal feedstock for achieving high oxidative stability in biodiesel, and therefore, glucose supplementation could be serve this purpose well (Ratha et al. 2013).
Another strategy proposed by Duong et al. (2015) was to target both algal lipid and protein simultaneously to improve the economic viability of algal biodiesel production. More specifically, they tried to isolate algal strains meeting three criteria of fast growth, high lipid content, and protein-rich biomass, while that last could be used for animal feed (Duong et al. 2015). Converti et al. (2009) explored the effects of temperature concentration on lipid content in Nannochloropsis oculata and Chlorella vulgaris. They argued that variations in the investigated factor strongly impacted lipid content. For instance, a temperature boost from 20 to 25 °C increased lipid content by 100%, while an opposite was observed for C. vulgaris when the temperature was increased from 25 to 30 °C (Converti et al. 2009).
Nitrogen concentration in the cultivation media is also an important parameter. It is well documented that nitrogen deprivation could result in increased lipid content but could also negatively affect algal growth. Therefore, a trade-off should be observed to achieve the highest lipid productivity. In-depth understanding of the relationships between cell nitrogen content, growth, and cell composition is essential in order to be able to identify an optimal nitrogen content required for most favorable lipid productivity in batch or continuous cultivation modes (Griffiths et al. 2014). It should be highlighted that nitrogen deprivation could also improve the fatty acid profile of algal oils leading to more favorable biodiesel properties (Griffiths et al. 2014).
Nitrogen-to-phosphorus (N/P) ratio could also have a crucial effect on the biomass growth (Alketife et al. 2017). Xin et al. (2010) showed that this ratio is significantly effective on biomass yield and lipid accumulation of a freshwater microalga Scenedesmus sp. LX1. They claimed that under nitrogen (2.5 mg L−1) or phosphorus (0.1 mg L−1) limitations, the microalgae under investigation could accumulate lipids to as high as 30 and 53% of its algal biomass, respectively and lipid productivity was not enhanced reportedly. Similar observations were made by Kalla and Khan (2016) who also studied the effect of decreasing nitrogen and phosphorus concentrations on growth, biomass, and lipid content of C. vulgaris. They argued that significant decreases were recorded in growth and by decreasing nitrogen and phosphorus concentrations in the medium from (1.5–0.0 g/l) and (0.04–0.0 g/l), respectively. On the contrary, lipid accumulation was enhanced under the phosphorus and nitrogen limitations.
Different ions could also impact algal growth and lipid production significantly. For instance, Huang et al. (2014) investigated the effects of ferric ion concentrations on three species of microalgae (Tetraselmis subcordiformis, Nannochloropsis oculata, and Pavlova viridis). They concluded that growth, lipid content, as well as the fatty acid profiles of the studied microalgae varied in response to changes in ferric ion concentrations and that an optimum ferric ion concentration can improve the properties of respective algal biodiesels.
In a different study performed in high-glycerol content media, the effect of calcium and magnesium ions supplementation was studied using two fast-growing algal strains of Aurantiochytrium sp. DBTIOC-18 and Schizochytrium sp. DBTIOC-1 for biomass and lipid production (Singh et al. 2016). It was revealed that increasing both calcium and magnesium ions’ concentration promoted glycerol utilization and resulted in a significant boost in biomass and lipid production. Such findings highlight the importance of calcium and magnesium ions’ concentrations in preventing substrate inhibition under high nutrient concentrations, especially carbon sources to achieve high biomass and lipid yields (Singh et al. 2016).
Sulfate ions are also effective on growth of microalgae. In a recent study, Lv et al. (2017) strived to look into the responses of the self-flocculating microalga Chlorococcum sp. GD to different sulfate concentrations in a synthetic municipal wastewater. Their results showed that the microalgal cells grew better in the synthetic municipal wastewaters containing 18, 45, 77, 136, and 271 mg/L \( {\text{SO}}_{4}^{2 - } \) than in the control wastewater without \( {\text{SO}}_{4}^{2 - } \). They argued that sulfate deprivation led to significant decreases in antioxidative enzymes and photosynthetic activities and that these in turn significantly weakened the growth and self-flocculation properties of the algal cells (Lv et al. 2017).
pH is also important for the microalgal growth and the accumulation of intracellular lipids. This was confirmed by the findings of Sakarika and Kornaros (2016) who investigated the impacts of various pH values on C. vulgaris cultivation. They also argued that the fatty acid composition of the algal cultures was not impacted by pH variations. Illumination, i.e., length of photoperiod and light intensity, could also result in changes in algal growth and lipid content and have to be optimized (Wahidin et al. 2013). Overall, producing high amounts of lipids while maintaining a high algal growth rate is critical for an economic algal biodiesel production simply because high algal biomass productivity would lead to high yield per harvest volume and high lipid content would decrease the cost of extraction per unit product (Tan and Lee 2016). On such basis and since high lipid content and high biomass growth rate basically contradict each other, efforts have been being made to construct algal strains capable of producing high amounts of lipids without sacrificing growth through genetic and metabolic engineering (Talebi et al. 2015).
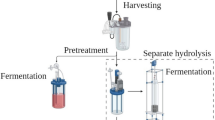
13.4 Harvesting and Drying of Microalgae
Harvesting in general constitutes a major fraction (20–30%) of the costs associated with microalgal production (Ndikubwimana et al. 2016). Two-step separation, i.e., thickening followed by dewatering, is usually practiced to decrease the cost of the final product. The concentration of the algal cells is increased to approx. 2–7 and 15–25% (TSS basis) through the two stages, respectively. There are several methods for harvesting algae including (Christenson and Sims 2011): (1) filtration—algae can be filtered out by passing through membranes; in this method, recovery rate is high and lower energy inputs are involved, but dewatering might be required; (2) centrifugation—a mechanical method for harvesting microalgae which does not involve contamination with chemicals and, like filtration, the rate of recovery is high; (3) flocculation, a method for separating algae using chemicals that lead to aggregation of algal cells. In this method, destruction of algal cells is less than in centrifugation and low energy is required; (4) floatation is a separation method in which algae are floated into the surface using bubbling, often used in combination with flocculation for wastewater treatment. No disturbance is made to the cells, and low-energy requirement is also considered as an advantage of this system; (5) ultrasonic separation in which sound waves cause the cells to agglomerate.
Choice of harvesting methods depends on the characteristics of microalgal strain/consortium, while the type and value of the end product are also of importance (Barros et al. 2015). Among different methods, bioflocculation, i.e., the use of microorganisms for the recovery of microalgae biomass, has been most widely used as it is accompanied with significantly less dewatering cost which is economically critical for their full-scale application (Ndikubwimana et al. 2016). A list of microorganisms used in bioflocculation is tabulated in Table 13.6 (Al Hattab et al. 2015; Kawaroe et al. 2016). These microorganisms when added to an algal culture lead to the settlement of the algal cells by adhering and consequent weight increase (Al Hattab et al. 2015). For instance, Ndikubwimana et al. (2014) claimed 98% removal efficiency when they use Bacillus licheniformis as bioflocculant for harvesting Desmodesmus sp. culture. In a different study, Zhang and Hu (2012) employed a co-culture of Chlorella vulgaris and filamentous fungi and successfully extracted the oil for biodiesel production.
In general, both algal oil extraction and its conversion into biodiesel are strongly negatively affected by the presence of water and, therefore, algal biomass should be effectively dried prior to the transesterification reaction (Kumar et al. 2010a, b, c). As a result, different drying methods are usually employed after secondary dewatering (Richmond 2008). Solar drying is the most economically viable drying method especially in places where abundant sunlight is available throughout the year (Sharma et al. 2013). On the contrary, drying methods which are dependent on fossil-oriented energy carriers for their operation, e.g., spray drying and drum drying, are economically and environmentally justified for microalgae biodiesel production (Zhang et al. 2014).
13.5 Lipid Extraction
Microalgal lipids are divided into nonpolar (hydrocarbons, waxes, eicosanoids, fatty acids, and acylglycerols) and polar (phospholipids and glycolipids). There are several methods for cell disruption and extracting microalgal lipids such as mechanical (expeller press), physical (decompression, microwave, freeze-drying, and thermolysis), chemical (organic solvent, chelating agent, supercritical CO2, detergent, and antibiotics), and enzymatic (lytic, autolysis, and phage) (Kumar et al. 2015).
Mechanical extraction methods offer a number of advantages over the other methods including less dependency on the type of microalgae species to be processed and no contamination of the extracted lipid (Ramesh 2013). Nevertheless, higher energy requirements are considered as a drawback of mechanical extraction methods. This is ascribed to the fact that heat is generated during mechanical extraction of lipids and, in order to prevent damages to the lipids, cooling needs to be performed whose energy and equipment costs negatively impact the overall economics of the process (Lee et al. 2012). Moreover, for a successful implementation of mechanical extraction methods, low-moisture-content algal biomass is required and, therefore, a drying stage needs to be included which could also considerably increase the overall extraction costs. It should also be noted that the amount of pressure employed during mechanical extraction is of critical importance. More specifically, increasing pressure to an optimal level could improve the extraction efficiency, while above-optimal pressure values could negatively affect the process leading to decreased lipid recovery and increased heat generation (Ramesh 2013). Expeller press is one of the simplest mechanical techniques for extracting various oil feedstocks including algae. Nevertheless, its major technical drawback is the presence of pigments along with oil. This method also requires huge amounts of energy, and its efficiency rate is low to moderate.
Among the physical extraction methods, microwave-assisted extraction has attracted a great deal of attention due to its effectiveness in disrupting algal cell walls, being non-toxic, and the possibility of reusing the media after extraction (Lee et al. 2010; Halim et al. 2012; Hattab and Ghaly 2015). Nevertheless, the high costs associated with its maintenance still limit its large-scale application. Freeze-drying and autoclave techniques are also classified among physical extraction methods. However, both these methods suffer from drawbacks such as high costs and long processing times (Hattab and Ghaly 2015).
Cell disruption and consequently extraction of lipids can be also achieved by using a large variety of chemical compounds including antibiotics, chelating agents, chaotropes, detergents, solvents, hypochlorites, acids, and alkali, through different mechanisms though (Günerken et al. 2015). For instance, basic compounds disrupt the cell membranes through saponification of the membrane lipids, while acidic compounds exert their disruptive properties through poration of the cell membrane/wall (Halim et al. 2012; Günerken et al. 2015). In general, lipid extraction from algal biomass is currently carried out using organic solvents such as chloroform, methanol, water, chloroform/methanol (1:2 v/v), chloroform/methanol/water (1:2:0.8 v/v/v), hexane, isopropanol, hexane/isopropanol (3:2 v/v), and ethanol (Zhang et al. 2014). It should be mentioned that organic solvent-based extraction is time- and labor-demanding and, more importantly, it is most efficient for lipid extraction from some algal strains, while it is not reportedly applicable for all algal strains (Ranjith Kumar et al. 2015).
Extracting oil from algal cells is generally limited due to the presence of algal cell wall (Johnson and Wen 2009). Therefore, the use of enzymes such as cellulase, neutral protease, alkaline protease, papain, and lysozyme has been practiced to facilitate cell disruption (Taher et al. 2014; Hattab and Ghaly 2015). Compared to mechanical and chemical methods, enzymatic extraction of algal lipids is very efficient and rapid while causing no corrosion as is the case when chemical extraction methods are used. However, the application of enzyme-based methods is limited owing to the high cost of enzymes.
13.6 Microalgae Biofuel Production Pathways
After oil extraction from microalgae for biodiesel production, the remaining biomass can be converted into different types of biofuels, i.e., biohydrogen (Fedorov et al. 2005; Kapdan and Kargi 2006), biomethane (Sialve et al. 2009), and bioethanol (Dexter and Fu 2009) (Table 13.7).
13.6.1 Biochemical Conversion of Algal Biomass
Technologies for biochemical conversion of algal biomass include anaerobic digestion (or biomethanation) and fermentation. More specifically, in biochemical conversion, carbohydrates are digested into sugars using bacteria, microorganisms, and enzymes, which are then transformed into gaseous or liquid fuels, such as biogas (biomethane and biohydrogen) and bioethanol (Zamalloa et al. 2012). For instance, Batista et al. (2015) converted the biomass of an algal consortium (Chlorella vulgaris, Scenedesmus obliquus) grown on wastewater into biohydrogen through dark fermentation by an Enterobacter aerogenes strain. The highest biohydrogen production yield achieved was 56.8 mL H2/gVS.
13.6.2 Thermochemical Conversion of Algal Biomass
Thermochemical conversion involves the use of heat to convert algal biomass into gaseous or liquid fuels. Thermochemical conversion can be classified according to the primary desired product (solid, liquid, gas) and the water content of the feedstock (dry or wet).
13.6.2.1 Biocrude Oil Production by Hydrothermal Liquefaction (HTL) of Wet Algal Biomass
The thermochemical conversion of wet algal biomass (75–98% moisture) into biocrude oil in the presence of a solvent at 200–350 °C temperatures and 5–25 MPa pressure to maintain water in the liquid state is called hydrothermal liquefaction (HTL) (Biller et al. 2011). In HTL, biomass is broken down into shorter carbon chains that have a higher energy density (Brennan and Owende 2010). Oxygen, sulfur, and water contents are very low in crude HTL oil. HTL oil recovers more than 70% of the feedstock carbon content. The product is a heavy oil or tarry material, which is called biocrude oil (Biller et al. 2011). The size of biomass particles, residence time, solvent media type, and hydrogen donor solvents are effective for the bio-oil yield and the product quality (Akhtar and Amin 2011). The basic reaction mechanisms involve: (a) depolymerization of the biomass, (b) decomposition of biomass monomers, and (c) recombination of reactive fragments (Toor et al. 2011).
13.6.2.2 Biofuel Production by Pyrolysis of Algal Biomass
Pyrolysis is one of the subclasses of thermochemical conversion in which dry algal biomass is decomposed in the absence of oxygen (or any halogen) and converted into biofuels such as bio-oil–charcoal–syngas. This conversion occurs in the temperature range of 401.85–701.85 °C and 0.1–0.5 MPa pressure (Demirbas 2006). On the basis of operation conditions, pyrolysis process is classified into: (1) slow pyrolysis with operation temperature of 286.75–676.85 °C (Bridgwater 2003), (2) fast pyrolysis with operation temperature of 577–977 °C under inert atmospheric conditions (Mohan et al. 2006), and (3) flash pyrolysis with operation temperature of 777–1027 °C (Balat et al. 2009).
13.6.2.3 Syngas Production Through Gasification of Microalgal Biomass
Syngas (a combination of hydrogen, carbon monoxide, and carbon dioxide) is usually produced through the gasification of different carbonous materials including algal biomass (Brown et al. 2010). Gasification process is in fact a partial oxidation process that converts dry algal biomass for instance into a mixture of gases. Gasification is classified into low temperature gasification (700–1000 °C) and high temperature gasification (1200–1600 °C) (McKendry 2002). Yield of syngas depends on various factors including microalgal biomass quality, the equipment (gasifier) used, as well as process parameters (e.g., temperature and catalysis used). In a study, Raheem et al. (2015a, b) reported that syngas yield increases from 28 to 57% by increasing temperature from 552 to 952 °C. The generated syngas could eventually be used for hydrogen production, liquid biofuels production, synthetic natural gas (SNG) production, etc. (Mondal et al. 2011).
13.6.3 Chemical Reaction
13.6.3.1 Biodiesel Production by Transesterification of Algal Oil
Biodiesel, also known as methyl or ethyl esters of long-chain fatty acids, is an alternative to mineral diesel fuel produced from vegetable oils, animal fats, and algal oil mainly through the transesterification reaction with an alcohol (methanol and/or ethanol) and in the presence of a catalyst (mostly NaOH or KOH). The main advantages of biodiesel as fuel include widespread availability, renewability, clean-burning features compared with mineral diesel, and lower sulfur and aromatic contents (Demirbas 2007). These are numerous reports confirming that biodiesel lowers exhaust emissions from diesel engines (Hayyan et al. 2010), i.e., particulate matter (PM) (Kolesárová et al. 2011), unburned hydrocarbons (HC), and carbon monoxide (CO). On the contrary, there is no consensus on the impact of biodiesel on nitrogen oxide (NO x ) emission as there are reports claiming increases in NO x due to the oxygen content of biodiesel (Sharma et al. 2008). There are four methods for biodiesel production and utilization, direct use and raw oils blending, microemulsions, pyrolysis, and transesterification. As mentioned earlier, the last procedure is most commonly used (Demirbas 2003). Through transesterification, biodiesel and its co-product, i.e., glycerin, is produced in several stages. Afterward, the excess methanol is recovered from the methyl esters through evaporation, and the final biodiesel is eventually washed with water, neutralized, and dried (Xu et al. 2006). Since fossil oil is derived from spores and planktonic algae that were under high pressure and temperature over millions of years, the chemical properties of microalgal lipids and the consequent biodiesel are also very similar to those of mineral diesel (Demirbas and Demirbas 2011).
Transesterification reaction can be acid/base/enzyme catalyzed. Alkaline catalysts include NaOH, NaO−, KOH, and KO−1, while acid catalysts include HCL and H2SO4. Enzymatic catalysts such as lipases that are able to catalyze the transesterification of triglycerides effectively in either aqueous or nonaqueous systems are more environmentally friendly than the other two groups as they result in no wastewater and the produced glycerin needs minimal purification (Fukuda et al. 2001). In another word, the weak points of transesterification reaction by alkaline catalysts are difficult recovery of glycerol, the need for alkaline wastewater treatment, free fatty acid and water interference with the reaction, energy intensity, and the necessity of removing the catalyst from the product (Meher et al. 2006). Some properties of diesel, biodiesel from various oil feedstocks, and microalgae biodiesel are shown in Table 13.8 (Kiss et al. 2007; Huang et al. 2010; Veillette et al. 2012).
13.7 Applications of Microalgae in Integrated Systems
Integration of algal biodiesel production with other activities such as wastewater treatment with an aim to enhance the economic viability of the whole process could be regarded as an efficient strategy to overcome most of the challenges faced. For instance, and as mentioned earlier, wastewater resources are rich in nutrients, such as nitrogen and phosphorus, and could be served microalgal growth as cultivation medium. In fact, using wastewater for microalgal biofuel production not only can reduce freshwater footprint and the cost of these fuels (Clarens et al. 2010) but also could offer new algal-based wastewater treatment systems (Table 13.5). It is worth mentioning that non-fuel products such as fertilizers, chemicals, pharmaceutics, dyes, paints, and animal feeds could also be obtained from microalgaes grown on wastewater (Bhatt et al. 2014).
Beside biofuel production, biofixation of carbon could also be the secondary objective of algal-based biofuel production systems. This is increasingly important given the criticality of climate change and the very recent international call for immediate action to address this crisis to the level that even the leader of the Catholic Church Pope Francis raised the issue during his visit to the USA in September 2015 (The Gurdian 2015).
The following sections summarize the efforts made during the last several years in order for integrating algal biofuel production systems with wastewater treatment and carbon biofixation.
13.7.1 Algal Biofuel Production and Wastewater Treatment
A major requirement of an efficient wastewater treatment is obviously the need to remove high concentrations of nutrients, in particular N and P. As mentioned earlier, microalgae are capable of uptaking such nutrients as well as heavy metals and organic pollutants from wastewater and producing biomass. Thus, it offers great promises for the treatment of various municipal, agricultural, and industrial wastewaters (Feng et al. 2011; Zhu et al. 2013). However, there are many reports indicating that most of the microalgal species with high lipid contents do not adapt well to grow in wastewater (Xin et al. 2010). Contrary to these reports, there are also a number of success stories through which efficient integration of algal biofuels production and wastewater treatment has been accomplished (Zhou et al. 2011a, b, 2013; de Alva et al. 2013; Hena et al. 2015). For instance, Zhou et al. (2011a, b) claimed that five species of microalgae isolated from Minnesota wastewaters including Chlorella sp., Heynigia sp., Hindakia sp., Micractinium sp., and Scenedesmus sp. showed high growth rate (0.455–0.498 d−1) and lipid productivities (74.5–77.8 mg L−1 d−1) on municipal wastewater.
In a more recent investigation, de Alva et al. (2013) also cultivated Scenedesmus acutus in pretreated municipal wastewater with a dual focus on biomass productivity and lipid accumulation. They argued that S. acutus could successfully remove nutrients from the wastewater and that they achieved 249.4 mg L−1 biodiesel from the referred algal oil. It should be pointed out that in a series of experimental surveys, Chinnasamy et al. (2010a, b), Kong et al. (2010), and Zhou et al. (2011a, b) revealed that municipal wastewater was a better option compared with industrial wastewater for algal biomass and lipid production. A comparison of biomass productivity, lipid content, and lipid productivity of different microalgae species grown on various wastewater is tabulated in Table 13.9. The following sections present the integration of biofuels production with algal-based treatment of different types of wastewaters, namely high N/P content wastewater, high heavy metal-content wastewater, and high organic-content wastewater (PAHs aromatic hydrocarbons and polychlorinated biphenyls (PCBs)).
Zhu et al. (2013) proposed Chlorella zofingiensis cultivation on piggery wastewater with a dual purpose of wastewater treatment and biodiesel production. In a different investigation, Maity et al. (2014) investigated the integration of biofuel and bioelectricity production with wastewater treatment using one species of microalgae, i.e., Leptolyngbya sp. JPMTW1 (KF977831). They argued that only after 7 d of cultivation, biomass production, rate of biomass production, lipid production, and rate of lipid production stood at 3300 mg L−1, 471.42 mg L−1 day−1, 1068.383 mg g−1 dry wt. biomass, 152.62 mg g−1 dry biomass/day, respectively. The also reported that over the same period, electrical conductivity (EC), chemical oxygen demand (COD), and total dissolved solid (TDS) decreased from 982 to 854 (mS/cm), 255 to 112 mg L−1, and 490–427 mg L−1, respectively. Overall, their findings were indicative of the possibility of the production of biofuel, bioelectricity, and wastewater treatment by Leptolyngbya sp. JPMTW1. In another study, Chen et al. (2014) produced biocrude oils from a mixed-culture algal biomass harvested from a functioning wastewater treatment system as well.
13.7.1.1 High N/P Content Wastewater
Nitrogen is a critical nutrient required for algal growth, and the application of nitrogen starvation for enhancing algal cell lipid content is well documented (Brennan and Owende 2010). Likewise, another key factor in algal energy metabolism is phosphorus which is found in a variety of biological substances, such as nucleic acids, lipid, proteins, and intermediates of carbohydrate metabolism. All eukaryotic algae require inorganic nitrogen, while some algal species are capable of using both inorganic and organic phosphorus (Liang 2013). In recent years, investigations into the ability of microalgae to simultaneously grow on wastewater streams and remove nutrients have revealed many microalgae species with high protectional for N and P removal from wastewaters (Table 13.10). For instance, Cai et al. (2013) achieved an N removal efficiency of 79–100% by S. obliquus from municipal wastewater. Earlier in the year 2010, Lim et al. made an attempt to treat textile wastewater medium using C. vulgaris and reportedly managed to remove N and P by 45 and 33%, respectively. A wide range of N (55–88%) and P (12–100%) removal has been reported when municipal wastewater was used as the waste stream (Khan and Yoshida 2008; Ruiz-Marin et al. 2010; Li et al. 2011). Mixed municipal and industrial wastewater was used by Gentili (2014) to produce Selenastrum minutum algal biomass and lipid, while effective wastewater treatment was also targeted. Their results showed that ammonium and phosphate contents were decreased from 96 to 99% and 91 to 99%, respectively, while the highest biomass and lipids yields (dry matter basis) reaching 37%.
Lu et al. (2015) used meat processing wastewater for the cultivation of the microalgae Chlorella sp. (UM6151) aiming at simultaneous biomass production, wastewater treatment, and nutrient removal. They implemented an innovative cultivation approach based on wastewater mixing to supply nutrients and improve biomass yield at economic rates. They claimed that algal biomass yield (0.675–1.538 g/L) achieved using mixed wastewater was much higher than those obtained using individual wastewater and synthetic medium. Moreover, they achieved improved ammonia nitrogen removal efficiencies (68.75–90.38%) and total nitrogen removal efficiencies (30.06–50.94%). Interestingly, by using wastewater mixing, algal protein content was also enhanced reaching as high as 60.87–68.65%.
In an effort, Abou-Shanab et al. (2013) strived to integrate biofuel production and the treatment of piggery wastewater (TN: 56 ± 2 and TP:13.5 ± 0.6 mg/L). They reported that six microalgal species including Ourococcus multisporus, Nitzschia cf. pusilla, Chlamydomonas mexicana, S. obliquus, Chlorella vulgaris, and Micractinium reisseri were capable of efficiently treating wastewater and producing high oil content for biodiesel production. Among the studied species, C. Mexicana was proven to have the highest removal rates, i.e., N (62%), phosphorus (28%), and inorganic carbon (29%). Hence and due to the higher lipid productivity and lipid content (0.31 ± 0.03 g/L and 33 ± 3%, respectively), compared with the other species, the authors suggested that C. mexicana could be a suitable candidate for integrated biodiesel production and wastewater treatment.
In a study, Min et al. (2014) suggested an efficient method, i.e., a pilot-scale stacked-tray bioreactor to increase nutrient removal rate from piggery wastewater coupled with biofuel production. Through their proposed cultivation system, algal biomass productivity (based on TSS) was enhanced from 19.15 to 23.19 g m−2 day−1 and they achieved lipid contents ranging between 1.77 and 3.55%. Wang et al. (2012) looked into the impact of dilution on algal biodiesel production and nutrient removal from high N/P content wastewater. In their study, primary piggery wastewater was used as the waste stream and was treated by mixotrophic cultivation of Chlorella pyrenoidosa. They stated that there was a positive linear correlation between algal biomass productivity and the initial COD values ranging from 250 to 1000 mg L−1. The maximal lipid productivity 6.3 mg L−1 day−1 was recorded with an initial COD of 1000 mg L−1, while nutrients such as ammonium were removed efficiently at rates as high as >90% in all diluted samples. Can et al. (2015) also explored the potential of microalgae Spirulina platensis for biofuel and biochemical production coupled with domestic wastewater treatment. Similar to Wang et al. (2012), in their experimental approach, wastewater was also diluted with distilled water to achieve different concentrations of 100, 75, 50, and 25%. Their findings were in line with those of Wang et al. (2012), revealing that the highest biomass yield was recorded when the wastewater without dilution (100%) was used. In terms of lipid production, however, the maximal value was measured in 25% wastewater. Therefore, a trade-off should be observed in order to maximize lipid productivity.
In a different study, Malla et al. (2015) also studied the potential of Chlorella minutissima for biodiesel production coupled with wastewater treatment. Their results indicated that after 12 d of the experiment, C. minutissima removed about 90–98% TDS, 70–80% N, 60–70% P, and 45–50% K from the high N/P content wastewater. They also converted the algal lipid extracted to biodiesel as part of the integrated system. Hena et al. (2015) investigated the potentials of a consortium of native microalgae species grown on a dairy farm treated wastewater for biodiesel production. The claimed that biomass production and lipid content of the consortium were 153.54 t ha−1 year−1 and 16.89%, respectively, and that 72.70% of the algal lipid obtained could be converted into biodiesel.
13.7.1.2 High Heavy Metal Content Wastewaters
Heavy metals mainly include transition metals, metalloids, lanthanides, and actinides. These metals have a highly specified gravity and are toxic to a level that even at low concentrations represents a significant environmental concern (Bhargava et al. 2012). Various methods have been investigated for heavy metal removal, which are tabulated in Table 13.11 (Fu et al. 2011). Among these methods, algae have been proposed as ideal candidates for heavy metal removal from various wastewaters through either uptake or accumulation of Hg, Cd, Zn, Au, Ag, Co, Mn, Cs, Ni, Fe, Cu, and Cr from their environment (Chekroun and Baghour 2013). In fact, algae produce polypeptides called chelating agents capable of binding to heavy metals. Apart from that, large surface area of algal cells is also effective in removing heavy metals (Kumar et al. 2015). More specifically, metal absorption by microalgae occurs at two stages: first, at the surface of algal cells through very quick physical adsorption or ion exchange. The second stage, also called chemisorption, takes place at a slower rate intracellularly and is driven by metabolic processes involving active binding groups (Zhou et al. 2012).
Richards and Mullins (2013) studied algal-based bioremediation of municipal leachate by using a consortium of four marine microalgae species, i.e., Pavlova lutheri, Tetraselmis chuii, Nannochloropsis, and Chaetoceros muelleri while also targeting enhanced lipid production. Their results revealed that algal-based bioremediation was a feasible method for simultaneous treatment of waste streams and lipid production. Yang et al. (2015) proposed an integration of heavy metal wastewater utilization and biofuel production as an alternative solution to address energy shortage and environmental concerns. They claimed that Chlorella minutissima UTEX 2341 had strong resistance to cadmium, copper, manganese, and zinc ions under heterotrophic culture condition and could efficiently remove these heavy metals through intracellular accumulation and extracellular immobilization. Moreover, lipid accumulation was not negatively affected by heavy metals. Heavy metal removal by some species of microalgae from various wastewater sources is depicted in Table 13.12.
13.7.1.3 High Organic-Content Wastewater
Organic pollutants are chemical substances that persist in an environment through industrial discharges and agricultural usages. They are also resistant to environmental degradation through chemical, biological, and photolytic processes and have harmful effects on human health. Among these organic pollutants, polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) are highly persistent compounds and, if introduced into the food chain, they have been proven to be carcinogenic (Gilden et al. 2010). Microalgae are capable of decomposing different kinds of organic pollutants including phenolics, pesticides, as well as PAHs and PCBs. Ankistrodesmus braunii, Scenedesmus quadricauda, Ochromonas danica, and Monoraphidium braunni are examples of microalgae species that can biodegrade phenolic and biophenolic compounds (Mukherjee et al. 2013; Gattullo et al. 2012). Ali et al. (2012) introduced microalgae such as chlorella vulgaris as a low-cost adsorbent for removing organic pollutants from wastewaters. Attempts for degradation of organic pollutants by some species of microalgae are summarized in Table 13.13.
-
PAHs aromatic hydrocarbons
PAHs and polyaromatic hydrocarbons are ubiquitous environmental pollutants which are found in petroleum and fossil fuels, or are formed during the incomplete combustion of these energy carriers (Chekroun et al. 2014). These are neutral and nonpolar hydrocarbons that are composed of two or more benzene rings or pentacyclic molecules. Certain types of PAHs including benzo [a] anthracene, chrysene, benzo [b] fluoranthene, benzo [a] pyrene, and benzo[ghi] perylene are potentially carcinogenic for human beings and, due to their carcinogenic and mutagenic characteristics, are dangerous air pollutants (Gariazzo et al. 2015). Some types of the PAHs such as fluoranthene (Fla), pyrene (Pyr), benz[a]anthracene (BaA), chrysene (Chr), benzo[a]pyrene (BaP), benzo[k]fluoranthene (BkF), and dibenzo[a,h] anthracene (DA)] have half-lives of about 1000–3000 h in aquatic environments (Luo et al. 2014). Absorption, chemical degradation, photolysis, and volatilization and microbial degradation are significant methods for PAH removal. Nevertheless, the major process of removing PAH contamination in the environment is microbial degradation and algae are no exception (Ukiwe et al. 2013).
Microalgae release biosurfactants that could further enhance phenanthrene degradation. Moreover, microalgae are able to produce the O2 required by acclimatized bacteria to biodegrade hazardous pollutants such as polycyclic aromatic hydrocarbons, phenolics, and organic solvents (Chekroun et al. 2014). For example, some kinds of marine algae such as cyanobacteria, Oscillatoria, and Agmenellum spp. are known to degrade naphthalene through pathways that are similar to fungus (Haritash and Kaushik 2009; Barrios et al. 2011). The capability of S. obliquus and Nitzschia linearis in removing n-alkanes and PAHs has also been reported (Subashchandrabose et al. 2013).
-
Polychlorinated biphenyls (PCBs)
PCBs; organic chemical compounds of chlorine attached to ‘biphenyl, are a class of the worst persistent organic pollutants (POPs) (Gauthier et al. 2014). Due to their characteristics such as high toxicity, carcinogenicity, and slow biodegradation, exposure to PCBs can cause neurological disorders, reproductive toxicity, endocrine disruption, cancer, and even at extremely low concentrations (Pandelova et al. 2010). There are several technologies for PCB remediation, including biological treatment (phytoremediation, aerobic biodegradation, anaerobic dechlorination), physical methods, thermal treatment, and chemical treatment (Gomes et al. 2013). Bioaccumulation of PCBs by algae has attracted a great deal of attention (Chekroun et al. 2014), while its integration with biodiesel production is also of interest (Usher et al. 2014). The efficiency of algal-based remediation of PCBs could be influenced by water quality, chlorination, phytoplankton composition, the structure of the PCBs, and the algal cell wall (Zhao et al. 2014).
13.7.2 Biofixation of Carbon and Biofuel Production Systems
Microalgae use inorganic carbon for growth, while they can also fix CO2 from industrial exhaust gases (Shilton et al. 2008). Utilization of microalgae for biofixation of carbon has numerous advantages as follows: (1) Microalgae have much higher CO2 fixation abilities compared with other crops, since they have a higher growth rate (Chisti 2007; Li et al. 2008), and (2) microalgae are able to convert CO2 into chemical energy through photosynthesis, which can then be converted into biofuels (Demirbas et al. 2004). Therefore, combination of wastewater treatment, biofuel production, and biofixation of CO2 and GHG may provide a very promising alternative to climate change mitigation strategies.
For instance, CO2 fixation rate (g/m3/h) by Chlorella vulgaris has been reported at 80–260 (Cheng et al. 2006). Yoo et al. (2010) studied three species of microalgae, Botryococcus braunii, Chlorella vulgaris, and Scenedesmus sp., cultivated with ambient air containing 10% CO2 and flue gas. Their results showed that the biomass and lipid productivity in flue gas condition rose by 1.9-fold (39.44 mg L−1 d−1) and 3.7-folds (20.65 mg L−1 d−1), for Scenedesmus sp and B. braunii, respectively. Moreover, they suggested that B. braunii was suitable for biodiesel production, due to its high lipid content, whereas Scenedesmus sp. was suitable for mitigating CO2 as a result of high biomass productivity. In another study by Tang et al. (2011), two species of microalgae, S. obliquus and Chlorella pyrenoidosa, were explored as suitable species for mitigating CO2 in the flue gases and biodiesel production.
CO2 removal efficiency (%) by Euglena gracilis, Porphyridium sp., S. platensis has also been recorded at 3.1, 3–18, 38.3–60, respectively (Chae et al. 2006; Shibata et al. 2004; Kumar et al. 2010a, b, c). Nayak et al. (2013) also demonstrated biomass productivity and CO2 biofixation of three strains of Scenedesmus sp. in the presence of different NaOH concentrations in algae cultivation media. They stated that under their experimental conditions, the algal lipids were mainly composed of C16/C18 fatty acids and were favorable for biodiesel production.
Exogenous CO2 concentration could also impact algal biomass yield, nutrients removal rate, as well as biodiesel production potentials. For instance, in a study, Li et al. (2011) looked into the effects of environmental factors including exogenous CO2 concentration on wastewater nutrient removal and biodiesel production using 14 strains of microalgae belonging to the genus of Chlorella, Haematococcus, Scenedesmus, Chlamydomonas, and Chloroccum cultivated. The results of this study proved that the environmental factors had effects on the yields of algal biomass and lipid accumulation which could consequently result in significantly different biodiesel production potentials. Among the algal strains investigated, Chlorella kessleri and Chlorella protothecoides represented the highest biomass accumulation of 2.01, 1.31 g/L, respectively. Overall, biomass accumulation, biodiesel production rate, and the removal rates of nitrogen and COD were increased by higher light intensity and exogenous CO2 concentration as well as longer lighting period, while higher phosphorus removal rates were achieved in lower exogenous CO2 concentrations.
13.8 Conclusions and Future Prospects
Widespread utilization of fossil fuels is among the major causes of GHG emissions and the resultant tragic environmental consequences such as global warming. Biofuels such as biodiesel produced from algae could be regarded as a promising solution to turn this scenario around. However and in spite of these attractive features of algal fuels, current technologies are yet to be further improved to lead to economically justified production of these alternative fuels. Accordingly, it seems that the integration of algal fuels production with wastewater treatment and/or carbon biofixation could potentially serve as cost-effective and eco-friendly platform to achieve the above-mentioned goals.
References
Abou-Shanab RA, Ji MK, Kim HC, Paeng KJ, Jeon BH (2013) Microalgal species growing on piggery wastewater as a valuable candidate for nutrient removal and biodiesel production. J Environ Manage 115:257–264
Ajayan K V, Selvaraju M, Thirugnanamoorthy K (2011) Growth and Heavy Metals Accumulation Potential of Microalgae Grown in Sewage Wastewater and Petrochemical Effluents. Pak J Bio Sci 14(16):805–811
Akhtar J, Amin NAS (2011) A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew Sustain Energy Rev 15(3):1615–1624
Al Hattab M, Ghaly A, Hammoud A (2015) Microalgae harvesting methods for industrial production of biodiesel: critical review and comparative analysis. J Fundam Renew Energy Appl 5:1000154
Ali I, Asim M, Khan TA (2012) Low cost adsorbents for the removal of organic pollutants from wastewater. J Environ Manage 113:170–183
Alketife AM, Judd S, Znad H (2017) Synergistic effects and optimization of nitrogen and phosphorus concentrations on the growth and nutrient uptake of a freshwater Chlorella vulgaris. Environ Technol 38(1):94–102
Ananadhi Padmanabhan MR, Stanley SA (2012) Microalgae as an oil producer for biofuel applications. Res J Recent Sci 1(3):57–62
Atabani AE, da Silva César A (2014) Calophyllum inophyllum L.-A prospective non-edible biodiesel feedstock. Study of biodiesel production, properties, fatty acid composition, blending and engine performance. Renew Sustain Energy Rev 37:644–655
Balat M, Balat M, KÄrtay E, Balat H (2009) Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 1: pyrolysis systems. Energy Convers Manage 50(12):3147–3157
Barros AI, Gonçalves AL, Simões M, Pires JC (2015) Harvesting techniques applied to microalgae: a review. Renew Sustain Energy Rev 41:1489–1500
Batista AP, Ambrosano L, Graça S, Sousa C, Marques PA, Ribeiro B … Gouveia L (2015) Combining urban wastewater treatment with biohydrogen production–an integrated microalgae-based approach. Bioresour Technol 184: 230–235
Bhargava A, Carmona FF, Bhargava M, Srivastava S (2012) Approaches for enhanced phytoextraction of heavy metals. J Environ Manage 105:103–120
Bhatt NC, Panwar A, Bisht TS, Tamta S (2014) Coupling of algal biofuel production with wastewater. Sci World J Article ID 210504
Biller P, Riley R, Ross A (2011) Catalytic hydrothermal processing of microalgae: decomposition and upgrading of lipids. Biores Technol 102(7):4841–4848
Bohutskyi P, Kula T, Kessler BA, Hong Y, Bouwer EJ, Betenbaugh MJ, Allnutt FT (2014) Mixed trophic state production process for microalgal biomass with high lipid content for generating biodiesel and biogas. BioEnergy Res 7(4):1174–1185
Brennan L, Owende P (2010) Biofuels from microalgaeâ-a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev 14(2):557–577
Bridgwater AV (2003) Renewable fuels and chemicals by thermal processing of biomass. Chem Eng J 91(2–3):87–102
Brown TM, Duan P, Savage PE (2010) Hydrothermal liquefaction and gasification of Nannochloropsis sp. Energy Fuels 24(6):3639–3646
Bwatanglang IB, Faruq M, Gupta AK, Yusof NA (2015) Algae-derived biomass for sustainable and renewable biofuel production. In: Agricultural biomass based potential materials. Springer International Publishing, Berlin, pp 341–373
Cai T, Park SY, Li Y (2013) Nutrient recovery from wastewater streams by microalgae: status and prospects. Renew Sustain Energy Rev 19:360–369
Carvalho P, Goder V, Rapoport TA (2006) Distinct ubiquitin-ligase complexes define convergent pathways for the degradation of ER proteins. Cell 126(2):361–373
Chae S, Hwang E, Shin H (2006) Single cell protein production of Euglena gracilis and carbon dioxide fixation in an innovative photo-bioreactor. Biores Technol 97(2):322–329
Chan A, Salsali H, McBean E (2013) Heavy metal removal (copper and zinc) in secondary effluent from wastewater treatment plants by microalgae. ACS Sustain Chem Eng 2(2):130–137
Chekroun KB, Baghour M (2013) The role of algae in phytoremediation of heavy metals: a review. J Mater Environ Sci 4(6):873–880
Chekroun KB, Sánchez E, Baghour M (2014) The role of algae in bioremediation of organic pollutants. Int Res J Public Environ Health 1(2):19–32
Chen WT, Zhang Y, Zhang J, Yu G, Schideman LC, Zhang P, Minarick M (2014) Hydrothermal liquefaction of mixed-culture algal biomass from wastewater treatment system into bio-crude oil. Biores Technol 152:130–139
Cheng L, Zhang L, Chen H, Gao C (2006) Carbon dioxide removal from air by microalgae cultured in a membrane-photobioreactor. Sep Purif Technol 50(3):324–329
Chevalier P, Proulx D, Lessard P, Vincent W, De la NoÃŒe J (2000) Nitrogen and phosphorus removal by high latitude mat-forming cyanobacteria for potential use in tertiary wastewater treatment. J Appl Phycol 12(2):105–112
Chinnasamy S, Bhatnagar A, Claxton R, Das K (2010a) Biomass and bioenergy production potential of microalgae consortium in open and closed bioreactors using untreated carpet industry effluent as growth medium. Biores Technol 101(17):6751–6760
Chinnasamy S, Bhatnagar A, Hunt RW, Das K (2010b) Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Biores Technol 101(9):3097–3105
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25(3):294–306
Chisti Y (2008) Biodiesel from microalgae beats bioethanol. Trends Biotechnol 26(3):126–131
Chisti Y (2013) Constraints to commercialization of algal fuels. J Biotechnol 167(3):201–214
Christenson L, Sims R (2011) Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol Adv 29(6):686–702
Clarens AF, Resurreccion EP, White MA, Colosi LM (2010) Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ Sci Technol 44(5):1813–1819
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process 48(6):1146–1151
Craggs R, Sutherland D, Campbell H (2012) Hectare-scale demonstration of high rate algal ponds for enhanced wastewater treatment and biofuel production. J Appl Phycol 24(3):329–337
de Alva MS, Luna-Pabello VM, Cadena E, Ortiz E (2013) Green microalga Scenedesmus acutus grown on municipal wastewater to couple nutrient removal with lipid accumulation for biodiesel production. Bioresour Technol 146:744–748
de-Bashan LE, Bashan Y (2010) Immobilized microalgae for removing pollutants: review of practical aspects. Biores Technol 101(6):1611–1627
Demirbaş A (2003) Biodiesel fuels from vegetable oils via catalytic and non-catalytic supercritical alcohol transesterifications and other methods: a survey. Energy Convers Manag 44(13):2093–2109
Demirbas A (2007) Converting biomass derived synthetic gas to fuels via fisher-tropsch synthesis. Energy Sour Part A 29(16):1507–1512
Demirbas A (2009) Biofuels securing the planet’s future energy needs. Energy Convers Manag 50(9):2239–2249
Demirbas A, Demirbas MF (2010) Algae energy: algae as a new source of biodiesel. Springer Science & Business Media, Berlin
Demirbas A, Demirbas MF (2011) Importance of algae oil as a source of biodiesel. Energy Convers Manag 52(1):163–170
Demirbas E, Kobya M, Senturk E, Ozkan T (2004) Adsorption kinetics for the removal of chromium (VI) from aqueous solutions on the activated carbons prepared from agricultural wastes. Water Sa 30(4):533–539
Deng DJ, Lu ZM (2013) Differentiation and adaptation epigenetic networks: translational research in gastric carcinogenesis. Chin Sci Bull 58(1):1–6
Dosnon-Olette R, Trotel-Aziz P, Couderchet M, Eullaffroy P (2010) Fungicides and herbicide removal in Scenedesmus cell suspensions. Chemosphere 79(2):117–123
Dexter J, Fu P (2009) Metabolic engineering of cyanobacteria for ethanol production. Energy Environ Sci 2(8):857–864
Duong VT, Ahmed F, Thomas-Hall SR, Quigley S, Nowak E, Schenk PM (2015) High protein-and high lipid-producing microalgae from northern australia as potential feedstock for animal feed and biodiesel. Front Bioeng Biotechnol 3:53
Eroglu E, Smith SM, Raston CL (2015) Application of various immobilization techniques for algal bioprocesses. In: Biomass and biofuels from microalgae. Springer International Publishing, Berlin, pp 19–44
Fedorov AS, Kosourov S, Ghirardi ML, Seibert M (2005) Continuous hydrogen photoproduction by Chlamydomonas reinhardtii. Paper presented at the twenty-sixth symposium on biotechnology for fuels and chemicals
Feng Y, Li C, Zhang D (2011) Lipid production of Chlorella vulgaris cultured in artificial wastewater medium. Biores Technol 102(1):101–105
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manage 92(3):407–418
Fukuda H, Kondo A, Noda H (2001) Biodiesel fuel production by transesterification of oils. J Biosci Bioeng 92(5):405–416
Gariazzo C, Lamberti M, Hänninen O, Silibello C, Pelliccioni A, Porta D … Forastiere F (2015) Assessment of population exposure to Polycyclic Aromatic Hydrocarbons (PAHs) using integrated models and evaluation of uncertainties. Atmos Environ 101:235–245
Gattullo CE, Bährs H, Steinberg CE, Loffredo E (2012) Removal of bisphenol a by the freshwater green alga Monoraphidium braunii and the role of natural organic matter. Sci Total Environ 416:501–506
Gavrilescu M (2010) Environmental biotechnology: achievements, opportunities and challenges. Dyn Biochem Process Biotech Mol 4(1):1–36
Gentili FG (2014) Microalgal biomass and lipid production in mixed municipal, dairy, pulp and paper wastewater together with added flue gases. Biores Technol 169:27–32
Gilden RC, Huffling K, Sattler B (2010) Pesticides and health risks. J Obstet Gynecol Neonatal Nurs 39(1):103–110
Gomes HI, Dias-Ferreira C, Ribeiro AB (2013) Overview of in situ and ex situ remediation technologies for PCB-contaminated soils and sediments and obstacles for full-scale application. Sci Total Environ 445:237–260
Griffiths MJ, van Hille RP, Harrison ST (2014) The effect of nitrogen limitation on lipid productivity and cell composition in Chlorella vulgaris. Appl Microbiol Biotechnol 98(5):2345–2356
Günerken E, d’Hondt E, Eppink MHM, Garcia-Gonzalez L, Elst K, Wijffels RH (2015) Cell disruption for microalgae biorefineries. Biotechnol Adv 33(2):243–260
Gupta VK, Ali I, Saleh TA, Nayak A, Agarwal S (2012) Chemical treatment technologies for waste-water recycling—an overview. Rsc Adv 2(16):6380–6388
Halim R, Harun R, Danquah MK, Webley PA (2012) Microalgal cell disruption for biofuel development. Appl Energy 91(1):116–121
Haritash A, Kaushik C (2009) Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater 169(1):1–15
Hattab MA, Ghaly A (2015) Microalgae oil extraction pretreatment methods: critical review and comparative analysis. J Fundam Renew Energy Appl 5:172
Hayyan A, Alam MZ, Mirghani ME, Kabbashi NA, Hakimi NINM, Siran YM, Tahiruddin S (2010) Sludge palm oil as a renewable raw material for biodiesel production by two-step processes. Biores Technol 101(20):7804–7811
Hena S, Fatimah S, Tabassum S (2015) Cultivation of algae consortium in a dairy farm wastewater for biodiesel production. Water Resour Ind 10:1–14
Hoffmann JP (1998) Wastewater treatment with suspended and nonsuspended algae. J Phycol 34(5):757–763
Hong YW, Yuan DX, Lin QM, Yang TL (2008) Accumulation and biodegradation of phenanthrene and fluoranthene by the algae enriched from a mangrove aquatic ecosystem. Marine Poll Bull 56(8):1400–1405
Hongyang S, Yalei Z, Chunmin Z, Xuefei Z, Jinpeng L (2011) Cultivation of Chlorella pyrenoidosa in soybean processing wastewater. Bioresour Technol 102(21):9884–9890
Hu B, Min M, Zhou W, Du Z, Mohr M, Chen P … Ruan R (2012) Enhanced mixotrophic growth of microalga Chlorella sp. on pretreated swine manure for simultaneous biofuel feedstock production and nutrient removal. Bioresour Technol 126:71–79
Huang G, Chen F, Wei D, Zhang X, Chen G (2010) Biodiesel production by microalgal biotechnology. Appl Energy 87(1):38–46
Huang X, Wei L, Huang Z, Yan J (2014) Effect of high ferric ion concentrations on total lipids and lipid characteristics of Tetraselmis subcordiformis, Nannochloropsis oculata and Pavlova viridis. J Appl Phycol 26(1):105–114
Islam MA, Magnusson M, Brown RJ, Ayoko GA, Nabi MN, Heimann K (2013) Microalgal species selection for biodiesel production based on fuel properties derived from fatty acid profiles. Energies 6(11):5676–5702
Johnson MB, Wen Z (2009) Production of biodiesel fuel from the microalga Schizochytrium limacinum by direct transesterification of algal biomass. Energy Fuels 23(10):5179–5183
Johnson MB, Wen Z (2010) Development of an attached microalgal growth system for biofuel production. Appl Microbiol Biotechnol 85(3):525–534
Kalla N, Khan S (2016) Effect of nitrogen, phosphorus concentrations, pH and salinity ranges on growth, biomass and lipid accumulation of Chlorella vulgaris. Int J Pharm Sci Res 7(1):397
Kapdan IK, Kargi F (2006) Bio-hydrogen production from waste materials. Enzyme Microb Technol 38(5):569–582
Karmakar A, Karmakar S, Mukherjee S (2010) Properties of various plants and animals feedstocks for biodiesel production. Biores Technol 101(19):7201–7210
Kawaroe M, Prartono T, Sunuddin A, Saputra D (2016) Marine microalgae Tetraselmis suecica as flocculant agent of bio-flocculation method. HAYATI J Biosci 23(2):62–66
Khan M, Yoshida N (2008) Effect of L-glutamic acid on the growth and ammonium removal from ammonium solution and natural wastewater by Chlorella vulgaris NTM06. Biores Technol 99(3):575–582
Kiss AA, Dimian AC, Rothenberg G (2007) Biodiesel by catalytic reactive distillation powered by metal oxides. Energy Fuels 22(1):598–604
Kolesárová N, Hutňan M, Bodík I, Špalková V (2011) Utilization of biodiesel by-products for biogas production. BioMed Res Int
Kong Q-X, Li L, Martinez B, Chen P, Ruan R (2010) Culture of microalgae Chlamydomonas reinhardtii in wastewater for biomass feedstock production. Appl Biochem Biotechnol 160(1):9–18
Kothari R, Pathak VV, Kumar V, Singh DP (2012) Experimental study for growth potential of unicellular alga Chlorella pyrenoidosa on dairy waste water: an integrated approach for treatment and biofuel production. Bioresour Technol 116:466–470
Krishna AR, Dev L, Thankamani V (2012) An integrated process for Industrial effluent treatment and Biodiesel production using Microalgae. Res Biotechnol 3(1)
Kumar A, Ergas S, Yuan X, Sahu A, Zhang Q, Dewulf J et al (2010a) Enhanced CO2 fixation and biofuel production via microalgae: recent developments and future directions. Trends Biotechnol 28:371–380. https://doi.org/10.1016/j.tibtech.2010.04.004
Kumar A, Ergas S, Yuan X, Sahu A, Zhang Q, Dewulf J, Van Langenhove H (2010b) Enhanced CO2 fixation and biofuel production via microalgae: recent developments and future directions. Trends Biotechnol 28(7):371–380
Kumar A, Yuan X, Sahu AK, Dewulf J, Ergas SJ, Van Langenhove H (2010c) A hollow fiber membrane photoâ-bioreactor for CO2 sequestration from combustion gas coupled with wastewater treatment: a process engineering approach. J Chem Technol Biotechnol 85(3):387–394
Kumar KS, Dahms H-U, Won E-J, Lee J-S, Shin K-H (2015) Microalgaeâ-A promising tool for heavy metal remediation. Ecotoxicol Environ Saf 113:329–352
Kumar P, Suseela MR, Toppo K (2011) Physico-chemical characterization of algal oil: a potential biofuel. Asian J Exp Biol Sci 2(3):493–497
Laghari SM, Isa MH, Abdullah AB, Laghari AJ, Saleem H (2015) Microwave individual and combined pre-treatments on lignocellulosic biomasses. IOSR J Comput Eng 4(2):14–28
Lee AK, Lewis DM, Ashman PJ (2012) Disruption of microalgal cells for the extraction of lipids for biofuels: processes and specific energy requirements. Biomass Bioenerg 46:89–101
Lee HJ, Lee SY (2001) Heat transfer correlation for boiling flows in small rectangular horizontal channels with low aspect ratios. Int J Multiph Flow 27(12):2043–2062
Lee JY, Yoo C, Jun SY, Ahn CY, Oh HM (2010) Comparison of several methods for effective lipid extraction from microalgae. Biores Technol 101(1):S75–S77
Lei AP, Hu ZL, Wong YS, Tam NFY (2007) Removal of fluoranthene and pyrene by different microalgal species. Bioresour Technol 98(2):273–280
Li Y, Horsman M, Wu N, Lan CQ, Dubois-Calero N (2008) Biofuels from microalgae. Biotechnol Prog 24(4):815–820
Li Y, Zhou W, Hu B, Min M, Chen P, Ruan RR (2011) Integration of algae cultivation as biodiesel production feedstock with municipal wastewater treatment: strains screening and significance evaluation of environmental factors. Biores Technol 102(23):10861–10867
Liang Y (2013) Producing liquid transportation fuels from heterotrophic microalgae. Appl Energy 104:860–868
Lim SL, Chu WL, Phang SM (2010) Use of Chlorella vulgaris for bioremediation of textile wastewater. Bioresour Technol 101(19):7314–7322
Lu Q, Zhou W, Min M, Ma X, Chandra C, Doan YT … Chen P (2015) Growing Chlorella sp. on meat processing wastewater for nutrient removal and biomass production. Bioresour Technol 198:189–197
Luo L, Wang P, Lin L, Luan T, Ke L, Tam NFY (2014) Removal and transformation of high molecular weight polycyclic aromatic hydrocarbons in water by live and dead microalgae. Process Biochem 49(10):1723–1732
Lv J, Guo J, Feng J, Liu Q, Xie S (2017) Effect of sulfate ions on growth and pollutants removal of self-flocculating microalga Chlorococcum sp. GD in synthetic municipal wastewater. Biores Technol 234:289–296
Mahapatra DM, Chanakya HN, Ramachandra TV (2013) Euglena sp. as a suitable source of lipids for potential use as biofuel and sustainable wastewater treatment. J Appl Phycol 25(3):855–865
Maity JP, Bundschuh J, Chen CY, Bhattacharya P (2014) Microalgae for third generation biofuel production, mitigation of greenhouse gas emissions and wastewater treatment: Present and future perspectives–a mini review. Energy 78:104–113
Malla FA, Khan SA, Sharma GK, Gupta N, Abraham G (2015) Phycoremediation potential of Chlorella minutissima on primary and tertiary treated wastewater for nutrient removal and biodiesel production. Ecol Eng 75:343–349
Mallick N (2002) Biotechnological potential of immobilized algae for wastewater N, P and metal removal: a review. Biometals 15(4):377–390
Markou G, Nerantzis E (2013) Microalgae for high-value compounds and biofuels production: a review with focus on cultivation under stress conditions. Biotechnol Adv 31(8):1532–1542
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sustain Energy Rev 14(1):217–232
McKendry P (2002) Energy production from biomass (part 2): conversion technologies. Biores Technol 83(1):47–54
Meher L, Sagar DV, Naik S (2006) Technical aspects of biodiesel production by transesterificationâ-a review. Renew Sustain Energy Rev 10(3):248–268
Mirghaffari N, Moeini E, Farhadian O (2015) Biosorption of Cd and Pb ions from aqueous solutions by biomass of the green microalga, Scenedesmus quadricauda. J Appl Phycol 27(1):311–320
Mittal A (2011) Biological wastewater treatment. Water Today 1:32–44
Mohan D, Pittman CU, Steele PH (2006) Pyrolysis of wood/biomass for bio-oil: a critical review. Energy Fuels 20(3):848–889
Mondal P, Dang GS, Garg MO (2011) Syngas production through gasification and cleanup for downstream applications—recent developments. Fuel Process Technol 92(8):1395–1410
Morita M, Watanabe Y, Okawa T, Saiki H (2001) Photosynthetic productivity of conical helical tubular photobioreactors incorporating Chlorella sp. under various culture medium flow conditions. Biotechnol Bioeng 74(2):136–144
Moser BR, Vaughn SF (2012) Efficacy of fatty acid profile as a tool for screening feedstocks for biodiesel production. Biomass Bioenerg 37:31–41
Mukherjee K, Saha R, Ghosh A, Saha B (2013) Chromium removal technologies. Res Chem Intermed 39(6):2267–2286
Narala RR, Garg S, Sharma KK, Thomas-Hall SR, Deme M, Li Y, Schenk PM (2016) comparison of Microalgae cultivation in Photobioreactor, open raceway pond, and a two-stage hybrid system. Front Energy Res 4:29
Nayak M, Thirunavoukkarasu M, Mishra BK (2013) Maximizing biomass productivity and CO2 biofixation of microalga, Scenedesmus sp. by using sodium hydroxide. J Microbiol Biotechnol 23(9):1260–1268
Niestroy J, Martínez AB, Band-Schmidt CJ (2014) Analysis of concentration-dependent effects of copper and PCB on different Chattonella spp. microalgae (raphidophyceae) cultivated in artificial seawater medium. EXCLI J 13:197
Ndikubwimana T, Zeng X, Liu Y, Chang JS, Lu Y (2014) Harvesting of microalgae Desmodesmus sp. F51 by bioflocculation with bacterial bioflocculant. Algal Res 6:186–193
Ndikubwimana T, Zeng X, Murwanashyaka T, Manirafasha E, He N, Shao W, Lu Y (2016) Harvesting of freshwater microalgae with microbial bioflocculant: a pilot-scale study. Biotechnol Biofuels 9(1):47
Pandelova M, Piccinelli R, Kasham S, Henkelmann B, Leclercq C, Schramm K-W (2010) Assessment of dietary exposure to PCDD/F and dioxin-like PCB in infant formulae available on the EU market. Chemosphere 81(8):1018–1021
Park J, Craggs R, Shilton A (2011) Wastewater treatment high rate algal ponds for biofuel production. Biores Technol 102(1):35–42
Park J, Seo J, Kwon EE (2012) Microalgae production using wastewater: effect of light-emitting diode wavelength on microalgal growth. Environ Eng Sci 29(11):995–1001
Pereira FV, Gurgel LVA, Gil LF (2010) Removal of Zn2+ from aqueous single metal solutions and electroplating wastewater with wood sawdust and sugarcane bagasse modified with EDTA dianhydride (EDTAD). J Hazard Mater 176(1):856–863
Pittman JK, Dean AP, Osundeko O (2011) The potential of sustainable algal biofuel production using wastewater resources. Biores Technol 102(1):17–25
Powell RJ, Hill RT (2013) Rapid aggregation of biofuel-producing algae by the bacterium Bacillus sp. strain RP1137. Appl Environ Microbiol 79(19):6093–6101
Raheem A, Azlina WW, Yap YT, Danquah MK, Harun R (2015a) Thermochemical conversion of microalgal biomass for biofuel production. Renew Sustain Energy Rev 49:990–999
Raheem A, WAKG WA, Yap YT, Danquah MK, Harun R (2015b) Optimization of the microalgae Chlorella vulgaris for syngas production using central composite design. RSC Adv 5(88):71805–71815
Ramesh D (2013) Lipid identification and extraction techniques. In: Biotechnological applications of microalgae: biodiesel and value-added products, pp 89–97
Ranjith Kumar R, Hanumantha Rao P, Arumugam M (2015) Lipid extraction methods from microalgae: a comprehensive review. Front Energy Res 2:61
Ratha SK, Babu S, Renuka N, Prasanna R, Prasad RBN, Saxena AK (2013) Exploring nutritional modes of cultivation for enhancing lipid accumulation in microalgae. J Basic Microbiol 53(5):440–450
Richards RG, Mullins BJ (2013) Using microalgae for combined lipid production and heavy metal removal from leachate. Ecol Model 249:59–67
Richmond A (ed) (2008) Handbook of microalgal culture: biotechnology and applied phycology. Wiley, New Jersey
Ruiz-Marin A, Mendoza-Espinosa LG, Stephenson T (2010) Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Biores Technol 101(1):58–64
Sakarika M, Kornaros M (2016) Effect of pH on growth and lipid accumulation kinetics of the microalga Chlorella vulgaris grown heterotrophically under sulfur limitation. Biores Technol 219:694–701
San Martín YB (2011) Bioremediation: a tool for the management of oil pollution in marine ecosystems. Biotecnol Apl 28(2):69–76
Scott SA, Davey MP, Dennis JS, Horst I, Howe CJ, Lea-Smith DJ, Smith AG (2010) Biodiesel from algae: challenges and prospects. Curr Opin Biotechnol 21(3):277–286
Sharma KK, Garg S, Li Y, Malekizadeh A, Schenk PM (2013) Critical analysis of current microalgae dewatering techniques. Biofuels 4:397–407
Sharma Y, Singh B, Upadhyay S (2008) Advancements in development and characterization of biodiesel: a review. Fuel 87(12):2355–2373
Shibata T, Kawaguchi S, Hama Y, Inagaki M, Yamaguchi K, Nakamura T (2004) Local and chemical distribution of phlorotannins in brown algae. J Appl Phycol 16(4):291–296
Shilton A, Mara D, Craggs R, Powell N (2008) Solar-powered aeration and disinfection, anaerobic co-digestion, biological CO2 scrubbing and biofuel production: the energy and carbon management opportunities of waste stabilisation ponds. Water Sci Technol 58(1):253
Sialve B, Bernet N, Bernard O (2009) Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol Adv 27(4):409–416
Singh D, Barrow CJ, Puri M, Tuli DK, Mathur AS (2016) Combination of calcium and magnesium ions prevents substrate inhibition and promotes biomass and lipid production in thraustochytrids under higher glycerol concentration. Algal Res 15:202–209
Subashchandrabose SR, Ramakrishnan B, Megharaj M, Venkateswarlu K, Naidu R (2013) Mixotrophic cyanobacteria and microalgae as distinctive biological agents for organic pollutant degradation. Environ Int 51:59–72
Taher H, Al-Zuhair S, Al-Marzouqi AH, Haik Y, Farid M (2014) Effective extraction of microalgae lipids from wet biomass for biodiesel production. Biomass Bioenerg 66:159–167
Talebi AF, Tohidfar M, Mousavi Derazmahalleh SM, Sulaiman A, Baharuddin AS, Tabatabaei M (2015) Biochemical modulation of lipid pathway in microalgae Dunaliella sp. for biodiesel production. BioMed Res Int
Tan KWM, Lee YK (2016) The dilemma for lipid productivity in green microalgae: importance of substrate provision in improving oil yield without sacrificing growth. Biotechnol Biofuels 9(1):255
Tang D, Han W, Li P, Miao X, Zhong J (2011) CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Biores Technol 102(3):3071–3076
The Guardian (2015) Pope Francis issues final call for unity at end of historic US visit. Available at https://www.theguardian.com/world/2015/sep/28/pope-francis-leaves-us-end-visit. Accessed on 19 June 2017
Toor SS, Rosendahl L, Rudolf A (2011) Hydrothermal liquefaction of biomass: a review of subcritical water technologies. Energy 36(5):2328–2342
Tsukahara K, Sawayama S (2005) Liquid fuel production using microalgae. J Jpn Pet Inst 48(5):251
Ugwu C, Aoyagi H, Uchiyama H (2008) Photobioreactors for mass cultivation of algae. Biores Technol 99(10):4021–4028
Ukiwe LN, Egereonu UU, Njoku PC, Nwoko CI, Allinor JI (2013) Polycyclic aromatic hydrocarbons degradation techniques: a review. Int J Chem 5(4):43
Ullah K, Ahmad M, Sharma VK, Lu P, Harvey A, Zafar M … Anyanwu CN (2014) Algal biomass as a global source of transport fuels: overview and development perspectives. Prog Nat Sci: Mater Int 24(4):329–339
Usher PK, Ross AB, Camargo-Valero MA, Tomlin AS, Gale WF (2014) An overview of the potential environmental impacts of large-scale microalgae cultivation. Biofuels 5(3):331–349
Uthman H, Saka AA (2013) Comparatives study of production biodiesel from soybean oil and Jatropha Curcas seeds oil. Distrib Gener Altern Energy J 28(2):31–42
Valiente Moro C, Bricheux G, Portelli C, Bohatier J (2012) Comparative effects of the herbicides chlortoluron and mesotrione on freshwater microalgae. Environ Toxicol Chem 31(4):778–786
Vardon DR, Sharma BK, Blazina GV, Rajagopalan K, Strathmann TJ (2012) Thermochemical conversion of raw and defatted algal biomass via hydrothermal liquefaction and slow pyrolysis. Biores Technol 109:178–187
Veillette M, Nikiema J, Heitz M, Chamoumi M, Faucheux N (2012) Production of biodiesel from microalgae. INTECH Open Access Publisher, London
Wahidin S, Idris A, Shaleh SRM (2013) The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Biores Technol 129:7–11
Wang B, Li Y, Wu N, Lan CQ (2008) CO2 bio-mitigation using microalgae. Appl Microbiol Biotechnol 79(5):707–718
Wang H, Xiong H, Hui Z, Zeng X (2012) Mixotrophic cultivation of Chlorella pyrenoidosa with diluted primary piggery wastewater to produce lipids. Biores Technol 104:215–220
Wang L, Min M, Li Y, Chen P, Chen Y, Liu Y, Ruan R (2010) Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl Biochem Biotechnol 162(4):1174–1186
Woertz I, Feffer A, Lundquist T, Nelson Y (2009) Algae grown on dairy and municipal wastewater for simultaneous nutrient removal and lipid production for biofuel feedstock. J Environ Eng 135(11):1115–1122
Wu X, Ruan R, Du Z, Liu Y (2012) Current status and prospects of biodiesel production from microalgae. Energies 5(8):2667–2682
Xie Y, Li H, Wang X, Ng I S, Lu Y, Jing K (2014) Kinetic simulating of Cr (VI) removal by the waste Chlorella vulgaris biomass. J Taiwan Inst Chem Eng 45(4):1773–1782
Xin L, Hong-ying H, Ke G, Ying-xue S (2010) Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Biores Technol 101(14):5494–5500
Xu H, Miao X, Wu Q (2006) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126(4):499–507
Yang J, Cao J, Xing G, Yuan H (2015) Lipid production combined with biosorption and bioaccumulation of cadmium, copper, manganese and zinc by oleaginous microalgae Chlorella minutissima UTEX2341. Biores Technol 175:537–544
Yoo C, Jun S-Y, Lee J-Y, Ahn C-Y, Oh H-M (2010) Selection of microalgae for lipid production under high levels carbon dioxide. Biores Technol 101(1):S71–S74
Zamalloa C, Boon N, Verstraete W (2012) Anaerobic digestibility of Scenedesmus obliquus and Phaeodactylum tricornutum under mesophilic and thermophilic conditions. Appl Energy 92:733–738
Zhang J, Hu B (2012) A novel method to harvest microalgae via co-culture of filamentous fungi to form cell pellets. Biores Technol 114:529–535
Zhang X (2015) Microalgae removal of CO2 from flue gas. IEA Clean Coal Centre, UK
Zhang X, Rong J, Chen H, He C, Wang Q (2014) Current status and outlook in the application of microalgae in biodiesel production and environmental protection. Front Energy Res 2:32
Zhao Z, Jiang Y, Xia L, Mi T, Yan W, Gao Y, Hussain J (2014) Application of canonical correspondence analysis to determine the ecological contribution of phytoplankton to PCBs bioaccumulation in Qinhuai River, Nanjing, China. Environ Sci Pollut Res 21(4):3091–3103
Zhou N, Zhang Y, Wu X, Gong X, Wang Q (2011a) Hydrolysis of Chlorella biomass for fermentable sugars in the presence of HCl and MgCl2. Biores Technol 102(21):10158–10161
Zhou W, Hu B, Li Y, Min M, Mohr M, Du Z, Ruan R (2012) Mass cultivation of microalgae on animal wastewater: a sequential two-stage cultivation process for energy crop and omega-3-rich animal feed production. Appl Biochem Biotechnol 168(2):348–363
Zhou W, Li Y, Min M, Hu B, Chen P, Ruan R (2011b) Local bioprospecting for high-lipid producing microalgal strains to be grown on concentrated municipal wastewater for biofuel production. Biores Technol 102(13):6909–6919
Zhou Y, Schideman L, Yu G, Zhang Y (2013) A synergistic combination of algal wastewater treatment and hydrothermal biofuel production maximized by nutrient and carbon recycling. Energy Environ Sci 6(12):3765–3779
Zhu L, Wang Z, Takala J, Hiltunen E, Qin L, Xu Z, Yuan Z (2013) Scale-up potential of cultivating Chlorella zofingiensis in piggery wastewater for biodiesel production. Biores Technol 137:318–325
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Madadi, R., Tabatabaei, M., Aghbashlo, M., Zahed, M.A., Pourbabaee, A.A. (2018). Biodiesel from Microalgae. In: Singhania, R., Agarwal, R., Kumar, R., Sukumaran, R. (eds) Waste to Wealth. Energy, Environment, and Sustainability. Springer, Singapore. https://doi.org/10.1007/978-981-10-7431-8_13
Download citation
DOI: https://doi.org/10.1007/978-981-10-7431-8_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7430-1
Online ISBN: 978-981-10-7431-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)