Abstract
Maintenance of agricultural productivity is currently based mainly on extraneous application of fertilizers and pesticides. However, indiscriminate use of agrochemicals for controlling the pests and diseases led to pollution of soil, water, and food sources, poisoning of nontarget beneficial insects, and development of insect population resistant to insecticides. To obviate the pollution problem and obtain higher yields in a sustainable manner, biological control of insect pests using specific antagonistic microorganisms is an effective alternate approach with minimum deleterious effects. Microorganisms have been obtained from the rhizosphere of different crop plants that inhibited insect pests by producing toxins, bacteriocins, siderophores, hydrolytic enzymes, and other secondary metabolites. Moreover, plant hormones salicylic acid, jasmonic acid, and ethylene orchestrate a complex transcriptional programming that eventually leads to pest-induced SAR (systemic acquired resistance) and ISR (induced systemic resistance) in many plant species. Microbial genes involved in the biosynthesis of secondary metabolites and enzymes have been cloned and transferred to other microorganisms and plants to enhance the suppression and killing of insects. The efficiency of these biocontrol products can be further increased through genetic improvement, manipulation of the soil and plant environment, using mixtures of biocontrol agents, and optimization of formulations and by integration of biocontrol agents with other alternative methods that provide additive and synergistic effects. Thus, the application of effective biocontrol agents may reduce the use of chemical insecticides and support sustainable agriculture in an eco-friendly manner in tandem with improved crop productivity.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Global crop yields are reduced by 20–40% annually due to pests and diseases (FAO 2012). For the control of insect pests in agriculture, farmers have mostly relied on the application of synthetic pesticides, and the global pesticide market is presently growing at a rate of 3.6% per year (Lehr 2010). However, indiscriminate use of chemical pesticides to control the pathogens/insects has generated several problems including resistance to insecticides/fungicides, outbreak of secondary pests, as well as safety risks for humans and domestic animals. Moreover, the long persistence of applied pesticides in soil leads to contamination of groundwater and soil, and the residual toxic chemicals enter into the food chain. Excessive pesticide application also decreases the biodiversity due to destruction of nontarget entomofauna. These problems have increased the interest of scientists for development of eco-friendly microbe-based insecticides or biocontrol agents, which act differently from known chemicals (Ruiu et al. 2013). Sustainable agriculture in the twenty-first century will rely increasingly on alternative interventions for pest management that are environment-friendly and will reduce the human contact with chemical pesticides. Therefore, microorganisms are currently being explored for their possible use as biocontrol agents in the integrated pest management programs.
Some of the microorganisms obtained from the rhizosphere of crop plants provide the frontline defense to plant roots against the attack by various plant pathogens and insects (Compant et al. 2005). Several microorganisms including bacteria, actinomycetes, fungi, viruses, protozoa, and nematodes have been identified to control various root, foliage, and postharvest diseases of agricultural crops (Glick and Bashan 1997), and many microorganisms have been found to act as potential entomopathogens (Vaga and Kaya 2012; Lacey et al. 2015; Mascarin and Jaronski 2016). Among the various microbial control agents (MCAs), Bacillus thuringiensis (Bt), Pseudomonas fluorescens, Serratia marcescens, Streptomyces sp., Lecanicillium lecanii, Trichoderma virens, Metarhizium sp., Beauveria bassiana, and nuclear polyhedrosis virus are popularly used in plant protection (Mascarin and Jaronski 2016; Sindhu et al. 2016). So far, about 175 biopesticide active ingredients and 700 products have been registered worldwide.
9.2 Characterization of Microorganisms Involved in Biological Control of Insect Pests
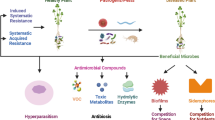
Several microorganisms inhabiting either the soil or plant rhizosphere have been identified to act as entomopathogens, and some of the microbes have also been found to suppress the diseases of agricultural crops (Borneman and Becker 2007; Lacey et al. 2015). The microorganisms isolated from the rhizosphere soil could be screened for their biocontrol activities for subsequent use as biocontrol agents (Fig. 9.1). Li et al. (2015) demonstrated distinct variations in the microbial community of cotton rhizosphere between monocropped (4 and 15 years) rhizosphere soils and fallow (control) agricultural soil. The monocropped soils significantly influenced the composition of root exudates and also reduced soil suppressiveness to Fusarium wilt. A significant correlation existed between the presence of certain amino acids (e.g., glutamic acid and alanine) and predominant bacterial taxa in the rhizosphere, indicating that some constituents in root exudates influenced the microbial compositions of the cotton rhizosphere to manage the disease status of plants in monocropped soils.
9.2.1 Antagonistic Bacteria
Rhizobacteria have been found to inhibit the growth of various pathogenic bacteria, fungi, and insects resulting in suppression of the diseases caused by different pathogens and insect pests (Sindhu et al. 2014). Bacillus thuringiensis (Bt) is the most studied entomopathogenic species for biological control of insect pests, and some of the toxin-producing strains have shown high mortality against specific insects compared to conventional insecticides used in the microbial pest management (Vega and Kaya 2012). The insecticidal proteins produced by B. thuringiensis are highly specific insect gut toxins and do not affect the nontarget organisms (Lacey and Goettel 1995). B. thuringiensis subspecies kurstaki strain HD-1 (De Barjac and Lemille 1970) is most widely used for the management of lepidopteran pests in agriculture and forestry, whereas B. thuringiensis subspecies israelensis and Lysinibacillus sphaericus are the pathogens used for medically important pests including dipteran vectors. Strains of B. thuringiensis subsp. aizawai (Bta) (i.e., ABTS-1857) are used against armyworms and diamondback moth larvae. Similarly, Bacillus strains belonging to the subsp. israelensis (Bti) and tenebrionis (Btt) have been employed for the management of mosquitoes and simulids and against coleoptera, respectively (Glare and O’Callaghan 2000).
Other Bacillus species have also shown potential for insect pest management. Spherical endospore-producing bacteria B. sphaericus (Alexander and Priest 1990) produce parasporal crystals located within the exosporium, and these strains showed toxicity against mosquitoes. The equimolar ratio of the two homologous binary protein toxins (Bin), BinA and BinB, found in parasporal bodies acts in a similar fashion as Cry proteins (Charles et al. 2000). Other entomopathogenic bacteria that possess potential against diverse insect pests include B. popilliae with B. lentimorbus, the causal agents of milky disease in phytophagous scarab larvae (Zhang et al. 1997). Serratia entomophila contains a specific plasmid (pADAP) encoding genes implicated in pathogenicity against the grass-grub, Costelytra zealandica (White) (Jackson et al. 1992). Clostridium bifermentans serovar malaysia has been found effective against mosquitoes and blackflies (Nicolas et al. 1990). The mosquitocidal activity in this bacterium was associated to the production of a protein with homology to Bt δ-endotoxins (Cbm71). The encoding gene of this protein was cloned and induced for expression by transformed Bt, which exhibited toxicity against mosquitoes (Barloy et al. 1996).
Another group of entomopathogenic bacteria includes the endosymbionts of insecticidal nematodes, especially the members of the genera Xenorhabdus and Photorhabdus (Burnell and Stock 2000). These entomopathogenic bacteria and the nematodes produce a variety of metabolites that enable them to colonize and reproduce in the insect host. The metabolites produced include enzymes such as proteases, lipases, and phospholipases to maintain a food supply during reproduction (Bowen et al. 2000). Antifungal and antibacterial agents prevent the degradation or colonization of the insect carcass, while the bacteria and nematodes reproduce. Bowen (1995) reported that a soluble protein fraction purified from P. luminescens culture medium possessed sufficient insecticidal activity to kill Manduca sexta upon injection. The novel protein toxin secreted by bacterium Xenorhabdus nematophila was found effective against Galleria mellonella and H. armigera, cabbage white caterpillar Pieris brassicae, mosquito larva Aedes aegypti, and mustard beetle Phaedon cochleariae (Sergeant et al. 2006). These bacteria were found effective on most of the economically important lepidopteran, dipteran, and coleopteran insect orders, suggesting wide scope of these organisms for application in insect pest management.
Common soil organism B. cereus has also been found pathogenic to insects and has been isolated from several insect species (Kuzina et al. 2001; Sezen et al. 2005). Various bacterial isolates, i.e., B. cereus (Ags1), Bacillus sp. (Ags2), B. megaterium (Ags3), Enterobacter aerogenes (Ags4), Acinetobacter calcoaceticus (Ags5), Enterobacter sp. (Ags6), Pseudomonas putida (Ags7), Enterococcus gallinarum (Ags8), and Stenotrophomonas maltophilia (Ags9), were identified from the flora of Agrotis segetum (Sevim et al. 2010), and these isolates caused 60% insect mortality after 8 days of application. B. cereus, B. sphaericus, Morganella morganii, Serratia marcescens, and Klebsiella species isolated from the predatory larvae of the antlion species Myrmeleon bore (Neuroptera, Myrmeleontidae) were found to kill 80% or more cutworms S. litura (Nishiwaki et al. 2007). The bacterial flora Leclercia adecarboxylata of Colorado potato beetle showed highest insecticidal effect (100% mortality) within 5 days (Muratoglu et al. 2009) and thus showed a potential for the control of several coleopteran pests.
Pseudomonas entomophila showed insecticidal properties against insects in different orders and triggered a systemic immune response in Drosophila melanogaster Meigen after ingestion (Vodovar et al. 2006). Similarly, biopesticidal potential of Brevibacillus laterosporus Laubach has been reported against insects in different orders, such as coleoptera (Boets et al. 2004), lepidoptera (Oliveira et al. 2004), mosquitoes and blackflies (Rivers et al. 1991), and houseflies (Ruiu et al. 2006), nematodes (Singer 1996), and against phytopathogenic fungi (Saikia et al. 2011). Chromobacterium subtsugae showed its insecticidal potential after ingestion against diverse insect species in different orders (i.e., coleoptera, lepidoptera, hemiptera) (Hoshino 2011). These insects included Colorado potato beetle (Leptinotarsa decemlineata Say), Western corn rootworm (Diabrotica virgifera Le Conte), Southern corn rootworm (Diabrotica undecimpunctata Mannerheim), small hive beetle (Aethina tumida Murray), diamondback moth (Plutella xylostella L.), sweet potato whitefly (Bemisia tabaci Gennadius), and Southern green stink bug (Nezara viridula L.) (Martin et al. 2007).
Subterranean termites have been found to cause extensive damage to major agricultural crops and forest plantation trees. Khan et al. (1985) reported that a commercial preparation of B. thuringiensis (Thuricide-HP concentrate) exhibited 100% mortality within 6 days of exposure against H. indicola, M. championi, and Bifiditermes beesoni (Gardner) (Kalotermitidae). Similarly, the colonies of M. championi, H. indicola, and B. beesoni exposed to suspensions of the spore-forming bacterium Serratia marcescens Bizio succumbed completely 7–13 days following infection (Khan et al. 1977). Khan et al. (1992) showed that mortality of M. championi, H. indicola, and Coptotermes heimi (Wasmann) (Rhinotermitidae) termites ranged from 25–52% after 7 days postinoculation to 84–100% 25 days after postinoculation due to the pathogenicity of Pseudomonas aeruginosa (Schroeter) in the laboratory. Osbrink et al. (2001) isolated biological control agents from dead termites and revealed the presence of 15 bacteria and 1 fungus in dead termites. Bacteria isolated from termite substrata included Corynebacterium urealyticum Pitcher, Acinetobacter calcoacet/baumannii/Gen2 (Beijerinck), S. marcescens, and Enterobacter gergoviae Brenner. Devi et al. (2007) observed killing of Odontotermes obesus subterranean termites under in vitro conditions by three HCN-producing rhizobacterial species, i.e., Rhizobium radiobacter, Alcaligenes latus, and Aeromonas caviae. Rakshiya et al. (2016) reported that 63 bacterial isolates out of 220 bacterial isolates obtained from the soil collected from termite mounds (along with 8 reference strains) killed the termites under Petri plate conditions at 2 days of observation. Killing frequency of different bacterial isolates was found to vary from 5 to 90%. Six bacterial isolates, i.e., PPM119, PPM123, PPM167, PPM194, PPM199, and PPM203, caused even 100% killing at 5 days of observation. Forty-eight bacterial isolates caused 90 to 100% killing of termites at 10 days of incubation.
9.2.2 Fungi
Fungi also play a prominent role in insect control, and over 700 species have been recorded as insect pathogens (Table 9.1.). Fungi invade directly through the cuticle and could be used for the control of all insects including sucking insects. Products based on Beauveria bassiana (Li et al. 2001), Metarhizium anisopliae, Isaria fumosorosea and B. brongniartii, Verticillium lecanii, Nomuraea rileyi, and Paecilomyces fumosoroseus are the most common fungal species among the 171 products currently used for insect control (de Faria and Wraight 2001; Lacey and Neven 2006). Some of these fungi are obligate for insects; for example, Aschersonia aleyrodes infects only scale insects and whiteflies, while other fungal species are facultative with individual isolates being more specific to target pests. The fungus B. bassiana is being developed as a biocontrol agent against soil-dwelling pests such as scarabs and weevils (Klingen et al. 1998; Keller 2000) with no effect on the nontargeted insects (Goettel and Hajek 2001).
Colorado potato beetle, the codling moth, several genera of termites, and American bollworm H. armigera are the hosts of entomopathogenic fungi of agricultural and forest significance (Thakur and Sandhu 2010). Hyblaeapara and Eutectona machaeralis, Ostrinia nubilalis, pine caterpillars Dendrolimus sp. and green leafhoppers Nephotettix sp., Lecanicillium (Verticillium) lecanii, and Paecilomyces fumosoroseus fungi mainly attack sucking pests such as aphids and whiteflies (Kim et al. 2002; Nunez et al. 2008). Isaria (Paecilomyces) fumosoroseus has strong epizootic potential against Bemisia and Trialeurodes sp. in both greenhouse and open-field environments (de Faria and Wraight 2001). Metarhizium sp. has been found effective in controlling the several economically important insect pests of global importance, viz., H. armigera and S. litura that attack crops such as groundnut, soybean, sunflower, cotton, and tomato (Revathi et al. 2011). M. anisopliae has been tested on teak skeletonizer, Eutectona machaeralis, and found to be a potential myco-biocontrol agent of teak pest (Sandhu et al. 2000).
Some of these entomopathogenic fungi, viz., B. bassiana, M. anisopliae, and Lecanicillium lecanii, have been found to colonize plant tissues as symptomless endophytes (Rodriguez et al. 2009; Vidal and Jaber 2015). Plants harboring these fungi as endophytes have shown detrimental effects against herbivorous insects (Azevedo et al. 2000) or plant parasitic nematodes (Waweru et al. 2014). The endophytic growth of B. bassiana Vuillemin (BB) is a common feature in corn cropping systems in the USA, and the natural colonization of corn stalks ranged from 0 to more than 60% of plants sampled in different US federal states (Arnold and Lewis 2005). Plant species harboring BB include coffee (Posada et al. 2007), banana (Akello et al. 2008), sorghum (Tefera and Vidal 2009), cotton, pumpkin and wheat (Gurulingappa et al. 2010), jute (Biswas et al. 2013), and common bean (Parsa et al. 2013). M. anisopliae and B. bassiana and their related species are also used as biological pesticides to control a number of pests such as termites (Maniania et al. 2002), thrips (Ekesi et al. 2012), locusts (Ouedraogo et al. 2003), and hazelnut weevil (Cheng et al. 2016). M. anisopliae and B. bassiana do not infect humans or other animals and are, therefore, considered safe for use as pesticide.
Twenty-two fungal species have been reported as obligate ectoparasites of termites (Blackwell and Rossi 1986). Leong (1966) demonstrated high pathogenicity of M. anisopliae to Coptotermes formosanus. Mortality exceeded 86% with short exposures (e.g., 5–35 min) and termites succumbed within 3–6 days posttreatment. Exposure over 40 min caused 100% mortality and longer exposure times caused death within 24 h. No significant difference between the pathogenicities of B. bassiana and M. anisopliae was found. Grace (1991) found that M. anisopliae caused greater and rapid mortality than B. bassiana in C. formosanus to low conidial concentrations. Wells et al. (1995) concluded that one isolate each of B. bassiana and M. anisopliae showed the greatest potential for control of C. formosanus populations, based on LD50 (median lethal dose, as conidia per insect), time to death, and conidial production. Similarly, different isolates of Conidiobolus coronatus were found pathogenic to C. formosanus, R. flavipes, and Nasutitermes exitiosus (Wells et al. 1995). Milner et al. (1998) tested 93 isolates of M. anisopliae (Metschnikoff) obtained from two species of termites. The direct inoculation of the most effective isolate FI-610 by applying 3 x 1011 conidia into termite mounds resulted in successful control of Coptotermes acinaciformis (Froggatt) in Australia. Wright et al. (2003) patented Paecilomyces sp. for controlling subterranean termites, and these Paecilomyces strains were transferred among termites and caused rapid mortality.
Grace and Zoberi (1992) found that living R. flavipes workers exposed to sporulating B. bassiana cultures effectively spread infection to unexposed nestmates, whereas introduction of fungus-killed workers did not result in sufficient spore transfer or mycelial growth to cause significant mortality. However, the level of mortality achieved in the laboratory was not considered sufficient to control a termite infestation in the field. Rath and Tidbury (1996) found that Coptotermes acinaciformis (Froggatt) (Rhinotermitidae) and N. exitiosus were equally susceptible to direct conidial applications of both Australian and American strains of M. anisopliae. The injection of large quantities of conidia directly into the termite nest had the greatest success in the field studies (Milner et al. 1996). Sun et al. (2002) quantified the sporulation of 22 isolates of M. anisopliae and B. bassiana on cadavers of the Formosan subterranean termite, C. formosanus. Conidial production increased significantly over 11 days post-death. Effects of M. anisopliae and B. bassiana isolates on in vivo sporulation were significant, and it differed by as much as 89x and 232x among the selected isolates of M. anisopliae and B. bassiana, respectively.
Wright et al. (2005) reported that M. anisopliae (Metschnikoff) isolate, C4-B, caused rapid mortality on Formosan subterranean termite alates. In initial experiments, C4-B was more lethal to both alates and workers as compared with M. anisopliae strains ESC 1, previously marketed as the termite biocontrol agent, BioBlast. Dose-response assays to a known concentration of C4-B spores revealed that 106 spores/μl killed 100% of the Formosan subterranean termite alates in 3 days, both 105 and 104 spores/μl in 6 days, 103 spores/μl in 9 days, and 102 spores/μl in 12 days. When the transfer of inoculum from infected workers to uninfected nestmates was tested, 62.8% of the workers died in 21 days when only 20% of the workers had been inoculated. Mortality of alates caused by M. anisopliae (Metschnikoff) isolate C4-B was studied at two field sites by dispersing fungal spores on grassy lawns and collecting the alates from the treated areas. Infected alates showed 100% mortality by day 5, whereas only 64.8% of untreated control alates from the same collection area were dead on that day. Thus, fungi have the potential for termite control of all the pathogens tested (Milner and Staples 1996).
9.2.3 Actinobacteria
Actinobacteria are important part of the microbial community in the rhizosphere soil and produce many secondary metabolites with different disease suppression effects. More than a 1000 secondary metabolites are produced by actinomycetes, which makes 45% of total microbial metabolites. Actinomycetes have been reported as biocontrol agents effective against numerous plant pathogens and insects (Snyder et al. 2007; Prapagdee et al. 2008; de Oliveira et al. 2010; Laid et al. 2016). The potential use of actinomycetes as a biocontrol agent has been reviewed recently (Sabaratnam and Traquair 2015), where inoculation with some of these microorganisms also promoted the growth of plants.
Actinomycetes were found effective against the housefly Musca domestica (Hussain et al. 2002), mosquito larvae (Dhanasekaran et al. 2010), and Drosophila melanogaster (Gadelhak et al. 2005). The mortality of insects by actinomycetes may be due to secretion of bioactive materials, which stimulate the gamma-aminobutyric acid (GABA) system or disruption of nicotinic acetylcholine receptors (Herbert 2010). Strains of Streptomyces inhibited the growth of S. exigua, Dendrolimus punctatus, Plutella xylostella, Aphis glycines, and Culex pipiens (Huamei et al. 2008). Spinosad is a novel insecticide produced from fermentation of the actinomycetes Saccharopolyspora spinosa (Snyder et al. 2007), and it has been accepted for application in organic farming. It is particularly toxic to lepidoptera and diptera insects. Avermectins (a series of 16-membered macrocyclic lactone derivatives) were obtained from the fermentation products by S. avermitilis that showed potent anthelmintic and insecticidal properties (Pitterna et al. 2009). Cholesterol oxidase derived from Streptomyces broth showed selective and high potency against cotton boll weevil and stunting effect on H. virescens, H. zea, and Pectinophora gossypiella, which might be due to disruption of the midgut epithelial membrane (Purcell et al. 1993).
9.2.4 Protozoa
Protozoan diseases of insects are ubiquitous and play an important regulatory role in insect populations (Brooks 1988). They are generally host specific and slow acting, most often producing chronic infections. Entomopathogenic protozoa develop only in living hosts, and many species require an intermediate host. Their main advantages are persistence and recycling in host populations and their debilitating effect on reproduction and overall fitness of target insects. Of the four groups of protozoa containing species parasitic to insects, the phylum Microspora includes species that are potentially most useful in applied insect control (Henry 1990). Desportes (1963) described a gregarine (phylum Apicomplexa) from the hemocoel of the damp-wood termite Zootermopsis nevadensis (Hagen) (Termopsidae). Microsporidians were found in the body cavity and proventriculus of Microcerotermes championi collected from the roots of Saccharum munja Roxburgh (Poaceae) (Jafri et al. 1976). The organisms attacked fat body tissues in the midgut after ingestion with food and caused death. Although protozoa are important biocontrol agents for many insects, they have not been used as soil-applied microbial insecticides because they tend to be slow acting and cause low levels of immediate mortality. Moreover, the protozoa populations are vulnerable to changes in environmental conditions (Klein 1988; Henry 1990).
9.2.5 Nematodes
A plethora of nematode species in more than 30 families is associated with insects and other invertebrates (Kaya and Gaugler 1993). They have been found parasitizing species in the orders Hemiptera, Diptera, Hymenoptera, Lepidoptera, Orthoptera, Coleoptera, Thysanoptera, Siphonaptera, as well as Isoptera (Nickle and Welch 1984). Four families of nematodes, i.e., the Mermithidae, Allantonematidae, Steinernematidae, and Heterorhabditidae, have found application in insect control programs (Popiel and Hominick 1992). After infection of the host by nematodes, symbiotic bacteria are released into the insect hemocoel, causing septicemia and death (Kaya and Gaugler 1993). These entomopathogenic nematodes have a number of characteristics that make them especially suitable for biological control and for commercial production as microbial insecticides. These characteristics include a broad host range, especially among soil-dwelling pests; an ease of production, easy to store, and easy in application; and a high degree of safety to vertebrates, plants, and other nontarget organisms and amenability to genetic selection (Kaya et al. 1993).
Fujii (1975) obtained 96% mortality in C. formosanus termites within 7 days of exposure to infective-stage Steinernema carpocapsae (Weiser) (Steinernematidae) in laboratory experiments. Mortality exceeding 95% was recorded by Georgis et al. (1982) for both Zootermopsis sp. and Reticulitermes sp. within 3 days after laboratory exposure to S. carpocapsae; termites were also found to carry infection back to their colonies. Under laboratory conditions, high rates of infection of Nasutitermes costalis and R. flavipes were reported with S. carpocapsae (Trudeau 1989). Yu et al. (2006) showed that different species of entomopathogenic nematodes, i.e., Steinernema riobrave Cabanillas, Poinar, and Raulston (355 strain), Steinernema carpocapsae (Weiser) (Mexican 33 strain), Steinernema feltiae (Filipjev) (UK76 strain), and Heterorhabditis bacteriophora Poinar (HP88 strain), were all capable of infecting and killing three termite species, Heterotermes aureus (Snyder), Gnathamitermes perplexus (Banks), and Reticulitermes flavipes (Kollar), in laboratory sand assays. S. riobrave and S. feltiae caused low levels of Reticulitermes virginicus (Banks) mortality under the same conditions. Nematode concentration and incubation time had significant effects on the mortality of worker H. aureus. S. riobrave consistently produced highest infection levels and mortality of H. aureus in sand assays.
Biocontrol efficacy of nematodes is usually inconsistent due to various abiotic and biotic factors and the complex interactions between these two after application. Cabanillas and Barker (1989) reported that Paecilomyces lilacinus was more effective in protecting tomato against M. incognita when it was delivered before transplanting or at transplanting stage than after plants were infected by nematodes. Similar results were obtained when tomato or banana plants were treated with the mutualistic endophyte Fusarium oxysporum Fo162 at transplanting (Vu 2005; Dababat and Sikora 2007). In comparison, post-planting application of biocontrol agents, especially in the case of endophytes, does not always lead to high levels of biocontrol since the establishment of a biocontrol agent in the rhizosphere is a prerequisite for the control of endoparasitic nematodes (Dababat and Sikora 2007).
9.2.6 Viruses
A large number of viruses offer potential as microbial control agents of plant pathogens and insects (Payne 1982). Baculoviruses are widely used as insect pest control agents (Payne 1982; Popham et al. 2016). More than 400 insect species, mostly in the Lepidoptera and Hymenoptera, have been reported to act as hosts for baculoviruses. These viruses are used extensively for control of insect pests in a diverse range of agricultural and forest habitats. Biotechnological techniques are being used for genetic enhancement of baculoviruses for improved insecticidal efficacy. Insects that feed openly on the foliage of host plants are most easily treated, and the most promising results have been obtained against pest of this type (e.g., caterpillars sawfly larvae) (Smith 1967). The efficacy, specificity, and production of secondary inoculum make baculoviruses attractive alternative to broad-spectrum insecticides and ideal components of integrated pest management (IPM) systems due to their lack of untoward effects on beneficial insects including other biological control organisms (Cunningham 1995).
Gibbs et al. (1970) isolated a virus infecting Coptotermes lacteus (Froggatt) (Rhinotermitidae), which was similar to acute paralysis virus of the honey bee Apis mellifera Linnaeus (Hymenoptera: Apidae). A nuclear polyhedrosis virus, obtained from caterpillars of Spodoptera littoralis Boisduval (Lepidoptera: Noctuidae), was infective to a laboratory colony of Kalotermes flavicollis (Fabricius) (Kalotermitidae) (Al Fazairy and Hassan 1988). Termites died 2–10 days postinfection of viruses under laboratory conditions. The viral insecticide Elcar™ (Heliothis zea nuclear polyhedrosis virus, NPV) introduced during the 1980s provided control of cotton bollworm. Commercial preparations based on Spodoptera NPV were used to protect cotton, corn, and vegetables globally (Moscardi 1999; Kumari and Singh 2009). Autographa californica and Anagrapha falcifera NPVs with relatively broad host spectrum activity were used on a variety of crops infested with Spodoptera and Helicoverpa. Amsacta moorei entomopoxvirus has been reported to infect agriculturally important lepidopteran pests such as Estigmene acrea and Lymantria dispar (Muratoglu et al. 2010).
Insertion of insect-specific toxin genes such as juvenile hormone esterase, diuretic hormone, and prothoracicotropic hormone and genes encoding enzyme inhibitors, neuropeptides, or toxins improved the efficiency of viruses in killing of the insects. Application of recombinant baculoviruses, vAPcmIT2 and vAP10IT2, against two major pesticide-resistant pests Plutella xylostella and S. exigua resulted in shortening of the lethal time (Tuan et al. 2007). Seo et al. (2005) documented higher pathogenicity for recombinant baculovirus containing a fusion protein with polyhedrin and Bt toxin than wild-type strains. Two recombinant baculoviruses containing the ScathL gene from Sarcophaga peregrina (vSynScathL) and the keratinase gene from the fungus Aspergillus fumigatus (vSynKerat), against third-instar and neonate S. frugiperda larvae, showed protease activity in the hemolymph and reduced the time of insect killing (Gramkow et al. 2010).
9.3 Mechanisms Involved in Biocontrol
The mechanisms by which antagonistic microorganisms kill the insects or inhibit the growth of phytopathogenic microorganisms include (1) toxin production, (2) antibiotic production, (3) production of siderophores, (4) production of hydrolytic enzymes, (5) production of secondary metabolites and volatile organic compounds, and (6) phytoalexin production and induction of systemic resistance. Some of the microbial strains produced a wide range of secondary compounds that include siderophores, antibiotics, volatile metabolites, enzymes, etc. (Saraf et al. 2014). Mode of action of different allelochemicals and molecular mechanisms involved in plant-microbe-pathogen/insect interactions will contribute to better disease and insect management.
9.3.1 Toxin Production
Bacillus thuringiensis has been found to produce an array of virulence factors including insecticidal parasporal crystal (Cry) toxins, δ-endotoxin, vegetative insecticidal proteins, phospholipases, immune inhibitors, and antibiotics (de Maagd et al. 2003). There are about 200 registered Bt products in the USA, and worldwide sales of the Bt products amounted to about 100 million dollars (about 2% of the total global insecticide market) (Anonymous 1998). Most commercially available formulations are prepared using spore-crystal mixtures with effectiveness against different pest species. Ultraviolet (UV)-resistant mutant strains with high melanin, which absorb light of any wavelength, were used for large-scale production of light-stable insecticides (Liu et al. 2013). Most of the insecticidal activity of Bacillus thuringiensis is associated with the proteinaceous toxins located in the parasporal inclusion bodies, also known as parasporal crystals. The toxins found in the parasporal crystals are collectively referred to as δ-endotoxins. The Cry1 proteins (protoxins) which are found in the crystals are biologically inactive. Following ingestion and solubilization in the alkaline midgut, cleavage of protoxins by gut proteases produces a 60- to 65-kDa activated protein that recognizes specific binding sites at the brush border membrane surface of epithelial columnar cells lining the gut lumen (van Rie et al. 1989). The activated protein subsequently causes pore formation, membrane transport disruption, and cell lysis leading to the death of the insect (Bravo et al. 2007).
Vegetative insecticidal proteins (Vips) produced by B. cereus and B. thuringiensis also showed similar activity to endotoxins. Vip1 and Vip2 are toxic to coleopteran insects, and Vip3 is toxic to lepidopteran insects (Zhu et al. 2006). VIPs have excellent activity against black cutworms and armyworms (Yu et al. 1997), S. frugiperda (Barreto et al. 1999), S. litura and Plutella xylostella (Bhalla et al. 2005), and Heliothis zea, Trichoplusia sp., and Ostrinia nubilalis (Fang et al. 2011; Sellami et al. 2011). The pathogenic action of the B. cereus bacterium normally occurs after ingestion of spores and crystalline inclusions containing insecticidal δ-endotoxins specifically interact with receptors in the insect midgut epithelial cells (Pigott and Ellar 2007). Lysinibacillus sphaericus (B. sphaericus) produced insecticidal toxins during the vegetative phase of growth, and mosquitoes have been found to be the major targets of bacterium. Sphaericolysin, a toxin from the L. sphaericus, was found lethal to the common cutworm S. litura (Nishiwaki et al. 2007).
In addition to endotoxins showing insecticidal properties in Bt, the exotoxins of microbial origin from Pseudomonas sp. were found toxic to larvae of mosquitoes as well as lepidopteran insects (Murty et al. 1994). P. aeruginosa oxyR mutant revealed its ability to kill the insect Drosophila melanogaster (Lau et al. 2003). P. aeruginosa strain also conferred an efficient protection against Galleria mellonella and Bactrocera oleae (Mostakim et al. 2012), and the potency was due to the presence of quantitatively as well as qualitatively different proportions of biosurfactants in the crude glycolipids (Desai and Banat 1997). B. subtilis, B. amyloliquefaciens, B. megaterium, and Pseudomonas sp. showed more than 50% mortality in S. litura and H. armigera (Gopalakrishnan et al. 2011).
Hurst et al. (2011) isolated Yersinia entomophaga from diseased larvae of the New Zealand grass-grub, Costelytra zealandica White (Coleoptera, Scarabaeidae). This bacterium secreted a multi-subunit toxin complex (Yen-Tc) that showed homology with toxin complexes produced by Photorhabdus sp. Tc-like proteins were also identified in other entomopathogenic bacteria such as Serratia entomophila and the nematode symbiont Xenorhabdus nematophila (Morgan et al. 2001). Histopathological studies on the effects caused by the ingestion of Yen-Tc revealed the progressive disorganization and deterioration of the midgut epithelium of C. zealandica. Recently, the insecticidal activity of formulations containing Y. entomophaga against the pasture pest porina (Wiseana sp. larvae) has been reported under the field conditions (Ferguson et al. 2012).
A mixture of soluble endotoxin, spores, and inclusion bodies of Bacillus thuringiensis (Berliner) caused greater than 95% mortality of the subterranean termite species, Reticulitermes flavipes (Kollar) and R. hesperus Banks (Rhinotermitidae), after 6 days of exposure of the laboratory colonies (Smythe and Coppel 1965). Khan et al. (1978, 1985) employed a commercial preparation of Bt (Thuricide-HP concentrate) which exhibited 100% mortality of H. indicola, M. championi, and Bifiditermes beesoni (Gardner) (Kalotermitidae) within 6 days of exposure. Grace and Ewart (1996) constructed recombinant cells of the bacterium Pseudomonas fluorescens that expressed the δ-endotoxin genes of Bacillus thuringiensis (Bt). Two commercial agricultural formulations prepared by the CellCap process were evaluated for palatability to the termite C. formosanus. The MVP formulation, active against Lepidoptera, contained the P. fluorescens encapsulated δ-endotoxin of Bt var. kurstaki. Similarly, the M-Trak™ formulation, active against Coleoptera, contained the δ-endotoxin of Bt var. san diego. The palatability of the CellCap formulations indicated that the host bacterium, P. fluorescens, is a suitable delivery system for genetically engineered termiticides. Gunner et al. (1994) reported that the spores of entomopathogenic fungi may contain toxins which may kill the termite host when ingested. Insecticidal cyclic depsipeptides were found to be produced by entomopathogenic fungi including the destruxins from M. anisopliae var. major (Kaijiang and Roberts 1986) and Aschersonia sp. (Krasnoff and Gibson 1996) and the beauvericins from B. bassiana (Jegorov et al. 1989). It has been suggested that depsipeptides are localized on the surface of Beauveria sp. spores (Jegorov et al. 1989), whereas Metarhizium destruxins are generally associated with in vivo or in vitro mycelial growth (Chen et al. 1999).
9.3.2 Production of Antibiotics
Antibiotic production by microrganisms is one of the major mechanisms postulated for disease control. These antimicrobial compounds may act on plant pathogenic fungi by inducing fungistasis, inhibition of spore germination, and lysis of fungal mycelia or by exerting fungicidal effects. A large number of antibiotics including diacetylphloroglucinol, oomycin A, phenazines, pyocyanin, pyrroles, pyoluteorin, pyrrolnitrin, etc. are produced by rhizobacteria (Bender et al. 1999), which help in suppression of pathogen growth. Kido and Spyhalski (1950) isolated antimycin A from cultures of an unidentified species of Streptomyces. The initial tests showed that the antibiotic caused mortality to some insects which ingested the material. The toxicity of antimycin A was not confined to members of the Insecta, as it showed efficacy for the control of the red spider mite, Tetranychys sp. Beck (1950) reported that antimycin A had insecticidal possibilities for some species of insects and mites. The antibiotic inhibited either the succinoxidase system or some other essential step in the oxidative metabolic cycle of cockroaches. Inhibition of the oxidative cycle readily explained the depression of oxygen consumption by the poisoned insects. Photorhabdus is a virulent pathogen that kills its insect host by overcoming immune responses (Eleftherianos et al. 2007). Photorhabdus produces a small-molecule antibiotic (E)-1,3-dihydroxy-2-(isopropyl)-5- (2 phenylethenyl)benzene (ST) that also acts as an inhibitor of phenoloxidase (PO) in the insect host Manduca sexta. The bacterium inhibits two key immune defenses of the insect: activity of the antimicrobial enzyme PO and formation of melanotic nodules. Due to production of antibiotics, different Brevibacillus laterosporus strains showed insecticidal action in different orders, including Coleoptera, Lepidoptera, and Diptera (Ruiu 2013).
9.3.3 Siderophore Production
Many microorganisms synthesize extracellular siderophores, in response to iron stress (Neilands 1981), which are involved in disease suppression and plant growth promotion. Extracellular siderophores of the brown-rot wood decay fungus Gloeophyllum arabeum (Persoon: Fries) Murnill (Polyporaceae) were found to inhibit feeding by C. formosanus termites (Grace et al. 1992). Siderophore-treated filter paper disks showed negligible feeding, whereas untreated disks were almost completely consumed over a 3-day test period.
9.3.4 Production of Extracellular Enzymes
Different extracellular enzymes are produced by rhizosphere microorganisms which contribute to killing of the pathogens and insects. Fungal isolates that produce extracellular enzymes to degrade the host cuticle have large scope in pest management. For example, M. anisopliae grown in optimum fermentation conditions produced host-degrading enzymes such as acid phosphatase and phosphatase isoenzymes (Strasser et al. 2000; Li et al. 2007). Trichoderma produced protease (31 kDa) and chitinase (44 kDa) during the growth phase (Shakeri and Foster 2007), and it also produced a number of antibiotics, such as trichodermin, trichodermol, harzianum A, harzianolide, and peptaibols (Hoell et al. 2005). The crude Alternaria alternata chitinase showed 82% mortality against fruitfly (Sharaf 2005). Quesada-Moraga et al. (2006) used the crude protein extracts of M. anisopliae for the control of S. littoralis. Tolypocladium and Isaria fumosorosea were found toxic to Plutella xylostella (Freed et al. 2012).
Different microorganisms including bacteria, fungi, and actinomycetes were found to produce proteases from various types of natural resources. Lysenko and Kucera (1971) showed that Serratia marcescens produced extracellular proteases that could be a mode of pathogenicity of these bacteria in termites. Osbrink et al. (2001) examined 15 bacteria and one fungus associated with dead termites as possible biological control agents against Formosan subterranean termites, Coptotermes formosanus Shiraki. Bacterial isolates obtained from dead termites were primarily Serratia marcescens Bizio that caused septicemia in C. formosanus and found to contain proteolytic enzymes. Singh (2007) reported chitinolytic activity in some of the bacterial isolates that killed the termites. Bahar et al. (2011) identified chitinase producing Serratia marcescens which were found effective in killing the coleopteran insects with more chitin in their exoskeleton. Jafri et al. (1976) found microsporidians in the body cavity and proventriculus of Microcerotermes championi collected from the roots of Saccharum munja. These organisms passed into the midgut after ingestion with the food, attacked fat body tissues, and caused death of termites, indicating the role of lipolytic enzymes in termite killing. Rakshiya et al. (2016) reported that some of the bacterial isolates were found effective in termite killing and possessed all the three enzyme activities, i.e., lipase, protease, and chitinolytic activity. Lack of correlation between enzyme activities and termite killing indicated that besides the production of three enzymes, some other metabolites (toxin or siderophore) could also be contributing to the killing of termites.
9.3.5 Production of Secondary Metabolites and Volatile Organic Compounds
A large number of secondary metabolites are produced by rhizosphere bacteria, which play important roles in disease control and plant growth promotion. Hydrogen cyanide (HCN) is known to be produced by many rhizosphere bacteria and has been demonstrated to play a role in the biological control of the pathogens and pests. HCN-producing P. aeruginosa was found to have lethal effects on nematodes (Darby et al. 1999; Gallagher and Manoil 2001). Devi et al. (2007) tested three different species of HCN-producing rhizobacteria for their potential to kill subterranean termite O. obesus. The three bacterial species, Rhizobium radiobacter, Alcaligenes latus, and Aeromonas caviae, were found effective in killing the termites under in vitro conditions. R. radiobacter and A. latus caused 100% mortality of the termites following 1-h incubation. A. caviae, which produced significantly lower amounts of HCN, caused only 70% mortality. Termites exposed to exogenous HCN showed 80% mortality at cyanide concentrations of up to 2 μg/ml. The observed HCN toxicity in termites could be correlated with the inhibition of the respiratory enzymes.
Daisy et al. (2002) showed that naphthalene, an insect repellent, is produced by a fungus, Muscodor vitigenus. Three species of Muscodor and one Gliocladium sp. that produce volatile organic compounds with biocidal activity were isolated from several host plants in geographically diverse areas. A large number of metabolites are produced by different fungi (Table 9.2), which showed adverse effects on the insects (Boonphong et al. 2001; Shakeri and Foster 2007). For example, bassianin, beauvericin, bassianolide, and bassiacridin were produced by Beauveria sp. that controlled the Culex pipiens, Aedes aegypti, Calliphora erythrocephala, and H. zea (Quesada-Moraga and Alain 2004). Similarly, Trichoderma sp. produced trichodermin, trichodermol, harzianum A, harzianolide, and peptaibol metabolites having deleterious effects on Tenebrio molitor (Shakeri and Foster 2007).
9.3.6 Induction of Systemic Resistance
Salicylic acid (SA) and jasmonic acid (JA) hormones control defense responses to different types of microbes, and they orchestrate a different and complex transcriptional reprogramming that eventually leads to plant resistance. Evidences indicate that cyclic precursors of jasmonic acid (JA), the cyclopentenones, can also function as potent signals of plant defense responses (Farmer and Ryan 1992). Similarly, volatile derivatives of JA, such as methyl jasmonate (meJA) and cis-jasmone, can act as airborne signals stimulating plant defenses and repelling insects (Birkett et al. 2000).
The attack of insect herbivores on the plant roots and leaves imposes different selection pressures on plants, which in turn produces contrasting responses in terms of changes in biomass, gene expression, production of secondary metabolites, and wound hormones (Johnson et al. 2016). Different kinds of plant defenses are reported against root herbivores as compared with foliar herbivores (Johnson and Rasmann 2015). Following herbivore recognition, plants configure their metabolism through changes in the phytohormonal networks (Johnson et al. 2016). Jasmonates, which are widely viewed as the master regulators of plant responses to herbivores, are less inducible in the roots than the leaves (Erb et al. 2012; Lu et al. 2015). Salicylic acid signaling can buffer the jasmonic acid response aboveground (Gilardoni et al. 2011). Root herbivores attack induces different signal signature compared with leaf attack. For instance, attacked rice roots do not increase the biosynthesis of abscisic acid and ethylene (Lu et al. 2015), two important synergistic signals in the wound response of leaves. The difference may be explained by the fact that both hormones strongly influence root growth and architecture; plants may therefore be able to maintain root development under herbivore attack by maintaining abscisic acid and ethylene homeostasis. Thus, it is apparent that roots respond to pathogen or insect attack differently than shoots and regulate the defenses through modulating their phytohormonal networks in a tissue-specific manner.
Biological control of termites may also be facilitated if their highly evolved immune systems can be suppressed (Connick et al. 2001). Eicosanoids (C20 polyunsaturated acids) have been found to play an important role in protecting insects from bacterial infections (Miller et al. 1994). In laboratory experiments, the eicosanoid biosynthesis inhibitors dexamethasone, ibuprofen, and ibuprofen sodium salt were each provided along with a red-pigmented isolate of Serratia marcescens Bizio to the Formosan subterranean termite, C. formosanus Shiraki, by means of treated filter paper (Stanley-Samuelson et al. 1991). The increased mortality was observed with dexamethasone and ibuprofen suggesting that the termites’ immune systems were suppressed by these compounds, making the insects more vulnerable to infection by S. marcescens (Connick et al. 2001). This effect on mortality was noted only at 3.4 x 1010 colony-forming units ml−1 treatment level. A significant amount of infection and subsequent mortality may have resulted from direct contact with the bacterium and the remainder from its ingestion.
9.4 Approaches to Increase the Efficiency of Biocontrol Agents
Various entomopathogenic microbial species are normally able to persist in the environment, multiply in the host, and spread to other susceptible hosts. These entomopathogens have developed different strategies to attack, enter, and kill the attacking insect. Mycopathogens enter through the cuticle, whereas virus, bacteria, and protozoa enter through the midgut. Connick et al. (2001) reported that Serratia marcescens isolate T8 was highly virulent to the Coptotermes formosanus and termite mortality was 24% by 2 days and 99% after 19 days of the exposure. Nematodes belonging to the two families, Steinernematidae and Heterorhabditidae, have shown promise for use in termite control programs (Kaya and Gaugler 1993; Yu et al. 2006). The limited numbers of field trials attempted on insect or pathogen control have failed largely in reducing pathogen densities below economically damaging levels. Another factor that can contribute to inconsistent performance of biocontrol agents is variable production or inactivation in situ of microbial metabolites responsible for killing of insect/pathogen. The inconsistency in performance is a major constraint to the widespread use of biocontrol agents in commercial agriculture. Future strategies are required to clone genes involved in the production of toxins, antibiotics, and other metabolites so that these cloned genes could be transferred into the microbial strains having good colonization potential (Grace and Ewart 1996). Biotechnological approaches used in manipulation of microbial traits could lead to improved biocontrol activity of pathogenic microorganisms in the control of insects. The development of more stable formulations, such as microencapsulation, would be necessary to ensure their long-term, residual action (Grace and Ewart 1996).
9.5 Constraints in Development and Application of Biocontrol Agents
Recently, various biocontrol agents have been tested under field conditions on different crops for controlling pests and diseases of crop plants, and some of the antagonistic bacterial strains have led to the development of commercial biocontrol products. The major disadvantages in using microbes as a biocontrol agent include variability of field performance and the necessity to ensure survival and delivery of the product. Moreover, the effectiveness of a given biocontrol agent may be restricted to a specific location due to the effects of soil and climate. Many soil edaphic factors including temperature, soil moisture, pH, clay content, and interactions of biological disease control microorganisms with other rhizosphere bacteria and with pathogens will also affect their viability and tolerance to adverse conditions once applied. During root colonization, introduced biocontrol agents have to compete with indigenous microflora for carbon source, mineral nutrients, and infection sites on the roots. Sometimes, this competition is so severe that introduced biocontrol agents fail to survive in the soil. Another factor that can contribute to inconsistent performance is variable production or inactivation in situ of bacterial metabolites responsible for killing of the pathogen/insect.
Biological control strategies are also emerging as promising alternatives to the use of synthetic pesticides in the preservation of fruits. Antagonists must survive after their exposure to both postharvest treatments and storage conditions. The limitations of these biocontrol products can be addressed by enhancing the biocontrol through manipulation of the environment, using mixtures of beneficial organisms, physiological and genetic enhancement of the biocontrol mechanisms, manipulation of formulations, and integration of biocontrol with other alternative methods that in combination with biocontrol agents may provide additive or synergistic effects for adequate protection.
9.6 Conclusion
The role of microbial pesticides in the integrated management of insect pests has been reviewed for agriculture, forestry, and public health. In most cases, no single microbial control agent provides sustainable control of an insect pest or complex pests. As components of an integrated approach in all agricultural practices, entomopathogens could provide significant and selective insect control without interfering with the effectiveness of other practices. In the near future, synergistic combination of microbial control agents with other technologies (in combination with semiochemicals, soft chemical pesticides, other natural enemies, resistant plants, remote sensing, etc.) may enhance the effectiveness and sustainability of integrated control strategies. Till now, the market for microbial insecticides hardly represents only 1% of the total crop protection market. In the near future, microbials will face even stiffer competition from new pesticide chemistries and transgenic plants. However, several microbial control agents have good potential for use in integrated pest management (IPM) programs.
Complete elimination of chemical pesticides for controlling plant pests and diseases in modern agriculture may be impossible, but a logical reduction in their application is absolutely feasible. Biopesticide use in combination or rotation with synthetic pesticides is likely to be enhanced in the near future, but more research is needed to come up with innovative solutions that can really meet farmer and regulator needs in terms of effectiveness and environmental sustainability (Glare et al. 2012). To have a sustainable agricultural system with minimum contamination and risks to the environment, a combination of all available methods should be applied to manage pest problems, and this can be achieved by integrated pest management. Implementation of IPM strategies may be the safest solution for management of pest problems including insect infestation in every cropping system. Biological control could be one of the most important components of integrated pest management, which can lead us toward a safe, sustainable, and environmentally sound agricultural system in the future.
References
Akbar W, Lord JC, Nechols JR, Loughin TM (2005) Efficacy of Beauveria bassiana for red flour beetle when applied with plant essential oils or in mineral oil and organosilicone carriers. J Econ Entomol 98:683–688. https://doi.org/10.1603/0022-0493-98.3.683
Akello J, Dubois T, Coyne D, Kyamanywa S (2008) Endophytic Beauveria bassiana in banana (Musa spp.) reduces banana weevil (Cosmopolites sordidus) fitness and damage. Crop Prot 27:1437–1441. https://doi.org/10.1016/j.cropro.2008.07.003
Al Fazairy AA, Hassan FA (1988) Infection of termites by Spodoptera littoralis nuclear polyhedrosis virus. Insect Sci Appl 9:37–39. doi: https://doi.org/10.1017/S1742758400009991
Alexander B, Priest FG (1990) Numerical classification and identification of Bacillus sphaericus including some strains pathogenic for mosquito larvae. J Gen Microbiol 136:367–376
Anonymous (1998) United States Environmental Protection Agency, R.E.D. Facts, Bacillus thuringiensis, prevention, pesticides and toxic substances (751 W), EPA-738-F-98-001
Arnold AE, Lewis LC. (2005) Ecology and evolution of fungal endophytes, and their roles against insects. Insect-fungal associations: ecology and evolution. Oxford University Press, New York 3:74–96
Askary H, Yarmand H (2007) Development of the entomopathogenic hyphomycete Lecanicillium muscarium (Hyphomycetes: Moniliales) on various hosts. Eur J Entomol 104:67
Askary H, Carriere Y, Belanger RR, Brodeur J. (1998) Pathogenicity of the fungus Verticillium lecanii to aphids and powdery mildew. Biocont Sci Technol 8:23–32. doi: https://doi.org/10.1080/09583159830405
Azevedo JL, Maccheroni Jr W, Pereira JO, de Araújo WL (2000) Endophytic microorganisms: a review on insect control and recent advances on tropical plants. Electron J Biotechnol 3:15–16
Bahar AA, Sezen K, Demirbağ Z, Nalçacioğlu R (2011) The relationship between insecticidal effects and chitinase activities of Coleopteran-originated entomopathogens and their chitinolytic profile. Ann Microbiol 62:647–653. https://doi.org/10.1007/s13213-011-0301-y
Barloy F, Delècluse A, Nicolas L, Lecadet MM (1996) Cloning and expression of the first anaerobic toxin gene from Clostridium bifermentans sub-sp. malaysia, encoding a new mosquitocidal protein with homologies to Bacillus thuringiensis delta-endotoxins. J Bacteriol 178:3099–3105. https://doi.org/10.1128/jb.178.11.3099-3105.1996
Barreto MR, Loguercio LL, Valicente FH, Paiva E (1999) Biological control insecticidal activity of culture supernatants from Bacillus thuringiensis Berliner strains against Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) larvae. Ann Soc Entomol Brasil 28:675. https://doi.org/10.1590/S0301-80591999000400010
Beck D (1950) The toxicology of antimycin A. J Econ Entomol 43:105–107
Bender CL, Rangaswamy V, Loper J (1999) Polyketide production by plant-associated pseudomonads. Annu Rev Phytopathol 37:175–196. https://doi.org/10.1146/annurev.phyto.37.1.175
Bhalla R, Dalal M, Panguluri SK, Jagadish B, Mandaokar AD, Singh AK, Kumar PA (2005) Isolation, characterization and expression of a novel vegetative insecticidal protein gene of Bacillus thuringiensis. FEMS Microbiol Lett 243:467–472
Birkett MA, Campbell CAM, Chamberlain K, Guerrieri E, Hick AJ, Martin JL, Matthes M, Napier JA, Pettersson J, Pickett JA, Poppy GM, Pow EM, Pye BJ, Smart LE, Wadhams GH, Wadhams LJ, Woodcock CM (2000) New roles for cis-jasmone as an insect semiochemical and in plant defense. Proc Natl Acad Sci U S A 97:9329–9334
Biswas C, Dey P, Satpathy S, Sarkar SK, Bera A, Mahapatra BS (2013) A simple method of DNA isolation from jute (Corchorus olitorius) seed suitable for PCR-based detection of the pathogen Macrophomina phaseolina (Tassi) Goid. Lett Appl Microbiol 56:105–110. https://doi.org/10.1111/lam.12020
Blackwell M, Rossi W (1986) Biogeography of fungal ectoparasites of termites. Mycotaxon 25:581–601
Boets A, Arnaut G, Van Rie J, Damme N (2004) Toxins United States Patent No 6,706,860
Boonphong S, Kittakoop P, Isaka M, Palittapongarnpim P, Jaturapat A, Danwisetkanjana K, Tanticharoen M, Thebtaranonth Y (2001) A new antimycobacterial, 3b-acetoxy-15a, 22-dihydroxyhopane, from the insect pathogenic fungus Aschersonia tubulata. Planta Med 67:279–281
Borneman J, Becker JO (2007) Identifying microorganisms involved in specific pathogen suppression in soil. Annu Rev Phytopathol 45:153–172. https://doi.org/10.1146/annurev.phyto.45.062806.094354
Bowen D (1995) Characterization of a high molecular weight insecticidal protein complex produced by the entomopathogenic bacterium Photorhabdus luminescens. Ph.D. thesis. University of Wisconsin, Madison
Bowen D, Blackburn M, Rocheleau T, Grutzmacher C, Ffrench-Constant RH (2000) Secreted proteases from Photorhabdus luminescens: separation of the extracellular proteases from the insecticidal tc toxin complexes. Insect Biochem Mol Biol 30:69–74. https://doi.org/10.1016/S0965-1748(99)00098-3
Bravo A, Gill SS, Soberon M (2007) Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 49:423–435
Brooks WM (1988) Entomogenous protozoa. Handbook of natural. pesticides 5:1–49
Burnell AM, Stock SP (2000) Heterorhabditis, Steinernema and their bacterial symbionts – lethal pathogens of insect. Nematology 2:31–42
Cabanillas E, Barker KR (1989) Impact of Paecilomyces lilacinus inoculum level and application time on control of Meloidogyne incognita on tomato. J Nematol 21:115–120
Charles JF, Silva-filha MH, Nielsen-leroux C (2000) Mode of action of Bacillus sphaericus on mosquito larvae: incidence on resistance. In: Charles JF, Delécluse A, Nielsen-le Roux C (eds) Entomopathogenic bacteria: from laboratory to field application. Kluwer Academic Publisher, London, pp 237–252
Chen JW, Liu BL, Tzeng YM (1999) Purification and quantification of destruxins A and B from Metarhizium anisopliae. J Chromatogr 830:115–125. https://doi.org/10.1016/S0021-9673(98)00849-8
Cheng Y, Liu T, Zhao Y, Geng W, Chen L, Liu J (2016) Evaluation of pathogenicity of the fungi Metarhizium anisopliae and Beauveria bassiana in hazelnut weevil (Curculio nucum L., Coleoptera, Curculionidae) larvae. Indian J Microbiol 56:405–410. https://doi.org/10.1007/s12088-016-0614-4
Compant S, Duffy B, Nowak J, Clément C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71(9):4951–4959
Connick WJ Jr, Osbrink WLA, Wright MS, Williams KS, Daigle DJ, Boykin WL, Lax AR (2001) Increased mortality of Coptotermes formosanus (Isoptera: Rhinotermitidae) exposed to eicosanoid biosynthesis inhibitors and Serratia marcescens (Eubacteriales: Enterobacteriaceae). Environ Entomol 30:449–455. doi: https://doi.org/10.1603/0046-225X-30.2.449
Cunningham JC (1995) Baculoviruses as microbial insecticides. In: Reuveni R (ed) Novel approaches to integrated pest management, Lewis, Boca Raton, pp 261–292
Dababat AE, Sikora RA (2007) Influence of the mutualistic endophyte Fusarium oxysporum 162 on Meloidogyne incognita attraction and invasion. Nematology 9:771–776
Daisy S, Strobel G, Ezra D, Castillo UF, Baird G, Hess WM (2002) Muscodor vitigenus anam. sp. nov. an endophyte from Paullinia paulliniodes. Mycotaxon 84:39–50
Darby C, Cosma CL, Thomas JH (1999) Lethal paralysis of Caenorhabditis elegans by Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 96:202–207. https://doi.org/10.1073/pnas.96.26.15202
de Barjac H, Lemille F (1970) Presence of flagellar antigenic subfactors in serotype 3 of Bacillus thuringiensis. J Invert Pathol 15:139–140
de Faria M, Wraight SP (2001) Biological control of Bemisia tabaci with fungi. Crop Prot 20:767–778. https://doi.org/10.1016/S0261-2194(01)00110-7
de Maagd RA, Bravo A, Berry C, Crickmore N, Schnepf HE (2003) Structure, diversity, and evolution of protein toxins from spore forming entomopathogenic bacteria. Annu Rev Gen 37:409–433. https://doi.org/10.1146/annurev.genet.37.110801.143042
de Oliveira FM, de Silva GM, van der Sand ST (2010) Anti-phytopathogen potential of endophytic actinobacteria isolated from tomato plants (Lycopersicon esculentum) in Southern Brazil and characterization of Streptomyces sp. R18(6), a potential biocontrol agent. Res Microbiol 161:565–572. https://doi.org/10.1016/j.resmic.2010.05.008
Desai JD, Banat IM (1997) Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev 61:414–764
Desportes I (1963) Cycle evolutif d’une nouvelle Gregarine parasite de Termites: Diplocystis zootermopsidis sp. n. (Eugregarina Diplocystidae). Comtes Rendus Hebdom des Seances de Acad des Sci 257:4013–4015
Devi K, Seth N, Kothamasi S, Kothamasi D (2007) Hydrogen cyanide-producing rhizobacteria kill subterranean termite Odontotermes obesus (Rambur) by cyanide poisoning under in vitro conditions. Curr Microbiol 54:74–78
Dhanasekaran D, Sakthi V, Thajuddin N, Panneerselvam A (2010) Preliminary evaluation of anopheles mosquito larvicidal efficacy of mangrove actinobacteria. Int J Appl Biol Pharm Technol 1:374–381
Ekesi S, Maniania NK, Lwande W (2012) Susceptibility of the legume flower thrips to Metarhizium anisopliae on different varieties of cowpea. BioControl 45:79–95. https://doi.org/10.1023/A:1009927302916
Eleftherianos I, Boundy S, Joyce SA, Aslam S, Marshall JW, Cox RJ, Simpson TJ, Clarke DJ, Reynolds SE (2007) An antibiotic produced by an insect-pathogenic bacterium suppresses host defenses through phenoloxidase inhibition. Proc Natl Acad Sci USA 104(7):2419–2424
Erb M, Glauser G, Robert CAM (2012) Induced immunity against belowground insect herbivores – activation of defences in the absence of jasmonate burst. J Chem Ecol 38:629–640
Fang J, XL X, Wang P, Zhao JZ, Shelton AM, Cheng J, Feng MG, Shen ZC (2011) Characterization of chimeric Bacillus thuringiensis Vip3 toxins. Appl Environ Microbiol 73:956–961
FAO (2012) http://www.fao.org/news/story/en/item/131114/icode/
Farmer EE, Ryan CA (1992) Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase-inhibitors. Plant Cell 4:129–134
Ferguson CM, Barton DM, Harper LA, Swaminathan J, Van Koten C, Hurst MRH (2012) Survival of Yersinia entomophaga MH96 in a pasture ecosystem and effects on pest and non-target invertebrate populations. New Zealand. Plant Prot 65:166–173
Freed S, Feng-Liang J, Naeem M, Shun-Xiang R, Hussian M (2012) Toxicity of proteins secreted by entomopathogenic fungi against Plutella xylostella (Lepidoptera: Plutellidae). Intern J Agric Biol 14:291–295
Fujii JK (1975) Effect of an entomogenous nematode Neoaplectana carpocapsae Weiser, on the Formosan subterranean termite, Coptotermes formosanus Shiraki, with ecological and biological studies on C. formosanus. Ph.D. dissertation, University of Hawaii, Honolulu, Hawaii, USA. 163 pp
Gadelhak GG, EL-Tarabily KA, AL-Kaabi FK (2005) Insect control using chitinolytic soil actinomycetes as biocontrol agents. Int J Agric Biol 7:627–633
Gallagher LA, Manoil C (2001) Pseudomonas aeruginosa PAO1 kills Caenorhabditis elegans by cyanide poisoning. J Bacteriol 183:6207–6214. https://doi.org/10.1128/JB.183.21.6207-6214.2001
Georgis R, Poinar GO Jr, Wilson AP (1982) Susceptibility of damp-wood termites and soil and wood-dwelling termites to the entomogenous nematode Neoaplectana carpocapsae. IRCS. Med Sci 10:563
Gibbs AJ, Gay FJ, Wetherly AH (1970) A possible paralysis virus of termites. Virology 40:1063–1065
Gilardoni PA, Hettenhausen C, Baldwin IT, Bonaventure G (2011) Nicotiana attenuata lectin receptor kinase 1 suppresses the insect-mediated inhibition of induced defence responses during Manduca sexta herbivory. Plant Cell 23:3512–3532
Glare TR, O’callaghan M (2000) Bacillus thuringiensis: biology, ecology and safety. Wiley, Chichester
Glare T, Caradus J, Gelernter W, Jackson T, Keyhani N, Kohl J, Marrone P, Morin L, Stewart A (2012) Have biopesticides come of age? Trends Biotechnol 30:250–258
Glick BR, Bashan Y (1997) Genetic manipulation of plant growth-promoting bacteria to enhance biocontrol of fungal phytopathogens. Biotechnol Adv 15:353–378. https://doi.org/10.1016/S0734-9750(97)00004-9
Goettel MS, Hajek AE (2001) Evaluation of non-target effects of pathogens used for management of arthropods. In: Wajnberg E, Scott JK, Quimby PC (eds) Evaluating indirect ecological effects of biological control. CABI Press, Wallingford, pp 81–97
Gopalakrishnan S, Ranga Rao GV, Humayun P, Rameshwar Rao V, Alekhya G, Simi J, Deepthi K, Sree Vidya M, Srinivas V, Mamatha L, Rupela O (2011) Efficacy of botanical extracts and entomopathogens on control of Helicoverpa armigera and Spodoptera litura. Afr J Biotechnol 10:16667–16673. https://doi.org/10.5897/AJB11.2475
Govindarajan M, Jebanesan A, Reetha D (2005) Larvicidal effect of extracellular secondary metabolites of different fungi against the mosquito, Culex quinquefasciatus say. Trop Biomed 22:1–3
Grace JK (1991) Termite-fungal associations and manipulations for termite control. In: Program and abstracts. 24th annual meeting, Society of Invertebrate Pathology, Northern Arizona University, Flagstaff, August 4–9, 1991. p 29
Grace JK, Ewart D (1996) Recombinant cells of Pseudomonas fluorescens: a highly palatable encapsulation for delivery of genetically engineered toxins to subterranean termite (Isoptera: Rhinotermitidae). Lett Appl Microbiol 23:183–186
Grace JK, Zoberi MH (1992) Experimental evidence for transmission of Beauveria bassiana by Reticulitermes flavipes workers (Isoptera: Rhinotermitidae). Sociobiology 20:23–28
Grace JK, Goodell BS, Jones WE, Chandhoke V, Jellison J (1992) Evidence for inhibition of termites (Isoptera: Rhinotermitidae) feeding by extracellular metabolites of a wood decay fungus. Proc Hawaiian Entomol Soc 31:249–252
Gramkow AW, Perecmanis S, Sousa RLB, Noronha EF, Felix CR, Nagata T, Ribeiro BM (2010) Insecticidal activity of two proteases against Spodoptera Frugiperda larvae infected with recombinant baculoviruses. Virol J 7:143. https://doi.org/10.1186/1743-422X-7-143
Gunner HB, Kane J, Duan H (1994) Biological control of termites. PCT Patent Application ~WO94 04034
Gurulingappa P, Sword GA, Murdoch G, McGee PA (2010) Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biol Control 55:34–41. https://doi.org/10.1016/j.biocontrol.2010.06.011
Henry JE (1990) Control of insects by protozoa. In: Baker RR, Dunn PE (eds) New directions in biological control: alternatives for suppressing agricultural pests and disease. Alan R Liss Inc, New York, pp 161–176
Herbert AK (2010) The spinosyn family of insecticides: realizing the potential of natural products research. J Antibiot 63:101–111. https://doi.org/10.1038/ja.2010.5
Hoell IA, Klemsdal SS, Vaaje-Kolstad G, Horn SJ, Eijsink VGH (2005) Overexpression and characterization of a novel chitinase from Trichoderma atroviride strain. Biochim Biophys Acta 1748:180–190. https://doi.org/10.1016/j.bbapap.2005.01.002
Hoshino T (2011) Violacein and related tryptophan metabolites produced by Chromobacterium violaceum: biosynthetic mechanism and pathway for construction of violacein core. Appl Microbiol Biotechnol 91:1463–1475. https://doi.org/10.1007/s00253-011-3468-z
Huamei L, Qf S, Yongxia W, Wenjun L, Jie Z (2008) Insecticidal action of quinomycin A from Streptomyces sp. KN-0647 isolated from a forest soil. World J Microbiol Biotechnol 24:2243–2248. https://doi.org/10.1007/s11274-008-9736-0
Hurst MRH, Becher SA, Young SD, Nelson TL, Glare TR (2011) Yersinia entomophaga sp. nov., isolated from the New Zealand grass grub Costelytra zealandica. Intern J Syst Evol Microbiol 61:844–849. https://doi.org/10.1099/ijs.0.024406-0
Hussain AA, Mostafa SA, Ghazal SA, Ibrahim SY (2002) Studies on antifungal antibiotic and bioinsecticidal activities of some actinomycete isolates. Afr J Mycol Biotechnol 10:63–80
Jackson TA, Pearson JF, O’Callaghan M, Mahanty HK, Willocks M (1992) Pathogen to product development of Serratia entomophila Enterobacteriaceae as a commercial biological control agent for the New Zealand grass grub Costelytra zealandica. In: Jackson TA, Glare TR (eds) Use of pathogens in scarab pest management. Intercept Ltd., Andover, pp 191–198
Jafri RH, Ahmad M, Idrees K (1976) Microsporidian infection in the workers of termite, Microcerotermes championi. Pak J Zool 8:234–236
Jegorov A, Kadlec J, Novak J, Matha V, Sedmera P, Triska J, Zahradnickova H (1989) Are the depsipeptides of Beauveria brongniartii involved in the entomopathogenic process? In: Jegorov A, Matha V (eds) Proceedings of the international conference on biopesticides, theory and practice. Ceske Budejovice, Czechoslovakia, pp 71–81
Johnson SN, Rasmann S (2015) Root-feeding insects and their interactions with organisms in the rhizosphere. Annu Rev Entomol 60:517–535
Johnson SN, Erb M, Hartley SE (2016) Roots under attack: contrasting plant responses to below- and above-ground insect herbivory. New Phytol 210:413–418. https://doi.org/10.1111/nph.13807
Kaijiang L, Roberts DW (1986) The production of destruxins by the entomogenic fungus, Metarhizium anisopliae var. major. J Invertebr Pathol 47:120–122. https://doi.org/10.1016/0022-2011(86)90170-9
Kaya HK, Gaugler R (1993) Entomopathogenic nematodes. Annu Rev Entomol 38:181–206. https://doi.org/10.1146/annurev.en.38.010193.001145
Kaya HK, Bedding RA, Akhurst RJ (1993) An overview of insect-parasitic and entomopathogenic nematodes. In: Bedding RA, Akhurst R, Kaya H (eds) Nematodes and the biological control of insect pest. CSIRO, East Melbourne, Australia, pp 1–10
Keller S (2000) Use of Beauveria brongniartii in Switzerland and its acceptance by farmers. Bull OILB/SROP 23:67–71
Khan KI, Fazal QA, Jafri RH, Ahmad MU (1977) Susceptibility of various species of termites to a pathogen, Serratia marcescens. Pak J Sci Res 29:46–47
Khan KI, Fazal QA, Jafri RH (1978) Development of Bacillus thuringiensis in a termite, Heterotermes indicola (Wassman) [sic]. Pak J Sci Res 30:117–119
Khan KI, Jafri RH, Ahmad M (1985) The pathogenicity and development of Bacillus thuringiensis in termites. Pak J Zool 17:201–209
Khan KI, Jafri RH, Ahmad M, Khan KMS (1992) The pathogenicity of Pseudomonas aeruginosa against termites. Pak J Zool 24:243–245
Kido GS, Spyhalski E (1950) Antimycin A, an antibiotic with insecticidal and miticidal properties. Science 112:172–173
Kim JJ, Lee MH, Yoon CS, Kim HS, Yoo JK, Kim KC (2002) Control of cotton aphid and greenhouse whitefly with a fungal pathogen. J Nat Inst Agri Sci Technol:7–14
Kim JJ, Goettel MS, Gillespie DR (2007) Potential of Lecanicillium species for dual microbial control of aphids and the cucumber powdery mildew fungus, Sphaerotheca fuliginea. BioControl 40:327–332. https://doi.org/10.1016/j.biocontrol.2006.12.002
Kim JJ, Goettel MS, Gillespie DR (2008) Evaluation of Lecanicillium longisporum, Vertalec® for simultaneous suppression of cotton aphid, Aphis gossypii, and cucumber powdery mildew, Sphaerotheca fuliginea, on potted cucumbers. BioControl 45:404–409. https://doi.org/10.1016/j.biocontrol.2008.02.003
Klein MG (1988) Pest management of soil-inhibiting insects with microorganisms. Agric Ecosyst Environ 24:337–349
Klingen I, Eilenberg J, Meadow R (1998) Insect pathogenic fungi from northern Norway baited on Delia floralis (Diptera, Anthomyiidae) and Galleria mellonella (Lepidoptera, Pyralidae). IOBC wprs Bull 21:121–124
Konstantopoulou MA, Mazomenos BE (2005) Evaluation of Beauveria bassiana and B. brongniartii strains and four wild-type fungal species against adults of Bactrocera oleae and Ceratitis capitata. BioControl 50:293–305. https://doi.org/10.1007/s10526-004-0458-4
Krasnoff SB, Gibson DM (1996) New destruxins from the entomopathogenic fungus, Aschersonia sp. J Natur Prod 59:485–489. https://doi.org/10.1021/np9601216
Kumari V, Singh NP (2009) Spodoptera litura nuclear polyhedrosis virus (NPV-S) as a component in Integrated Pest Management (IPM) of Spodoptera litura (Fab.) on cabbage. J Biopest 2:84–86
Kuzina LV, Peloquin JJ, Vacek DC, Miller TA (2001) Isolation and identification of bacteria associated with adult laboratory Mexican fruit flies Anastrepha ludens (Diptera: Tephritidae). Curr Microbiol 42:290–294. https://doi.org/10.1007/s002840110219
Lacey LA, Goettel M (1995) Current developments in microbial control of insects, pests and prospects for the early 21st century. Entomophaga 40:3–27. https://doi.org/10.1007/BF02372677
Lacey LA, Neven LG (2006) The potential of the fungus, Muscodor albus, as a microbial control agent of potato tuber moth (Lepidoptera: Gelechiidae) in stored potatoes. J Invertebr Pathol 91:195–198. https://doi.org/10.1016/j.jip.2006.01.002
Lacey LA, Grzywacz D, Shapiro-Ilan DI, Frutos R, Brownbridge M, Goettel MS (2015) Insect pathogens as biological control agents: back to the future. J Invertebr Pathol 132:1–41. https://doi.org/10.1016/j.jip.2015.07.009
Laid B, Kamel K, Mouloud G, Manel S, Walid S, Amar B, Hamenna B, Faiçal B (2016) Effects of plant growth promoting rhizobacteria (PGPR) on in vitro bread wheat (Triticum aestivum L.) growth parameters and biological control mechanisms. Adv Microbiol 6:677–690. https://doi.org/10.4236/aim.2016.69067
Lau GW, Goumnerov BC, Walendziewicz CL, Hewitson J, Xiao W, Mahajan-Miklos S, Tompkins RG, Perkins LA, Rahme LG (2003) The Drosophila melanogaster toll pathway participates in resistance to infection by the Gram-negative human pathogen Pseudomonas aeruginosa. Infect Immun 71:4059–4066. https://doi.org/10.1128/IAI.71.7.4059-4066.2003
Lehr P (2010) Biopesticides: the global market, report code CHM029B, BCC Research
Leong KLH (1966) Infection of the Formosan subterranean termite, Coptotermes formosanus Shiraki, by the fungus Metarhizium anisopliae (Metsch.) Sorok. M. Sc. thesis, University of Hawaii, Honolulu
Li Z, Li CR, Huang B, Meizhen MZ (2001) Discovery and demonstration of the teleomorph of Beauveria bassiana (Bals.) Vuill. An important entomogenous fungus. Chin Sci Bull 46:751–753. https://doi.org/10.1007/BF03187215
Li Z, Wang Z, Peng G, Yin Y, Zhao H, Cao Y, Xia Y (2007) Regulation of extracellular acid phosphatase biosynthesis by culture conditions in entomopathogenic fungus Metarhizium anisopliae strain. Ann Microbiol 57:565–570. https://doi.org/10.1007/BF03175356
Li X, Zhang Y, Ding C, Jia Z, He Z, Zhang T, Wang X (2015) Declined soil suppressiveness to Fusarium oxysporum by rhizosphere microflora of cotton in soil sickness. Biol Fertil Soils 51:935–946. https://doi.org/10.1007/s00374-015-1038-8
Liu F, Yang W, Ruan L, Sun M (2013) A Bacillus thuringiensis host strain with high melanin production for preparation of light-stable biopesticides. Ann Microbiol 63:1131–1135. https://doi.org/10.1007/s13213-012-0570-0
Lu J, Robert CAM, Riemann M, Cosme M, Mene-Saffrane L, Massana J, Stout MJ, Lou Y, Gershenzon J, Erb M (2015) Induced jasmonate signaling leads to contrasting effects on root damage and herbivore performance. Plant Physiol 167:1100–1116
Lysenko O, Kucera M (1971) Microorganisms as sources of new insecticidal chemicals; toxins. In: Burges HD, Hussey NW (eds) Microbial control of insects and mites. Academic Press, London/New York, pp 205–227
Maniania NK, Ekesi S, Songa JM (2002) Managing termites in maize with the entomopathogenic fungus Metarhizium anisopliae. Int J Trop Insect Sci 22:41–46. https://doi.org/10.1017/S1742758400015046
Martin PAW, Shropshire ADS, Gundersen-Rindal DE, Blackburn MB (2007) Chromobacterium subtsugae sp. nov. and use for control of insect pests. US Patent Application Publication, 2007/0172463 A1
Mascarin GM, Jaronski ST (2016) The production and uses of Beauveria bassiana as a microbial insecticide. World J Microbiol Biotechnol 32:177–188. https://doi.org/10.1007/s11274-016-2131-3
Mazet I, Hung SY, Boucias DG (1995) Hirsutellin A, a toxic protein produced in vitro by Hirsutella thompsonii. J Invertbr Pathol 64:200–207. https://doi.org/10.1099/13500872-141-6-1343
Miller JS, Nguyen T, Stanley-Samuelson DW (1994) Eicosanoids mediate insect modulation responses to bacterial infections. Proc Natl Acad Sci U S A 91:2418–2422
Milner RJ, Staples JA (1996) Biological control of termites: results and experiences within a CSIRO project in Australia. Biocontrol Sci Tech 6:3–9
Milner RJ, Staples JA, Lenz M (1996) Options for termite management using the insect pathogenic fungus Metarhizium anisopliae. International group on wood preservation. Document No. IRG/WP9610142, pp 1–5
Milner RJ, Staples JA, Lutton GG (1998) The selection of an isolate of the hyphomycete fungus, Metarhizium anisopliae, for control of termites in Australia. Biol Control 11:240–247. https://doi.org/10.1006/bcon.1997.0574
Morgan JAW, Sergeant M, Ellis D, Ousley M, Jarrett P (2001) Sequence analysis of insecticidal genes from Xenorhabdus nematophilus PMFI296. Appl Environ Microbiol 67:2062–2069. https://doi.org/10.1128/AEM.67.5.2062-2069.2001
Moscardi F (1999) Assessment of the application of baculoviruses for control of Lepidoptera. Annu Rev Entomol 44:257–289. https://doi.org/10.1146/annurev.ento.44.1.257
Mostakim M, Mohammed IH, Ibnsouda SK (2012) Biocontrol potential of a Pseudomonas aeruginosa strain against Bactrocera oleae. Afr J Microbiol Res 6:5472–5478. https://doi.org/10.5897/AJMR11.1598
Muratoglu H, Kati H, Demibag Z (2009) High insecticidal activity of Leclercia adecarboxylata isolated from Leptinotarsa decemlineata (Col.: Chrysomelidae). Afr J Biotechnol 8:7111–7115
Muratoglu H, Nacacioglu R, Demibag Z (2010) Transcriptional and structural analyses of Amsacta moorei entomopoxvirus protein kinase gene (AMV197, pk). Ann Microbiol 60:523–530. https://doi.org/10.1007/s13213-010-0082-8
Murty MG, Srinivas G, Sekar V (1994) Production of mosquitocidal exotoxin by a Pseudomonas fluorescens strain. J Invert Pathol 64:68–70. https://doi.org/10.1006/jipa.1994.1071
Neilands JB (1981) Microbial iron compounds. Annu Rev Biochem 50:715–731. https://doi.org/10.1146/annurev.bi.50.070181.003435
Nickle WR, Welch HE (1984) History, development and importance of insect nematology. In: Nickle WR (ed) Plant and insect nematodes. Marcel Dekker Inc, New York, pp 627–653
Nicolas L, Hamon S, Frachon E, Sebald M, De Barjac H (1990) Partial inactivation of the mosquitocidal activity of Clostridium bifermentans serovar malaysia by extracellular proteinases. Appl Microbiol Biotechnol 34:36–41. https://doi.org/10.1007/BF00170920
Nishiwaki H, Nakashima K, Ishida C, Kawamura T, Matsuda K (2007) Cloning, functional characterization, and mode of action of a novel insecticidal pore-forming toxin, sphaericolysin, produced by Bacillus sphaericus. Appl Environ Microbiol 73:3404–3411. https://doi.org/10.1128/AEM.00021-07
Nunez E, Iannacone J, Gomez H (2008) Effect of two entomopathogenic fungi in controlling Aleurodicus cocois (Curtis 1846) (Hemiptera: Aleyrodidae). Chil J Agric Res 68:21–30. https://doi.org/10.4067/S0718-58392008000100003
Oliveira EJ, Rabinovitch L, Monnerat RG, Passos LK, Zahner V (2004) Molecular characterization of Brevibacillus laterosporus and its potential use in biological control. Appl Environ Microbiol 70:6657–6664. https://doi.org/10.1128/AEM.70.11.6657-6664.2004
Osbrink LAW, Williams KS, Connick WJ Jr, Wright MS, Lax AR (2001) Virulence of bacteria associated with the Formosan subterranean termite (Isoptera: Rhinotermitidae) in New Orleans, LA. Environ Entomol 76:443–448
Ouedraogo RM, Cusson M, Goettel MS, Brodeur J (2003) Inhibition of fungal growth in thermoregulating locusts, Locusta migratoria, infected by the fungus Metarhizium anisopliae var. acridum. J Invert Pathol 82:103–109. https://doi.org/10.1016/S0022-2011(02)00185-4
Parsa S, Ortiz V, Vega FE (2013) Establishing fungal entomopathogens as endophytes: towards endophytic biological control. J Vis Exp (74):e50360. doi: https://doi.org/10.3791/50360
Payne CC (1982) Insect viruses as control agents. Parasitology 84:35–77. https://doi.org/10.1017/S0031182000053609
Pigott CR, Ellar DJ (2007) Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol Mol Biol Rev 71:255–281. https://doi.org/10.1128/MMBR.00034-06
Pitterna T, Cassayre J, Huter O (2009) New ventures in the chemistry of Avermectins. Bioorg Med Chem 17:4085–4095. https://doi.org/10.1016/j.bmc.2008.12.069
Popham HJR, Nusawardani T, Bonning BC (2016) Introduction to the use of baculoviruses as biological insecticides. Methods Mol Biol 1350:383–392. https://doi.org/10.1007/978-1-4939-3043-2-19
Popiel I, Hominick WH (1992) Nematodes as biological control agents: Part II. Adv Parasitol 31:381–433. https://doi.org/10.1016/S0065-308X(08)60025-1
Posada F, Aime MC, Peterson SW, Rehner SA, Vega FE (2007) Inoculation of coffee plants with the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales). Mycol Res 111:748–757. http://www.scielo.org.co/pdf/rudca/v13n2/v13n2a09.pdf
Prapagdee B, Kuekulvong C, Mongkolsuk S (2008) Antifungal potential of extracellular metabolites produced by Streptomyces hygroscopicus against phytopathogenic fungi. Intern J Biol Sci 4:330–337. https://doi.org/10.7150/ijbs.4.330
Purcell JP, Greenplate JT, Jennings MG, Ryerse JS, Pershing JC, Sims SR, Prinsen MJ, Corbin DR, Tran M, Sammons RD, Stonard RJ (1993) Cholesterol oxidase: a potent insecticidal protein active against boll weevil larvae. Biochem Biophys Res Commun 196:1406–1413. https://doi.org/10.1006/bbrc.1993.2409
Quesada-Moraga E, Alain VE (2004) Bassiacridin, a protein toxic for locusts secreted by the entomopathogenic fungus Beauveria bassiana. Mycol Res 108:441–452
Quesada-Moraga E, Carrasco-diaz JA, Santiago-Alvarez C (2006) Insecticidal and antifeedant activities of proteins secreted by entomopathogenic fungi against Spodoptera littoralis (Lep., Noctuidae). J Appl Entomol 130:442–452
Rakshiya YS, Verma MK, Sindhu SS (2016) Efficacy of antagonistic soil bacteria in management of subterranean termites (Isoptera). Res Environ Life Sci 9:949–955
Rath AC, Tidbury CA (1996) Susceptibility of Coptotermes acinaciformis (Isoptera: Rhinotermitidae) and Nasutitermes exitiosus (Isoptera: Termitidae) to two commercial isolates of Metarhizium anisopliae. Sociobiology 28:67–72
Revathi N, Ravikumar G, Kalaiselvi M, Gomathi D, Uma C (2011) Pathogenicity of three entomopathogenic fungi against Helicoverpa armigera. J Plant Pathol Microbiol 2:114. https://doi.org/10.4172/2157-7471.1000114
Rivers DB, Vann CN, Zimmack HL, Dean DH (1991) Mosquitocidal activity of Bacillus laterosporus. J Invert Pathol 58:444–447. https://doi.org/10.1016/0022-2011(91)90191-R
Rodriguez RJ, White Jr JF, Arnold AE, Redman RS (2009) Fungal endophytes: diversity and functional roles. New Phytol 182:314–330. https://doi.org/10.1111/j.1469-8137.2009.02773.x
Ruiu L (2013) Brevibacillus laterosporus, a pathogen of invertebrates and a broad-spectrum antimicrobial species. Insects 4:476–492
Ruiu L, Delrio G, Ellar DJ, Floris I, Paglietti B, Rubino S, Satta A (2006) Lethal and sub-lethal effects of Brevibacillus laterosporus on the housefly (Musca domestica). Entomol Exp Appl 118:137–144. https://doi.org/10.1111/j.1570-7458.2006.00370.x
Ruiu L, Satta A, Floris I (2013) Emerging entomopathogenic bacteria for insect pest management. Bull Insectol 66:181–186
Sabaratnam S, Traquair JA (2015) Mechanism of antagonism by Streptomyces grisecarneous (strain Di944) against fungal pathogens of green house-grown tomato transplants. Can J Plant Pathol 37:197–211. https://doi.org/10.1080/07060661.2015.1039062
Saikia R, Gogoi DK, Mazumder S, Yadav A, Sarma RK, Bora TC, Gogoi BK (2011) Brevibacillus laterosporus strain BPM3, a potential biocontrol agent isolated from a natural hot water spring of Assam, India. Microbiol Res 166:216–225. https://doi.org/10.1016/j.micres.2010.03.002. Epub 2010 Jul 13
Sandhu SS, Rajak RC, Hasija SK (2000) Potential of entomopathogens for the biological management of medically important pest: progress and prospect. In: Glimpses in plant sciences, pp 110–117
Saraf M, Pandya U, Thakkar A (2014) Role of allelochemicals in plant growth promoting rhizobacteria for biocontrol of phytopathogens. Microbiol Res 169:18–29. https://doi.org/10.1016/j.micres.2013.08.009
Seleena P, Lee HL (1994) Insecticidal activity of a Malaysian isolate of Aspergillus niger. Asian J. Sci Technol Dev 11:47–53
Sellami S, Jamoussi K, Dabbeche E, Jaoua S (2011) Increase of the Bacillus thuringiensis secreted toxicity against lepidopteran larvae by homologous expression of the vip3LB gene during sporulation stage. Curr Microbiol 63:289–294
Seo JH, Yeo JS, Cha HJ (2005) Baculoviral Polyhedrin- Bacillus thuringiensis toxin fusion protein: a protein-based bio-insecticide expressed in Escherichia coli. Biotechnol Bioeng 92:166–172
Sergeant M, Baxter L, Jarrett P, Shaw E, Ousley M, Winstanley C, Alun J, Morgan W (2006) Identification, typing and insecticidal activity of Xenorhabdus isolates from entomopathogenic nematodes in United Kingdom soil and characterization of the xpt toxin loci. Appl Environ Microbiol 72:5895–5907. https://doi.org/10.1128/AEM.00217-06
Sevim A, Demirbag Z, Demirturk I (2010) A new study on the bacteria of Agrotis segetum Schiff. (Lepidoptera: Noctuidae) and their insecticidal activities. Turk J Agric For 34:333–342
Sezen K, Demir I, Demirbag Z (2005) Investigations on bacteria as a potential biological control agent of summer chafer Amphimallon solstitiale L. (Coleoptera: Scarabaeidae). J Microbiol 43:463–468
Shakeri J, Foster HA (2007) Proteolytic activity and antibiotic production by Trichoderma harzianum in relation to pathogenicity to insects. Enzym Microbiol Technol 40:961–968
Sharaf EF (2005) A potent chitinolytic activity of Alternaria alternata isolated from Egyptian black sand. Pol J Microbiol 54:145–151
Sindhu SS, Parmar P, Phour M, Kumari K (2014) Rhizosphere microorganisms for improvement in soil fertility and plant growth. In: Nagpal R, Kumar A, Singh R (eds) Microbes in the service of mankind: tiny bugs with huge impact. JBC Press, New Delhi, pp 32–94
Sindhu SS, Sehrawat A, Sharma R, Dahiya A (2016) Biopesticides: use of rhizosphere bacteria for biological control of plant pathogens. Def Life Sci J 1:135–148
Singer S (1996) The utility of morphological group II Bacillus. Adv Appl Microbiol 42:219–261
Singh Y (2007) Isolation and identification of bacteria having pathogenic interactions with termites (Isoptera). M.Sc. thesis submitted to CCS Haryana Agricultural University, Hisar, p 104
Smith KM (1967) Insect virology. Academic, New York, p 256
Smythe RV, Coppel HC (1965) The susceptibility of Reticulitermes flavipes (Kollar) and other termite species to an experimental preparation of Bacillus thuringiensis Berliner. J Invertebr Pathol 7:423–426
Snyder D, Meyer J, Zimmerman AG, Qiao M, Gissendanner SJ, Cruthers LR, Slone RL, Young DR (2007) Preliminary studies on the effectiveness of the novel pulicide, spinosad, for the treatment and control of fleas on dogs. Vet Parasitol 150:345–351
Srivastava JN, Prakash S (2001) Chrysosporium tropicum efficacy against Anopheles stephensi larvae in the laboratory. J Am Mosquito Contr Assoc 17:127–130
Stanley-Samuelson DW, Jensen E, Nickerson KW, Tiebel K, Ogg CL, Howard RW (1991) Insect immune response to bacterial infection is mediated by eicosanoids. Proc Natl Acad Sci U S A 88:1064–1068
Strasser H, Vey A, Butt TM (2000) Are there any risks in using entomopathogenic fungi for pest control, with particular reference to the bioactive metabolites of Metarhizium, Tolypocladium and Beauveria species? Biocontrol Sci Tech 10:717–735
Sun J, Fuxa JR, Henderson G (2002) Sporulation of Metarhizium anisopliae and Beauveria bassiana on Coptotermes formosanus and in vitro. J Invertebr Pathol 81:78–85
Tefera T, Vidal S (2009) Effect of inoculation method and plant growth medium on endophytic colonization of sorghum by the entomopathogenic fungus Beauveria bassiana. Biol Control 54:663–669
Thakur R, Sandhu SS (2010) Distribution, occurrence and natural invertebrate hosts of indigenous entomopathogenic fungi of central India. Indian J Microbiol 50:89–96
Trudeau D (1989) Selection of entomophilic nematodes for control of the eastern subterranean termite, Reticulitermes flavipes (Kollar) (Isoptera: Rhinotermitidae). Master’s thesis, University of Toronto, Toronto, Ontario, Canada, 93 pp
Tuan SJ, Hou RF, Lee CF, Chao YC (2007) High level production of polyhedral in a scorpion toxin containing recombinant baculovirus for better control of insect pests. Bot Stud 48:273–281
van Rie J, Jansens S, Hofte H, Degheele D, van Mellaert H (1989) Specificity of Bacillus thuringiensis δ-endotoxins. Importance of specific receptors on the brush-border membrane of the mid-gut of target insects. Eur J Biochem 186:239–247
Vandermeer J, Perfecto I, Liere H (2009) Evidence for hyperparasitism of coffee rust (Hemileia vastatrix) by the entomogenous fungus, Lecanicillium lecanii, through a complex ecological web. Plant Pathol 58:636–641
Vega FE, Kaya HK (2012) Insect pathology. Academic, San Diego
Vega FE, Posada F, Aime MC, Pava-Ripoll M, Infante F, Rehner SA (2008) Entomopathogenic fungal endophytes. Biol Control 46:72–82
Vidal S, Jaber LR (2015) Entomopathogenic fungi as endophytes: plant-endophyte-herbivore interactions and prospects for use in biological control. Curr Sci 109:46–54
Vimala Devi PS (2001) Prospects of using Nomuraea rileyi for the management of crop pests. In: Rabindra RJ, Kennedy JS, Sainath N, Rajsekaran B, Srinivasan MR (eds) Microbial control of crop pests. Graphic Skill Publisher, Coimbatore, pp 80–94
Vodovar N, Vallenet D, Cruveiller S, Rouy Z, Barbe V, Acosta C, Cattolico L, Jubin C, Lajus A, Segurens B, Vacherie B, Wincker P, Weissenbach J, Lemaitre B, Médigue C, Boccard F (2006) Complete genome sequence of the entomopathogenic and metabolically versatile soil bacterium Pseudomonas entomophila. Nature Biotechnol 24:673–679
Vu TT (2005) Modes of action of non-pathogenic Fusarium oxysporum endophytes for bio-enhancement of banana toward Radopholus similis. Ph.D. thesis, University of Bonn, Germany
Waweru B, Turoop L, Kahangi E, Coyne D, Dubois T (2014) Non-pathogenic Fusarium oxysporum endophytes provide field control of nematodes, improving yield of banana (Musa sp). Biol Control 74:82–88
Wells JD, Fuxa JR, Henderson G (1995) Virulence of four fungal pathogens to Coptotermes formosanus (Isoptera: Rhinotermitidae). J Entomol Sci 30:208–215
Wright MS, Connick WJ, Jackson MA (2003) Use of Paecilomyces sp. as pathogenic against termites. US Patent 6660291
Wright MS, Raina AK, Lax AR (2005) A strain of the fungus Metarhizium anisopliae for controlling subterranean termites. J Econ Entomol 98:1451–1458
Yankouskaya A (2009) Application of biological insecticide Pecilomicine-B for greenhouse pest control. Scientific works of the Lithuanian Institute of Horticulture and Lithuanian University of Agriculture Sodininkystė Ir Daržininkystė 28:249–258
Yu CG, Mullins MA, Warren GW, Koziel MG, Estruch JJ (1997) The Bacillus thuringiensis vegetative insecticidal protein Vip3A lyses midgut epithelium cells of susceptible insects. Appl Environ Microbiol 63:532–536
Yu H, Gouge DH, Baker P (2006) Parasitism of subterranean termites (Isoptera: Rhinotermitidae: Termitidae) by entomopathogenic nematodes (Rhabditida: Steinernematidae; Heterorhabditidae). J Econ Entomol 99:1112–1119
Zhang J, Hodgman TC, Krieger L, Schnetter W, Schairer HU (1997) Cloning and analysis of the cry gene from Bacillus popilliae. J Bacteriol 179:4336–4341
Zhu C, Ruan L, Peng D, Yu Z, Sun M (2006) Vegetative insecticidal protein enhancing the toxicity of Bacillus thuringiensis subsp kurstaki against Spodoptera exigua. Kett Appl Microbiol 42:109–114. https://doi.org/10.1111/j.1472-765X.2005.01817.x
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sindhu, S.S., Sehrawat, A., Sharma, R., Khandelwal, A. (2017). Biological Control of Insect Pests for Sustainable Agriculture. In: Adhya, T., Mishra, B., Annapurna, K., Verma, D., Kumar, U. (eds) Advances in Soil Microbiology: Recent Trends and Future Prospects. Microorganisms for Sustainability, vol 4. Springer, Singapore. https://doi.org/10.1007/978-981-10-7380-9_9
Download citation
DOI: https://doi.org/10.1007/978-981-10-7380-9_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7379-3
Online ISBN: 978-981-10-7380-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)