Abstract
Solid tumor tissues often have functional and phenotypical heterogeneities, arising at least in part from the local hypoxic tumor microenvironment (generally O2 concentration is less than 2%). The elevated level of hypoxia is tightly associated with genetic instability, tumor progression, drug resistance, and/or poor clinical outcome after treatment, indicating that hypoxia exerts a strong selection pressure for the survival of cancer stem cells (CSCs) within tumors and also permits their maintenance. Thus, it has become urgent to precisely clarify the molecular basis of how hypoxia could contribute to the acquisition and/or maintenance of the aggressive phenotypes of this deadly disease. Meanwhile, cells keep genomic integrity to avoid genetic instability-mediated tumorigenesis through the proper stress response under normoxia. Upon hypoxia, hypoxia-inducible factor-1α (HIF-1α) which has an O2-sensing ability accumulates and then facilitates tumor development through an induction of vascular endothelial growth factor (VEGF)-dependent angiogenesis. Therefore, the hypoxic HIF-1α/VEGF regulatory axis plays a vital role during the malignant tumor progression. Intriguingly, pro-oncogenic runt-related transcription factor 2 (RUNX2) has an ability to stimulate HIF-1α-mediated induction of VEGF. Recently, we have found for the first time that RUNX2 contributes to the acquisition of drug-resistant phenotype of malignant tumor cells. In this review, we focus on the functional interplay between HIF-1α/VEGF and RUNX2 within the hypoxic tumor microenvironment. Finally, we would like to discuss the potential therapeutic strategy targeting this tumor hypoxia.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
Accumulating evidence strongly suggests that tumor cell microenvironments including hypoxia play a pivotal role in the acquisition and/or maintenance of malignant phenotypes of advanced tumors. Although tumor cells with a higher proliferation rate consume a large amount of oxygen and nutrients, they are exposed to a serious hypoxic condition. To survive under these severe conditions, hypoxic response takes place within tumors [1, 2].
One of the initial molecular events in response to hypoxia is the stabilization and activation of hypoxia-inducible factor-1α (HIF-1α). HIF-1α, which recognizes and binds to HRE (hypoxia-responsive element) within its target gene promoter/enhancer, is a basic helix-loop-helix family of transcription factor. Under normoxia, the amount of HIF-1α is kept at an extremely low level within cell nucleus through von Hippel-Lindau (VHL) E3 ubiquitin ligase-mediated proteasomal degradation system [3]. Upon hypoxia, VHL is dissociated from HIF-1α, and thereby HIF-1α becomes stable without proteolytic degradation. Stabilized and activated HIF-1α then induces the expression of its numerous target gene products such as pro-angiogenic vascular endothelial growth factor (VEGF), erythropoietin, and enolase [4, 5].
As expected, VEGF plays a central role in hypoxic response to assist the survival of a certain population of tumor cells exposed to serious hypoxic conditions. VEGF promotes the formation of new blood vessels around hypoxic areas, which supply hypoxic tumor cells with the enough amount of oxygen as well as nutrients, and then a certain subset of tumor cells acquires much more malignant phenotypes including the enhanced drug resistance and the increased metastatic potential [6,7,8,9]. Indeed, it has been shown that the aberrant overexpression of VEGF is closely associated with poor clinical prognosis of the patients with a variety of aggressive tumors [10, 11]. With these in mind, HIF-1α/VEGF regulatory axis in response to hypoxia has been considered to be one of the promising molecular targets for cancer therapy [12].
The evolutionarily conserved small noncoding RNAs, termed microRNAs (miRNAs), regulate their target gene expression primarily through the posttranscription level in a sequence-specific manner [13]. Of interest, increasing evidence strongly indicates that miRNAs are implicated in the regulation of various cellular processes such as hypoxic response [14]. For example, it has been described that miR-622 directly targets HIF-1α and then significantly impedes HIF-1α-mediated tumor cell migration as well as invasion in response to hypoxia [15]. On the other hand, miR-630, a HIF-1α-induced miRNA, contributed to the promotion of tumor growth and metastasis [16]. Thus, it is likely that a certain set of miRNAs involved in hypoxic response might serve as critical prognostic indicators and also potential molecular targets for future cancer therapy.
Runt-related transcription factor 2 (RUNX2) is one of the RUNX family members composed of RUNX1, RUNX2, and RUNX3. It has been well established that RUNX2 is a master regulator of osteoblast differentiation and bone formation. RUNX2-deficient mice displayed a complete loss of bone formation [17, 18]. Consistent with these observations, RUNX2 transactivates a variety of its target genes implicated in osteogenesis such as type I collagen, osteopontin, and osteocalcin [19]. However, this previous point of view has been challenged by the findings showing that, in addition to osteogenesis, RUNX2 has a strong oncogenic potential. Firstly, it has been shown that aberrant overexpression of RUNX2 is detectable in numerous tumor tissues [20,21,22]. Secondly, RUNX2 has an ability to transactivate several tumor cell invasion-related genes including MMP-9 and MMP-13 [23, 24]. Lastly, we have found that depletion of RUNX2 significantly improves drug sensitivity of various tumor cells ([25, 26]).
In the present review article, we describe the basic background of hypoxic response of tumor cells and then discuss the potential therapeutic strategies which might overcome hypoxic response-mediated malignant progression of tumor cells based on our current observations.
5.2 Hypoxia-Inducible Factor-1 (HIF-1) and Hypoxic Response of Tumor Cells
To survive, the majority of solid tumor cells exhaust a lot of nutrients and oxygen provided from the surrounding normal vasculature, and thereby tumor tissues become hypoxic (insufficient oxygen levels). Hypoxic tumors appear to become much more aggressive (the reduced cell death, the enhanced drug resistance, the higher resistance to radiotherapy, and the increased metastatic potential) [27,28,29,30]. Consistent with these observations, tumor hypoxia is an independent poor prognostic factor for the survival of tumor patients irrespective of treatment modality [31, 32].
To increase their mass under this serious hypoxic condition, tumor cells require the additional nutrients and oxygen. The angiogenesis (the formation of new blood vessels) which plays a pivotal role in malignant tumor progression as well as metastasis is a multistage biological process tightly regulated by a balance between pro-angiogenic and anti-angiogenic signalings [33, 34]. The tumor angiogenesis, which is one of the hallmarks of the hypoxic tumors, is implicated in the accelerated proliferation rate of the vascular endothelial cells [8].
The vascular endothelial growth factor (VEGF) (also known as VEGF-A), which is the most important angiogenic secreted dimeric glycoprotein, promotes these angiogenic processes such as the vascular endothelial cell proliferation and the development of the tumor vessels under the hypoxic condition [35]. As described [36], VEGF family is composed of several structurally and functionally related proteins including VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and placental growth factor. Among them, VEGF-A has been the most studied member of VEGF family. Since the secretion of VEGF from the hypoxic tumor cells has been shown to trigger the advanced tumor development [6, 7, 9], VEGF-mediated formation of tumor vessels is one of the essential hypoxic responses of tumor cells. Therefore, preventing these processes might contribute at least in part to prohibit both aggressive tumor progression and metastasis [10, 11].
Transcriptionally active hypoxia-inducible factor-1 (HIF-1), a basic helix-loop-helix family of transcription factor, is a heterodimer made up of α- and β-subunits. Under normal conditions, HIF-1α is continuously produced and simultaneously degraded through ubiquitin-proteasome breakdown system driven by von Hippel-Lindau (VHL) E3 ubiquitin ligase, whereas HIF-1β is constitutively expressed and kept at constant level within cell nucleus [3]. The immediate molecular response to hypoxia is stabilization of HIF-1α. Upon hypoxia or loss of functional VHL, HIF-1α is stabilized, forms a heterodimeric complex with HIF-1β, and binds to hypoxia-responsive elements (HREs) within its target gene promoters to trigger a concerted transcriptional response [5]. Among HIF-1-target genes, VEGF is the extensively studied HIF-1-regulated gene [4]. HIF-1-mediated deregulated expression of VEGF leads to the development of hypoxic tumors through the promotion of the angiogenesis as mentioned above. As expected, the aberrant expression of VEGF has been shown to be associated with the poor prognosis of various types of human tumors [37, 38] (Fig. 5.1). Therefore, prohibition of VEGF-mediated pro-angiogenic pathway improved the efficacy of various anticancer drugs on breast, cervix, stomach, lung, colon, rectum, and ovary carcinomas [39,40,41].
5.3 Maintenance and Propagation of Cancer Stem Cells (CSCs) Under Hypoxia
It has been well known that phenotypic and functional heterogeneity is a common property of a variety of solid tumors, arising at least in part from tumor cell microenvironments. According to cancer stem cell (CSC) theory, which might recapitulate the primary tumors, only a small subset of tumor cell population has a strong pro-oncogenic potential. A subset of tumor cells which expresses cell surface normal stem cell markers such as CD24 and CD44 has been shown to be much more tumorigenic [42]. In support of these findings, it has been described that malignant glioma and colon cancer include CSCs [43, 44]. Growing evidence indicates that CSCs cause the acquisition of malignant phenotypes of advanced tumors including drug resistance and recurrence after therapy [45].
Since hypoxia is one of the critical properties of tumor cell microenvironments, it is possible that hypoxia-induced HIF-1α might contribute to the maintenance and/or survival of CSCs. Bos et al. demonstrated that the expression level of HIF-1α protein in breast cancer tissues is larger in poorly differentiated than well-differentiated lesions, indicating that HIF-1α plays a role in the maintenance of the undifferentiated state of aggressive tumors [46]. Soeda et al. found that hypoxia promotes cell survival of undifferentiated CD133-positive glioma-initiating CSCs through the activation of HIF-1α, whereas CSC differentiation is markedly prohibited under hypoxia [47]. CD133 has been considered to be one of the molecular markers of CSC population [48]. Based on their results, depletion of HIF-1α led to a massive reduction in the sphere-forming ability of glioma CSCs. Collectively, CSCs are obviously dependent on HIF-1α for their survival, self-renewal, and tumor growth.
Moreover, Heddleston et al. found that hypoxia increases number of CSC population and enhances the stem-like phenotype of the established tumor cell lines [49]. Similarly, Dong et al. described that hypoxia potentiates CSC sphere formation and causes an increase in number of CD44-positive colon cancer CSC subpopulations [50]. From their observations, the expression level of inhibitor of DNA-binding protein 2 (Id2) in colon cancer CSCs was significantly elevated in response to hypoxia through the activation of pro-oncogenic Wnt/β-catenin signaling pathway. Consistently, silencing of Id2 markedly attenuated hypoxia-mediated CSC sphere formation and also tumor metastasis in vivo. Intriguingly, highly aggressive pancreatic and colon cancer cells expressed a large amount of Id2 [51, 52].
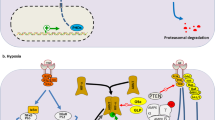
Together, in addition to HIF-1α, Wnt/β-catenin-dependent augmentation of Id2 might be involved in the maintenance and/or survival of CSCs in response to hypoxia (Fig. 5.2).
5.4 Regulatory Role of MicroRNAs in Response to Hypoxia
MicroRNAs (miRNAs) are evolutionarily conserved small noncoding regulatory RNAs of around 22 nucleotides in length, which recognize and bind to the 3′-untranslated regions (3′-UTR) of their target mRNAs, repressing their translation [13]. Bioinformatic analysis indicates that more than 30% of protein-coding genes might be regulated by miRNAs [53]. A growing body of evidence strongly suggests that numerous siRNAs act as tumor suppressors or oncogene products. For example, Cheng et al. demonstrated that miR-622 has an ability to downregulate HIF-1α and then prohibits metastatic spread of lung cancer in mouse xenograft model [15]. Xue et al. found that hypoxia-mediated repression of miR-15-16, which targets angiogenic FGF2, promotes tumor angiogenesis and metastasis [54].
In contrast, Rupaimoole et al. found that miR-630 reduces the expression level of Dicer, which is involved in miRNA biogenesis, and stimulates tumor growth as well as metastasis [16]. Ge et al. described that miR-421 targets E-cadherin as well as caspase-3 and then stimulates metastasis [55]. According to their results, the expression level of miR-421 was significantly higher in advanced gastric cancer tissues than localized ones. Similarly, Devlin et al. revealed that miR-210 is upregulated in response to hypoxia and contributes to tumor metastasis [56]. In addition, miR-382 has been shown to be angiogenic miRNA targeting tumor suppressor PTEN (phosphatase and tensin homolog) [57]. Since the expression of miR-630, miR-421, miR-210, and miR-382 has been shown to be regulated by HIF-1α, it is worth noting that a certain subset of miRNAs responsible for hypoxia-induced tumor invasion/metastasis participates in HIF-1α-mediated oncogenic pathway. In this connection, several miRNAs implicated in hypoxic response might provide potential therapeutic clues to overcome malignant phenotypes of advanced tumors.
5.5 Pro-oncogenic Property of Runt-Related Transcription Factor 2 (RUNX2)
Runt-related transcription factor 2 (RUNX2) is a nuclear sequence-specific transcription factor responsible for the induction of bone formation and osteoblast differentiation. Indeed, RUNX2-deficient mice died just after birth and exhibited a complete loss of bone formation [17, 18]. In support of these observations, RUNX2 transactivates a number of osteogenic indicators such as type I collagen, osteopontin, and osteocalcin through RUNX2-responsive elements within their promoter regions [19].
Meanwhile, accumulating evidence demonstrated that RUNX2 is highly expressed in a variety of tumor tissues as compared to their corresponding normal ones. For example, Pratap et al. described that RUNX2 is aberrantly overexpressed in breast and prostate cancers [21]. Kayed et al. demonstrated that pancreatic ductal adenocarcinoma tissues highly express RUNX2 as compared to the normal pancreas [20]. In addition, Wang et al. revealed that the expression level of RUNX2 is higher in ovarian cancer tissues than normal ovarian ones [22]. Lastly, Boregowda et al. found that RUNX2 is highly expressed in melanoma tissues relative to melanocytes [58]. Accordingly, it has been shown that the expression level of RUNX2 is employed as a prognostic indicator of non-small cell lung carcinoma patients [59]. These observations imply that, in addition to osteoblast differentiation and bone formation, RUNX2 has a strong pro-oncogenic potential in vivo.
Recently, we have found for the first time that RUNX2 is tightly linked to the drug-resistant phenotype of malignant tumor-derived cells such as osteosarcoma U2OS cells and pancreatic cancer AsPC-1 cells ([25], [26]). The drug resistance has been considered to be one of the hallmarks of advanced tumors [30]. From our observations, siRNA-mediated knockdown of RUNX2 remarkably improved adriamycin (ADR) and gemcitabine (GEM) sensitivity of U2OS and AsPC-1 cells, respectively.
5.6 Functional Implication of RUNX2 in the Regulation of the Hypoxic Response
Since RUNX2 has a pro-oncogenic function, it is conceivable that RUNX2 might be closely implicated in the regulation of hypoxic response. Of interest, Pratap et al. described that RUNX2 transactivates tumor cell invasion-related MMP-9 gene in bone metastatic tumor cells [24]. Similarly, Mendoza-Villanueva et al. found that RUNX2 directly regulates the expression of MMP-13 and promotes the malignant invasion of breast cancer cells [23]. Forced expression of RUNX2 in prostate cancer cells stimulated their invasiveness [60]. In accordance with these observations, depletion of RUNX2 resulted in a significant reduction of migration and invasion rate of colon cancer cells [61]. Furthermore, it has been described that pro-oncogenic PI3K/AKT pathway directly or indirectly augments the expression and the transcriptional activity of RUNX2, while RUNX2 also stimulates PI3K/AKT pathway, indicating that this positive feedback loop regulatory mechanism is one of the major driving forces during the malignant tumor cell progression [62]. Thus, it is possible that RUNX2 participates in the acquisition of the malignant phenotypes of the aggressive tumors such as invasion and metastasis.
Consistent with the above-mentioned findings, it has been also shown that RUNX2 has an ability to enhance VEGF transcription [63]. Of note, Lee et al. revealed that RUNX2 stabilizes HIF-1α through the inhibition of VHL-mediated ubiquitination of HIF-1α and stimulates the transcriptional activity of HIF-1α [64]. Kwon et al. reported that RUNX2 forms a complex with HIF-1α in cell nucleus and is then efficiently recruited onto VEGF promoter region [65]. Additionally, it has been described that hypoxia-mediated induction of RUNX2 drives the malignant progression of numerous tumors through the direct upregulation of anti-apoptotic Bcl-2 [66]. Together, it is likely that RUNX2 potentiates HIF-1α and thus promotes angiogenesis-mediated tumor cell invasion and/or metastasis in response to hypoxia (Fig. 5.3).
5.7 Future Therapeutic Strategy Targeting Hypoxic Response
As described above, hypoxia-dependent tumor angiogenesis plays a critical role in the promotion of invasion and/or metastasis. These findings prompted us to develop anti-tumor drugs which block HIF-1α-/VEGF-induced angiogenesis. To our knowledge, a number of VEGF-targeting drugs have been produced and approved for clinical treatment. For example, bevacizumab, a monoclonal antibody against VEGF-A, has been approved for the treatment of the patients with renal cancer [67]. In addition to VEGF, blocking VEGF receptor (VEGFR)-mediated signaling might be a promising approach to develop anti-tumor drugs. Recently, Li et al. described that a small chemical compound termed DW10075 selectively prohibits kinase activity of VEGFR [68]. However, chemotherapeutic drugs targeting HIF-1α/VEGF/VEGFR have limited efficacy against malignant tumors and sometimes cause adverse effects [69].
Given that RUNX2 further stimulates HIF-1α-mediated induction of VEGF [63], it is highly possible that depletion of RUNX2 suppresses an aggressive progression of malignant tumors through the downregulation of VEGF. Intriguingly, several lines of evidence imply that certain miRNAs might regulate the expression of RUNX2. For example, the diminished expression of miR-135 or miR-203 led to the enhancement of RUNX2 expression in metastatic breast cancer cells [70]. According to their results, miR-135 and miR-203 were highly expressed in normal breast epithelial cells, whereas RUNX2 was undetectable. In contrast, metastatic breast cancer tissues expressed RUNX2 but not miR-135 or miR-203.
Similarly, exogenous expression of miR-34c in osteosarcoma cells decreased the expression level of RUNX2 [71]. Thus, it is likely that miRNA-mediated downregulation of RUNX2 causes the efficient suppression of malignant phenotypes of the aggressive tumors.
Alternatively, we have found that siRNA-mediated silencing of RUNX2 improves the efficacy of anti-tumor drugs [25, 26]. Unfortunately, it has been well known that siRNA is extremely unstable and thus its effect is transient. To overcome this weakness, Zorde Khvalevsky et al. developed a local prolonged siRNA delivery system (termed LODER) [72]. According to their results, LODER system blocked the degradation of siRNA and released intact siRNA slowly into tumor cells over a few months. Collectively, it is suggestive that LODER system overcomes the present siRNA delivery obstacles, and thus siRNA-mediated knockdown of RUNX2 by employing LODER as a delivery system is an attractive therapeutic strategy for the treatment of the patients with malignant tumors.
5.8 Conclusion
A growing body of evidence implies that tumor microenvironments composed of several cell populations including tumor cells play vital roles in the regulation of the malignant tumor progression under severe hypoxic condition. Hypoxia-dependent stabilization and nuclear access of HIF-1α stimulate the expression of VEGF, which contributes to tumor cell survival, invasion, and metastasis through the formation of new blood vessels. Meanwhile, RUNX2 is associated with HIF-1α and further potentiates its sequence-specific transactivation ability. Thus, RUNX2/HIF-1α regulatory axis is essential for VEGF-mediated malignant tumor progression in response to hypoxia, and RUNX2 might be one of the attractive molecular targets for cancer therapy.
References
Biddlestone J, Bandarra D, Rocha S. The role of hypoxia in inflammatory disease. Int J Mol Med. 2015;35:859–69.
Span PN, Bussink J. Biology of hypoxia. Sem Nucl Med. 2015;45:101–9.
Kaelin WG Jr. Treatment of kidney cancer: insights provided by the VHL tumor-suppressor protein. Cancer. 2009;(115):2262–72.
Forsythe JA, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–13.
Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2012;12:9–22.
Claffey KP, Robinson GS. Regulation of VEGF/VPF expression in tumor cells: consequences for tumor growth and metastasis. Cancer Metastasis Rev. 1996;15:165−176.
Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669−676.
Hu K, et al. Hypoxia-inducible factor 1 upregulation of both VEGF and ANGPTL4 is required to promote the angiogenic phenotype in uveal melanoma. Oncotarget. 2016;7(7):7816–28. 10.18632/oncotarget.6868.
Price DJ, et al. Role of vascular endothelial growth factor in the stimulation of cellular invasion and signaling of breast cancer cells. Cell Growth Differ. 2001;12:129–−135.
Grothey A, Galanis E. Targeting angiogenesis: progress with anti-VEGF treatment with large molecules. Nat Rev Clin Oncol. 2009;6:507−518.
Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–39.
Ulivi P, Marisi G, Passardi A. Relationship between hypoxia and response to antiangiogenic therapy in metastatic colorectal cancer. Oncotarget. 2016;7:46678–91. 10.18632/oncotarget.8712.
Shivdasani RA. MicroRNAs: regulators of gene expression and cell differentiation. Blood. 2006;108:3646–53.
Zhao Y, et al. miRNA-directed regulation of VEGF in tilapia under hypoxia condition. Biochem Biophys Res Commun. 2014;454(1):183–8.
Cheng S, et al. Global microRNA depletion suppresses tumor angiogenesis. Genes Dev. 2014;28:1054–67.
Rupaimoole R, et al. Hypoxia-upregulated microRNA-630 targets Dicer, leading to increased tumor progression. Oncogene. 2016;35(33):4312–20. https://doi.org/10.1038/onc.2015.492.
Komori T, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–64.
Otto F, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–71.
Karsenty G. The genetic transformation of bone biology. Genes Dev. 1999;13:3037–51.
Kayed H. Regulation and functional role of the Runt-related transcription factor-2 in pancreatic cancer. Br J Cancer. 2007;97(8):1106–15.
Pratap J, et al. Regulatory roles of Runx2 in metastatic tumor and cancer cell interactions with bone. Cancer Metastasis Re. 2006;25(4):589–600.
Wang ZQ, et al. Inhibition of RUNX2 transcriptional activity blocks the proliferation, migration and invasion of epithelial ovarian carcinoma cells. PLoS One. 2013;8(10):e74384.
Mendoza-Villanueva D, et al. The Runx transcriptional co-activator, CBFbeta, is essential for invasion of breast cancer cells. Mol Cancer. 2010;9:171.
Pratap J, et al. The Runx2 osteogenic transcription factor regulates matrix metalloproteinase 9 in bone metastatic cancer cells and controls cell invasion. Mol Cell Biol. 2005;25(19):8581–91.
Ozaki T, et al. Runt-related transcription factor 2 (RUNX2) inhibits p53-dependent apoptosis through the collaboration with HDAC6 in response to DNA damage. Cell Death Dis. 2013;4:e610.
Sugimoto K, et al. Silencing of RUNX2 enhances gemcitabine sensitivity of p53-deficient human pancreatic cancer AsPC-1 cells through the stimulation of TAp63-mediated cell death. Cell Death Dis. 2015;6:e1914.
Carlson DJ, Yenice KM, Orton CG. Tumor hypoxia is an important mechanism of radioresistance in hypofractionated radiotherapy and must be considered in the treatment planning process. Med Phys. 2011;38(12):6347–50.
Graeber TG, et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91.
Subarsky P, Hill RP. The hypoxic tumour microenvironment and metastatic progression. Clin Exp Metastasis. 2003;20:237–50.
Wartenberg M, et al. Regulation of the multidrug resistance transporter P-glycoprotein in multicellular tumor spheroids by hypoxia-inducible factor (HIF-1) and reactive oxygen species. FASEB J. 2003;17:503–5.
McKeown SR. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br J Radiol. 2014;87:20130676.
Nordsmark M, Overgaard J. Tumor hypoxia is independent of hemoglobin and prognostic for loco-regional tumor control after primary radiotherapy in advanced head and neck cancer. Acta Oncol. 2004;43(4):396–403.
McDougall SR, Anderson ARA, Chaplain MAJ. Mathematical modelling of dynamic adaptive tumour-induced angiogenesis: clinical implications and therapeutic targeting strategies. J Theor Biol. 2006;7:564–89.
Nishida N, et al. Angiogenesis in cancer. Vasc Health Risk Manag. 2006;2:213–9.
Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579−591.
Otrock ZK, Makarem JA, Shamseddine AI. Vascular endothelial growth factor family of ligands and receptors: review. Blood Cells Mol Dis. 2007;38(3):258–68.
Hirayama N, et al. Pleural effusion VEGF levels as a prognostic factor of malignant pleural mesothelioma. Respir Med. 2011;105:137–42.
Hsu IL, et al. Angiogenetic biomarkers in non-small cell lung cancer with malignant pleural effusion: Correlations with patient survival and pleural effusion control. Lung Cancer. 2009;65:371–6.
Blagosklonny MV. Antiangiogenic therapy and tumor progression. Cancer Cell. 2004;5:13–7.
Bottsford-Miller JN, Coleman RL, Sood AK. Resistance and escape from antiangiogenesis therapy: clinical implications and future strategies. J Clin Oncol. 2012;30:4026–34.
Jayson GC, Hicklin DJ, Ellis LM. Antiangiogenic therapy-evolving view based on clinical trial results. Nat Rev Clin Oncol. 2012;9:297–303.
Al-Hajj M, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8.
Ricci-Vitiani L, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–5.
Singh SK, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8.
Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10(6):717–28.
Bos R, et al. Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. J Natl Cancer Inst. 2001;93(4):309–14.
Soeda et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1α. Oncogene. 2009;28:3949–59.
Jung P, et al. Isolation and in vitro expansion of human colonic stem cells. Nat Med. 2011;17:1225–7.
Heddleston JM, et al. Hypoxia inducible factors in cancer stem cells. Br J Cancer. 2010;102(5):789–95.
Dong et al. The Wnt/β-catenin signaling/Id2 cascade mediates the effects of hypoxia on the hierarchy of colorectal-cancer stem cells. Sci Rep. 2016;6:22966.
Benezra R, Rafii S, Lyden D. The Id proteins and angiogenesis. Oncogene. 2001;20(58):8334–41.
Kleeff J, et al. The helix-loop-helix protein Id2 is overexpressed in human pancreatic cancer. Cancer Res. 1998;58:3769–72.
Drakaki A, Iliopoulos D. MicroRNA gene networks in oncogenesis. Curr Genomics. 2009;10:35–41.
Xue G, et al. c-Myc-mediated repression of miR-15-16 in hypoxia is induced by increased HIF-2α and promotes tumor angiogenesis and metastasis by upregulating FGF2. Oncogene. 2015;34(11):1393–406.
Ge X, et al. MicroRNA-421 regulated by HIF-1α promotes metastasis, inhibits apoptosis, and induces cisplatin resistance by targeting E-cadherin and caspase-3 in gastric cancer. Oncotarget. 2016;7(17):24466–82. 10.18632/oncotarget.
Devlin C, et al. miR-210: More than a silent player in hypoxia. IUBMB Life. 2011;63:94–100.
Seok JK, et al. MicroRNA-382 induced by HIF-1alpha is an angiogenic miR targeting the tumor suppressor phosphatase and tensin homolog. Nucleic Acids Res. 2014;42:8062–72.
Boregowda RK, et al. RUNX2 is overexpressed in melanoma cells and mediates their migration and invasion. Cancer Lett. 2014;348(1–2):61–70.
Li H, et al. Clinical significance of RUNX2 expression in patients with nonsmall cell lung cancer: a 5-year follow-up study. Tumour Biol. 2013;34(3):1807–12.
Baniwal SK, et al. Runx2 transcriptome of prostate cancer cells: insights into invasiveness and bone metastasis. Mol Cancer. 2010;9:258.
Sase T, et al. Runt-related transcription factor 2 in human colon carcinoma: a potent prognostic factor associated with estrogen receptor. Int J Cancer. 2012;131:2284–93.
Cohen-Solal KA, Boregowda RK, Lasfar A. RUNX2 and the PI3K/AKT axis reciprocal activation as a driving force for tumor progression. Mol Cancer. 2015;14:137.
Zelzer E, et al. Tissue specific regulation of VEGF expression during bone development requires Cbfa1/Runx2. Mech Dev. 2001;106:97–106.
Lee SH, et al. Runx2 protein stabilizes hypoxia-inducible factor-1α through competition with von Hippel-Lindau protein (pVHL) and stimulates angiogenesis in growth plate hypertrophic chondrocytes. J Biol Chem. 2012;287(18):14760–71.
Kwon TG, et al. Physical and functional interactions between Runx2 and HIF-1α induce vascular endothelial growth factor gene expression. J Cell Biochem. 2011;112(12):3582–93.
Browne G, et al. Bicalutamide-induced hypoxia potentiates RUNX2-mediated Bcl-2 expression resulting in apoptosis resistance. Br J Cancer. 2012;107(10):1714–21.
Willett CG, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–7.
Li MY, et al. DW10075, a novel selective and small-molecule inhibitor of VEGFR, exhibits antitumor activities both in vitro and in vivo. Acta Pharmacol Sin. 2016;37(3):398–407.
Zhang Y, et al. The DEK oncogene activates VEGF expression and promotes tumor angiogenesis and growth in HIF-1α-dependent and -independent manners. Oncotarget. 2016;7(17):23740–56. 10.18632/oncotarget.8060.
Taipaleenmäki H, et al. Targeting of Runx2 by miRNA-135 and miRNA-203 impairs progression of breast cancer and metastatic bone disease. Cancer Res. 2015;75(7):1433–44.
van der Deen M. MicroRNA-34c inversely couples the biological functions of the runt-related transcription factor RUNX2 and the tumor suppressor p53 in osteosarcoma. J Biol Chem. 2013;288(29):21307–19.
Zorde Khvalevsky E, et al. Mutant KRAS is a druggable target for pancreatic cancer. Proc Natl Acad Sci U S A. 2013;100:20723–8.
Acknowledgments
The authors are grateful to Dr. Hiroki Nagase for his helpful discussions.
Conflicts of Interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ozaki, T., Nakamura, M., Ogata, T., Sang, M., Shimozato, O. (2018). The Functional Interplay Between Pro-oncogenic RUNX2 and Hypoxia-Inducible Factor-1α (HIF-1α) During Hypoxia-Mediated Tumor Progression. In: Shinomiya, N., Kataoka, H., Xie, Q. (eds) Regulation of Signal Transduction in Human Cell Research. Current Human Cell Research and Applications. Springer, Singapore. https://doi.org/10.1007/978-981-10-7296-3_5
Download citation
DOI: https://doi.org/10.1007/978-981-10-7296-3_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7295-6
Online ISBN: 978-981-10-7296-3
eBook Packages: MedicineMedicine (R0)