Abstract
Commercial activated charcoal was investigated for removal of Cr(VI) from aqueous solutions. The effects of altering the initial Cr(VI) concentration, pH, contact time and amount of activated charcoal were studied. Maximum adsorption of Cr(VI) was achieved between pH 1–3 and after a contact time of 120 min. The percentage of Cr(VI) removed decreased from 99.99–90.83% when the initial Cr(VI) concentration was increased from 0.05–0.5 mg ml−1 at pH 2 and 26 ± 2 °C. Various kinetic models such as pseudo first-order and pseudo second-order models were used to evaluate the mechanism of Cr(VI) adsorption on activated charcoal. The Cr(VI) removal process was found to be governed by second-order kinetics and the rate constant of the adsorption (k2) was 0.1800 g kg−1 min−1 for an initial Cr(VI) concentration of 0.1 mg ml−1. The adsorption of Cr(VI) was evaluated using Langmuir, Freundlich, and Temkin isotherms and their constants were determined. The maximum adsorption capacity obtained using the Langmuir isotherm model was 45.24 g kg−1 at pH 2.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
All over the world industry is forced to diminish down to acceptable level contents of heavy metal in water and industrial waste waters. Conventional methods like precipitation are a favorable especially when dealing with large volume of matter which contains heavy metal ions in low concentration. Typically these ions are precipitated as hydrated metal oxides or hydroxides using calcium oxide. Precipitation is accompanied by flocculation or coagulation, and one major problem is the formation of large amounts of sediments containing heavy metal ions. In recent years, considerable attention has been devoted to fine new adsorbents. One of the suitable methods for removing heavy metals from water and waste water is using surface adsorption process. Activated carbon is the most widely used adsorbent for this purpose because it has a high capacity for adsorption of organic matter, but its use is limited because of its high cost (Battacharya and Venkobachar 1984; Khare et al. 1987). This has led to search for cheaper substitutes. Coal, fly ash, wood, silica gel, clay materials (bentonite, montmorillonite, etc.), agricultural wastes (bagasse pith, maize cob, coconut shell, rice husk, etc.), and cotton wastes have been tried with varying success for metal ions removal (Mande Seyf-Laye et al. 2009; Singh and Rawat 1994; Theng and Wells 1995).
The objective of this study was to investigate the use of activated charcoal as an adsorbent material for removing Cr(VI) from wastewater.
Batch experiments were carried out for kinetic studies on the removal of Cr(VI) from aqueous solutions. The influence of various important parameters such as the pH, contact time, adsorbent amount, and initial Cr(VI) concentration were investigated by varying any one of the process parameters and holding the other parameters constant. The Langmuir and Freundlich equations were used to fit the equilibrium isotherm models. Pseudo first-order and second-order kinetic models were used to evaluate the mechanism of adsorption.
2 Experimental
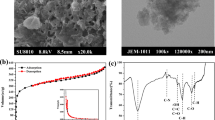
Commercial activated carbon in granular form (CAC) (Table 1) was obtained from Sinopharm Chemical Reagent Co. Ltd (Beijing, China) and crushed and sieved to a particle size of 450 μm. A Cr(VI) stock solution (1 mg.ml−1) was prepared by dissolving 99.9% K2Cr2O7 (2.8287 g) in distilled water (1 L). This solution was diluted as required to obtain 0.05–0.5 mg.ml−1 Cr(VI) standard solutions. The initial pH of the solution was adjusted using either 0.5 M NaOH or 0.5 M H2SO4. The batch experiments were carried out in 100 ml conical flasks by agitating a pre-weighed amount of the activated charcoal with 50 ml of the aqueous Cr(VI) solution for a predetermined period of time (based on prior kinetic studies) at 26 ± 2 °C on a water bath-mechanical shaker (198 rpm). The effect of altering the initial pH was studied with 0.05 mg.ml−1 Cr(VI). The effect of altering the contact time was studied with 0.1–0.4 mg.ml−1 Cr(VI) solutions at pH 2. Experiments were also conducted to investigate the effect of varying the amount of activated charcoal from 2–24 g.L−1 with a Cr(VI) concentration of 0.250 mg.ml−1 at pH 2. Adsorption isotherm studies were carried out with the different standard solutions (0.05–0.5 mg.ml−1 Cr(VI)) while maintaining the adsorbent dosage at 10 g.L−1 and the pH at 2. An UV-visible spectrophotometer was employed with 1,5-diphenylcarbazide in acid medium to determine the concentrations of Cr(VI) remaining in the sample. The absorbance of the purple-violet colored solution was recorded at 540 nm. The filtrate was analyzed for the remaining Cr(VI) concentration. The amount of Cr(VI) adsorbed (g kg−1) at time t was calculated using Eq. (1):
Where \( C_{0} \) and \( C_{t} \) are the Cr(VI) concentrations in g L−1 at time 0 and time t, respectively, V is the volume of the Cr(VI) solutions in L, and \( m_{s} \) is the weight of activated charcoal in kg.
3 Results and Discussion
3.1 Effect of Initial PH
The percentage of Cr(VI) adsorbed by activated charcoal decreased from 99.9–39.54% when the pH was increased from 1–7 (Fig. 1). The maximum percent removal of Cr(VI) was obtained between pH 1–3, and therefore pH 2 was selected for the rest of the experiments. Chromium exists mostly in two oxidation states which are Cr(VI) and Cr(III) and the stability of these forms is dependent on the pH of the system (Cimino et al. 2000; Selomulya et al. 1999; Shama and Forster 1994). It is well known that the dominant form of Cr(VI) at pH 2 is \( HCrO_{4}^{ - } \). Increasing the pH will shift the concentration of \( HCrO_{4}^{ - } \) to other forms; \( CrO_{4}^{2 - } \) and \( Cr_{2} O_{7}^{2 - } \). Maximum adsorption at pH 1.0 indicates that it is the \( HCrO_{4}^{ - } \) form of Cr(VI), which is the predominant species between pH 1 and 4, which is adsorbed preferentially on the activated charcoal.
3.2 Effect of Contact Time
The contact time was found to be an important parameter for the adsorption of Cr(VI) on activated charcoal (Fig. 2). Over the first 20 min the percent removal of Cr(VI) from the aqueous solution increased rapidly and reached 60.21–97.43% (for 0.4–0.1 mg.ml−1 Cr(VI) solutions. After this the percent removal tapered off until at 120 min it reached 99.76% and 90.83% for solutions containing 0.1 and 0.4 mg.ml−1 of Cr(VI), respectively. Further increase in the contact time had a negligible effect on the percent removal. Therefore, a contact time of 120 min was used for Cr(VI) adsorption on activated charcoal in all the batch studies.
3.3 Effect of Initial Cr(VI) Concentration
When the initial Cr(VI) concentration was varied from 0.05–0.5 mg.ml−1, the percentage of Cr(VI) removed decreased from 99.99–88.21% (Fig. 3). However, the absolute amount of Cr(VI) removed per unit mass of activated charcoal (or absorption capacity) significantly increased from 4.99–44.10 g.kg−1 with the increase in initial Cr(VI) concentration.
3.4 Effect of Adsorbent Amount on Cr(VI) Removal
When the amount of activated charcoal was increased from 2–24 g L−1, the percentage of Cr(VI) removed increased from 56–99.44% (Fig. 4). The amount of activated charcoal selected for subsequent experiments was 10.0 g L−1 as above this amount the removal of Cr(VI) remained stable.
3.5 Adsorption Isotherm
The equilibrium of adsorption is an important physicochemical parameter for evaluation of the adsorption process. The adsorption isotherm (\( q_{e} \) versus \( C_{e} \)) obtained in this study showed that the adsorption capacity (g kg−1) increased with increasing equilibrium Cr(VI) concentrations (\( C_{e} \) ) and eventually attained a constant value (Fig. 5).
To model the adsorption behavior, three adsorption isotherms were studied and their correlation with the experimental data was assessed. These included the Freundlich and Langmuir isotherms, which are the earliest and simplest known relationships describing the adsorption equation (Muhamad et al. 1998; Jalali et al. 2002). Table 1 shows the adsorption capacities of various adsorbents. It is clear from this table that the adsorption capacity of the commercial activated carbon used in this study far exceeded that of other activated carbons prepared from different materials.
-
Langmuir isotherm
The linear form of the Langmuir equation is shown in Eq. (2) (Bulut and Aydin 2006; Langmuir 1996; Ravikumar et al. 2007).
Where b and \( q_{m} \) are constants related to the apparent energy of adsorption and the adsorption capacity, respectively; and \( q_{e} \) is the amount adsorbed per unit mass of the adsorbent (g kg−1) with an equilibrium concentration of \( C_{e} \) (mg ml−1). A plot of (\( C_{e} \)/\( q_{e} \)) vs. \( C_{e} \) was linear and the constants \( q_{m} \) and b were determined from the slope and intercept of the plot (Table 3). The correlation coefficient obtained with the Langmuir equation was high (\( R^{2} \) = 0.98), which indicated a good fit between the parameters. The dimensionless parameter (\( R_{L} = 1/1 + bC_{0} \)), which is a measure of adsorption favorability, was found to be in the range of 0.00653–0.06174 (0 < \( R_{L} \) < 1) and confirmed that Cr(VI) removal using activated charcoal at pH 2 and 26 ± 2 °C was a favorable adsorption process.
-
Freundlich isotherm
The Freundlich isotherm is expressed by Eq. (3) (Freundlich 1906)
Where K f (g1−1/n l1/n kg−1) is the Freundlich constant, which indicates the relative adsorption capacity of the adsorbent related to the bonding energy, and n f is the heterogeneity factor representing how the absorption deviates from linearity. Values of nf less than one are an indication that significant adsorption takes place at low concentration, while high K f values indicate greater adsorption intensity. The linear form of the Freundlich isotherm is Eq. (4):
The Freundlich coefficients were determined from a plot of log \( \varvec{q}_{\varvec{e}} \) versus log \( (\varvec{C}_{\varvec{e}} \)) and are given in Table 2. The Freundlich model clearly agreed very well with the experimental data.
3.6 Adsorption Kinetics
-
Pseudo first-order kinetics
Kinetic modeling of the removal of Cr(VI) by activated charcoal was carried out using the well-known Lagergren model (Hameed 2009; Tabak et al. 2009).
Where \( q_{e} \) and \( q_{t} \) are the amounts of Cr(VI) adsorbed (g kg−1) at equilibrium and time t, respectively, and k ad (L min−1) is the rate constant of the pseudo first-order adsorption operation.
A plot of log \( \left( {q_{e} - q_{t} } \right) \) versus t was linear and represents the pseudo first-order kinetics for the removal of Cr(VI) using activated charcoal. The first-order rate constants \( k_{1} \) and \( q_{e} \) were calculated for a range of initial Cr(VI) concentrations (0.1–0.4 mg L−1) with a constant amount of activated charcoal (10 g L−1) (Table 2). The regression correlation coefficient was in the range 0.97–0.982, which is good and shows the applicability of the pseudo first-order kinetic model to the removal of Cr(VI) using activated charcoal. The experimental values of \( q_{e} \) obtained using initial Cr(VI) concentrations of 100, 200, 300, and 400 mg L−1 were 9.97, 19.73, 28.81, and 36.33 g kg−1, respectively, which do not agree with the values predicted by the pseudo first-order model.
-
Pseudo second-order kinetics
The pseudo second-order adsorption kinetic rate equation is expressed as Eq. (6) (Ho et al. 2000):
Where \( k_{2} \) is the rate constant of pseudo second-order adsorption (\( g\, kg^{ - 1} \, min^{ - 1} \)). Equation (6) can be rearranged to obtain Eq. (7), which has the linear form:
With h (\( g\, kg^{ - 1} \, min^{ - 1} \)), the initial adsorption rate, expressed by: Eq. (8)
The plot of \( \left( {t/q_{t} } \right) \) and t of Eq. (9) was linear (Fig. 10), and \( q_{e} \) and \( k_{2} \) were determined from the slope and intercept, respectively. Because pseudo first-order kinetics were not applicable to the adsorption of Cr(VI) on activated charcoal, the second-order kinetic model was evaluated. The calculated \( q_{e} \) values agreed with the experimental values, and the values obtained for the regression correlation coefficients were more than 0.9998. These results indicate that the kinetics of Cr(VI) adsorption using activated charcoal are explained better by a second-order kinetic model than a first-order one.
3.7 Comparison of Cr(VI) Removal with Different Adsorbents Reported in Literature
The adsorption capacities of the adsorbents for the removal of Cr(VI) have been compared with those of others reported in literature and the values of adsorption capacities have been presented in Table 3. The values reported ares in the form of monolayer adsorption capacity. The experimental data of the present investigations are comparable with the reported values.
4 Conclusion
Activated charcoal has been successfully used to remove Cr(VI) from aqueous solution. The adsorption of Cr(VI) fitted well at low pH values, and the removal of Cr(VI) depended on the initial concentration, contact time and amount of activated charcoal. The Freundlich and Langmuir isotherm models agreed with the experimental data. Cr(VI) adsorption obeyed a second order rate equation, and the rate constant was found to be 0.0032 kg.g−1 min−1 with an initial Cr(VI) concentration 0.4 mg.ml−1.
References
Mande Seyf-Laye, A.-S., Liu, M., Liu, F., Chen, H.: Kinetics of the adsorption of chromium (VI) ions from water by vegetable precursors treated with sulphuric acid. Adsorp. Sci. Technol 27(10), 965–974 (2009)
Poojari, A.C., Maind, S.D., Bhalerao, S.A.: Effective removal of Cr(VI) from aqueous solutions using rind of Orange (Citrus sinensis), (L.) Osbeck. Int. J. Curr. Microbiol. App. Sci. 4(4), 653–671 (2015)
Battacharya, A., Venkobachar, C.: Removal of cadmium (II) by low cost adsorbents. J. Environ. Eng. ASCE 110, 110–122 (1984)
Bhattacharya, A.K., Mandal, S.N., Das, S.K.: Removal of Cr(VI) from aqueous solution by adsorption onto low cost non-conventional adsorbents. Indian J. Chem. Technol. 13, 576–583 (2006)
Bulut, Y., Aydin, H.: A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 194, 259–267 (2006)
Cimino, G., Passerini, A., Toscano, G.: Removal of toxic cations and Cr(VI) from aqueous solution by hazelnut shell. Water Res. 34, 2955–2962 (2000)
Freundlich, H.M.F.: Uber die adsorption in losungen. Zeitschrift fur Physikalische Chemie (Leipzig) 57A, 385–470 (1906)
Hameed, B.H.: Spent tea leaves: a new non-conventional and lowcost adsorbent for removal of basic dye from aqueous solutions. J. Hazard. Mater. 161, 753–759 (2009)
Ho, Y., Mckay, G., Wase, D., Foster, C.F.: Study of the sorption of divalent metal ions on to peat. Adsorp. Sci. Technol. 18, 639–650 (2000)
Jalali, R., Ghafourian, H., Aself, Z., Davarpanah, S.J., Sepehr, S.: Removal and recovery of lead using nonliving biomass of marine algae. J. Harzard. Mater. 92(3), 253–262 (2002)
Khare, S.K., Panday, K.K., Srivastava, R.M., Singh, V.N.: Removal of victoria blue from aqueous solution by fly ash. J. Chem. Technol. Biotechnol. 38, 99–104 (1987)
Langmuir, I.: The constitution and fundamental properties of solids and liquids. Am. J. Chem. Soc. 38, 2221–2295 (1996)
Muhamad, N., Parr, J., Smith, D.M., Wheatley, D.A.: Adsorption of heavy metals in slow sand filters. In: Proceeding of the WEDC Conference on Sanitation and Water for all Islamabad, Pakistan, pp. 346–349 (1998)
Ravikumar, K., Krishnan, S., Ramalingnam, S., Balu, K.: Optimization of process variables by the application of response surface methodology for dye removal using a novel adsorbent. Dyes Pigm. 72, 66–69 (2007)
Selomulya, C., Meeyoo, V., Amal, R.: Mechanisms of Cr(VI) removal from water by various types of activated carbons. J. Chem. Technol. Biotechnol. 74, 111–122 (1999)
Selvi, K., Pattabhi, S., Kadirvelu, K.: Removal of Cr(VI) from aqueous solution by adsorption onto activated carbon. Bioresour. Technol. 80, 87–89 (2001)
Sharma, D.C., Forster, C.F.: The treatment of chromium wastewaters using the sorptive potential of leaf mould. Bioresour. Technol. 49, 31–40 (1994)
Singh, B.K., Rawat, N.S.: Comparative sorption equilibrium studies of toxic phenols on fly ash and impregnated fly ash. J. Chem. Technol. Biotechnol. 61, 307–317 (1994)
Tabak, A., Eren, E., Afsin, B., Caglar, B.: Determination of adsorptive properties of a Turkish sepiolite for removal of reactive blue 15 anionic dye from aqueous solutions. J. Hazard. Mater. 161, 1087–1094 (2009)
Theng, B.K.G., Wells, N.: Assessing the capacity of some New Zealand clays for decolourizing vegetable oil and butter. Appl. Clay Sci. 9, 321–326 (1995)
Acknowledgments
This work was supported by 863 Program (2007AA06A410) from the Chinese Ministry of Science and Technology. The analytical data were supplied by the Lab of Water Resources and Environmental Engineering in the China University of Geosciences, Beijing.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Mande Seyf-Laye, AS., Ibrahim, T., Gbandi, DB., Honghan, C. (2018). Parameter Study on Remediating Cr(VI) in Water Using Activated Charcoal. In: Tran-Nguyen, HH., Wong, H., Ragueneau, F., Ha-Minh, C. (eds) Proceedings of the 4th Congrès International de Géotechnique - Ouvrages -Structures. CIGOS 2017. Lecture Notes in Civil Engineering , vol 8. Springer, Singapore. https://doi.org/10.1007/978-981-10-6713-6_103
Download citation
DOI: https://doi.org/10.1007/978-981-10-6713-6_103
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-6712-9
Online ISBN: 978-981-10-6713-6
eBook Packages: EngineeringEngineering (R0)