Abstract
Hydrogels are primarily synthesized to retain large amounts of aqueous solution. Depending upon modes of synthesis, the hydrogel materials develop various types of network structure. Recently, popular techniques have been developed for synthesis of hydrogels in the presence of crosslinking agents or multifunctional co-monomer which acts as a crosslinker. It can be categorized according to synthesis techniques, bio-degradability, response to environment and their intended applications. These applications may vary from water retention, conditioner to different aspects of biomedical applications and tissue engineering. Hydrogels contain a number of functional groups which may be utilized as such or modified and used to suit our requirements. In this review article, we have focused on the available synthesis techniques of hydrogels along with their inevitable properties and applications.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Polymer hydrogels are well-known materials with widespread usage in many fields, particularly in biomedical, pharmaceutical and agricultural sector. The first water-absorbent polymer was synthesized in 1938 after thermal polymerization of acrylic acid and divinylbenzene in an aqueous medium (Zohuriaan-Mehr 2006). Significant advancement in this field was marked in 1960 when these polymeric hydrogels were widely worked upon exploring their hidden potential (Kumar et al. 2006).
From the era of 1970s, available literature reports polymeric hydrogels with new chemical structure and resultant better physical properties as well as innovative applications. Advancements in hydrogel properties led to increase its suitability for design and operation of some sophisticated system, like automatic drug delivery systems. Hydrogels need to be smarter to perform necessary function to achieve desired applications. The development of novel hydrogels with their potential applications is in huge demand to suit our requirements.
For example, there are a number of wound-care products available in market today, from injectable foam to hydrocolloid dressings. Recently, hydrogels have found application in wound management systems. Since blood is indispensable for healing process and wounds can damage these blood vessels which may turn fatal, it is important to hold it at the affected areas. For this purpose, the researchers started developing an approach to wound-care management that would rebuild those damaged or dead vessels, creating a matrix of stem cell sponges and hydrogel that develops like skin of a growing foetus. Now, scientists are searching for the best source of such cell sponges, bone marrow and fats seem to be the best options so far. Soon, the clinical trials will be done in which participants will use this advanced hydrogel on diabetic foot ulcers.
More than one million people are suffering from inflammatory bowel disease (IBD) worldwide. The population of patients suffering from this disease is increasing continuously around the world, but its treatment is very limited. People have to depend on daily enemas as a part of therapy which is not comfortable, totally impractical and has great side effects on health. In this regard, clinical development for the effective treatment of chronic and debilitating inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis, is necessary. So, scientists have tried to develop an alternative therapy using disease targeted hydrogel that rapidly sticks to the ulcers and slowly releases the drug to the targeted area only. It is a great breakthrough for the patient for more targeted enema-based therapy in future.
The need to develop smarter hydrogels which can simulate the physiological system to address the biomedical issues is one of the primary objectives of contemporary research. Yet, it is important to understand the fundamental similarities and differences between the two. Such understanding will lead to development of multifunctional hydrogels to suit our requirements. Thus, it is important to review the available literature on synthesis, characterization and available applications of hydrogels along with patents and commercial products.
1.1 What Is Hydrogel?
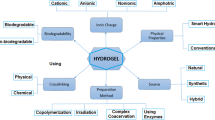
The term ‘hydrogel’ bears no specific definition as per updated medical and pharmaceutical encyclopaedia. Hydrogels are three-dimensional materials having capacity to hold excess amount of water to maintain the stability of its dimension. It maintains its 3D integrity in its swollen phase by the virtue of crosslinking (Williams 1990). Polymer hydrogels are either synthetic or of natural origin, homopolymers or co-polymers (Langer and Peppas 2003). It can also be classified on the basis of nature of crosslinked junction.
-
(a)
Chemically crosslinked with stable networks
-
(b)
Physically bonded networks owing to ionic interactions, hydrogen bonds or hydrophobic interactions, or even polymeric chain entanglements (Jen et al. 1996).
Chemically, hydrogels are synthesized by two different techniques: ‘three-dimensional polymerization’ occurs in the presence of hydrophilic monomer in addition with either a crosslinking agent indirectly or by direct crosslinking shown in (Fig. 1). Alternatively, polymerization is carried out by employing free radical initiators like benzoyl peroxide, ammonium peroxodisulphate, 2,2-azo-isobutyronitrile (AIBN). This may also be achieved by irradiation with UV rays, gamma rays or electron beam shown in (Fig. 2). In three-dimensional polymerization, product is obtained along with considerable amount of residual monomers. These unreacted monomers are often toxic so they need to be separated out; otherwise, there remains a possibility of leaching from the hydrogels (Khutoryanskiy et al. 2013; Montoro et al. 2014; Mathur et al. 1996).
Rosiak used biopolymers like agar and gelatin as well as synthetic polymers like polyvinyl pyrrolidone (PVP) or polyvinyl alcohol (PVA) which have been crosslinked in the presence of gamma radiation and give sterile hydrogels for wound care (Rosiak et al. 1989). Nowadays, these hydrogels are available in market under brand name ‘Kikgel’ and ‘Aqua-gel’ used for dressing of wounds (Rosiak et al. 1989).
Another method for hydrogel synthesis is suggested by Khutoryanskiy. His method synthesizes water-soluble polymers using thermal and microwave radiation in aqueous medium (Khutoryanskiy et al. 2013; Cook et al. 2012). In this process, the aqueous solutions of PVA and poly(methyl vinyl ether-alt-maleic anhydride) are homogenized well at room temperature and kept under microwave radiation and thermal treatment under high pressure through autoclave. These synthesis techniques using irradiation as well as thermal procedures are cheap, safe and free from purification steps. They result in formation of hydrogels in the presence of hydrophilic polymers in suitable combination.
1.2 History of Hydrogel
According to Lee, Kwon and Park, the word ‘hydrogel’ was used in a number of articles since 1894 (Wei and Charlotte 2014). The material ascribed at that time was a colloidal gel made up of inorganic salts rather than a hydrogel. In 1960, the first developed crosslinked network material having hydrogel properties as well as high water affinity was polyhydroxyethylmethacrylate (PHEMA) hydrogel. This was intended to be used in a much-awaited project of permanent contact lens production. Hydrogel was the first soft material developed to be used inside any patient (Nierzwicki and Prins 1975; Wichterle and Lim 1960). Broadly, the history of hydrogels can be categorized (Buwalda et al. 2014) as follows:
-
The first era of hydrogel development history involved a wide range of random crosslinking experimental procedures. These involved using initiators for the chemical modifications. The primary objective was to synthesize materials with high swelling index and reasonable mechanical properties.
-
The second generation of materials involved optimizing response to specific stimuli like variation in pH, temperature, pressure or even concentration of solvent molecules. These stimuli were used to initiate specific phenomenon like polymerization of monomer, drug delivery. (Buwalda et al. 2014).
-
Finally, the third generation of hydrogels led to further advancement. It focused on the development of stereo-complexed materials, e.g. PEG-PLA interaction (Yom-Tov et al. 2014; Abebe and Fujiwara 2012), and hydrogels crosslinked with other physical interaction, e.g. cyclodextrins (Chung et al. 2008; Kirakci et al. 2014).
In 1980, Lim and Sun modified hydrogels and designed calcium alginate microcapsules for cell engineering. Later, Yanna and co-workers (Lim and Sun 1980) worked upon collagen and shark cartilage tissues as natural raw materials for novel dressing which reduced the healing burn. Both natural and synthetic hydrogel generated an interest for encapsulation of cells (Yannas et al. 1989) and have become a popular choice in the field of ‘tissue engineering’. Here, they serve as matrices for repairing of tissues and regeneration of tissues and organs (Sefton et al. 2000). It is helpful in the prevention of thrombosis, in drug delivery systems, as biosensor coatings as well as in cell transplants (Nguyen and West 2002; Peppas and Bures 2000; Sawhney and Pathak 1994; Miyata et al. 2002; Chang et al. 2010).
The tunable properties, hydrophilic character, biocompatibility and response to stimuli focus the interest of scientists in the development of the ‘smart hydrogels’ for many years (Park et al. 1993).
1.3 Types of Hydrogel
Hydrogels can be broadly categorized on the basis of various features. This may include their synthesis procedures and routes, biodegradable properties, applications or their response to various stimuli and some other parameters. Hydrogels are found to exhibit variations with respect to changes in pH, temperature, light, enzyme, electric and other stimuli. Hydrogels showing some environmentally sensitive properties along with their parent polymers are shown in Table 1.
2 Synthesis Procedures of Hydrogels
Therefore, hydrogels may be categorized as homo-polymers, co-polymers, interpenetrating networks and semi-interpenetrating network based on synthesis techniques and routes.
2.1 Homopolymers
Homopolymers are developed from single monomer. It provides a basic structural and functional unit to any polymeric network (Iizawa et al. 2007). Homopolymer crosslinked frame structure depends upon polymerization technique and nature of the monomer. The possible way to prepare homopolymeric hydrogel is by use of PHEMA {poly(2-hydroxyethyl methacrylate)} as monomer, polyethylene glycol dimethacrylate as crosslinking agent and benzoin isobutyl ether as a UV-sensitive initiator. This film was kept in de-ionized water under UV radiation (λ = 253.7 nm). The film was immersed in water to remove toxic or unreacted substances. PHEMA is used in artificial skin manufacturing and wound dressing besides the contact lenses to provide good healing conditions. PHEMA can also be synthesized by low molecular weight crosslinking agents like trimethylolpropane trimethacrylate. These hydrogels are soft in nature like PMMA and have high oxygen permeability. So, they can also be used for manufacture of contact lenses, as drug delivery matrices and tissue implants. Its application can further be improved upon by increasing its mechanical strength. Cretu has improved the hydrogel properties by introducing hydrophobic compound (caprolactone) into its structures or synthesizing amphiphilic material (Cretu et al. 2004).
The technique for the synthesis of these macromolecular architectures by an elegant utilization of ‘click chemistry’ was first made by Hilborn and co-workers in 2006 (Ossipov and Hilborn 2006). They synthesized poly(vinyl alcohols) functionalized with acetylene or azide groups. Hydrogel was formed immediately after addition of CuSO4/Na ascorbate. Lin and Anseth also synthesized PEG hydrogel through click chemistry (Benamer et al. 2006). This technique is based on a step-growth synthesis process. In this reaction, monomers having azide and alkyne functional groups remain bonded with each other by the virtue of catalysts to form stable covalent bonds. PEG hydrogel obtained by this method has good mechanical properties and permits modulation of physicochemical characteristics of the PEG hydrogels.
Polyvinylpyrrolidone hydrogels are synthesized by irradiation technique (Benamer et al. 2006). This is done by irradiating the reaction mixture with 60 Co source at a dose rate of 3.2 Gy/min. It can also be used for wound healing.
Polyacrylic acid (PAA) is also a homopolymeric hydrogel (Christensen et al. 2006). It contains 2.5% PAA and 97.5% water in its commercially available grade. It is stable and possesses fairly elastic properties. It is non-toxic, non-inflammatory and simulates the human physiological environment of soft tissue for the application in endoprosthesis.
2.2 Co-polymers
Co-polymers consist of multimonomer systems with one hydrophilic monomer, managed in any standard arrangement like random, block or alternating along with the polymer chain (Kopecek and Yang 2009). Ring opening co-polymerization of a-caprolactone to poly(ethylene glycol)-poly(a-caprolactone)-poly(ethylene glycol) co-polymeric hydrogel was given by Gong for establishment of drug delivery system (Gong et al. 2009; Kim and Peppas 2003). In triblock synthesis mPEG, stannous octoate and hexamethylene diisocyanate were used as initiator, catalyst and coupling agent. When this co-polymeric block is applied in situ, it is capable to form hydrogel. This hydrogel can release both hydrophilic and hydrophobic drugs including proteins.
Carboxymethyl cellulose (CMC) is known to be water soluble and bio-compatible. Wang suggested blending CMC with cellulose or with PVP to form PVP/CMC hydrogel (Wang et al. 2007). The blended PVP/CMC hydrogels possessed good mechanical properties. Its high water retention capacity improved its bio-degradability and also made it suitable as dressing material.
Later on, Thomas developed free radical co-polymerization. This hydrogel has been synthesized using acrylamide and acrylic acid as monomers and N,N-methylene bisacrylamide and potassium persulfate in aqueous medium (Thomas et al. 2007). This hydrogel was transparent and embedded silver nanoparticles. This silver embedded nano-composite hydrogel possessed antimicrobial activity which could be used further in various applications.
Synthesis of a thermoplastic hydrogel was reported by polymerization of a-benzyl l-glutamate (BLG) N-carboxyanhydride. This reaction was carried out using diamine groups placed at the ends of poly(ethylene oxide) chains of the poloxamer. This hydrogel was pH-sensitive and thermosensitive in nature and used for drug delivery applications (Oh et al. 2003).
Multistep gelation process synthesizes hydrogel with multimembrane ‘onion-like structure’ under controlled physicochemical conditions by Ladet as shown in Fig. 3. He reported synthesis of a multilayered material based on physical hydrogels of amphiphilic polymers. These were synthesized without any external crosslinker by using chitosan and alginate. These novel three-dimensional multimembrane structures are useful in biomedical applications (Ladet et al. 2008).
2.3 Semi-interPenetrating Networks (Semi-IPNs)
Semi-interpenetrating networks are formed without any chemical bonds between them. Here, (Semi-IPN) one straight chain polymer penetrates another crosslinked network (Zhang et al. 2009). Semi-IPNs are reported to have fast response rates towards pH and temperature as given by their kinetic studies. The polymerization of cationic polyallylammonium chloride (linear) in acrylamide or acrylic acid is an example of (Semi-IPN) co-polymer hydrogel. It has higher mechanical strength which led to theophylline release by pH variation. This type of semi-IPN was prepared by using N,N-methylene bisacrylamide as a crosslinker by means of template co-polymerization. (Zhang et al. 2005). The IPN network consists of both covalent and ionic bonds, and 3D structure is formed due to covalent bonds. The ionic bonds were responsible for better mechanical properties and pH reversible property.
Also, a semi-IPN hydrogel network was synthesized for its suitability in nano-level systems. Here, production and stabilization of silver nanoparticles were achieved (3–5 nm size) (Murthy et al. 2008). PVP chains were dispersed physically throughout PAA network. This silver nano-architecture was found to have significant antibacterial effects.
Crosslinked co-polymer of PHEMA and semi-IPN of gum arabic was prepared by the use of ammonium persulfate as initiator and N,N-methylene bisacrylamide as crosslinker (Gils et al. 2010). The hydrogel was embedded with silver nanoparticles using silver nitrate with trisodium citrate as reducing agent. This led to appreciable antibacterial activity due to the presence of silver nanoparticles inside the hydrogel network.
Some semi-IPN hydrogels of alginate and poly(N-isopropylacrylamide) (PNIPAAm) were synthesized using calcium chloride as crosslinker (Ju et al. 2002). Swelling behaviour of semi-IPNs was observed at different pH at higher temperatures, and the formation of a polyelectrolyte complex was reported. It forms by the reaction of carboxyl groups of alginate and amino groups of modified PNIPAAm. It was sensitive towards pH, temperature and ionic strength of solvent.
Semi-IPN hydrogels have also been investigated for delivery in stomach. They show more swelling index in acidic conditions. These studies have been carried out for the delivery of amoxicillin and metronidazole antibiotics. These drugs are used for the treatment of helicobacter pylori infestation in stomach. A guar gum (GG)-based semi-IPN hydrogel and poly(methacrylic acid) are also reported (Li and Liu 2008). The minimum swelling index was observed in acidic pH condition for hydrogel loaded with 5-aminosalicylic acid (5-ASA).
In acidic pH conditions, the model drug 5-ASA formed complex hydrogen-bonded structure with the polymeric network structure. The maximum swelling index is observed for these hydrogels at pH 7.4 due to the electrostatic repulsion of the carboxylic groups. It resulted in minimum release of 5-ASA from the hydrogel network at pH 2.2. In vitro study showed that concentration of crosslinker and amount of GG affected degradation. This enzymatic degradation of hydrogels by cecal bacteria accelerates the release of 5-ASA from hydrogel at pH 7.4.
PVP-based hydrogel (Lu et al. 2010a, b) was synthesized as very promising thermosensitive material. The most vital shortcoming of PVP hydrogel as thermosensitive material is that it did not exhibit thermosensitivity under normal conditions. PVP and CMC resulted in the formation semi-IPN hydrogel having application as volume-phase transition temperature (VPTT). This study explained that the VPTT was significantly dependent on CMC content and pH of the swelling medium. VPTT behaviour is reflected in buffer solution of pH 1.2 but not in alkaline medium. In vitro drug release was studied at various buffer solutions using bovine serum albumin (BSA) as a model drug. These studies suggested that PVP/CMC semi-IPN hydrogels could work as suitable candidates for the delivery of protein drug in the intestine.
2.4 InterPenetrating Networks (IPNs)
An interpenetrating polymer network (IPN) consists of two or more polymeric chains in any network. The synthesis is done by immersing a pre-polymerized hydrogel into a solution of monomers and a polymerization initiator. These are not covalently bonded to each other but partially interconnected with each other on a polymer scale (Lipatov 2002). These networks cannot be distributed unless chemical bonds are broken. In this hydrogel, two or more networks can be arranged in such a manner that they are coupled and cannot be separated from each other. These hydrogels are often referred as ‘intelligent polymers’ or ‘hungry network’ (Shivashankar and Mandal 2012). The benefits of using IPNs are its compact matrices, strong mechanical properties, modifiable physical properties and fairly good candidature for drug delivery systems as compared to conventional hydrogels (Mohamadnia et al. 2007). As of now, it is the centre of immense interest for scientific research due to its unexplored potential in a large number of applications, particularly in medicine, industry, biology and environmental areas.
IPN has tailor-made properties. Its pore size and surface chemistry can be tuned for specific drug release kinetics. This is a result of interaction between hydrogel and the surrounding tissues (Li et al. 2007). Chivukula synthesized highly crosslinked IPN hydrogel network that restricts the swelling response of pH-sensitive hydrogel. It allows linear swelling with an abrupt pH change from 7.4 to 2 suited for oral drug delivery application (Chivukula et al. 2006). Chitosan crosslinked/PNIPAM IPN network has been studied with diclofenac (Alvarez et al. 2005).
Polyurethane (PU) is also a biomaterial. IPN of PU and polyacrylamide controlled water absorption (Abraham et al. 2001). They are mixed together by addition of crosslinking agents vinyl pyrrolidone and methylenebisacrylamide. Then, the reaction mixture is exposed to UV radiation. This type of IPN hydrogels finds application as wound dressing material, artificial muscles, sensor systems and bio-separators.
Series of IPN hydrogels were reported to affect sensitivity towards temperature and pH fluctuations (Liu et al. 2012). This synthesis involved incorporation of polyaspartic acid which is a pH-sensitive polymer, into PNIPAAm hydrogel system. The swelling mechanism confirmed that IPN hydrogels possessed swift shrinking and re-swelling properties with respect to the concentration ratio of two components. These fast responsive properties of hydrogels enhance its applications in biomedical fields.
Next series of hydrogels include in situ polymerization approaches. Calcium alginate (Ca–Alg) and dextran methacrylate derivative (Dex-MA) hydrogels showed potential application in pharmaceutical field (Matricardi et al. 2008). The semi-IPN synthesized by the dispersion of Dex-MA chains into Ca–Alg hydrogel forms a hydrogel having different rheological properties than original Ca–Alg precursor. This enhanced its chances through use in injection of the semi-IPN through hypodermic needle. The IPN synthesized by UV treatment of semi-IPN forms hydrogels which are strong enough for delivery of bioactive molecules like protein.
3 Significant Properties of Hydrogel
3.1 Swelling Properties
The swelling properties of hydrogel matrix are important features that govern its future applications in pharmaceutical, biomedical, ophthalmology and tissue engineering fields. The polymer chains present in hydrogel interact with solvent molecules and start expanding to the fully relaxed and solvated state till they attain equilibrium state. The crosslinked network, on the other hand, applies an opposite force that pulls the chains inside. When these two forces acting in opposite directions balance each other, equilibrium is attained. This equilibrium ratio (Eq. 1.) generally illustrates the swelling behaviour of hydrogels.
where
- W swollen :
-
weight of the swollen hydrogel
- W dry :
-
weight of the dry gel
The swelling kinetics of the hydrogels can be determined from the swelling kinetics. First, the weight of the dry hydrogel (Wdry) is determined. Then, this dried hydrogel is immersed in water until the swelling equilibrium is reached. This is followed by weighing the swollen hydrogel (Wswollen) after removing the excess water. The swelling ratio is given as (Eq. 2).
Many research groups have assessed the swelling/shrinking kinetics of PNIPAAm hydrogel with temperature variations. Yoshida and co-workers have discussed the shrinking kinetics of PNIPAAm hydrogel. They explained that comb-type PNIPAAm hydrogels collapsed fist followed by the hydrogels without grafted side chains. (Yoshida et al. 1995; Kaneko et al. 1995). They also mentioned a comb-type grafted hydrogel synthesized by PEO graft chains in the crosslinked PNIPAAm network (Kaneko et al. 1998). The swelling characteristics are of utmost importance for hydrogels in biomedical and pharmaceutical applications. The equilibrium swelling ratio is an important parameter which affects the solute diffusion coefficient, surface wettability and mechanical properties of the hydrogel. The swelling properties are affected by many factors like nature of monomer, crosslinker concentration and other environmental factors like temperature, pH and ionic strength.
Kiil developed a mathematical model to explain the water-induced swelling, drug dissolution, external and internal mass transport resistance of dissolved drug in an HPMC matrix (Kiil and Dam-Johansen 2003). The main purpose of this model was to determine the position of three distinct moving fronts (Fig. 3).
3.2 Mechanical Properties of Hydrogel
The mechanical strength of the hydrogel network depends upon the composition and structure of hydrogels (Shibayama 2012). In general, the polymer hydrogels are very weak, soft, brittle and cannot withstand large deformation. These mechanical properties of hydrogels are very important parameters for pharmaceutical and biomedical applications. The assessment of mechanical property is important for biomedical applications, e.g. ligament and tendon repair, wound dressing material, matrix for drug delivery, tissue engineering and as cartilage replacement material. The mechanical properties are suitable to maintain its physical texture during delivery of therapeutic moieties. Hydrogel is a tailor-made material which can be optimized mechanically by tuning its crosslinking parameters. Higher degree of crosslinking results in formation of a strong hydrogel, but it reduces the percentage elongation of hydrogel and forms more brittle structure. Therefore, optimum degree of crosslinking has been achieved for relatively strong and elastic hydrogel. The mechanical characterization involved relaxation experiments based on generalized Maxwell model. Crosslinking density of hydrogel was determined by Flory’s theory and Young modulus. This value was then used to determine the average polymeric mesh size according to the equivalent network theory (Schurz 1991).
Recently, a hydrogel having capacity for the large deformation and development of a slide-ring (SR) gel was given by Okumura and Ito. The process involved the crosslinking of polyrotaxane (Okumura et al. 2001).
3.3 Bio-compatible Properties
Bio-compatibility and non-toxicity is an important property for the hydrogel to make it appreciable in the field of biomedical studies. Most of the hydrogels used for this application have to pass cytotoxicity and in-vivo toxicity tests (Grodzinski 2009). Biocompatibility of the material signifies its suitable response in a specific application. It is divided into two components:
-
(a)
bio-safety: the absence of cytotoxicity, mutagenesis carcinogenesis and related things
-
(b)
bio-functionality: ability of material to perform the specified task for which it is intended (Grodzinski 2009)
This is relevant for tissue engineering applications since the tissue continuously interacts with the body. In case of synthetic hydrogel synthesis, any chemical used in polymerization may cause challenge for in vivo biocompatibility. Further, initiators, organic solvents, stabilizers, emulsifiers, unreacted monomers and crosslinkers may also be toxic to host cells (Bryant et al. 2000). Thus, removal of hazardous chemicals from preformed hydrogels is important. This may involve a number of purification processes like solvent washing or dialysis. In situ gelation of scaffolds presents a unique challenge since reactants which are used to synthesize the gel are injected into the body in the pre-polymer solution form. The utilization of this technique is ideal but needs precaution that all components used in the reaction are safe and non-toxic. Earlier, natural polymers were believed to be better than synthetic ones in terms of biocompatibility; still the presence of synthetic crosslinkers and initiators seems to have the same toxicity concerns as purely synthetic hydrogels.
4 Characterization of Hydrogels
Different types of characterization techniques have been developed for understanding the hydrogel network structure and physical or chemical properties. The physical properties of hydrogels network structure depend upon the equilibrium and dynamic swelling ratios. It depends on volume fraction of polymer, effective molecular weight of the polymer chain in between two crosslinking points and the correlation between two adjacent crosslinks (Lin and Metters 2006; Peppas et al. 2006). Flory (1942) and Huggins (1942) were the first to develop independently the theoretical base 70 years ago for understanding the polymer solutions.
Hydrogels have numerous properties, like absorption capacity, swelling behaviour, permeability, surface properties, optical properties and mechanical properties. All these properties make hydrogel a promising material for a wide variety of applications. The properties of the polymer chains and the crosslinking structures in these aqueous solutions play a vital role in the outcome of the properties of the hydrogel. Hydrogels are characterized by following methods/tests.
4.1 Fourier Transform Infrared Spectroscopy
FTIR analysis provides a reliable information about the crosslinking which is confirmed by appearance of IR bands near 1648 cm−1 region. IR absorption spectra give an idea about morphology of hydrogels.
4.2 Atomic Force Microscopy (AFM)
A multimode atomic force microscope helps to examine the surface morphology of the hydrogels.
4.3 Network Pore Size
Pore size determination is an important technique for hydrogel characterization. Different techniques like Quasi-elastic laser light scattering, electron microscopy, mercury porosimetry, rubber elasticity measurements, and equilibrium swelling experiments are used to determine the network pore size of hydrogel.
4.4 X-ray Diffraction
X-ray diffraction studies provide useful insight into the crystalline nature of hydrogel, whether the hydrogels retain their crystallinity or they get deformed during the synthesis procedures
4.5 Swelling Behaviour
Data of specific swelling studies is a must to determine its potential use as a hydrogel, and many researchers have successfully worked upon it.
4.6 Crosslinking and Mechanical Strength
The mechanical strength of the hydrogel depends upon the crosslinker density inside the network structure. Generally, the mechanical strength of the hydrogel increases with increase in the crosslinker concentration.
4.7 Rheology
It depends on the type of interactions based on structural properties (i.e. association, entanglement and crosslinks) present in the system. Polymer solutions are essentially viscous at low frequencies and tend to fit the scaling laws: G′ ~ ω2 and G″ ~ ω. At high frequencies, elasticity dominates (G′ > G″). This corresponds to Maxwell-type behaviour with a single relaxation time, which may be determined from the crossover point, and this relaxation time increases with concentration. Crosslinked microgel dispersions exhibit G′ and G″ that are almost independent of oscillation frequency.
All these characterization techniques give an account for the confirmation of intended crosslinking results, formation of hydrogel, used further for different applications.
5 Application of Smart Hydrogel
In the last few decades due to its hydrophilic character, biocompatibility and initiate stimuli hydrogels received considerable attention towards several applications. These applications are discussed in the following subsections.
5.1 Biomaterial Application
5.1.1 Soft Contact Lenses
Soft contact lenses remain one of the most widely used applications of hydrogels. Hydrogels have the adaptability towards the global ocular curvature and permit the atmospheric oxygen to reach up to cornea by dissolving the water of the lens (Lum et al. 2013). PHEMA was the first ever synthetic hydrogel prepared by DuPont scientists in 1936 (Strain et al. 1939). It was established as a promising and wonderful candidate for manufacture of contact lens by Wichterle and Lim (1960) due to their biocompatibility and mechanical properties. Nowadays, a number of hydrogel contact lens materials have been developed containing various monomers such as N-VP, MAA, MMA and glyceryl methacrylate. These are incorporated to increase the water content of hydrogel contact lens and also to enhance its mechanical properties to allow them to hold the force of the eyelid along with an elevated permeability to oxygen (Lum et al. 2013) (Fig. 4).
5.1.2 Tissue Regeneration and Tissue Engineering Applications
Globally, many people suffer from the loss of an organ or chronic failure of any organ function as a result of some severe disease, accident, etc., every year. This necessitates the need of tissue and organ transplantations, but they are difficult to be carried out due to lesser availability of donors, legal norms, social norms, etc. (Lee and Mooney 2001).
The term ‘tissue engineering’ came in practice in 1988 as an engineered application. The beautiful combination of fundamental principles of basic engineering and life sciences towards understanding human physiology and solving its problems by developing substitutes for tissues or organ system was a major breakthrough in the history of mankind. This helped in reviving a hope towards extension of life by regenerating specific tissues and organ systems using engineered materials and suitable synthetic strategies (Chapekar 2000).
Thus, tissue engineering has developed a hope for design of an ideal living substitute which mimics the properties of living tissues in human system (Langer and Vacanti 1993). Scaffolds act as three-dimensional artificial templates in which the tissue targeted for reconstruction is cultured to grow. The highly porous nature of hydrogel allows it for the diffusion of cells during migration. It also transfers the nutrients and excludes the waste products from cellular membranes (Loh and Choong 2013).
Now, both synthetic as well as natural hydrogels are used as scaffolds for various tissue engineering applications. These include repair of tendon, ligament, cartilage, blood vessels, skin and even heart valves (Ma 2004). The synthetic hydrogels targeted to be used as scaffolds are polyurethanes (PU), PVA, PEO, PNIPAAm, PAAc and poly(propylene fumarate-co-ethylene glycol) [P(PFco-EG)]. The natural hydrogels to be used in these applications include agarose, alginate, collagen, chitosan, gelatin, fibrin and hyaluronic acid (HA).
The micron-sized hydrogels (microgels) are also used to deliver macromolecules like phagosomes into cytoplasm of antigen-presenting cells. This release is triggered on due to acidic conditions within the tissues. These hydrogels mould themselves according to the pattern of membranes, tissues and possess considerable mechanical strength. This property of hydrogels is also used in cartilage repair (Bindu et al. 2012). Presently, the following hydrogels have been successfully used in tissue engineering applications:
-
(a)
Collagen-coated tissue culture: They are used for implant of cornea, trachea gland cells, etc. (Pal et al. 2009).
-
(b)
Poly(lactic-co-glycolic acid) (PLGA) polymer: They are used in conjunction with preadipocytes for epithelial cell culture of breast cells (Pal et al. 2009).
-
(c)
Porous scaffolding: These are coated with fibrillar collagen, used for the culture of liver cells, to be used in liver implants (Pal et al. 2009).
Nowadays, scientists are expanding the application of hydrogels in the regeneration of the central nervous system. For this application, chemically crosslinked PHEMA tubes have been developed by synthesizing centrifugal force; the outer diameter of these tubes is 2.4 mm, and wall thickness is 40–400 μm, which could be used for guided regeneration in the nervous system (Lum et al. 2013).
5.1.3 Wound Dressing
From early 1980s investigations are going on to promote the skin healing potential of hydrogels and its application in clinical setting. At first, the hydrogels absorb and retain wound exudates. Afterwards, fibroblast proliferation and keratinocyte migration are done for completion of epithelialization of the wound or wound healing. [Bullock et al. 2010; Ribeiro et al. 2009) The dense matrices of hydrogels (100 nm in swollen state) not only check the entry of bacteria but also permit transport of bioactive molecules (e.g. antimicrobial agents or drugs) to the targeted wound site to be healed (Drury and Mooney 2003). Such molecules can be easily entrapped in the polymeric network during the gel formation process, which is gradually released on the wound site as hydrogel absorbs its exudate and swells (Burd 2007; Boonkaew et al. 2014; Chakavala et al. 2012; Kumar et al. 2012a, b; Cui et al. 2011). The unique and tunable mechanical properties of hydrogels increase its suitability towards elasticity and flexibility to adapt with wounds caused in different body sites.
Hydrogels bring immediate relief to patients in distress, as compared to conventional bandages, pads or gauzes. Even, in case of burns, hydrogels are good alternatives for running water. It acts as a coolant to localized wound also reduces the pain and recovers the extent of resultant damage and reduces pain (Cuttle et al. 2009; Coats et al. 2002). The high water retention ability of hydrogels makes them particularly soothing on the wound. Non-adhesive nature of hydrogels causes less pain and discomfort to patient, as cells do not attach firmly to hydrophilic surfaces. Transparency of hydrogel provides an advantage over traditional bandages causing less pain during peeling it off.
A wide variety of hydrogels for wound dressings is commercially available in the market for treatment of minor burns and other skin wounds. They are available in numerous forms like amorphous gels, gel-impregnated gauzes, sheets or plasters (Burd 2007; Grippaudo et al. 2010). Amorphous gels are generally prescribed for superficial burn like cavity wounds, sheets and gel-impregnated gauzes (Winter et al. 1962). Plaster-like hydrogel dressings (e.g. MySkin®) are very user-friendly and attractive, as it can be well positioned on the wound without the use of adhesives and bandages. The development of hydrogel formulations is attaining new heights (Table 2), to address different aspects of wound healing and management (e.g. reduction in infection control, easy dressing).
5.1.4 Drug Delivery
Hydrogels have unique properties that make it useful in drug delivery application. Due to its hydrophilicity, it can hold excessive amounts of water. Hence, it can be used for design of drug delivery systems that control release of solute over a given time period. Many biomaterials have been explored for this purpose, which act by two mechanisms: (1) controlled release may be achieved by varying the crosslinker concentration and controlling the ratio of hydrophilic to hydrophobic monomers. (2) The interaction of hydrogels with drugs is very less, so it is better to release large fraction of active molecules drugs (protein and peptides) through hydrogel carriers. Controlled and targeted drug delivery would decrease the unwanted side effects and aid the recovery aspects. Several mechanisms describe the release of drug from hydrogel: (a) diffusion, (b) chemical control, (c) swelling and environmentally responsive release.
The diffusion-controlled release systems can be represented by matrix devices. This may be available as vacant chambers in form of a capsule, cylinders or sphere. This system carries drugs covered with a hydrogel membrane as shown in Fig. 5.
In this system, a continuous drug release system is maintained due to high concentration of drug in the centre of system (Peppas and Lowman 1999). Other matrix systems include dispersion or uniform dissolution of drug throughout the three-dimensional space of hydrogel as shown in Fig. 5. Here, drug release is carried out by the macromolecular pores. In this system, the initial rate of drug release is proportional to the square root of time (Peppas and Lowman 1999).
In the swelling-controlled release devices, the drug is loaded in a glassy polymer and it starts swelling on contact with a bio-fluid. Afterwards, hydrogel starts expanding beyond its boundary and allows the drug to diffuse with the relaxation of polymeric chains. This is called Case II transport mechanism, and it shows constant, time-independent release kinetics. It is called as ‘anomalous transport’, combining swelling-controlled release with diffusion (Peppas and Lowman 1999).
‘Smart’ hydrogels are promising materials for controlled release of drug, since they change their properties in response to specific stimuli. Right after the discovery of hydrogels, it has been used as anticancer and antibiotic delivery. Various research experiments have focus on the variety of drugs that can be delivered efficiently through the hydrogel based on the delivery systems shown in Table 3.
5.2 Industrial Applications
Hydrogels are significantly used for the adsorption of methylene blue dye from the industrial effluent. Hydrogels beads are used for the adsorption of dioxins.
A numbers of researchers are trying to develop techniques to sieze the metal ions. Irani and the co-workers synthesized a polyethylene-g-poly(acrylic acid)-co-starch/OMMT (LLDPE-g-PAA-co-starch/OMMT) hydrogel composite for PbI(II) removal (Joint et al. 2010; Irani et al. 2015).
Yan and co-workers performed etherification and functionalization of chitosan beads resulting in carboxymethylated chitosan having adsorption capacity of metal ions. These beads selectively adsorb specific ions like Cu(II), Pu(II) and Mg(II) (Yan et al. 2011). This property increases its potential towards dye removal after magnetic doping of hydrogel microsphere with IPN structures (Yan et al. 2011).
Novel and porous bio-absorbent xylan-based hydrogel has been developed by Xin after graft co-polymerization of acrylic acid (AA) and xylan-rich hemicelluloses. It has many applications towards adsorption of heavy metal ions (Pd2+, Cd2+ and Zn2+) from aqueous solutions.
The maximum adsorption capacities of Pd2+, Cd2+ and Zn2+ were reported to be 859, 495 and 274 mg/g, respectively (Peng et al. 2012).
5.3 Environmental Applications
Hydrogels are polymeric chains of repeating units with high water absorbing capability. Some hydrogels have capability to absorb water 500 times more than their weight. These superabsorbent properties of hydrogel enhance its potential to conserve water and to solve other environmental issues.
5.3.1 Hydrogels for the Prevention of Soil Erosion
Soil erosion has been controlled since a decade by reducing erosion and increasing water retention and permeability of finely textured agricultural soils. Water-soluble hydrogels have been used to reduce erosion and improve water infiltration among fine-textured agricultural soils. The water-soluble PAM hydrogels play an important role in preventing soil erosion by forming a thin film that covers the soil surface. During irrigation, this film protects the soil from washing away and maintains the optimum moisture conditions within the soil system, so that irrigation water can permeate easily. Several studies have proved that PAM hydrogels are efficient agents used to combat soil erosion.
5.3.2 Use of Hydrogels for Agricultural Purposes
Hydrogel uniqueness enhances its potential towards the field of agriculture also. It is reported that anionic herbicide 2,4-D encapsulated in carboxymethylcellulose (CMC) gel is suitable for the controlled release of herbicide. More perfection in this process has been attained by the addition of bentonite in the gel formulation by insinuating inorganic or organic cations in between of Na+ saturated bentonite for the slow release rate of herbicide in water and soil (Li et al. 2009).
Nowadays, clay-loaded hydrogels are also gaining more interest because of its unique properties and broad applications. Nano-composite-based hydrogels have been developed to release macro- and micronutrients in slow and controlled manner into independent or concurrent systems. Its physicochemical properties and water uptake capacity improved 5000 times more than its weight by producing nanocomposite by underwent hydrolysis treatment. These nanocomposites had great swelling degree, so they were formulated with high calcium montmorillonite (MMt) contents for agricultural applications (e.g. carriers for nutrient release) (Bortolin et al. 2016).
6 Patents on Hydrogels
As a result of high potential and tailor-made properties of hydrogels, researchers have obtained various patents in its various spheres of utilization. The recent available patents are listed in Table 4.
7 Conclusion
The hydrogel materials have a great ability to serve as response to stimuli materials. Hydrogels have immense hidden potential in various applications. They are successfully being used in biomedical field in adverse environmental conditions like low pH and high temperatures, in human metabolism. A large numbers of hydrogel materials have been investigated for controlled drug release for drug formulation purpose. This bio-compatible nature of hydrogels makes them a promising candidate for future applications as well as for the development of next generation of materials for biomedical application also environmental field.
A major weakness of all these hydrogels is the need for optimization of their response time, which is either too slow or too fast. These optimized hydrogels as per their response time are in huge demand for various emerging applications. Future hydrogels are in the process of development with perfect control over their structure–property relationships even at micro- and nano-levels. So, practically they are used as smart materials. This relationship is further expected to result in a new range of hydrogels which are specifically suited for an application and unlock a new era of human imagination.
References
Abebe DG, Fujiwara T (2012) Controlled thermoresponsive hydrogels by stereocomplexed PLA-PEG-PLA prepared via hybrid micelles of premixed copolymers with different PEG lengths. Biomacromol 13:1828–1836
Abraham GA, de Queiroz AAA, San RJS (2001) Hydrophilic hybrid IPNs of segmented polyurethanes and copolymers of vinyl pyrrolidone for applications in medicine. Biomaterials 22:1971–1985
Aimetti AA, Machen AJ, Anseth KS (2009) Poly (ethylene glycol) hydrogels formed by thiolene photopolymerization for enzyme-responsive protein delivery. Biomaterials 30(30):6048–6054
Alvarez LC, Concheiro A, Dubovik AS, Grinberg NV, Burova TV, Grinberg VY (2005) Temperature-sensitive chitosan-poly N-isopropylacrylamide interpenetrated networks with enhanced loading capacity and controlled release properties. J Control Release 102:629–641
Amiji M (1997) Gelatin-poly(ethylene oxide) semi interpenetrating polymer network with pH-sensitive swelling and enzyme-degradable properties for oral drug delivery. Drug Dev Ind Pharm 23:575–582
Aqua Source Inc (1993) United State Patent US 5185024
Arthur R, Barry JJ, Sahatjian R (2006) US Patent US706690
Benamer S, Mahlous M, Boukrif A, Mansouri B, Youcef SL (2006) Synthesis and characterisation of hydrogels based on poly(vinyl pyrrolidone). Nucl Instrum Methods Phy Res Section B: Beam Interact Mater Atoms 248(2):284–290
Bindu SM, Ashok V, Chatterjee A (2012) Review article as a review on hydrogels as drug delivery in the pharmaceutical field. Int J Pharm Chem Sci 1:642–661
Boonkaew B, Kempf M, Kimble R, Supaphol P, Cuttle L (2014a) Antimicrobial efficacy of a novel silver hydrogel dressing compared to two common silver burn wound dressings: Acticoat™ and PolyMem Silver®. Burns 40:89–96
Boonkaew B, Barber PM, Rengpipat S, Supaphol P, Kempf M, He J et al (2014b) Development and characterization of a novel antimicrobial, sterile hydrogel dressing for burn wounds: single-step production with gamma irradiation creates silver nanoparticles and radical polymerization. J Pharm Sci 103:3244–3253
Boppana R, Kulkarni RV, Mutalik SS, Biswanath S (2010) Interpenetrating network hydrogel beads of carboxymethylcellulose and egg albumin for controlled release of lipid lowering drug. J Microencap 27:337–344
Bortolin A, Serafim AR, Aouada FA, Mattoso LH, Ribeiro C (2016) Macro- and micronutrient simultaneous slow release from highly swellable nanocomposite hydrogels. J Agric Food Chem 64(16):3133–3140
Brandt KA, Goldman SA, Inglin TA, Procter and Gamble Co (1987) Hydrogel-forming polymer compositions for use in absorbent structures. U.S. Patent 4,654,039
Brown LR, Edelman ER, Fischel‐Ghodsian F, Langer R (1996) Characterization of glucose-mediated insulin release from implantable polymers. J Pharm Sci 85 (12):1341–1345
Bryant SJ, Nuttelman CR, Anseth KS (2000) Cytocompatibility of UV and visible light photo initiating systems on cultured NIH/3T3 fibroblasts in vitro. J Biomater Sci Polym Ed 11:439–457
Bullock AJ, Pickavance P, Haddow DB, Rimmer S, MacNeil S (2010) Development of a calcium-chelating hydrogel for treatment of superficial burns and scalds. Regen Med 5:55–64
Burd A (2007) Evaluating the use of hydrogel sheet dressings in comprehensive burn wound care. Ostomy Wound Manage 53:52–62
Buwalda SJ, Boere KW, Dijkstra PJ, Feijen J, Vermonden T (2014) Hydrogels in a historical perspective: from simple networks to smart materials. J Control Release 190:254–273
Cartmell JV, Sturtevant WR, Bausmith WE, Wolf ML (1995) US Patent US5423737
Chakavala SR, Patel NG, Pate NV, Thakkar VT, Patel KV, Gandhi TR (2012) Development and in vivo evaluation of silver sulfadiazine loaded hydrogel consisting of polyvinyl alcohol and chitosan for severe burns. J Pharm Bioallied Sci 4:S54–S56
Chang C, Duan B, Cai J, Zhang L (2010) Hydrogels based on cellulose for smart swelling and controllable delivery. Eur Polym J 46:92–100
Chapekar MS (2000) J Biomed Mater Res 53:617–620
Chivukula P, Dusek K, Wang D, Duskova SM, Kopeckova P, Kopecek J (2006) Synthesis and characterization of novel aromatic azo bond-containing pH-sensitive and hydrolytically cleavable IPN hydrogels. Biomaterials 27:1140–1151
Christensen L, Breiting V, Vuust J, Hogdall E (2006) Adverse reactions following injection with a permanent facial filler polyacrylamide hydrogel (aquamid): causes and treatment. Eur J Plast Surg 28:464–471
Chung HJ, Lee Y, Park TG (2008) Thermo-sensitive and biodegradable hydrogels based on stereocomplexed Pluronic multi-block copolymers for controlled protein delivery. J Control Release 127:22–30
Coats TJ, Edwards C, Newton R, Staun E (2002) The effect of gel burn dressings on skin temperature. Emerg Med J 19:224–225
Cook JP, Goodall GW, Khutoryanskaya OV, Khutoryanskiy VV (2012) Microwave‐assisted hydrogel synthesis: a new method for crosslinking polymers in aqueous solutions. Macromol Rapid Commun 33:332–336
Cretu A, Gattin R, Brachais L, Barbier-Baudry D (2004) Synthesis and degradation of poly(2-hydroxyethyl methacrylate)-graft-poly (ε-caprolactone) copolymers. Polym Degrad Stab 83:399–403
Cui F, Li G, Huang J, Zhang J, Lu M, Lu W (2011) Development of chitosan-collagen hydrogel incorporated with lysostaphin (CCHL) burn dressing with anti-methicillin-resistant Staphylococcus aureus and promotion wound healing properties. Drug Deliv 18:173–180
Cuttle L, Pearn J, McMillan JR, Kimble RM (2009) A review of first aid treatments for burn injuries. Burns 35:768–775
Damodaran S, Hwang DC (1998) United State Patent US 5847089
Derwent JJK, Mieler WF (2008) Thermoresponsive hydrogels as a new ocular drug delivery platform to the posterior segment of the eye. Trans Am Ophthalmol Soc 106:206–214
Drury JL, Mooney DJ (2003) Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 24(24):4337–4351
Ferreira L (2000) Evaluation of poly(2-hydroxyethyl methacrylate) gels as drug delivery systems at different pH values. Int J Pharm 194:169–180
Flory PJ (1942) Thermodynamics of high polymer solutions. J Chem Phys 10:51–61
Gaylord NG (1974) US Patent US3808178
Gemeinhart AR, Chen J, Park H, Park K (2000) pH sensitive of fast responsive superporous hydrogels. J Biomater Sci Plymer Edn 11(12):1371–1380
Ghadiri M, Chrzanowski W, Rohanizadeh R (2014) Antibiotic eluting clay mineral (Laponite®) for wound healing application: an in vitro study. J Mater Sci Mater Med 25(11):2513–2526
Gils PS, Ray D, Sahoo PK (2010) Designing of silver nanoparticles in gum arabic based semi-IPN hydrogel. Int J Biol Macromol 46:237–244
Gong CY, Shi S, Dong PW, Kan B, Gou ML, Wang XH (2009) Synthesis and characterization of PEG-PCL-PEG thermosensitive hydrogel. Int J Pharm 365:89–99
Grippaudo FR, Carini L, Baldini R (2010) Procutase versus 1% silver sulphadiazine in the treatment of minor burns. Burns 36:871–875
Grodzinski J (2009) Polymeric gels and hydrogels for biomedical and pharmaceutical application. Polym Adv Technol 21:27–47
Gunasekaran S, Chai WTC (2006) Swelling of pH-sensitive chitosan-poly(vinyl alcohol) hydrogels. J Appl Polym Sci 102:4665–4671
Hendriks BHW, Deladi S, Kurt R, Suijver JF (2012) United State Patent US 8244078
Huggins ML (1942) Some properties of solutions of long-chain compounds. J Phys Chem 46:151–158
Iizawa T, Taketa H, Maruta M, Ishido T, Gotoh T, Sakohara S (2007) Synthesis of porous poly(N-isopropylacrylamide) gel beads by sedimentation polymerization and their morphology. J Appl Polym Sci 104:842–850
Iordanov VP, Krijnsen HC, Van Bruggen MPB, Janner AM, Kurt R, Koninklijke Philips NV (2014) Hydrogel based device for detecting an environmental state. United State Patent 8,840,839
Irani M, Ismail H, Ahmad Z, Fan M (2015) Synthesis of linear low density polyethylene-g-poly(acrylic acid)-co starch/organomontmorillonite hydrogel composite as an adsorbent for removal of Pb(II) from aqueous solutions. J Environ Sci 27:9–20
Jen AC, Wake MC, Mikos AG (1996) Review: hydrogels for cell immobilization. Biotechnol Bioeng 50:357–364
Johannsmann D, Bunsow J (2008) Electrochemically produced responsive hydrogel films: influence of added salt on thickness and morphology. J Colloid Interface Sci 326:61–65
Joint I, Mühling M, Querellou J (2010) Culturing marine bacteria—an essential prerequisite for biodiscovery. Microb Biotechnol 3:564–575
Ju HK, Kim SY, Kim SJ, Lee YM (2002) pH/temperature-responsive semi-IPN hydrogels composed of alginate and poly(N-isopropylacrylamide). J Appl Polym Sci 83:1128–1139
Kaneko Y, Yoshida R, Sakai K, Sakurai Y, Okano T (1995) Temperature-responsive shrinking kinetics of poly(N-isopropylacrylamide) copolymer gels with hydrophilic and hydrophobic comonomers. J Membr Sci 101:13–22
Kaneko Y, Nakamura S, Sakai K, Aoyagi T, Kikuchi A, Sakurai Y, Okano T (1998) Rapid deswelling response of poly(N-isopropylacrylamide) hydrogels by the formation of water release channels using poly(ethylene oxide) graft chains. Macromolecules 31:6099–6105
Kellenberger SR (1992) Absorbent products containing hydrogels with ability to swell against pressure. U.S. Patent 5,147,343 issued September 15, 1992
Ketelson HA, Meadows DL, Stone RP (2005) Dynamic wettability properties of a soft contact lens hydrogel. Colloid Surf B. 40:1–9
Khutoryanskiy VV, Khutoryanskaya OV, Cook JP, Goodall GW (2013) US Patent 0018110
Kiil S, Dam-Johansen K (2003) Controlled drug delivery from swellable hydroxypropylmethyl cellulose matrices: model-based analysis of observed radial front movements. J Control Rel 90:1–21
Kim B, Peppas NA (2003) Poly(ethylene glycol)-containing hydrogels for oral protein delivery applications. Biomed Microdevices 5:333–341
Kirakci K, Šícha V, Holub J, Kubà P, Lang K (2014) Luminescent hydrogel particles prepared by self-assembly of Î2-cyclodextrin polymer and octahedral molybdenum cluster complexes. Inorg Chem 53:13012–13018
Kopecek J, Yang J (2009) Peptide directed self assembly of hygrogels. Acta Biomater 5:805–816
Kumar HGS, Satish CS, Satish KP (2006) Hydrogels as controlled drug delivery systems synthesis, crosslinking, water and drug transport mechanism. Ind J Pharm Sci 68:133–280
Kumar PT, Lakshmanan VK, Biswas R, Nair SV, Jayakumar R (2012a) Synthesis and biological evaluation of chitin hydrogel/nano ZnO composite bandage as antibacterial wound dressing. J Biomed Nanotechnol 8:891–900
Kumar PT, Lakshmanan VK, Anilkumar TV, Ramya C, Reshmi P, Unnikrishnan AG (2012b) Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: in vitro and in vivo evaluation. ACS Appl Mater Interfaces 4:2618–2629
Ladet S, David L, Domard A (2008) Multi-membrane hydrogels. Nature 452(7183):76–79
Langer R, Vacanti J (1993) Tissue engineering. Science 260:920–926
Langer R, Peppas NA (2003) Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J 49(12):2990–3006
Lee KY, Mooney DJ (2001) Chem Rev 101:1869–1880
Lee SD, Kim BS, Nguyen MK (2013) US Patent US8383153
Li S, Liu X (2008) Synthesis, characterization and evaluation of semi-IPN hydrogels consisted of poly(methacrylic acid) and guar gum for colon-specific drug delivery. Polym Adv Technol 19:371–376
Li Y, Zhou J (2007) United State Patent US 7304098
Li J, Jiang M, Wu H, Li Y (2009) Addition of modified bentonites in polymer gel formulation of 2,4-D for its controlled release in water and soil. J Agric Food Chem 57:2868–2874
Li S, Yajiang Y, Haibing Li, Xiangliang Y, Huibi Xu (2007) pH-responsive semi-interpenetrating networks hydrogels of poly(acrylic acid-acrylamide-methacrylate) and amylose. I. Synthesis and characterization. J Appl Polym Sci 106(6):3792–3799
Lim F, Sun AM (1980) Microencapsulated islets as bioartificial endocrine pancreas. Science 210:908–910
Lin CC, Metters AT (2006) Hydrogels in controlled release formulations: network design and mathematical modeling. Adv Drug Del Rev 58:1379–1408
Lipatov YS (2002) Polymer blends and interpenetrating polymer networks at the interface with solids. Prog Polym Sci 27:1721–1801
Liu M, Su H, Tan T (2012) Synthesis and properties of thermo and pH-sensitive poly(N-isopropylacrylamide)/polyaspartic acid IPN hydrogels. Carbohydr Polym 87:2425–2431
Loh QL, Choong C (2013) Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B: Rev 19(6):485–502
Lowman AM, Morishita M, Nagai T, Peppas NA (1998) US Patent WO1998043615
Lu G, Ling K, Zhao P, Xu Z, Deng C, Zheng H (2010a) A novel in situ-formed hydrogel wound dressing by the photocross-linking of a chitosan derivative. Wound Repair Regen 18:70–79
Lu S, Liu M, Ni B, Gao C (2010b) A novel pH- and thermo-sensitive PVP/CMC semi-IPN hydrogel: swelling, phase behavior, and drug release study. J Polym Sci Part B Polym Phys 48:1749–1756
Lum E, Golebiowski B, Gunn R, Babhoota M, Swarbrick H (2013) Corneal sensitivity with contact lenses of different mechanical properties. Optom Vis Sci 90(9):954–960
Ma PX (2004) Scaffolds for tissue fabrication. Mater Today 7:30–40
Masteikova R, Chalupova Z, Sklubalova Z (2003) Stimuli-sensitive hydrogels in controlled and sustained drug delivery. Medicina 39:19–24
Mather P, Wu J, Ren D, Hou S (2013) US Patent US8431151
Mathur AM, Moorjani SK, Scranton AB (1996) Methods for synthesis of hydrogel networks: a review. J Macromolecular Sci Part C: Polym Rev 36(2):405–430
Matricardi P, Pontoriero M, Coviello T, Casadei MA, Alhaique F (2008) In Situ Cross-Linkable Novel Alginate-Dextran Methacrylate IPN Hydrogels for Biomedical Applications: Mechanical Characterization and Drug Delivery Properties. Biomacromolecules 9(7):2014–2020
Mistry KK, Hussain AW, Beck PH, Palmer DV, Sales BR, Mint A (2013) European Patent EP2646002
Miyaji N, Iwama T, Gotoh M, Maruyama T, Kohda D (2015) European Patent EP2172475
Miyata T, Uragami T, Nakamae K (2002) Biomolecules sensitive hydrogels. Adv Drug Deliv Rev 54:79–98
Mohamadnia Z, Zohuriaan-Mehr MJ, Kabiri K, Jamshidi A, Mobedi H (2007) pH-sensitive IPN hydrogel beads of carrageenan-alginate for controlled drug delivery. J Bioact Compat Polym 22:342–356
Mohd ZR, Abu BZZ, Yusof N, Mohamed MN, Abdullah MN (2012) Gelam (Melaleuca spp.) honey based hydrogel as burn wound dressing. Evid Based Complement Alternat Med 2012:843025
Montoro SR, Medeiros SDF, Alves GM (2014) Chapter 10—nanostructured hydrogels. In: Nanostructured polymer blends. William Andrew, Elsevier, Oxford, pp 325–355
Murdan S (2003) Electro-responsive drug delivery from hydrogels. J Control Release 92:1–17
Murthy PSK, Murali Mohan Y, Varaprasad K, Sreedhar B, Mohana RK (2008) First successful design of semi-IPN hydrogel-silver nanocomposites: a facile approach for antibacterial application. J Colloid Interface Sci 318:217–224
Nalampang K, Panjakha R, Molloy R, Tighe BJ (2013) Structural effects in photopolymerized sodium AMPS hydrogels cross-linked with poly(ethylene glycol) diacrylate for use as burn dressings. J Biomater Sci Polym 24:1291–1304
Natesan S, Zamora DO, Wrice NL, Baer DG, Christy RJ (2013) Bilayer hydrogel with autologous stem cells derived from debrided human burn skin for improved skin regeneration. J Burn Care Res 34:18–30
Neefe CW (1984) US Patent US4472327
Nguyen K, West J (2002) Biomaterials. Photopolymerizable hydrogels for tissue engineering applications 23:4307
Nierzwicki W, Prins W (1975) Hydrogels of crosslinked poly(1-glyceryl methacrylate) and poly(2-hydroxypropyl methacrylamide). J Appl Poly Sci 19(7):1885–1892
Oh SB, Choi YK, Cho CS (2003) Thermoplastic hydrogel based on pentablock copolymer consisting of poly(γ-benzyl l-glutamate) and poloxamer. J Appl Polym Sci 88:2649–2656
Okay O, Ozturk V (2002) Temperature sensitive poly(N-t-butylacrylamide-co-acrylamide) hydrogels: synthesis and swelling behavior. Polymer 43:5017–5026
Okumura Y, Ito K (2001) The polyrotaxane gel: a topological gel by figure-of-eight cross-links. Adv Mater 13:485–487
Oliveira RN, Rouzé R, Quilty B, Alves GG, Soares GD, Thiré RM (2014) Mechanical properties and in vitro characterization of polyvinyl alcohol-nano-silver hydrogel wound dressings. Interface Focus 4:20130049
Omidian H, Qiu Y, Yang S, Kim D, Park H, Park K (2005) Purdue Research Foundation. Hydrogels having enhanced elasticity and mechanical strength properties. U.S. Patent No. 6,960,617
Ossipov DA, Hilborn J (2006) Macromolecules 39:1709
Pal K, Banthia AK, Majumdar DK (2009) Polymeric hydrogels: characterization and biomedical applications. A mini review. Des Monom Polym 12:197–220
Park K, Shalaby WSW, Park H (1993). Biodegradable hydrogels for drug delivery. Technomic
Peng XW, Zhong LX, Ren JL, Sun RC (2012) Highly effective adsorption of heavy metal ions from aqueous solutions by macroporous xylan-rich hemicelluloses-based hydrogel. J Agric Food Chem 60(15):3909–3916
Peppas N, Bures P (2000) Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm 50:27–46
Peppas NA, Lowman AM (1999) Hydrogels. In: Mathiowitz E (ed) Encyclopedia of controlled drug delivery. Wiley, New York, pp 397–418
Peppas NA, Hilt JZ, Khademhosseini A, Langer R (2006) Hydrogels in biology and medicine: from molecular principles to bio nanotechnology. Adv Mater 18:1345–1360
Ribeiro MP, Ana E, Daniela S, Patrícia B, Joaquim H, Catarina F, Jorge CS, João PB, Eduardo P, Paula C, Ilídio JC (2009) Development of a new chitosan hydrogel for wound dressing. Wound Repair Regeneration 17(6):817–824
Ribeiro MP, Morgado PI, Miguel SP, Coutinho P, Correia IJ (2013) Dextran-based hydrogel containing chitosan microparticles loaded with growth factors to be used in wound healing. Mater Sci Eng C Mater Biol Appl 33:2958–2966
Rosiak J, Ruciska-Rybus A, Pekala W (1989) United State Patent US Patent 4871490
Saleem MA, Azharuddin SK, Ali S, Patil CC (2012) Studies on different chitosan polyelectrolyte complex hydrogel for modified release of diltiazem hydrochlorid. Int J Pharm and Pharm Sci 2(4):64–67
Sannino A, Ambrosio L, Nicolasis L (2012) European Patent EP 2535359
Sawhney A, Pathak C (1994) Adhesion prevention. J Biomed Mat Res 28:831–838
Sawhney AS, Jarrett P, Bassett M, Blizzard C (2013) US Patent US8409606
Schurz J (1991) Rheology of polymer solutions of the network type. Prog Polym Sci 16:1–53
Sefton MV, May MH, Lahooti S, Babensee JE (2000) Making microencapsulation work: conformal coating, immobilization gels and in vivo performance. J Control Release 65:173–186
Şen M, Uzun C, Güven O (2000) Controlled release of terbinafine hydrochloride from pH sensitive poly(acrylamide/maleic acid) hydrogels. Int J Pharm 203(1–2):149–157
Segundo EP, Guerrero DQ, Cornejo BNZ, Rondero AG, Arzaluz MGN, Jose Manuel Cornejo-Bravo JMC (2008) Controlled release of model substances from pH-sensitive hydrogels. J Mex Chem Soc 52(4):272–278
Shibayama M (2012) Structure-mechanical property relationship of tough hydrogels. Soft Matter 8:8030–8038
Shivashankar M, Mandal BK (2012). A review on interpenetrating polymer network. Int J Pharm Pharm Sci 4(5):1–7
Sidney RS, Lousis LW, Gray JC (1993) US Patent US5185024
Song SS, Kim HH, Yi YW (1996) US Patent US5514380
Strain DE, Kennelly RG, Dittmar HR (1939) Methacrylate resins. Ind Eng Chem 31:382–387
Sun G, Zhang X, Shen YI, Sebastian R, Dickinson LE, Fox-Talbot K (2011) Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc Natl Acad Sci U S A 108:20976–20981
Thomas V, Yallapu MM, Sreedhar B, Bajpai SK (2007) A versatile strategy to fabricate hydrogel-silver nanocomposites and investigation of their antimicrobial activity. J Colloid Interface Sci 315:389–395
Turner DC, Steffen RB, Wildsmith C, Matiacio TA (2005) US patent 6861123
Wang S, Lu L, Yaszemski MJ (2016) Mayo Foundation for Medical Education. Photocrosslinkable poly (caprolactone fumarate). U.S. Patent 9,255,178
Wang M, Xu L, Hu H, Zhai M, Peng J, Nho Y, Li J, Wei G (2007) Radiation synthesis of PVP/CMC hydrogels as wound dressing. Nucl Instr Meth Phys Res B 265:385–389
Wei YS, Charlotte AEH (2014) Short to ultrashort peptide hydrogels for biomedical uses. Mater Today 17:381–388
Wichterle O, Lim D (1960) Hydrophilic gels for biological use. Nature 185:117–118
Williams DF (1990) Concise encyclopedia of medical and dental materials. Pergamon Press, Oxford, England (1990)
Winter GD (1962) Formation of the scab and the rate of re-epithelialisation in the skin of the young domestic pig. Nature 193:293–294
Yan H, Dai J, Yang Z, Yang H, Cheng R (2011) Enhanced and selective adsorption of copper(II) ions on surface carboxymethylated chitosan hydrogel beads. Chem Eng J 174:586–594
Yannas IV, Lee E, Orgill DP, Skrabut EM, Murphy GF (1989) Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci U S A 86:933–937
Yom-Tov O, Neufeld L, Seliktar D, Bianco-Peled H (2014) A novel design of injectable porous hydrogels with in situ pore formation. Acta Biomater 10:4236–4246
Yoshida R, Uchida K, Kaneko Y, Sakai K, Kikuchi A, Sakurai Y, Okano T (1995) Comb-type grafted hydrogels with rapid deswelling response to temperature changes. Nature 374:240–242
Zeng X, Jiang H (2008) Tunable liquid microlens actuated by infrared light-responsive hydrogel. Appl Phys Lett 93:151101–151103
Zhang YX, Wu FP, Li MZ, Wang EJ (2005) pH switching on-off semi-IPN hydrogel based on cross-linked poly (acrylamide-co-acrylic acid) and linear polyallyamine. Polymer 46:7695–7700
Zhang JT, Bhat R, Jandt KD (2009) Temperature-sensitive PVA/PNIPAAm semi-IPN hydrogels with enhanced responsive properties. Acta Biomater 5:488–497
Zhang L, Wang L, Guo B, Ma PX (2014) Cytocompatible injectable carboxymethyl chitosan/N-isopropylacrylamide hydrogels for localized drug delivery. Carbohydr Polym 103:110–118
Zohuriaan-Mehr MJ (2006) Super-absorbents. Iran Polym Soc, 2–4
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mishra, S., Rani, P., Sen, G., Dey, K.P. (2018). Preparation, Properties and Application of Hydrogels: A Review. In: Thakur, V., Thakur, M. (eds) Hydrogels. Gels Horizons: From Science to Smart Materials. Springer, Singapore. https://doi.org/10.1007/978-981-10-6077-9_6
Download citation
DOI: https://doi.org/10.1007/978-981-10-6077-9_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-6076-2
Online ISBN: 978-981-10-6077-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)