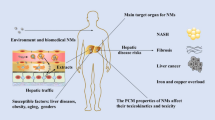
Abstract
The production, usage, and disposal of engineered nanomaterial (ENM)-based products inevitably increased their environmental accumulation and human exposures. Liver is the major organ for deposition of ENMs after their clearance from the circulation system. Accumulation of ENMs in liver may cause hepatic oxidative stress, inflammation, DNA damage, hepatocyte death, as well as liver fibrosis in healthy populations. In subpopulations with various liver diseases, such effects may be aggravated. Critical factors such as properties of ENMs, animal experimental protocols, and status of liver are discussed, as well as possible future directions.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
Liver is the major organ for xenobiotic chemicals metabolism and excretion. Liver injury induced by therapeutic drugs (troglitazone, bromfenac and pemoline for instance) and industrial chemicals (such as carbon tetrachloride and vinyl chloride) and the underlying mechanisms have been discussed [1,2,3,4,5]. With the global production, usage, and disposal of engineered nanomaterials (ENMs) and ENM-based products, the release of ENMs into the environment is inevitable. Therefore, ENMs have become a new environmental threat that may cause both acute and chronic hepatotoxicity.
12.1.1 Liver Function
Liver is a multifunctional organ involved in nutrient metabolism, proteins synthesis, hormone production, glycogen storage and release, and detoxification (Table 12.1). Blood coming from the stomach and the intestine flows through the liver prior to entering the systemic circulation. Therefore, liver is the first organ encountering absorbed nutrients. In liver, nutrients absorbed by intestines were metabolized into forms that can be utilized by human body. Liver also stores some nutrients such as vitamins (including vitamin A, vitamin D, and vitamin B12) and minerals (including iron and copper). Moreover, liver is the major organ involved in detoxification where toxic substances, such as drugs, alcohol, and environmental toxins, are broken down to less harmful or sometimes more toxic metabolites [6, 7].
12.1.2 Liver as the Major Organ for ENM Accumulation
As an important part of mononuclear phagocyte system, also known as reticuloendothelial system, liver is a major organ for xenobiotic ENMs accumulation and clearance. Gold NPs (Au NPs) can be accumulated in liver in a size-dependent manner. After intravenous (i.v.) injection into rats, about 50% of Au NPs with a diameter of 1.4 nm are accumulated in liver, while >99% of Au NPs with a diameter of 200 nm are accumulated [8]. Amorphous silica particles of 70 nm accumulate mainly in liver regardless of surface modifications [9]. Entering liver, ENMs may be taken up by hepatocytes or Kupffer cells. The selectivity may depend on the properties of the nanoparticles. For example, positively charged nanoparticles are accumulated more in hepatocytes, whereas negatively charged nanoparticles are rapidly taken up by Kupffer cells in liver sinusoids (Fig. 12.1) [10, 11]. Purposeful liver accumulation of nano-drug carrier or diagnostic agents may facilitate development of passive liver-targeting nanomedicines [12].
Imaging results for the liver tissues. a Optical and b quantitative LA-ICP-MS images of AuNP 1; c zoomed-in area illustrating the amount of AuNP 1 in a selected area of the liver tissue with a blood vessel, hepatoctyes, and Kupffer cells indicated in yellow-, white-, and black-dotted lines, respectively; d optical image after H&E staining of the same region shown in (c), indicating the blood vessel, hepatoctyes, and Kupffer cells in yellow-, white-, and black-dotted lines. e Optical and f quantitative LA-ICP-MS images of AuNP 2; g optical and h quantitative LA-ICP-MS images of AuNP 3; i optical and j quantitative LA-ICP-MS images of AuNP 4. All scale bars correspond to 0.5 mm. Reprinted with permission from [11]. Copyright (2016) American Chemical Society
Inert ENMs such as Au NPs, fullerenes, and carbon nanotubes (CNTs) can hardly be metabolized effectively [13]. Biliary route is a pathway for ENM excretion from the liver. ENMs in the liver that are not immediately internalized by Kupffer cells are translocated through the fenestrated vascular endothelium into the Dissé spaces where they are taken up by hepatocytes and processed into biliary canaliculi [14]. Then, ENMs are drained through the biliary duct and excreted in feces [15, 16].
Even though ENMs may be cleared from the liver via the biliary pathway, their long-term retention increases the risk of hepatotoxicity. For example, ENMs such as SWCNTs (a single i.v. injection of ~0.02 mg per mouse) [15] and superparamagnetic iron oxide nanoparticles (a single injection at a dose of 50 μmol/kg via a retro-orbitary route) [17] could still be detected in the liver of mouse models after 3 months.
12.2 ENM-Induced Hepatic Injuries in Healthy Population
Long-term ENM accumulation in the liver may affect its functions in various aspects as discussed below.
12.2.1 Hepatic Oxidative Stress
ENMs induce excess reactive oxygen species (ROS) via several mechanisms [18]. A low level of ROS in cells plays an important role in various physiological processes including antibacterial, anti-inflammatory, and tumor suppression. Meanwhile, excessive ROS could break the balance of the oxidant/antioxidant system in the liver, resulting in lipid peroxidation damage and hepatocyte toxicity.
Hepatic ROS level is reflected by malondialdehyde (MDA) activity, levels of several antioxidant enzymes such as glutathione peroxidase (GSHPx), catalase (CAT), and superoxide dismutase (SOD), as well as non-enzyme antioxidant, e.g., glutathione (GSH). In human liver cancer cells (HepG2), exposure of zinc oxide nanoparticles (ZnO NPs, 30 nm) [19], silver nanoparticles (Ag NPs, 5–10 nm) [20], silica nanoparticles (15 nm) [21], titanium dioxide nanoparticles (TiO2 NPs, 30–70 nm) [22], and single-walled carbon nanotubes (SWCNTs, 1–8 nm in diameter and 2000–6000 nm in length) [23] can induce oxidative cell damage, as indicated by increased ROS production, reduced level of GSH, increased MDA content, hydroperoxide level, as well as level of lipid peroxidation. Similar ENM-induced production of ROS was also observed in BRL 3A rat liver cells [24]. Ag NP (15 and 100 nm) exposure induces ROS-mediated cytotoxicity with depletion of GSH level and reduced mitochondrial membrane potential in these cells. In a murine hepatocyte cell line AML 12, cadmium telluride quantum dots (CdTe QDs) induce a dose-dependent generation of ROS. An antioxidant, tert-butyl Hydroquinone, can reduce intracellular ROS production [25].
ENM administration can also increase the production of ROS in liver tissues of animal models. For instance, subacute oral exposure of ZnO NPs (30 nm) for 14 consecutive days at a daily dose of 300 mg/kg induces hepatic oxidative stress in male Swiss albino mice, as indicated by an increase in lipid peroxidation [19]; Dermal exposure of TiO2 NPs (10 nm, 400 μg TiO2 per cm2) for 60 days caused oxidative stress-mediated liver injury with an increased MDA content and a reduced SOD activity in liver tissues of BALB/c hairless mice [26]; Intraperitoneal (i.p.) injection of TiO2 NPs (5 nm) daily for 14 days increases hepatic lipid peroxidation and O ·−2 and H2O2 generation, and reduced activities of hepatic antioxidative enzymes such as SOD, CAT, GSHPx, and ascorbic acid peroxidase (APx) in CD-1 (ICR) mice [27]; I.v. administration of Na-oleate-coated Fe3O4 nanoparticles (8 nm) at a dose equivalent to 10% of the LD50 (3.64 mg/kg) significantly increases hepatic mitochondrial respiration in female outbred Wistar rats [28].
On the contrast, several ENMs including fullerene [29, 30] and cerium oxide nanoparticles [31, 32] are able to efficiently scavenge ROS, protecting the liver from chemical-induced hepatic injury.
12.2.2 Liver Inflammation
The absorption and deposition of ENMs in the liver may stimulate the activation of immune responses. Besides, excessive ROS could activate ROS-sensitive pathways such as the mitogen-activated protein kinase (MAPK) and nuclear factor κB (NF-κB) cascades, resulting in pro-inflammatory responses [18].
Kupffer cells are resident liver macrophages responsible for removal of nanoparticles from the system [33]. They are major immune effector cells in the liver. Liver accumulation of ENMs activates Kupffer cells, resulting in hepatic inflammation. Single i.v. injection of silica NPs (15 nm) at a dose of 50 mg/kg caused Kupffer cell hyperplasia and hepatic inflammation in male Sprague Dawley (SD) rats [34]. A single i.v. administration of hydroxyapatite nanoparticles (HA NPs, needlelike, long diameter of 80 nm and short diameter of 20 nm) at a dose of 50 mg/kg induced oxidative stress-associated inflammatory cell infiltration at the portal area in the liver of SD rats [35]. Oral administration of Ag NPs (60 nm, 300 or 1000 mg/kg) for 28 days also induces hepatic inflammation in SD rats, as indicated by an increased level of alkaline phosphatase and infiltration of inflammatory cells in the liver [36].
Similar ENM-induced hepatic inflammation was also observed in mice. A single oral dose of Ag NPs (13 nm, 2.5 g per mouse) induced acute liver inflammation in male BALB/c mice, as evidenced by altered expression of genes related to inflammation and lymphocyte infiltration [37]. Anatase TiO2 NPs (21 nm) administrated (150 mg/kg/day) orally for 2 weeks triggered an inflammatory response in the liver of male albino mice, as indicated by the activation of Kupffer cells and increased production of TNF-α and interleukin-6 [38]. Moreover, these NPs upregulated the mRNA expression of Nrf2 and NF-κB. In female ICR mice, i.p. administration of Eu3+-doped gadolinium oxide nanotubes (Gd2O3:Eu3+ nanotubes, 400 mg/kg daily) for 35 days induced oxidative stress-related liver injury and increased the production of several inflammatory cytokines including TNF-α, interleukin-1β (IL-1β), and interleukin-8 (IL-8) [39]. In another study, intragastric administration of TiO2 NPs (5 nm, 5, 10, and 50 mg/kg) for 60 days increased the mRNA and protein levels of Toll-like receptor-2 (TLR2) and TLR-4, as well as several inflammatory cytokines, such as IKK1, IKK2, NF-κB, NF-κBP52, NF-κBP65, TNF-α, and NIK [40]. Meanwhile, TiO2 NPs exposure decreases the mRNA and protein expression of IκB and IL-2. This suggested a potential TiO2 NPs-stimulated TLRs- and NF-κB-mediated hepatic inflammation.
12.2.3 Hepatic DNA Damage
ENMs may impact DNA molecules directly by forming covalent linkages with them. By computational modeling, Al12X (X = Al, C, N, and P) nanoparticles are able to tightly bind to Watson–Crick DNA base pairs to form stable complexes, suggesting the potential adverse impacts of Al nanoparticles on the structure and stability of DNA [41]. I.p. injection of TiO2 NPs (5 nm) for 14 days resulted in a dose-dependent accumulation of NPs in the liver of ICR mice [42]. TiO2 NPs were able to bind DNA base pairs by interacting with three oxygen or nitrogen atoms and two phosphorous atoms on the DNA molecules. Lengths of Ti–O(N) and Ti–P bonds were 1.87 and 2.38 Å, respectively. The combination of TiO2 NPs with DNA altered the secondary structure of DNA, resulting in perturbations of genetic information transmission.
To induce genotoxicity, ENMs do not need to bind DNA directly. ENMs exposure may cause genotoxicity by interacting with nuclear proteins [44] or disturbing cell cycle checkpoint functions [45]. In most cases, ENM-induced oxidative stress leads to hepatic DNA damage indirectly (Fig. 12.2). ROS generated in cells may cause oxidative DNA damage through free radicals. For example, metal oxide NPs such as TiO2 NPs (30–70 nm) [22], ZnO NPs (30 nm) [19, 46], and nickel oxide nanoparticles (NiO NPs, 44 nm) [47] induce DNA damage in HepG2 cells through ROS generation. Furthermore, ZnO NPs [19], TiO2 NPs [48], CuO NPs [49], C60 fullerenes, and SWCNTs [50] induce oxidative DNA damage in the liver of rodents. Ag NPs (5–20 nm) administration induced hepatic DNA damage in adult zebrafish [51].
Indirect mechanisms that can lead to genotoxicity. Nanomaterials may result in oxidative stress or inflammatory responses that in turn have the potential to damage DNA and alter transcriptional patterns. Reprinted with permission from [43]. Copyright © 2009 Elsevier Ltd.
12.2.4 Hepatocyte Death
Death of hepatocytes typically follows one of two patterns: necrosis and apoptosis [52]. Necrosis is the consequence of acute metabolic injury with ATP depletion. ENMs could induce necrotic cell death of hepatocytes both in vitro and in vivo. For instance, ZnO NPs (47–106 nm) increase the number of necrotic catfish primary hepatocytes in a dose-dependent manner [53]; Chitosan nanoparticle (18 nm) exposure induces a dose-dependent increase in CYP3A4 enzyme activity and necrotic or autophagic cell death of human liver cells [54]; Administration of several ENMs including MWCNTs (20–30 nm in diameter, 5–50 μm in length) [55], Ag NPs (<100 nm) [56], Au NPs (10 and 20 nm) [57], TiO2 NPs (42 nm) [58], and silica NPs (20 and 80 nm) [59] all induce hepatocyte necrosis in livers of various animal models.
Apoptosis, on the other hand, is a form of programmed cell death. Apoptosis is initiated through one of the two main pathways: the extrinsic pathway (death receptor pathway) or the intrinsic pathway (mitochondrial pathway) [60]. ENM-induced apoptosis in hepatocytes is more likely through a mitochondrial pathway. For example, ENMs, such as copper oxide NPs (CuO NPs, 22 nm) [61], silica NPs (15 nm) [21], HA NPs (26, 45 and 78 nm) [62], CdTe QDs (2.2 nm) [25], and Ag NPs (5–10 nm) [20], can induce mitochondria-dependent apoptosis of hepatocytes, as shown by upregulated expression of apoptotic genes (caspase-3, p53, Bax, Bid, p21, etc.), downregulated expression of anti-apoptotic gene (Bcl-2), decreased mitochondrial membrane potential, and the release of cytochrome c from mitochondria into cytoplasm. Ag NPs (5–20 nm) induce oxidative stress and apoptosis in the liver of adult zebrafish by the upregulation of p53-related pro-apoptotic genes Bax, Noxa, and p21 [51].
12.2.5 Hepatic Fibrosis
Hepatic fibrosis is an excessive deposition of extracellular matrix (ECM) proteins including glycoproteins, collagen, and proteoglycans [63]. It is a response of the liver to chronic damages. The cellular mechanisms of liver fibrosis are highly complex and sophisticated, with several different hepatic cell types involved. ENMs can induce liver fibrosis in mouse models. Repeated i.v. injection of silica NPs (70 nm, 10 or 30 mg/kg) twice a week for 4 weeks causes hepatic fibrosis in male BALB/c mice, as evidenced by elevated hepatic hydroxyproline levels 1.6- or 3.5-fold over the control value [64]. Significant hepatic fibrosis around the central vein in ICR mice can be observed 7 days after a single i.p. injection of TiO2 NPs (100 nm) at a dose of 1944 mg/kg [65]. When female ICR mice are i.p. injected with silica NPs (110 nm, 10, 25, and 50 mg/kg) twice a week for 6 weeks [66], a dose-dependent hepatocyte fibrosis and collagen fibers accumulation around silicotic nodular-like lesions in the liver will occur. Activation of Kupffer cells may play a key role in these injuries.
In brief, various ENMs are able to cause hepatic DNA damage, necrosis, apoptosis of hepatocytes, and liver fibrosis. ENMs induce liver injuries by producing ROS and activating pro-inflammatory responses in the liver (Fig. 12.3). Excess ROS and pro-inflammatory cytokines disturb the oxidant/antioxidant equilibrium as well as the balance between pro-inflammatory and anti-inflammatory cytokines, resulting in hepatic toxicity.
12.3 ENM-Induced Hepatic Injuries in Diseased Populations
Liver diseases of both acute and chronic nature are common and the rate of liver diseases is steadily increasing over the years. Liver diseases are recognized as the fifth most common cause of death in the UK [42]. They are ranked as the second leading cause of death (after colorectal cancer) among all digestive diseases in the US [67]. In China, liver diseases affect approximately 300 million people, exerting a significant impact on the global burden of liver diseases [68]. The three major aetiologies of liver failure are nonalcoholic fatty liver disease (NAFLD), viral hepatitis, and alcoholic liver disease. It has been well known that populations with liver diseases were more susceptible to environmental pollutants [69, 70]. The potentially more severe toxicity of ENMs to hepatic diseased population needs to be addressed.
12.3.1 Chemical-Induced Hepatitis Animal Models
Hepatitis is an inflammation of the liver caused by virus, drugs, or other factors. Animal models of both acute and chronic hepatitis have been established for the investigation of the pathogenesis of liver disease and development of novel diagnostic, and therapeutic tools [71, 72]. Several hepatitis animal models were used to study the toxicity of ENMs. These models include concanavalin A (ConA)-induced hepatic injury model [73], carbon tetrachloride (CCl4)-induced hepatitis animal model [32], monocrotaline-induced liver injury model [31], and alcohol-induced liver injury model [30].
Gold nanorods (AuNR) exposure at a dose showing no toxicity in healthy mice (12 μg/kg body weight via i.v. administration) causes exacerbated liver damage by inducing pre-activation of hepatic macrophages in ConA-induced acute C57BL/6 hepatitis mouse model [73]. Meanwhile, such AuNR shows no effects on liver scarring or termed fibrosis in CCl4-induced chronic hepatic injury model.
As mentioned above, some ENMs exhibit efficient ROS scavenging. They may protect the liver from ROS-related liver injury. For example, C60 accumulated in the liver of Wistar rats efficiently scavenges radicals including CCl ·3 and CCl3OO· and improved the antioxidant status of rats [29]. As a result, C60 protects liver in a dose-dependent manner against CCl4-induced free-radical damage. C60 nanoparticles also exhibit potential hepatoprotective effects against alcohol-induced liver injuries by scavenging intracellular ROS induced by ethanol [30]. Similarly, cerium oxide nanoparticles also protect the liver from CCl4- [32] or monocrotaline-induced [31] liver injury by reducing oxidative stress in vivo. Besides, i.v. injected graphene quantum dots (40 nm, 50 mg/kg) are accumulated in the liver and reduced Con A-induced mouse hepatitis by interfering with T cell and macrophage activation [74].
12.3.2 Diet-Induced Fatty Liver Animal Models
Among various liver diseases, the most prevalent liver condition is fatty liver disease, which affects one-third of the population in the United States [75]. I.v. injection of Au NPs (5 mg/kg) in methionine- and choline-deficient diet-fed mice results in a higher level of liver injury, as evidenced by elevated the serum ALT and AST levels, severe hepatic cell damage, acute inflammation, and increased apoptosis and ROS production, compared to that in mice fed a normal chow diet [76]. In a high-fat diet (HFD)-induced overweight mouse model, HFD feeding results in hepatic steatosis. Oral administration of Ag NPs (30 nm) daily for 14 days at a dose (300 mg/kg) showing no toxicity to normal weight mice aggravates the progression of fatty liver disease in overweight mice, as evidenced by focal inflammation, hydropic degeneration, and enhanced steatosis [77]. Ag NPs, rather than Ag+ ions, are responsible for such a disease progression because Ag+ ions are reduced to Ag NPs in the liver of overweight mice and the liver doses of Ag NPs, not Ag ions are correlated to the toxic effects. Further mechanistic study reveals that pro-inflammatory activation of Kupffer cells and suppression of fatty acid oxidation play critical roles in the Ag NP-induced fatty liver disease progression. Meanwhile, orally co-exposure of ZnO NPs (14 or 58 nm, 200 mg/kg) and Pb2+ (150 mg/kg) for 14 days increases the Pb deposition in various organs (the liver, the kidneys, and the spleen) in overweight mice, compared to that in normal weight mice [78]. The ZnO NP-enhanced liver deposition of Pb causes hepatic ROS and increases the release of pro-inflammatory cytokines, resulting in enhanced liver injury in overweight mice.
ROS and inflammation play vital roles in the progression of various liver diseases including alcoholic liver disease, nonalcoholic fatty liver disease, virus hepatitis, and cirrhosis [79, 80]. When the liver is under diseased conditions, it is more sensitive to environmental toxicants including ENMs. ENM-induced additional oxidative stress and inflammation would definitely aggravate the disease progression.
12.4 Critical Factors in ENM-Induced Liver Injuries
ENMs can cause hepatic injuries in both healthy population and population with liver diseases. Even though our understanding of ENMs-induced liver injury is incomplete, several factors are of obvious importance, according to recent studies.
12.4.1 Physicochemical Properties of ENMs
Physicochemical properties of ENMs determine what the cells see and how the cells interact with them. These properties mainly refer to the primary characters of an ENM including composition [49], crystal structure [81], size [82], shape [83], surface coating [84], and agglomeration state [85], which further determine their secondary characters (protein adsorption and in situ characterization in cells or tissues, etc.). Physicochemical properties of ENMs not only affect their cellular uptake and sub-organ distribution and clearance [11], but also altered their hepatic toxicity. For example, surface chemical modifications are able to alter the interaction between CNTs and CYP450 enzymes in human liver microsomes, resulting in various perturbations on CYP3A4 (Fig. 12.4) [86]. Certain surface chemical structures responsible for inducing the inhibitory effects of CNTs are identified using a cheminformatics analysis.
CYP3A4 activity is modulated by f-MWCNTs from a combinatorial MWCNT library. The CYP3A4 activity in the HLM-only group was defined as 100%, and that in the ketoconazole group was defined as 0%. The activity of CYP3A4 in f-MWCNT-treated groups was calculated according to the following equation: f-MWCNT’s effect on CYP3A4 activity = (peak area of NFP in ketoconazole group—peak area of NFP in f-MWCNT group)/(peak area of NFP in ketoconazole group—peak area of NFP in HLM-only group). Reprinted with permission from [86]. Copyright © 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
12.4.2 Administration Procedures
Various administration methods such as dermal [26], intragastric (or oral) [40], i.v. [87], i.p. [66], or respiratory [88] exposure routes have been used in nanotoxicity investigations, mimicing the different ways of human exposures. Administration routes influence the amount of absorption and deposition of ENMs in animal models, resulting in different toxicities. For instance, the blood Ag content in lactating mice i.v. administrated with Ag NPs is twice as high as that exposed orally, even though the dose in the former is only 15% of the latter [89]. I.p. exposure causes greater hepatic accumulation of Ag NPs than i.v. administration, resulting in disordered hepatic cord alignment and enlarged central veins in livers of BALB/c mice [90]. Dosing and duration of exposure, on the other hand, are more direct factors determining ENM-induced hepatic injuries. In rat models, oral administration of Ag NPs at a dose >125 mg/kg for 90 days [91], or >300 mg/kg for 28 days [36] induces slight liver damages. Meanwhile, Ag NPs administration causes no hepatotoxicity or immunotoxicity after orally exposed for 28 days at a dose of 90 mg/kg [92].
12.4.3 Physiology of the Liver
The liver is the major organ for metabolism of xenobiotic chemicals and detoxification. A good redox and immunological status of the liver is essential for the operation of normal functions. Knowing that populations with hepatic diseases may be more sensitive to ENM exposures, hepatic toxicity of ENMs to aged population characterized by reduced metabolic activity, oxidative stress, and chronic inflammation is reported recently. Oral administration of ZnO NPs at 300 mg/kg for 14 days increases the liver accumulation of Zn in aged mice, which may be mainly attributed to the increased intestinal permeability and decreased hepatic metabolic capability [93]. Together with higher levels of oxidative stress and inflammation in livers of aged mice, ZnO NPs cause additional hepatic oxidative stress and inflammation and result in acute liver injury.
12.5 Concluding Remarks
In conclusion, ENMs can induce hepatic ROS and inflammation, resulting in hepatotoxicity in both healthy and diseased populations. Generally, populations with liver diseases are more susceptible to nanotoxicity because the additional ROS and inflammation induced by ENMs accelerate the progression of various liver diseases. Relating factors include the properties of the ENMs, amounts of liver deposition of ENMs, and the physiological state of the liver.
Investigations on hepatic nanotoxicity still need to be continued and intensified. A better characterization of ENMs is crucial. Both primary (composition, crystal structure, size, shape, surface coating, agglomeration state, etc.) and secondary (protein adsorption and in situ characterization in cells or tissues) characterizations of tested ENMs are very important for toxicity investigations. Hepatic toxicity assessment of ENMs should mimic the environmental exposure scenarios. Current studies use much higher doses of ENMs and quite different exposure periods. Therefore, low-dose and long-term exposures of ENMs will be much more helpful for the determination of the hepatic toxicity of ENMs. Furthermore, only limited studies have been carried out on ENM-induced hepatic injuries in diseased populations. Such understandings are very important for the safe applications of nanomaterial-based therapy and products. Toxicological data should be analyzed by computational and modeling approaches to uncover the general regularity of ENM-induced hepatic injuries. This will also guide ENMs’ safe applications in advanced biomedical fields, as well as many other applications.
References
Kaplowitz N (2005) Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov 4(6):489–499
Lee WM (2003) Medical progress: drug-induced hepatotoxicity. N Engl J Med 349(5):474–485
Navarro VJ, Senior JR (2006) Current concepts—drug-related hepatotoxicity. N Engl J Med 354(7):731–739
Recknagel RO (1967) Carbon tetrachloride hepatotoxicity. Pharmacol Rev 19(2):145–208
Thomas LB, Popper H, Berk PD, Selikoff I, Falk H (1975) Vinyl-chloride-induced liver disease. From idiopathic portal hypertension (Banti’s syndrome) to Angiosarcomas. N Engl J Med 292(1):17–22
Percival M (1997) Phytonutrients and detoxification. Clin Nutr Insights 5(2):1–4
Vander A, Sherman J, Luciano D (1994) Nonimmune metabolism of foreign chemicals. Hum Physiol 738–740
Hirn S, Semmler-Behnke M, Schleh C, Wenk A, Lipka J, Schäffler M, Takenaka S, Möller W, Schmid G, Simon U (2011) Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. Eur J Pharm Biopharm 77(3):407–416
Lu X, Ji C, Jin T, Fan X (2015) The effects of size and surface modification of amorphous silica particles on biodistribution and liver metabolism in mice. Nanotechnology 26(17):175101
Cheng S-H, Li F-C, Souris JS, Yang C-S, Tseng F-G, Lee H-S, Chen C-T, Dong C-Y, Lo L-W (2012) Visualizing dynamics of sub-hepatic distribution of nanoparticles using intravital multiphoton fluorescence microscopy. ACS Nano 6(5):4122–4131
Elci SG, Jiang Y, Yan B, Kim ST, Saha K, Moyano DF, Yesilbag Tonga G, Jackson LC, Rotello VM, Vachet RW (2016) Surface charge controls the sub-organ biodistributions of gold nanoparticles. ACS nano
Wang H, Thorling CA, Liang X, Bridle KR, Grice JE, Zhu Y, Crawford DH, Xu ZP, Liu X, Roberts MS (2015) Diagnostic imaging and therapeutic application of nanoparticles targeting the liver. J Mater Chem B 3(6):939–958
Hamidi M, Azadi A, Rafiei P, Ashrafi H (2013) A pharmacokinetic overview of nanotechnology-based drug delivery systems: an ADME-oriented approach. Critical Reviews™ in Therapeutic Drug Carrier Systems 30(5)
Johnston HJ, Hutchison G, Christensen FM, Peters S, Hankin S, Stone V (2010) A review of the in vivo and in vitro toxicity of silver and gold particulates: particle attributes and biological mechanisms responsible for the observed toxicity. Crit Rev Toxicol 40(4):328–346
Liu Z, Davis C, Cai W, He L, Chen X, Dai H (2008) Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc Natl Acad Sci USA 105(5):1410–1415
Wang H, Wang J, Deng X, Sun H, Shi Z, Gu Z, Liu Y, Zhaoc Y (2004) Biodistribution of carbon single-wall carbon nanotubes in mice. J Nanosci Nanotechnol 4(8):1019–1024
Levy M, Luciani N, Alloyeau D, Elgrabli D, Deveaux V, Pechoux C, Chat S, Wang G, Vats N, Gendron F, Factor C, Lotersztajn S, Luciani A, Wilhelm C, Gazeau F (2011) Long term in vivo biotransformation of iron oxide nanoparticles. Biomaterials 32(16):3988–3999
Nel A, Xia T, Madler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311(5761):622–627
Sharma V, Anderson D, Dhawan A (2012) Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis 17(8):852–870
Piao MJ, Kang KA, Lee IK, Kim HS, Kim S, Choi JY, Choi J, Hyun JW (2011) Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol Lett 201(1):92–100
Ahmad J, Ahamed M, Akhtar MJ, Alrokayan SA, Siddiqui MA, Musarrat J, Al-Khedhairy AA (2012) Apoptosis induction by silica nanoparticles mediated through reactive oxygen species in human liver cell line HepG2. Toxicol Appl Pharmacol 259(2):160–168
Shukla RK, Kumar A, Gurbani D, Pandey AK, Singh S, Dhawan A (2013) TiO2 nanoparticles induce oxidative DNA damage and apoptosis in human liver cells. Nanotoxicology 7(1):48–60
Yuan J, Gao H, Sui J, Chen WN, Ching CB (2011) Cytotoxicity of single-walled carbon nanotubes on human hepatoma HepG2 cells: an iTRAQ-coupled 2D LC–MS/MS proteome analysis. Toxicol In Vitro 25(8):1820–1827
Hussain SM, Hess KL, Gearhart JM, Geiss KT, Schlager JJ (2005) In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol In Vitro 19(7):975–983
Zhang T, Hu Y, Tang M, Kong L, Ying J, Wu T, Xue Y, Pu Y (2015) Liver toxicity of cadmium telluride quantum dots (CdTe QDs) due to oxidative stress in vitro and in vivo. Int J Mol Sci 16(10):23279–23299
Wu J, Liu W, Xue C, Zhou S, Lan F, Bi L, Xu H, Yang X, Zeng F-D (2009) Toxicity and penetration of TiO2 nanoparticles in hairless mice and porcine skin after subchronic dermal exposure. Toxicol Lett 191(1):1–8
Liu H, Ma L, Liu J, Zhao J, Yan J, Hong F (2010) Toxicity of nano-anatase TiO2 to mice: liver injury, oxidative stress. Toxicol Environ Chem 92(1):175–186
Volkovova K, Handy RD, Staruchova M, Tulinska J, Kebis A, Pribojova J, Ulicna O, Kucharská J, Dusinska M (2015) Health effects of selected nanoparticles in vivo: liver function and hepatotoxicity following intravenous injection of titanium dioxide and Na-oleate-coated iron oxide nanoparticles in rodents. Nanotoxicology 9(sup1):95–105
Gharbi N, Pressac M, Hadchouel M, Szwarc H, Wilson SR, Moussa F (2005) [60] fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett 5(12):2578–2585
Li Y, Luo HB, Zhang HY, Guo Q, Yao HC, Li JQ, Chang Q, Yang JG, Wang F, Wang CD, Yang X, Liu ZG, Ye X (2016) Potential hepatoprotective effects of fullerenol nanoparticles on alcohol-induced oxidative stress by ROS. RSC Adv 6(37):31122–31130
Amin KA, Hassan MS, Awad E-ST, Hashem KS (2011) The protective effects of cerium oxide nanoparticles against hepatic oxidative damage induced by monocrotaline. Int J Nanomed 6:143–149
Hirst SM, Karakoti A, Singh S, Self W, Tyler R, Seal S, Reilly CM (2013) Bio-distribution and in vivo antioxidant effects of cerium oxide nanoparticles in mice. Environ Toxicol 28(2):107–118
Sadauskas E, Wallin H, Stoltenberg M, Vogel U, Doering P, Larsen A, Danscher G (2007) Kupffer cells are central in the removal of nanoparticles from the organism. Part Fibre Toxicol 4(1):10
Chen Q, Xue Y, Sun J (2013) Kupffer cell-mediated hepatic injury induced by silica nanoparticles in vitro and in vivo. Int J Nanomed 8:1129–1140
Chen Q, Xue Y, Sun J (2014) Hepatotoxicity and liver injury induced by hydroxyapatite nanoparticles. J Appl Toxicol 34(11):1256–1264
Kim YS, Kim JS, Cho HS, Rha DS, Kim JM, Park JD, Choi BS, Lim R, Chang HK, Chung YH (2008) Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague–Dawley rats. Inhalation Toxicol 20(6):575–583
Cha K, Hong H-W, Choi Y-G, Lee MJ, Park JH, Chae H-K, Ryu G, Myung H (2008) Comparison of acute responses of mice livers to short-term exposure to nano-sized or micro-sized silver particles. Biotechnol Lett 30(11):1893–1899
Azim SAA, Darwish HA, Rizk MZ, Ali SA, Kadry MO (2015) Amelioration of titanium dioxide nanoparticles-induced liver injury in mice: possible role of some antioxidants. Exp Toxicol Pathol 67(4):305–314
Liu H, Jia G, Chen S, Ma H, Zhao Y, Wang J, Zhang C, Wang S, Zhang J (2015) In vivo biodistribution and toxicity of Gd2O3: Eu3+ nanotubes in mice after intraperitoneal injection. RSC Adv 5(90):73601–73611
Cui Y, Liu H, Zhou M, Duan Y, Li N, Gong X, Hu R, Hong M, Hong F (2011) Signaling pathway of inflammatory responses in the mouse liver caused by TiO2 nanoparticles. J Biomed Mater Res, Part A 96(1):221–229
Jin P, Chen Y, Zhang SB, Chen Z (2012) Interactions between Al12X (X = Al, C, N and P) nanoparticles and DNA nucleobases/base pairs: implications for nanotoxicity. J Mol Model 18(2):559–568
Li N, Ma L, Wang J, Zheng L, Liu J, Duan Y, Liu H, Zhao X, Wang S, Wang H (2009) Interaction between nano-anatase TiO2 and liver DNA from mice in vivo. Nanoscale Res Lett 5(1):108
Singh N, Manshian B, Jenkins GJS, Griffiths SM, Williams PM, Maffeis TGG, Wright CJ, Doak SH (2009) NanoGenotoxicology: the DNA damaging potential of engineered nanomaterials. Biomaterials 30(23–24):3891–3914
Baweja L, Gurbani D, Shanker R, Pandey AK, Subramanian V, Dhawan A (2011) C60-fullerene binds with the ATP binding domain of human DNA topoiosmerase II alpha. J Biomed Nanotechnol 7(1):177–178
Huang S, Chueh PJ, Lin Y-W, Shih T-S, Chuang S-M (2009) Disturbed mitotic progression and genome segregation are involved in cell transformation mediated by nano-TiO2 long-term exposure. Toxicol Appl Pharmacol 241(2):182–194
Sharma V, Anderson D, Dhawan A (2011) Zinc oxide nanoparticles induce oxidative stress and genotoxicity in human liver cells (HepG2). J Biomed Nanotechnol 7(1):98–99
Ahamed M, Ali D, Alhadlaq HA, Akhtar MJ (2013) Nickel oxide nanoparticles exert cytotoxicity via oxidative stress and induce apoptotic response in human liver cells (HepG2). Chemosphere 93(10):2514–2522
Trouiller B, Reliene R, Westbrook A, Solaimani P, Schiestl RH (2009) Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res 69(22):8784–8789
Song M-F, Li Y-S, Kasai H, Kawai K (2012) Metal nanoparticle-induced micronuclei and oxidative DNA damage in mice. J Clin Biochem Nutr 50(3):211–216
Folkmann JK, Risom L, Jacobsen NR, Wallin H, Loft S, Møller P (2009) Oxidatively damaged DNA in rats exposed by oral gavage to C60 fullerenes and single-walled carbon nanotubes. Environ Health Perspect 117(5):703
Choi JE, Kim S, Ahn JH, Youn P, Kang JS, Park K, Yi J, Ryu D-Y (2010) Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish. Aquat Toxicol 100(2):151–159
Malhi H, Gores GJ, Lemasters JJ (2006) Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology 43(S1)
Wang Y, Aker WG, H-m H, Yedjou CG, Yu H, Tchounwou PB (2011) A study of the mechanism of in vitro cytotoxicity of metal oxide nanoparticles using catfish primary hepatocytes and human HepG2 cells. Sci Total Environ 409(22):4753–4762
Loh JW, Yeoh G, Saunders M, Lim L-Y (2010) Uptake and cytotoxicity of chitosan nanoparticles in human liver cells. Toxicol Appl Pharmacol 249(2):148–157
Awasthi KK, John PJ, Awasthi A, Awasthi K (2013) Multi walled carbon nano tubes induced hepatotoxicity in Swiss albino mice. Micron 44:359–364
Korani M, Rezayat S, Gilani K, Bidgoli SA, Adeli S (2011) Acute and subchronic dermal toxicity of nanosilver in guinea pig. Int J Nanomed 6(1):855–862
Abdelhalim MAK, Jarrar BM (2011) Gold nanoparticles induced cloudy swelling to hydropic degeneration, cytoplasmic hyaline vacuolation, polymorphism, binucleation, karyopyknosis, karyolysis, karyorrhexis and necrosis in the liver. Lipids Health Dis 10(1):166
Xu J, Shi H, Ruth M, Yu H, Lazar L, Zou B, Yang C, Wu A, Zhao J (2013) Acute toxicity of intravenously administered titanium dioxide nanoparticles in mice. PLoS ONE 8(8):e70618
Xie G, Sun J, Zhong G, Shi L, Zhang D (2010) Biodistribution and toxicity of intravenously administered silica nanoparticles in mice. Arch Toxicol 84(3):183–190
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516
Siddiqui MA, Alhadlaq HA, Ahmad J, Al-Khedhairy AA, Musarrat J, Ahamed M (2013) Copper oxide nanoparticles induced mitochondria mediated apoptosis in human hepatocarcinoma cells. PLoS ONE 8(8):e69534
Yuan Y, Liu C, Qian J, Wang J, Zhang Y (2010) Size-mediated cytotoxicity and apoptosis of hydroxyapatite nanoparticles in human hepatoma HepG2 cells. Biomaterials 31(4):730–740
Bataller R, Brenner DA (2005) Liver fibrosis. J Clin Invest 115(2):209–218
Nishimori H, Kondoh M, Isoda K, S-i T, Tsutsumi Y, Yagi K (2009) Silica nanoparticles as hepatotoxicants. Eur J Pharm Biopharm 72(3):496–501
Chen J, Dong X, Zhao J, Tang G (2009) In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitioneal injection. J Appl Toxicol 29(4):330–337
Liu T, Li L, Fu C, Liu H, Chen D, Tang F (2012) Pathological mechanisms of liver injury caused by continuous intraperitoneal injection of silica nanoparticles. Biomaterials 33(7):2399–2407
Everhart JE, Ruhl CE (2009) Burden of digestive diseases in the United States part III: liver, biliary tract, and pancreas. Gastroenterology 136(4):1134–1144
Wang F-S, Fan J-G, Zhang Z, Gao B, Wang H-Y (2014) The global burden of liver disease: the major impact of China. Hepatology 60(6):2099–2108
Ruzzin J, Petersen R, Meugnier E, Madsen L, Lock E-J, Lillefosse H, Ma T, Pesenti S, Sonne SB, Marstrand TT (2010) Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ Health Perspect 118(4):465
Wahlang B, Falkner KC, Gregory B, Ansert D, Young D, Conklin DJ, Bhatnagar A, McClain CJ, Cave M (2013) Polychlorinated biphenyl 153 is a diet-dependent obesogen that worsens nonalcoholic fatty liver disease in male C57BL6/J mice. J Nutr Biochem 24(9):1587–1595
Rahman TM, Hodgson HJF (2000) Animal models of acute hepatic failure. Int J Exp Pathol 81(2):145–157
Liu Y, Meyer C, Xu C, Weng H, Hellerbrand C, ten Dijke P, Dooley S (2013) Animal models of chronic liver diseases. Am J Physiol Gastrointest Liver Physiol 304(5):G449
Bartneck M, Ritz T, Keul HA, Wambach M, Jr B, Gbureck U, Ehling J, Lammers T, Heymann F, Gassler N (2012) Peptide-functionalized gold nanorods increase liver injury in hepatitis. ACS Nano 6(10):8767–8777
Volarevic V, Paunovic V, Markovic Z, Simovic Markovic B, Misirkic-Marjanovic M, Todorovic-Markovic B, Bojic S, Vucicevic L, Jovanovic S, Arsenijevic N (2014) Large graphene quantum dots alleviate immune-mediated liver damage. ACS Nano 8(12):12098–12109
Ogden CL, Carroll MD, Kit BK, Flegal KM (2014) Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 311(8):806–814
Hwang JH, Kim SJ, Kim Y-H, Noh J-R, Gang G-T, Chung BH, Song NW, Lee C-H (2012) Susceptibility to gold nanoparticle-induced hepatotoxicity is enhanced in a mouse model of nonalcoholic steatohepatitis. Toxicology 294(1):27–35
Jia J, Li F, Zhou H, Bai Y, Liu S, Jiang Y, Jiang G, Yan B (2017) Oral exposure to silver nanoparticles or silver ions may aggravate fatty liver disease in overweight mice. Environ Sci Technol
Jia J, Li F, Zhai S, Zhou H, Liu S, Jiang G, Yan B (2017) Susceptibility of overweight mice to liver injury as a result of the ZnO nanoparticle-enhanced liver deposition of Pb2+. Environ Sci Technol 51(3):1775–1784
Cichoż-Lach H, Michalak A (2014) Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol: WJG 20(25):8082–8091
Dirchwolf M, Ruf AE (2015) Role of systemic inflammation in cirrhosis: from pathogenesis to prognosis. World J Hepatol 7(16):1974–1981
PetkoviĆ J, Žegura B, StevanoviĆ M, Drnovšek N, UskokoviĆ D, Novak S, FilipiČ M (2011) DNA damage and alterations in expression of DNA damage responsive genes induced by TiO2 nanoparticles in human hepatoma HepG2 cells. Nanotoxicology 5(3):341–353
Mu Q, Su G, Li L, Gilbertson BO, Yu LH, Zhang Q, Sun Y-P, Yan B (2012) Size-dependent cell uptake of protein-coated graphene oxide nanosheets. ACS Appl Mater Interfaces 4(4):2259–2266
Zhang Y, Tekobo S, Tu Y, Zhou Q, Jin X, Dergunov SA, Pinkhassik E, Yan B (2012) Permission to enter cell by shape: nanodisk vs nanosphere. ACS Appl Mater Interfaces 4(8):4099–4105
Wu L, Zhang Y, Zhang C, Cui X, Zhai S, Liu Y, Li C, Zhu H, Qu G, Jiang G (2014) Tuning cell autophagy by diversifying carbon nanotube surface chemistry. ACS Nano 8(3):2087–2099
Lankoff A, Sandberg WJ, Wegierek-Ciuk A, Lisowska H, Refsnes M, Sartowska B, Schwarze PE, Meczynska-Wielgosz S, Wojewodzka M, Kruszewski M (2012) The effect of agglomeration state of silver and titanium dioxide nanoparticles on cellular response of HepG2, A549 and THP-1 cells. Toxicol Lett 208(3):197–213
Zhang Y, Wang Y, Liu A, Xu SL, Zhao B, Zou H, Wang W, Zhu H, Yan B (2016) Modulation of carbon nanotubes’ perturbation to the metabolic activity of CYP3A4 in the liver. Adv Funct Mater 26(6):841–850
Bai Y, Zhang Y, Zhang J, Mu Q, Zhang W, Butch ER, Snyder SE, Yan B (2010) Repeated administrations of carbon nanotubes in male mice cause reversible testis damage without affecting fertility. Nat Nanotechnol 5(9):683–689
Adamcakova-Dodd A, Stebounova LV, Kim JS, Vorrink SU, Ault AP, O’Shaughnessy PT, Grassian VH, Thorne PS (2014) Toxicity assessment of zinc oxide nanoparticles using sub-acute and sub-chronic murine inhalation models. Part Fibre Toxicol 11(1):1
Morishita Y, Yoshioka Y, Takimura Y, Shimizu Y, Namba Y, Nojiri N, Ishizaka T, Takao K, Yamashita F, Takuma K (2016) Distribution of silver nanoparticles to breast milk and their biological effects on breast-fed offspring mice. ACS Nano 10(9):8180–8191
Wang Z, Qu G, Su L, Wang L, Yang Z, Jiang J, Liu S, Jiang G (2013) Evaluation of the biological fate and the transport through biological barriers of nanosilver in mice. Curr Pharm Des 19(37):6691–6697
Kim YS, Song MY, Park JD, Song KS, Ryu HR, Chung YH, Chang HK, Lee JH, Oh KH, Kelman BJ (2010) Subchronic oral toxicity of silver nanoparticles. Part Fibre Toxicol 7(1):1
van der Zande M, Vandebriel RJ, Van Doren E, Kramer E, Herrera Rivera Z, Serrano-Rojero CS, Gremmer ER, Mast J, Peters RJ, Hollman PC (2012) Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure. ACS Nano 6(8):7427–7442
Wei Y, Li Y, Jia J, Jiang Y, Zhao B, Zhang Q, Yan B (2016) Aggravated hepatotoxicity occurs in aged mice but not in young mice after oral exposure to zinc oxide nanoparticles. NanoImpact 3:1–11
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2016YFA0203103), the National Natural Science Foundation of China (91543204 and 91643204), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB14030401) and the Natural Science Foundation of Shandong Province (ZR2014BM026).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Jia, J., Yan, B. (2017). Hepatic Injuries Induced by Engineered Nanomaterials. In: Yan, B., Zhou, H., Gardea-Torresdey, J. (eds) Bioactivity of Engineered Nanoparticles. Nanomedicine and Nanotoxicology. Springer, Singapore. https://doi.org/10.1007/978-981-10-5864-6_12
Download citation
DOI: https://doi.org/10.1007/978-981-10-5864-6_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-5863-9
Online ISBN: 978-981-10-5864-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)