Abstract
Acrylics and polyolefins are widely used synthetic plastics in daily consumer products which are non-biodegradable in nature. An accumulation of these solid wastes in the environment poses ecological threats and requires novel management techniques. Researchers have now focussed their work on developing novel biodegradable polymer materials and isolating and identifying microorganisms which have the potential to degrade these polymeric materials. Isolating and identifying these microorganisms having potential to degrade polymers and polymer composites are required for developing newer biotechnological techniques for management of these solid wastes in the environment. The present work studies the biodegradation behaviour of PMMA and micro-/nano-cellulose-reinforced PMMA (polymethyl methacrylate) composites in pond water. The weight loss data revealed improved biodegradability in cellulose-reinforced PMMA composites in comparison to the synthetic PMMA. Scanning electron microscopy (SEM) images revealed effective biodegradability of the composites in pond water. The microorganism (fungus) was isolated, and its biodegradation behaviour was studied.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Plastic materials are used widely in our lives. These materials have their sources in petroleum products and are thus environmentally hazardous. Plastic wastes like carrier bags, refused sacks and other packaging materials are mainly buried in soil. They are resistant to biodegradation and result in serious environmental pollution. In order to solve this environmental problem, development of biodegradable polymers is the need of the hour. Plant fibres, agricultural and forest products are often used as alternative resources for product development in industries, in order to achieve environmentally sustainable biomaterials (Tudorachi et al. 2000). These cellulosic materials are widely used in the development of various products (e.g. twines, ropes, insulating materials, felts, fleece, non-woven materials, geotextile, fillers in polymer composites, etc.). Cellulose in plant fibres is often used as reinforcing filler in polymers. Cellulose (α-cellulose) is a major component of plant fibres. It is one of the most important natural polymer, a renewable material and a source for development of sustainable materials in industries. Incorporation of natural renewable fillers like cellulose in synthetic polymers is expected to enhance its properties and also improve its biodegradability. As cellulose is susceptible to degradation by microorganisms, its incorporation in synthetic polymers also renders biodegradability to it. Few research works have been carried out on biodegradability of synthetic polymers on incorporation of cellulose as fillers. Among the synthetic polymers, polymethyl methacrylate (PMMA) is widely used for manufacturing of various products, automotive parts, patio roofs, aircraft windscreens, etc (Sain and Khatua 2011). There are also reports on the development of cellulose-reinforced polymer composites having various application potentials. However, studies on biodegradability of the cellulose-reinforced PMMA composites are very few. Isolation of microorganisms is important for more effective degradation of the polymer composites. Specific microorganisms with ability to degrade polymers can be isolated from soil, compost, sewage, sludge, etc., through comprehensive screening and can thereafter be utilised for bioaugmentation in the context of solid disposal. Therefore, disposal of a specific polymer into a field enriched with microorganisms specific for the degradation of that polymer can significantly improve the rate of biodegradation.
2 Literature Review
There are few research works on the biodegradability of PMMA. Biodegradation studies were reported to have been performed in enzymatic route for PMMA grafted onto sago starch (Qudeseih et al. 2007). In this study, α-amylase, which was specific for starch degradation, was used. So, little degradation was achieved for the PMMA component. Considerable degradation was also achieved in the soil environment by the incorporation of starch cinnamate in PMMA (Thakore et al. 2001) and also with cellulose fibres in aerobic compost environment (Maity et al. 2013). Bhat et al. (Maity et al. 2013; Sain et al. 2014) had studied blend miscibility and biodegradability of polymer blends (PMMA with cellulose acetate, CA, and cellulose acetate phthalate, CAP). In this study along with the blends, films of the blends were fabricated through solution casting method using the solvents acetone and dimethylformamide (DMF), respectively. They were subjected to four different biodegradability methods (soil burial test, degradation in activated sludge, enzymatic degradation and degradation in phosphate) followed by water absorption tests. It was observed that blends were degraded effectively as was revealed by the weight loss data. It was further analysed that increase in CA and CAP content in the blend compositions increased the degradability. Maity et al. (2013) had also previously studied biodegradation of PMMA/cellulose nanocomposites by degrading the composites in an aerobic compost. In situ suspension polymerisation technique was deployed along with ex situ solution dispersion technique. The degradation was studied for a period of 60 days. Higher weight loss was observed in the in situ prepared composite films. Gel permeation chromatography (GPC) and nuclear magnetic resonance (NMR) studies also showed significant changes in the chemical structure of the biodegraded films.
Again, isolation and identification of polymer-degrading microorganisms are important as this knowledge provides valuable information for their use in bioaugmentation processes. The isolation, identification and study of metabolism of polymers by fungi have been well reported by some researchers (Cook et al. 1981). Fungi, due to their wide availability, faster growth rate in soil, robust nature and being a source of diverse enzymes, are widely used in bioremediation (Artham and Doble 2010). Sahoo et al. (Sahoo and Samal 2007) investigated the biodegradability of PMMA/montmorillonite nanocomposites. Unreinforced PMMA showed poor biodegradation behaviour due to its hydrophobic nature. The addition of MMT as a nanofiller in the PMMA polymer matrix increased the rate of degradation due to their hydrophilic nature of MMT . The specific microorganism, B. cereus, helped to increase the rate of biodegradation of PMMA/MMT nanocomposite than unreinforced PMMA. Prasantha et al. (2005) studied the biodegradability of chitosan-grafted PMMA films. They found that 50% weight loss was due to biodegradation evidenced by A. flavus. This microorganism helped in biodegradation by consuming chitosan, but it could not break the PMMA chains even after 25 days of degradation. Aspergillus niger was found to degrade starch grafted with PMMA (Moreno-Chulim et al. 2003).
In this study for the first time , biodegradation of PMMA composites was carried out in pond water, and their biodegradation behaviour was subsequently studied.
3 Materials and Methods
3.1 Materials
The raw material jute was procured from the local market for extracting cellulose nanofibre. Tung oil was bought from the local market. The monomer methyl methacrylate (purity of ≥99%), benzoyl peroxide (BPO, 98% purity), polyvinyl alcohol (PVOH, 96–99% hydrolysed) and sodium chlorite (NaClO2), NaOH, acetone, maleic anhydride (MA), sulphuric acid and chloroform (>99% purity) were purchased from Merck, Germany.
3.2 Extraction of the CNF from Jute
One gram of jute was treated with 0.7% of sodium chlorite (NaClO2) to remove the lignin fraction. The lignin removed fraction was treated with 17.5% NaOH solution for 15 min and then macerated. Then alkali was removed, and the jute was subjected to acid hydrolysis in 47% sulphuric acid for 3 h under constant stirring. The prepared cellulose nanofibre was washed repeatedly, and the concentrated mass was freeze-dried at −20 °C at a pressure of 15 Pa using Eyela Freeze Dryer FD-5N, Japan (Sain et al. 2012).
3.3 Fabrication of PMMA/Cellulose Composite Films
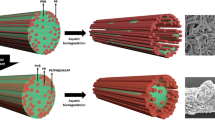
To prepare the PMMA /cellulose composite films, PMMA granules were first dissolved in chloroform, and a measured amount of cellulose nanofibre (10 wt% with respect to polymer weight) was dispersed and sonicated for 2–3 h and then solution casted in petri dishes and dried at 50 °C for 30 min in hot air oven.
3.4 Collection of Pond Water
The pond water was collected from three sites of a pond in Ballygunge Science College, Kolkata. This pond was subjected to a lot of solid waste dumping including plastic wastes.
3.5 Weight Loss
The collected pond water was poured in equal amounts in glass vials and PMMA film, and the composites were kept in it, and weight loss was recorded after 10, 20 and 30 min respectively. The loss in weight of the degraded samples was obtained following the equation, %Weight loss = {(W o − W t )/W o } × 100, where W o is the weight of the films before biodegradation and W t is the weight of the films after biodegradation at time ‘t’.
3.6 Scanning Electron Microscopy (SEM)
The composite film surface morphology before and after 30 days of biodegradation was examined under SEM (Zeiss EVO 18, Carl Zeiss, Germany) at an accelerating voltage of 5 kV. The 2.5% glutaraldehyde fixed fungus on the composite film surface was also observed under SEM (accelerating voltage of 15 kV).
3.7 Isolation of the Fungal Strain
3.7.1 Media Composition and Preparation
Potassium di-hydrogen phosphate (KH2PO4), ferrous sulphite (FeSO2.7H2O), sodium nitrate (NaNO3), potassium chloride (KCl), magnesium sulphate (MgSO4.7H2O) and ammonium chloride (NH4Cl) were obtained from Merck, Germany, in order to prepare the minimal media in the composition (Sain et al. 2014) . Agar powder (Merck, Germany) was used in the minimal broth for agar media. Nutrient agar media was also procured from Merck, Germany, for preparing the nutrient agar media.
3.7.2 Isolation of Fungal Strain
The fungal strain from pond water was isolated. The film samples were taken out from the pond water after 30 days and immersed in 10 ml of physiological saline. This was shaken for 1 h at room temperature and then several dilutions were made to isolate distinct fungal colonies. The distinct fungal colonies were replica plated on the minimal agar media where the sole source of carbon was the composite sample which was cut in even squares of 15 × 15 mm dimensions and embedded in the media. A control with only the minimal media was also kept. The colony which grew encircling the composite sample was isolated for further studies.
3.8 Weight Loss Study of the Composites in Liquid Culture Shaking Method by the Fungal Strain
The isolated pure fungal culture’s biodegradation behaviour was evaluated, where the composites were added to a conical flask containing previously sterilised minimal media. The fungal isolate spore suspension was added (2 μl in 50 ml) to the test medium, properly stoppered and incubated at 37 °C with reciprocal shaking for 7, 14 and 21 days. The degradation was monitored by determining the weight loss of the composite films at the stated intervals.
4 Results and Discussion
4.1 Weight Loss
The extent of the biodegradation of the samples at three sites of the pond was evaluated by measuring their weight loss as a function of the time of biodegradation, as shown in Fig. 1. All the samples showed weight loss especially at 30 days. Spot 3 recorded the highest weight loss percentage . Therefore, in the presence of the pond water microorganisms, the cross linking between the polymers was broken. The PMMA films did not show any weight loss. The cellulose contained in the composites thus facilitated the route effective biodegradation by breaking of the polymer chains and their subsequent uptake as the sole source of carbon. As the composite showed the best result in spot 3, further studies were conducted with water collected from that spot.
4.2 Scanning Electron Microscopy (SEM)
The PMMA films and the composite kept in pond water collected from spot 3 for 30 days were evaluated under SEM (Fig. 2). The PMMA film showed roughness of surface, but the SEM micrograph also revealed great extent of degradation of the polymer composite surface in the presence of cellulose filled. Its presence rendered pronounced biodegradability of the composites. Growth of fungal hypha was also seen, which indicated degradation of polymer composites by the fungal microbiota.
4.3 Weight Loss Study of the Composites in Liquid Culture Shaking Method by the Fungal Strain
In Fig. 3, the inoculation of the pure culture of the fungal strain in liquid minimal media revealed higher percentage of weight loss. Thus this result indicates that the isolated fungal strain isolated from spot 3 of the pond water was responsible for the biodegradation of the composite film.
5 Conclusion
The biodegradation of the ex situ prepared PMMA composite was studied. Being close to a solid waste dumping ground, it was found to contain microorganisms especially capable of degrading a PMMA reinforced with cellulose fillers. The presence of cellulose fillers facilitated the biodegradability of the composites. The SEM micrographs also revealed fungal growth on the surface revealing the microorganism responsible for the degradation. This study has provided a platform for identifying fungi in pond water which are capable of degrading polymer wastes. As a lot of polymer wastes are dumped in freshwater bodies, identifying microorganisms adapted to degrade them provides an interesting research idea towards effective bioaugmentation of polymeric materials.
References
Artham T, Doble M (2010) Biodegradation of physiochemically treated polycarbonate by fungi. Biomacromolecules 6:612–626
Cook WJ, Cameron JA, Bell JP, Huang SJ (1981) Scanning electron microscopic visualization of biodegradation of polycaprolactones by fungi. J Polym Sci B Polym Phys 19:159–165
Maity S, Sain S, Ray D, Mitra D (2013) Biodegradation behaviour of PMMA/cellulose nanocomposites prepared by in-situ polymerization and ex-situ dispersion methods. Polym Degrad Stab 98:635–642
Moreno-Chulim MV, Barahona-Perez F, Canche-Escamillia G (2003) Biodegradation of starch and acrylic-grafted starch by Aspergillus niger. J Appl Polym Sci 89:2764–2770
Prasantha KVH, Lakshman K, Shamlal TR, Tharananthan RN (2005) Biodegradation chitosan-graft-polymethylmethacrylate film. Int Biodeterior Biodegrad 56:115–120
Qudeseih IY, Kabbashi NA, Al-Khatib MF, Alam MZ, Ateih MA, Fandi KG, Mamun A, Rahman MZ, Muyibi SA (2007) Enzymatic biodegradability behaviour of poly methyl methacrylated grafted sago starch biopolymer. IIUM Eng J 8:37–45
Sahoo PK, Samal R (2007) Fire retardancy and biodegradability of polymethylmethacrylate/montmorillonite nanocomposite. Polym Degrad Stab 92:1700–1707
Sain S, Khatua BB (2011) Synthesis of highly exfoliated PS/Na+-MMT nanocomposites by suspension polymerization using Na+-MMT clay platelets as suspension stabilizer. Macromol Res 19(1):44–52
Sain S, Ray D, Mukhopadhyay A, Sengupta S, Kar T, Ennis JC, Rahman KSMP (2012) Synthesis and characterization of PMMA-cellulose nanocomposites by in situ polymerization technique. J Appl Polym Sci 126:E127–E134
Sain S, Sengupta S, Kar A, Mukhopadhyay A, Sengupta S, Kar T, Ray D (2014) Effect of modified cellulose fibres on the biodegradation behaviour of in-situ formed PMMA/cellulose composites in soil environment: isolation and identification of the composite degrading fungus. Polym Degrad Stab 99:156–165
Thakore IM, Desai S, Devi S (2001) Compatibility and biodegradability of PMMA/starch cinnamate blends in various solvents. J Appl Polym Sci 79:488–496
Tudorachi N, Cascaval CN, Rusu M (2000) Biodegradable polymer blends based on polyethylene and natural polymers: degradation in soil. J Polym Eng 20:287–304
Acknowledgement
S. Sengupta is thankful to UGC for granting her the UGC D. S. Kothari Postdoctoral Fellowship [Sanction No.: CH/15-16/0163 (S-63)]. Authors are also thankful to Ms. Priya Banerjee, Research Scholar, University of Calcutta, for her help in conducting the SEM studies and also to Mrs. Rimpa Majumder, student, Rabindra Bharati University, for her help in the work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Sengupta, S., Das, P., Datta, S., Sain, S., Mukhopadhyay, A., Ray, D. (2018). Biodegradation Behaviour of Cellulose-Reinforced PMMA Composites in Pond Water. In: Ghosh, S. (eds) Utilization and Management of Bioresources. Springer, Singapore. https://doi.org/10.1007/978-981-10-5349-8_6
Download citation
DOI: https://doi.org/10.1007/978-981-10-5349-8_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-5348-1
Online ISBN: 978-981-10-5349-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)