Abstract
Signaling events between rhizosphere microbes and plants substantially contribute to establish different qualities of microbe-plant interactions from beneficial cooperativity to pathogenicity. In addition to the pathogen-associated molecular patterns (PAMPs), like exo- and lipopolysaccharides or flagellins, which are effectively recognized by the plants’ innate immune system, various secondary metabolites, such as antibiotics or the so-called autoinducers involved in the quorum sensing response of bacteria, are additional modulators of plants’ perception of associated microbes. In Gram-negative bacteria, N-acyl homoserine lactones (AHLs) are the major quorum sensing autoinducing molecules, which have a central role in the differentiation of specific phenotypes of sessile cells, living in root-attached microcolonies or biofilm consortia. AHLs turned out to have profound effects on plant development and/or defense priming and development of systemic resistance against pathogens. AHLs have different structural modifications (e.g., short or long hydrocarbon chain residues). While the hydrophilic ones can be taken up by plants, the lipophilic stay in the roots. Different modes of plant growth promotion by these AHL types in various plants are summarized in this chapter. We hypothesize, that in the absence of pathogenic patterns, AHLs support a beneficial to symbiotic interaction with plants. In cases when plant pathogens use AHLs for virulence development, AHLs reinforce plant’s defense. Alternatively, AHL degradation activities of certain rhizosphere bacteria can be used to suppress the pathogenic attack. To foster beneficial interactions of rhizotrophs with plants, consortia of bacteria using the same autoinducers could be developed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
10.1 Introduction
The plant microbiome – the community of microbes, associated with plant surfaces and tissues – is currently in the focus of plant biotechnology, since molecular characterization technologies, so-called omic approaches, are providing almost unbiased insights into the diversity in the community structure and its activities (Turner et al. 2013). Plant-colonizing microbes exert great influences on plant health and development. This had been already recognized more than hundred years ago by the pioneers of rhizosphere microbiology like Lorenz Hiltner (Hartmann et al. 2008). Since the genetic capacity of the microbes associated with all higher organisms is by far higher than and complementary to the hosts’ genetic repertoire, the importance of prokaryote-eukaryote interactions has led to the metagenome or holobiont view of higher organisms and their associated microbiomes (Zilber-Rosenberg and Rosenberg 2008). The hypothesis was created that these assemblages/symbioses are the true, evolutionary superior life strategies for accommodating with rapidly changing and challenging environmental conditions. There is much to discover regarding prokaryotic diversity and their function within the plant microbiome, since many microbes are difficult to isolate and to grow in pure culture. Microbe-plant interactions cover a wide spectrum, from pathogenic to beneficial and even symbiotic interactions in plant and animal/human hosts (Berg et al. 2005; Mendes et al. 2013). Plant growth promotion by rhizosphere-associated, root-colonizing microbes is a well-documented phenomenon (Dessaux et al. 2010). It can be considered as a symbiotic and synergistic microbe-plant interaction, although no particular symbiotic organs are visible. Benefits of these associations can be observed particularly when the plant is challenged by limiting nutrient supply, by abiotic stresses like hypersaline conditions or lack of water, or when attacked by pathogens (Raaijmakers et al. 2009).
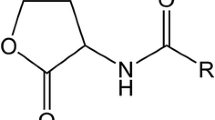
While the discovery and description of the microbial diversity within the plant microbiome has made substantial progress, only comparably little details are understood about signaling mechanisms involved in microbe-host interactions. Several groups of signaling molecules are known, like a multitude of bacterial volatile substances (Ryu 2015) or bacterial-synthesized phytohormones (Spaepen 2015). The autoinducing small molecules (N-acyl-L-homoserine lactones; Fig. 10.1) of the quorum sensing response in Gram-negative bacteria are only recently discovered signals between bacteria and plants. They are in the focus of this chapter.
Structure of N-acyl homoserine lactones (AHLs), the autoinducer molecule of the quorum sensing (QS) response in many Gram-negative bacteria. HSL homoserine lactone residue, R acyl chain residue, ranging from 4 to 14 carbon atoms. Further variations: hydroxyl group or carbonyl group at C3-atom and/or double bond in the acyl chain R
10.2 Bacteria as Social Organisms
Bacteria rarely live as a single dispersed organism; mostly they live in communities and colonize surfaces. The ability of bacterial populations to form biofilms is often under the quorum sensing (QS) control. Quorum sensing describes the phenomenon that bacteria produce and perceive signal molecules to coordinate their behavior in a population-dependent manner; thus QS is considered a social trait (Parsek and Greenberg 2005). Biofilm formation is essential to colonize surfaces of minerals and higher organisms, such as roots of plants. Biofilms are highly structured in which the metabolic activities are distributed between the different bacteria of the consortium. This requires a high degree of coordination and is quite similar to the situation in multicellular organisms. In bacteria, several types of autoinducers (AIs) are known. While in Gram-negative bacteria, frequently N-acyl-L-homoserine lactones (AHL) and occasionally 4-hydroxyl 2-alkyl quinolones (HAQ) are found; Gram-positive bacteria mainly use cyclic oligopeptides. A furanon derivative, borate complex (the so-called AI-2), seems to act as a more general AI in a variety of Gram-negative and Gram-positive bacteria. In addition, other molecules with unknown structures might be involved in unidentified QS systems. Usually, the signaling molecules are produced in an autoinducing process: it consists of a very low constitutive biosynthesis and release of the autoinducer (AI) and a receptor protein (R), which senses the overall concentration of the AI within the cells. Beyond a certain threshold level of AI, the AI/R complex binds to the promoter of the biosynthesis gene I, which is then greatly stimulated; also other AI/R-regulated promoters are activated. This regulatory cascade finally leads to the expression of a whole set of genes and operons depending on the QS activation which drastically alters the overall behavior of the population.
The QS mechanism of Gram-negative bacteria using N-acyl homoserine lactones (AHLs) is established since some time (Fuqua and Greenberg 2002), and until now more than 200 species are known to produce different AHLs (Kimura 2014). However, there are bacteria which do not produce AHLs but harbor so-called LuxR solos (Patel et al. 2013). It could be demonstrated that these receptors evolved in some cases to respond to different molecules including signals from plant origin (Patel et al. 2013). N-Acyl-L-homoserine lactones are known to have a wide variety of chemical structures (Fig. 10.1). They can carry a hydroxy- or oxo-function at the C3 atom of the hydrocarbon side chain and occur as quite hydrophilic molecules with a length of the CH chain from C4-, C6-, and C8-HSLs (hydrophilic) to C12- and C14-HSLs (hydrophobic). QS regulation is of high ecophysiological and evolutionary importance because it optimizes gene expression under changing environmental conditions. This led to the suggestion of the QS autoinducing regulation as an “efficiency sensing” mechanism (Hense et al. 2007). Important physiological traits, like expression of virulence, chemotaxis and swarming, siderophore, antibiotic, and exoenzyme production, are regulated by quorum sensing (Eberl 1999). In developing microcolonies and biofilms on root surfaces, the production of AHL substances has been demonstrated using AHL biosensor bacteria (Steidle et al. 2001; Gantner et al. 2006; Fekete et al. 2007), which indicate the presence of AHLs by the activation of AHL regulated promoters fused to a gene for fluorescence protein synthesis, like the green fluorescence gene (GFP). These colonies and biofilms of Gram-negative bacteria are the source for the AHL signaling molecules to interact with plant roots. Recently, it was discovered that these AHLs are also active as direct signal to the plant, stimulating different plant responses (see below). This may be regarded as a consequence of the coevolution of plants with the omnipresent microbiota. Since Gram-negative bacterial pathogens also coordinate their colonization and virulence using these signals, it is highly advantageous for plants to recognize and perceive these signals. However, the AI signals also play important roles in the coordination mechanisms of interactions of plant hosts with beneficial bacteria within holobionts (see below).
10.3 Perception of AHL Molecules by Plants
The first study reporting evidences for the impact of bacterial AHLs on plant physiological activities was published by Joseph and Phillips (2003). They measured the influence of several water-soluble compounds taken up by roots with the natural transpiration stream on stomatal conductance and transpiration of bean (Phaseolus vulgaris L.) plants. In these experiments, 10 nM homoserine lactones as well as the respective homoserine had similar stimulating effects. Shortly afterward, the group of Ulrike Mathesius at the University of Brisbane and our research unit at the Helmholtz Zentrum München communicated detailed plant responses toward different AHLs applied to roots. Using a differential proteome analysis approach, Mathesius et al. (2003) revealed that defense- and stress-related proteins of Medicago truncatula to be regulated in their expression by the addition of 3-oxo-C12-L-homoserine lactone. Proteins associated with flavonoid metabolism, several regulatory proteins (including protein degradation and synthesis), and an auxin-responsive promoter were within this AHL regulon. The first demonstration of C6 and C8 AHL-induced systemic resistance development was reported for tomato plants by Hartmann et al. (2004) and Schuhegger et al. (2006). Upon the addition of C6- and C8-HSL-producing Serratia liquefaciens MG1 to roots of the tomato variety MicroTom, growing in regular soil, the plant developed increased resistance in the leaves against the attack by the fungal pathogen Alternaria alternata. In a clean and axenic quartz sand-based hydroponic system, also the addition of pure C6- and C8-HSLs to the rooting solution of tomato seedlings caused induction of the expression of the pathogen defense genes PR1 and chitinase in the leaves. This supported the primary finding of a systemic induction due to inoculation with AHL-producing Serratia liquefaciens, especially because an AHL-deficient mutant was unable to prevent the pathogen attack like the wild type. It was very remarkable that salicylic acid was increased in AHL-treated tomato plants, which is known as a transmitter of induced systemic resistance (Schuhegger et al. 2006). When the same hydrophilic autoinducers C6- or C8-HSL were added to Arabidopsis thaliana, no induced resistance but an increased auxin/cytokinin ratio and an increased root growth response were observed (von Rad et al. 2008). It could be demonstrated that a diversity of phytohormone-regulated genes was up- or downregulated after addition of C6- or C8-HSL but no defense-regulated genes were upregulated (von Rad et al. 2008).
The root stimulatory effect of C6- and C8-HSLs was corroborated by Liu et al. (2012), who found that Arabidopsis mutants in the G-protein receptors GCR1 and GAP1 lost this effect, while constructs with increased levels of the G-protein-related receptor showed increased root stimulation. In contrast, when oxo- or hydroxyl-C14-HSL was added to the rooting solution of Arabidopsis thaliana or barley seedlings, a clear induction of systemic resistance responses was found (Schikora et al. 2011). Based on these observations, two contrasting effects of AHL molecules with different hydrophilicities (due to short versus long hydrocarbon chains) were hypothesized for Arabidopsis thaliana (Hartmann and Schikora 2012; Schenk et al. 2012) (Fig. 10.2). However, the impact of AHLs on plant growth and pathogen defense in different plant species seems to be quite diverse (Hartmann et al. 2014). For example, it was found for Medicago truncatula that the long-chain 3-oxo-C14-HSL produced by Sinorhizobium meliloti enhanced the nodulation in roots (Veliz-Vallejos et al. 2014). Very striking was the observation that only 3-oxo-C14-HSL, the predominant AHL of symbiotic bacterium S. meliloti, increased the number of roots but no other AHLs. In mung bean plants, an induction of the growth of adventitious roots was stimulated specifically only by 3-oxo-C10-HSL but failed to be increased by unsubstituted C10-HSL or C12-HSL (Bai et al. 2012). Palmer and coworkers (2014) proposed that a change in transpiration rate induced by the hydrolysis of AHLs to L-homoserines increased stomatal opening fostering water and mineral flow through the plant, which has been already described by Joseph and Philipps (2003).
10.4 Uptake and Physiological Interactions of AHL with Plants
The strong impact of the addition of AHLs to the rooting solution of plants could be either caused by the initiation of a systemic signal (or signals) which would be triggered after the perception of AHLs on the root surface or whether they are taken up by the roots and transported to the shoots and then act locally at the plant tissues. Using highly resolving chemical analysis equipment, like a 12 Tesla Fourier Transform Ion Cyclotron Resonance Mass Spectrometer (FTICR-MS), which can resolve the molecular weights of small molecules with highest accuracy, the identification of different AHL molecules could be achieved even within plant tissues (Fekete et al. 2007; Götz et al. 2007). The identification and quantification was further supported and confirmed by ultrahigh performing liquid chromatography coupled with MS detection (Fekete et al. 2010; Buddrus-Schiemann et al. 2014; Rothballer et al. 2016). When different short and long carbon chain AHLs were applied to the rooting solution of barley plants, the C4- to C8-HSLs were clearly identified in the roots and even in the shoots. Only traces of C10-HSL and no AHLs with longer carbon chains were detectable in shoots (Fig. 10.2). Obviously, their hydrophobicity prevented the transport. In contrast to barley plants, in the leguminous yam beans (Pachyrhizus erosus), no uptake of AHLs was detectable (Götz et al. 2007). Obviously, these plants had highly active AHL lactonases, which effectively destroyed the signal molecule. When the AHL transport in barley was measured using AHL-specific monoclonal antibodies for short carbon chain HSLs (Chen et al. 2010a), the uptake into the shoots could be quantified using a quantitative enzyme-linked immunosorbent assay (ELISA) (Chen et al. 2010b), when 60 μM of C6-HSL was added to roots. The quantification gave a concentration of 30 μM of antigen in the phloem liquid of cut barley stalks. To prove that the transported AHL molecule in the plant sap still is in the active form, a C6-HSL-specific biosensor bacterium was applied to this plant liquid, resulting in the quantification of a similar concentration of authentic C6-HSL (Fekete et al. 2010; Sieper et al. 2013). Finally, radioactively labeled C8- and C10-HSLs were applied by Sieper et al. (2013) to derive further details about the AHL transport in barley plants. Since the uptake was completely blocked in the presence of the energy poison iodic acetate, an ATP-dependent uptake of these AHLs into the vascular system was suggested. The further transport along the barley roots could be blocked by vanadate, which argues for a symplasmic transport of the majority of AHLs in the vascular system. The transport rate of the AHL molecules was quantified in excised roots in a Pitman chamber, using radiolabeled 3H-AHLs. The transport rate along the root was higher in the more hydrophilic AHLs, favoring short-chain AHLs (Sieper et al. 2013). Autoradiography clearly showed that the highest concentration of 3H-AHL was concentrated in the vascular system of roots. In Arabidopsis, the short-chain AHL, C6-HSL, was also taken up into the shoot after application to roots, while the long-chain AHL, oxo-C14-HSL, was not transported (Schikora et al. 2011) (Fig. 10.2).
In barley, which had taken up C6-, C8-, and C10-HSLs, specific detoxification enzymes, like glutathione S-transferase and dihydro-ascorbate reductase, were affected by AHLs. When the most hydrophilic compound C6-HSL was applied to roots, the largest influence on the leaf-located enzyme was found (Götz-Rösch et al. 2015). In yam beans, which have a high lactonase activity, no influences of AHL application on foliar enzyme activities were found. Since these short-term effects on enzyme activities were not accompanied by a concomitant increase of transcription level of the respective genes, it was hypothesized that the AHL compounds exert a direct modulation of certain enzyme activities (Götz-Rösch et al. 2015).
An influence of bacterial AHLs and the plant reproduction was found in the study of Singh et al. (2015) with the green macroalga Ulva and the red macroalga Gracilaria. The short-chain C4- and C6-HSLs produced by bacterial biofilms colonizing the algae stimulated the release of carpospores (Singh et al. 2015). Interestingly, the protein patterns of both the cystocarps and the cystocarp-bearing plantlets treated with AHLs had protein patterns different from the pattern in control algae. Another very interesting finding was the influence of bacterial QS signals on herbivore defense of plants, since the jasmonic acid (JA)-mediated herbivore resistance was reduced in tobacco plants by AHLs (Heidel et al. 2010).
10.5 AHLs and Priming/Induction of Induced Resistance
In addition to the induction of a fast and highly sensitive innate immune response to microbial pathogens, which are recognized by their microbe-associated molecular patterns (MAMPs), also other additional mechanisms, like AHL signals, may be integrated into a network of interactive perceptions of environmental signals to further specify and strengthen the plant defense response. At situations of biotic or abiotic stresses, plants can further induce steps toward defense stimulation with a sensibilization mechanism, called priming (Jung et al. 2005). A diversity of metabolites which induce priming also include a diversity of volatile substances, known to be produced by a number of biocontrol bacteria, which can prepare the plant for an upcoming pathogen attack (Ryu et al. 2003; Ryu 2015).
Using a set of Arabidopsis thaliana mutants in the signaling chain and downstream response effects, the specific effects of AHL compounds on the induction of resistance responses could be revealed (Schikora et al. 2011). When the major innate immune elicitor protein flg22 was added to the model plant Arabidopsis thaliana, the phosphorylation of mitogen-activated protein kinases MPK 3 and MPK 6 and an activation of the transcription factors WRKY 18, WRKY 22, and WRKY 29 occurred. In the presence of C12- and C14-HSL compounds in the rooting solution, MPK 3 and MPK 6 phosphorylation was modified and the expression of WRKY transcription factors was increased. In the presence of long carbon chain AHLs, the expression of pathogenesis-related proteins and accumulation of H2O2 was increased (Fig. 10.3). Barley plants also showed an enhanced production of ROS. In the presence of C12- and C14-HSLs, an increased level of hypersensitive response (HR) occurred after infection with Blumeria graminis.
Effects of C12- and C14-HSL on resistance response in Arabidopsis thaliana (according to Schikora et al. 2011) Flg22, flagellin as elicitor of microbe-associated molecular pattern (MAMP) response; MPK 3 and MPK 6, mitogen-associated protein kinases (MAPKs)
Also using the Arabidopsis model, further details of the molecular mechanism of AHL-induced priming were revealed by Schenk and Schikora (2015). Using the pathogen Pseudomonas syringae pathovar tomato (Pst), the treatment with oxo-C14-HSL resulted in increased pathogen resistance in accordance with SA- and oxylipin-dependent signaling (Schenk et al. 2014). Another AHL resistance phenotype could be the increased amount of closed stomata in response to Pst challenge in oxo-C14-HSL-pretreated plants (Schenk et al. 2014). This AHL-induced stomata defense response seems similar to the flg22-induced stomata closure, described as RES oxylipin and SA-dependent by Montillet et al. (2013). Another very striking similarity to other cellular signaling and response cascades was the demonstration of the increase of intracellular levels of calcium in Arabidopsis root cells (Song et al. 2011) and the involvement of calmodulin in the primary root elongation response to 3-oxoC6-HSL in Arabidopsis (Zhao et al. 2015). The occurrence of priming events as part of the response patterns of plants toward AHLs was recently reviewed in detail by Schikora et al. (2016).
10.6 Role of AHLs in the Integrated Plant Perception of Rhizobacteria
In most experiments about the effects of AHL autoinducers on plants, commercially available AHLs of different structures were applied to roots of plant seedlings in a clearly defined axenic hydroponic system. This approach has the great advantage that disturbances with the effects of other organic compounds from the soil solution or other components of the inoculated bacterium are avoided, which certainly also affect the response of the plant. However, the more realistic approach to investigate the role of AHLs and to learn about the true integrated role of AHLs of a certain beneficial rhizosphere bacterium on its perception by plants is to apply wild-type rhizosphere bacteria and compare their effects on plants with an AHL-deficient mutant. In the case of development of systemic resistance, against plant pathogens, induced by certain rhizosphere bacteria, specific AHL deletion mutants or phenotypic variants devoid of AHL production were already used and clearly showed the importance of the AHL production to achieve this trait (Schuhegger et al. 2006; Pang et al. 2009). However, in a general beneficial endophytic bacterium, the influence of AHL production on the detailed transcriptional response by plants was not investigated. Therefore, the Gram-negative bacterium Acidovorax radicis N35, characterized as a plant beneficial endophytic bacterium in wheat and barley (Li et al. 2011), was studied in detail in this respect. This bacterium produces only 3-OH-C10-HSL and according to its genome analysis harbors only one AHL-biosynthesis (araI) gene (Fekete et al. 2007). Therefore, an araI mutant was constructed, devoid of any AHL production (Han et al. 2016). When the wild type and the araI mutant, labeled differently with GFP and YFP, were compared for their colonization abilities, the mutant was less competitive when both strains were inoculated in a 1:1 mixture to barley roots. The wild type showed a biofilm-like colonization pattern, while the mutant occurred at the root surface only as dispersed single cells. However, both bacteria could finally exert a comparable plant growth-promoting effect (Han et al. 2016). When the transcription profile of the barley seedlings was tested by RNA sequencing, the wild-type bacteria showed several priming effects and only very weak induction of early defense responses, while the araI mutant caused severely increased expression of flavonoid biosynthesis genes among other defense genes. This was corroborated by directed qPCR quantification and the accumulation of the flavonoids, lutonarin and saponarin, and related compounds in barley leaves, which had been inoculated with the araI mutant (Han et al. 2016). These flavonoids were not or much less found after inoculation with the AHL-producing A. radicis wild type. Therefore, the production of AHL has pronounced implications on the perception by the host plant and contributes to the better establishment of the endophytic bacterium. AHL-producing bacteria may be used as co-inoculants to better pave the way for other inoculants. However, the compatibility of these bacteria in colonization has to be checked for each combination.
10.7 Possible Future Use of Quorum Sensing Mechanisms in Sustainable Agriculture
The ongoing and even increased application of chemicals in industrialized agriculture and the possible consequences on food quality are key arguments toward changes to more sustainable agricultural practices. Among biology-based plant protection methods, the use of biologicals or biocontrol agents is increasing in agriculture, but their application by far did not reach its full potential. Today, several products based on bacterial inocula mainly consisting of several strains of different Bacillus spp., Pseudomonas spp., or Serratia spp. are successfully used by farmers. The application of bacteria, producing specific AHLs, could enhance the beneficial effects of other rhizosphere bacteria, especially bacterial inocula, and enlarge the impact to plant species usually not associated with the particular strain (Zarkani et al. 2013; Hernández-Reyes et al. 2014). Furthermore, the potential of AHLs or AHL-producing bacteria which are able to prime or induce several immune responses could open new possibilities in the prevention of pathogen infections also in field crops. The possibility to interfere with bacterial QS mechanism, via mimicry or enzymatic degradation of QS molecules by the plant or rhizosphere microbes, provides additional possible strategies to compete with pathogen attack, since such approaches lower the virulence by pathogenic bacteria. A variety of rhizosphere bacteria have quorum-quenching activities, like many bacilli (Dong et al. 2007), which harbor efficient AHL lactonases and are therefore good candidates for practical pathogen control in the field. QS mechanisms are therefore in the center of new strategies against different infectious diseases (LaSarre and Federle 2013; Kusari et al. 2015).
References
Bai X, Todd CD, Desikan R, Yang Y, Hu X (2012) N-3-oxo-decanoyl-L-homoserine-lactone activates auxin-induced adventitious root formation via hydrogen peroxide- and nitric oxide-dependent cyclic GMP signaling in mung bean. Plant Physiol 158:725–736
Berg G, Eberl L, Hartmann A (2005) The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ Microbiol 7:1673–1685
Buddrus-Schiemann K, Rieger M, Mühlbauer M et al (2014) Analysis of N-acyl-homoserine lactone dynamics in continuous culture of Pseudomonas putida IsoF using ELISA and UPLC/qTOF-MS-related measurements and mathematical models. Anal Bioanal Chem 406:6373–6383
Chen X, Kremmer E, Gouzy MF et al (2010a) Development and characterization of rat monoclonal antibodies for N-acylated homoserine lactones. Anal Bioanal Chem 398:2655–2667
Chen X, Buddrus-Schiemann K, Rothballer M, Krämer P, Hartmann A (2010b) Detection of quorum sensing molecules in Burkholderia cepacia culture supernatants with enzyme-linked immunosorbent assays. Anal Bioanal Chem 398:2669–2676
Dessaux Y, Hinsinger P, Lemanceau P (eds) (2010) Rhizosphere: achievements and challenges. Springer-Science-Press, Berlin
Dong YH, Wang LH, Zhang LH (2007) Quorum-quenching microbial infections: mechanisms and implications. Philos T Roy Soc B 362:1201–1211
Eberl L (1999) N-acyl-L-homoserine lactone-mediated gene regulation in Gram-negative bacteria. Syst Appl Microbiol 22:493–506
Fekete A, Frommberger M, Rothballer M et al (2007) Identification of bacterial N-acyl homoserine lactones (AHL) using ultra performance liquid chromatography (UPLC™), ultrahigh resolution mass spectrometry and in situ biosensor constructs. Anal Bioanal Chem 387:455–467
Fekete A, Rothballer M, Hartmann A, Schmitt-Kopplin P (2010) Identification of bacterial autoinducers. In: Kraemer R, Jung K (eds) Bacterial signalling. Wiley Publishers, Weinheim, pp 95–111
Fuqua C, Greenberg EP (2002) Listening in on bacteria: acyl homoserine lactone signalling. Nat Rev Mol Cell Biol 3:685–695
Gantner S, Schmid M, Dürr C et al (2006) In situ spatial scale of calling distances and population density-dependent N-acyl homoserine lactone mediated communication by rhizobacteria colonized on plant roots. FEMS Microbiol Ecol 56:188–194
Götz C, Fekete A, Gebefügi I et al (2007) Uptake, degradation and chiral discrimination of N-acyl-D/L-homoserine lactones by barley (Hordeum vulgare) and yam bean (Pachyrhizus erosus) plants. Anal Bioanal Chem 389:1447–1457
Götz-Rösch C, Sieper T, Fekete A, Schmitt-Kopplin P, Hartmann A, Schröder P (2015) Influence of bacterial N-acyl-homoserine lactones on growth parameters, pigments, antioxidative capacities and the xenobiotic phase II detoxification enzymes in barley and yam bean. Front Plant Sci 6:205
Han, S, Li D, Trost E et al (2016) Systemic response of barley to the 3-hydroxy-C10-homoserine lactone producing plant beneficial endophyte Acidovorax radicis N35. Front Plant Sci 7:1868
Hartmann A, Schikora A (2012) Quorum sensing of bacteria and trans-kingdom interactions of N-acyl homoserine lactones with eukaryotes. J Chem Ecol 38:704–713
Hartmann A, Gantner S, Schuhegger R et al (2004) N-acyl homoserine lactones of rhizosphere bacteria trigger systemic resistance in tomato plants. In: Lugtenberg B, Tikhonovich I, Provorov N (eds) Biology of plant-microbe interactions. APS Press, St. Paul, pp 554–556
Hartmann A, Rothballer M, Schmid M (2008) Lorenz Hiltner, a pioneer in rhizosphere microbial ecology and soil bacteriology research. Plant Soil 312:7–14
Hartmann A, Rothballer M, Hense BA, Schröder P (2014) Bacterial quorum sensing compounds are important modulators of microbe-plant interactions. Front Plant Sci 5:131
Heidel A, Barazani O, Baldwin I (2010) Interaction between herbivore defense and microbial signaling: bacterial quorum-sensing compounds weaken JA-mediated herbivore resistance in Nicotiana attenuata. Chemoecology 20:149–154
Hense BA, Kuttler C, Mueller J, Rothballer M, Hartmann A, Kreft JU (2007) Does efficiency sensing unify diffusion and quorum sensing? Nat Rev Microbiol 5:230–239
Hernández-Reyes C, Schenk ST, Neumann C, Kogel KH, Schikora A (2014) N-acyl-homoserine lactones-producing bacteria protect plants against plant and human pathogens. Microb Biotechnol. doi:10.1111/1751-7915.12177
Joseph CM, Phillips DA (2003) Metabolites from soil bacteria affect plant water relations. Plant Physiol Biochem 41:189–192
Kimura N (2014) Metagenomic approaches to understanding phylogenetic diversity in quorum sensing. Virulence 5:433–442
Kusari P, Kusari S, Spiteller M, Kayser O (2015) Implications of endophyte-plant crosstalk in light of quorum responses for plant biotechnology. Appl Microbiol Biol 99:5383–5390
LaSarre B, Federle MJ (2013) Exploiting quorum sensing to confuse bacterial pathogens. Microbiol Mol Biol Rev 77:73–111
Li D, Rothballer M, Schmid M, Esperschütz J, Hartmann A (2011) Acidovorax radicis sp. nov., a rhizosphere bacterium isolated from wheat roots. Int J Syst Evol Microbiol 61:2589–2594
Liu F, Bian Z, Jia Z, Zhao Q, Song S (2012) The GCR1 and GPA1 participate in promotion of Arabidopsis primary root elongation induced by N-acyl-homoserine lactones, the bacterial quorum-sensing signals. Mol Plant-Microbe Interact 25:677–683
Mathesius U, Mulders S, Gao M et al (2003) Extensive and specific responses of a eukaryote to bacterial quorum-sensing signals. Proc Natl Acad Sci U S A 100:1444–1449
Mendes R, Garbeva P, Raaijmakers JM (2013) The rhizosphere microbiome. Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev 37:634–663
Montillet JL, Leonhardet N, Mondy S et al (2013) An abscisic acid-independent oxylipin pathway controls stomatal closure and immune defense in Arabidopsis. PLoS Biol 11(3):e1001513. doi:10.1371/journal.pbio.1001513
Pang YD, Liu XG, Ma YX, Chernin L, Berg G, Gao KX (2009) Induction of systemic resistance, root colonisation and biocontrol activities of the rhizospheric strain of Serratia plymuthica are dependent on N-acyl homoserine lactones. Eur J Plant Pathol 124:261–268
Parsek MR, Greenberg EP (2005) Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol 13:27–33
Patel HK, Suarez-Moreno ZR, Degrassi G, Subramoni S, Gonzalez JF, Venturi V (2013) Bacterial LuxR solos have evolved to respond to different molecules including signals from plants. Front Plant Sci 4:447
Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moenne-Loccoz Y (2009) The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321:341–361
Rothballer M, Uhl J, Kunze J, Schmitt-Kopplin P, Hartmann A (2016) Detection of the bacterial quorum sensing molecules N-acyl homoserine lactones (HSL) and N-acyl-homoserines (HS) with the enzyme-linked immunosorbent assay (ELISA) and via ultrahigh performance liquid chromatrography coupled to mass spectrometry (UPLC-MS). In: Leoni L, Ramioni G (eds) Quorum sensing: methods and protokolls, Springer series methods in molecular biology. Springer International Publishing, Cham. (in press)
Ryu CM (2015) Bacterial volatiles as airborne signals for plants and bacteria. In: Lugtenberg B (ed) Principles of plant-microbe interactions. Springer International Publishing, Cham, pp 53–64
Ryu CM, Farag MA, Hu CH et al (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci U S A 100:4927–4932
Schenk ST, Schikora A (2015) AHL-priming functions via oxylipin and salicylic acid. Front Plant Sci 5:784
Schenk ST, Stein E, Kogel KH, Schikora A (2012) Arabidopsis growth and defense are modulated by bacterial quorum sensing molecules. Plant Signal Behav 7:178–181
Schenk ST, Hernandez-Reyes C, Samans B et al (2014) N-acyl-homoserine lactone primes plants for cell wall reinforcement and induces resistance to bacterial pathogens via the salicylic acid/oxylipin pathway. Plant Cell 26:2708–2723
Schikora A, Schenk ST, Stein E, Molitor A, Zuccaro A, Kogel KH (2011) N-acyl-homoserine lactone confers resistance towards biotrophic and hemibiotrophic pathogens via altered activation of AtMPK6. Plant Physiol 157:1407–1418
Schikora ST, Schenk ST, Hartmann A (2016) Beneficial effects of bacteria-plant communication based on quorum sensing molecules of the N-acyl-homoserine lactone group. Plant Mol Biol 90:605–612
Schuhegger R, Ihring A, Gantner S et al (2006) Induction of systemic resistance in tomato by N-acyl-L-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ 29:909–918
Sieper T, Forczek S, Matucha M, Kramer P, Hartmann A, Schröder P (2013) N-acyl-homoserine lactone uptake and systemic transport in barley rest upon active parts of the plant. New Phytol 201:545–555
Singh RP, Baghel RS, Reddy CR, Jha B (2015) Effect of quorum sensing signals produced by seaweed-associated bacteria on carpospore liberation from Gracilaria dura. Front Plant Sci 6:117
Song S, Jia Z, Xu J, Zhang Z, Bian Z (2011) N-butyryl-homoserine lactone, a bacterial quorum-sensing signaling molecule, induces intracellular calcium elevation in Arabidopsis root cells. Biochem Biophys Res Commun 414:355–360
Spaepen S (2015) Plant hormones produced by microbes. In: Lugtenberg B (ed) Principles of plant-microbe interactions. Springer, Berlin, pp 247–256
Steidle A, Sigl K, Schuhegger R et al (2001) Visualization of N-acyl-L-homoserine lactone-mediated cell-cell communication between bacteria colonizing the tomato rhizosphere. Appl Environ Microbiol 67:5761–5770
Turner TR, James EK, Poole PS (2013) The plant microbiome. Genome Biol 14:209
Veliz-Vallejos DF, van Noorden GE, Yuan M, Mathesius U (2014) A Sinorhizobium meliloti-specific N-acyl-L-homoserine lactone quorum-sensing signal increases nodule numbers in Medicago truncatula independent of autoregulation. Front Plant Sci 5:551
von Rad U, Klein I, Dobrev PI et al (2008) Response of Arabidopsis thaliana to N-hexanoyl-L-homoserine-lactone, a bacterial quorum sensing molecule produced in the rhizosphere. Planta 229:73–85
Zarkani AA, Stein E, Rohrich CR et al (2013) Homoserine lactones influence the reaction of plants to rhizobia. Int J Mol Sci 14:17122–17146
Zhao Q, Zhang C, Jia Z, Huang Y, Li H, Song S (2015) Involvement of calmodulin in regulation of primary root elongation by N-3-oxo-hexanoyl homoserine lactone in Arabidopsis thaliana. Front Plant Sci 5:807
Zilber-Rosenberg I, Rosenberg E (2008) Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. FEMS Microbiol Rev 32:732–735
Acknowledgments
This chapter is dedicated to Dr. Michael Schmid, who unexpectedly died in June 2016. His contributions as commission head of the Research Unit Microbe-Plant Interactions and as expert on molecular microbial taxonomy and ecology are greatly appreciated. In addition, the fruitful cooperation with Burkhard Hense and Philippe Schmitt-Kopplin (Helmholtz Zentrum München), Leo Eberl (University of Zürich), Karl-Heinz Kogel (University of Giessen), and Adam Schikora (presently at Julius-Kühn Institute Braunschweig) over the years to discover more and more details about the perception of AHLs by plants is deeply acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Hartmann, A., Rothballer, M. (2017). Role of Quorum Sensing Signals of Rhizobacteria for Plant Growth Promotion. In: Mehnaz, S. (eds) Rhizotrophs: Plant Growth Promotion to Bioremediation. Microorganisms for Sustainability, vol 2. Springer, Singapore. https://doi.org/10.1007/978-981-10-4862-3_10
Download citation
DOI: https://doi.org/10.1007/978-981-10-4862-3_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4861-6
Online ISBN: 978-981-10-4862-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)