Abstract
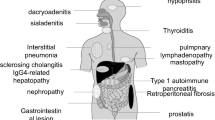
Autoimmune pancreatitis (AIP) was reported in 2001 to be associated with high serum immunoglobulin (Ig)G4 levels [1]. Thereafter, elevated serum IgG4 was also detected in other diseases, such as dacryoadenitis/sialadenitis, retroperitoneal fibrosis, tubulointerstitial nephritis, lung disease, and sclerosing cholangitis (SC), which led to the proposal of a new disease concept called IgG4-related disease (IgG4-RD). Roughly 60–90% of patients with IgG4-related SC (IgG4-SC) have concomitant AIP and are relatively easy to identify. However, those with no increase in serum IgG4 and/or biliary lesions only can be difficult to diagnose. In particular, differentiation from hilar cholangiocarcinoma and primary SC (PSC) is challenging in IgG4-SC patients with biliary stenosis, which encompasses the hepatic portal and intrahepatic regions. While imaging and histopathological findings remain the most important means of diagnosing each disease, increased serum IgG4 levels have also become a useful diagnostic aid. In this chapter, we describe the roles and properties of serum IgG4, the relevance of serum IgG4 in various diseases, and finally outline the diagnostic value of serum IgG4 for IgG4-SC.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Increased Serum IgG
- Intrahepatic Region
- Autoimmune Pancreatitis (AIP)
- IgG4-related Disease
- Hilar Cholangiocarcinoma
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Autoimmune pancreatitis (AIP) was reported in 2001 to be associated with high serum immunoglobulin (Ig)G4 levels [1]. Thereafter, elevated serum IgG4 was also detected in other diseases, such as dacryoadenitis/sialadenitis, retroperitoneal fibrosis, tubulointerstitial nephritis, lung disease, and sclerosing cholangitis (SC), which led to the proposal of a new disease concept called IgG4-related disease (IgG4-RD). Roughly 60–90% of patients with IgG4-related SC (IgG4-SC) have concomitant AIP and are relatively easy to identify. However, those with no increase in serum IgG4 and/or biliary lesions only can be difficult to diagnose. In particular, differentiation from hilar cholangiocarcinoma and primary SC (PSC) is challenging in IgG4-SC patients with biliary stenosis, which encompasses the hepatic portal and intrahepatic regions. While imaging and histopathological findings remain the most important means of diagnosing each disease, increased serum IgG4 levels have also become a useful diagnostic aid. In this chapter, we describe the roles and properties of serum IgG4, the relevance of serum IgG4 in various diseases, and finally outline the diagnostic value of serum IgG4 for IgG4-SC.
What Is the IgG4 Antibody?
IgG accounts for 70–75% of all human Igs and comprises four subclasses: G1, G2, G3, and G4. IgG4 normally represents only 3–6% of total IgG levels in the serum [2]. During immune responses, B cells that have reacted with antigens are activated and proliferate via antigen receptor-mediated signaling and CD40-mediated costimulation [3]. The B cells then differentiate into plasma cells to produce antibodies outside of follicles or remain inside follicles to form germinal centers differentiating into long-lived plasma cells or memory B cells. In activated B cells, the Ig heavy chain constant gene undergoes recombination for the production of IgG, IgE, and IgA while retaining antigenic specificity. This phenomenon is called class switching and occurs early in B cell activation. Cytokines such as interleukin (IL)-4, IL-10, and IL-21, which are produced by type 2 helper T (Th) cells, along with Th17 cells, regulatory T cells, and T follicular helper (Tfh) cells, may be involved in class switching to IgG4. Tfh cells are present in human peripheral blood and can be classified into Tfh1, Tfh2, and Tfh17 subsets. Tfh2 is closely related to IgG4 production and pathology in IgG4-RD [4].
Roles of the IgG4 Antibody
Functions of IgG by Subclass
Different IgG subclasses have high homology in the primary structure of the heavy chain constant region but possess distinct structural, chemical, and biological properties (Table 6.1). In healthy adults, the serum levels of IgG subclasses are maintained at a relatively constant ratio. IgG3 is slightly larger in molecular weight than the other subclasses and has a half-life of approximately 1 week, which is shorter than 3 weeks for the IgG1, IgG2, and IgG4 subclasses. Several similarities exist between IgG1 and IgG3 and between IgG2 and IgG4 with respect to biological and immunological properties: IgG1 and IgG3 bind strongly to complements, while IgG2 exhibits weak, and IgG4 virtually no, complement binding. IgG4 secretion is notably induced against parasites and allergens.
Functions of IgG4
Fc–Fc Binding (Fig. 6.1)
The fragment crystallizable (Fc) region of IgG4 binds to the Fc region of other IgG subclasses to form IgG4–IgG complexes with an apparent structure in which Igs are polymerized around IgG4. IgG4 aggregated through this IgG4 FC–IgG Fc binding may elicit local inflammation in lesions via integrin. Alternatively, IgG4 can bind to IgG1-type immune complexes to form larger complexes, thereby facilitating immune complex clearance from affected local sites to end the pathological state. The Fc region of Igs also plays important roles in complement activation, and IgG4 binding to the Fc region of other IgG subclasses can block the Fc-mediated inflammation process [5].
Fab Arm Exchange (Fig. 6.2)
IgG4 is typically secreted in the form of a dimer of weakly binding subunits. Unlike other IgG subclasses, the fragment antigen-binding (Fab) arms of IgG4 are exchanged with those of other IgG4 molecules, and the resulting antibody can recognize two different antigens. This so-called Fab arm exchange creates bispecific IgG4 that does not cross-link antigens and exhibits anti-inflammatory effects by decreasing the formation of immune complexes [6].
Diseases with Possible Pathological Involvement of IgG4
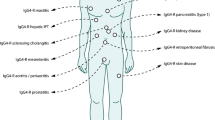
With its unique features of Fc–Fc binding, Fab arm exchange, and very low complement binding ability, IgG4 generally acts in immune response suppression. However, IgG4 also reportedly plays a pathogenic role in several diseases, as listed in Table 6.2 and described as follows:
Pemphigus Vulgaris and Pemphigus Foliaceus
Pemphigus vulgaris and pemphigus foliaceus are autoimmune skin diseases caused by antibodies against desmoglein 3 and 1. The anti-desmoglein autoantibodies are predominantly of the IgG4 subclass and are considered to be pathogenic after disease reproduction in mice receiving autoantibodies [7].
Rheumatoid Arthritis
IgG4 rheumatoid factors (RFs) are present in the serum and synovial fluid of patients with rheumatism. Although conventional RF binds via variable regions, IgG4 RF is reportedly mediated by nonspecific Fc–Fc binding. Elevated IgG4 levels in joints may be responsible for persistent, chronic synovial inflammation in patients with rheumatoid arthritis [8].
Thrombotic Thrombocytopenic Purpura
IgG subclass analysis of anti-ADAMTS13 antibodies has revealed the highest frequencies (90%) for IgG4 class autoantibodies, which may therefore play a central role in the immune response to this disease. Thrombotic thrombocytopenic purpura recurrence is more likely to occur in patients with high IgG4 and low IgG1 levels [9].
Anti-Muscle-Specific Kinase (MuSK) Antibody-Positive Myasthenia Gravis
IgG4 antibodies against the MuSK found in neuromuscular junctions are believed to be critical in disease pathology. Patient anti-MuSK antibodies are predominantly IgG4, and injected IgG from anti-MuSK-positive patients can reproduce the pathology of myasthenia gravis in mice [10].
Allergic Diseases
In allergic diseases, IgG4 is produced along with antigen-specific IgE because the IL-4 derived from allergen-specific T cells acts as a class-switching factor for IgE and IgG4. After regulatory T cells are induced, antigen-specific IgG4 titer becomes increased by hyposensitization therapy [11]. However, there remains debate on whether IgG4 enhances or suppresses allergic reactions.
IgG4-RD
Although IgG4-RD is considered by most to be an autoimmune disease, the precise role of IgG4 in IgG4-RD remains elusive. Shiokawa et al. [12] demonstrated the pathogenicity of IgG4 by showing that lesions were induced in the pancreas and salivary glands in mice injected with IgG, IgG1, and IgG4 from patients with IgG4-RD. They also witnessed that when IgG1 was coadministered with IgG4, it tended to show a reduced pathogenicity level than when injected alone. This result uncovered two sides of IgG4 in IgG4-RD; although IgG4 has weak pathogenicity, it can nonetheless suppress the pathogenicity of IgG1.
Serum IgG4 in IgG4-SC
Diagnostic Value of IgG4 in IgG4-SC
The disease concept of IgG4-RD includes elevated serum IgG4 levels as a hallmark diagnostic criterion. However, apart from AIP, there have been insufficient studies to establish precise cutoff values for IgG4-RD. Based on the clinical diagnostic criteria of IgG4-SC published in 2012 [13], hyper-IgG4-emia, defined as IgG4 levels of ≥135 mg/dl using nephelometry, has been accepted as a diagnostic item. In IgG4-SC, high serum IgG4 levels (i.e., ≥135 mg/dl) were present in 90% of patients [14, 15], but the reported sensitivity (64–90%) and specificity (87–93%) of hyper-IgG4-emia for diagnosing IgG4-SC have varied widely, presumably due to differences in assay methods and measurement kits, frequency of complicating type 1 AIP, and control group variance worldwide. Thus, although hyper-IgG4-emia is frequent in IgG4-SC and useful in diagnosis, it is not sufficiently specific such that cholangiocarcinoma (CC) and PSC can be ruled out solely on the basis of serological findings.
Differentiation from Cholangiocarcinoma
High serum IgG4 levels are absent in 10% of IgG4-SC patients and exist in 8–14% of patients with CC [14, 16]. Hence, there is a high risk of misdiagnosis when differentiating between IgG4-SC and CC using hyper-IgG4-emia alone as a benchmark. Conventional cutoff values have been determined on the basis of investigations of CC and multiple other diseases, including pancreatic cancer, PSC, and primary biliary cirrhosis. Serum IgG4 levels differ slightly from one disease to another, and IgG4 elevation (≥135 mg/dl) can be found at certain frequencies among each. Accordingly, a broad serum IgG4 cutoff value of >135 mg/dl may be useful for distinguishing IgG4-SC from CC. To achieve more accurate differentiation, the cutoff value needs to be set to at least twice the upper limit of the normal range, while a value of four times the upper limit of normal range yields a specificity of 99–100% [14, 16]. Meanwhile, IgG4-SC has been classified into four types based on cholangiographic findings [17] that are differentiated depending on the site of the lesion: type 1 IgG4-SC should be distinguished from chronic pancreatitis, distal CC, and pancreatic cancer when stenosis is present in the lower biliary duct, type 2 IgG4-SC from PSC when a broad internal region is affected, and type 3 and 4 IgG4-SC from hilar CC when stenosis is present in the hepatic portal region. An IgG4 cutoff value of 207 mg/dl is reportedly useful for the differential diagnosis of type 3 and 4 IgG4-SC with lesions in the hepatic portal region, where CC is an important disease to rule out [14]. However, differentiating IgG4-SC from CC based only on serum IgG4 levels remains difficult, and thus comprehensive diagnosis with consideration of imaging and histopathological findings is ultimately necessary.
Differentiation from PSC
High serum IgG4 levels are also found in 9–26% of PSC patients [14, 15, 18, 19]. The reported sensitivity and specificity of differentiating IgG4-SC from PSC at an IgG4 cutoff value of 117 mg/dl were 91.5% and 87.6%, respectively [14], which changed when different cutoffs were adopted (e.g., 90% and 85%, respectively, with 140 mg/dl; 70% and 98%, respectively, with 280 mg/dl; and 42% and 100%, respectively, with 560 mg/dl) [15]. As some studies have demonstrated that a serum IgG4 cutoff value of four times the upper limit of normal range can be useful for accurate differentiation, IgG4 appears to be of greatest utility for cases of severely increased serum levels. However, it may not have sufficient reliability for diagnosing patients with moderately increased serum IgG4; a cutoff value of 250 mg/dl afforded a sensitivity and specificity of 89% and 95%, but sensitivity fell to 67% in a different cohort. The sensitivity and specificity of diagnosing various diseases using serum IgG4 levels are summarized in Table 6.3. The use of the IgG4:IgG1 ratio has also been recommended for patients with IgG4 levels of less than twice the upper limit of normal, with a sensitivity and specificity of 86% and 95%, respectively, using an IgG4:IgG1 ratio cutoff value of 0.24 in patients with clinically elevated serum IgG4 [15]. IgG4-SC and PSC exhibit similar bile duct images but differ substantially in their treatment and prognosis. While corticosteroids elicit a good response in IgG4-SC, liver transplantation is the only therapeutic treatment option for advanced PSC. Accurate differential diagnosis is also important for determining appropriate care plans, for which data obtained on the basis of serum IgG4 levels are valuable. However, there are clear limitations to diagnoses based on serum IgG4 levels alone, and thus additional data, such as the presence or absence of lesions suggestive of IgG4-RD in other organs, histopathological findings from liver or bile duct biopsy, and biliary duct and other imaging findings, are crucial.
IgG4 Levels in Bile
Several recent pilot studies have reported that IgG4 levels in the bile are significantly elevated in patients with IgG4-SC and can be useful for distinguishing IgG4-SC from lower biliary strictures owing to other causes, such as SC, cholangiocarcinoma, and pancreatic cancer [19, 20]. This method of measuring IgG4 in the bile appears to be more effective for differential diagnosis in patients with low-to-moderately increased serum IgG4 than in those with abnormally high values. Moreover, bile can be safely collected without any special procedures in patients undergoing endoscopic retrograde cholangiopancreatography and IgG4 levels assessed inexpensively using conventional methods. Once the efficacy of this method is validated in larger cohorts, bile-based measurement of IgG4 is expected to contribute greatly to the timely and accurate diagnosis of IgG4-SC.
References
Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344(10):732–8.
Ochs HD, Wedgwood RJ. IgG subclass deficiencies. Annu Rev Med. 1987;38:325–40.
Crotty S. A brief history of T cell help to B cells. Nat Rev Immunol. 2015;15(3):185–9.
Akiyama M, Suzuki K, Yamaoka K, Yasuoka H, Takeshita M, Kaneko Y, et al. Number of circulating follicular helper 2 T cells correlates with IgG4 and Interleukin-4 levels and Plasmablast numbers in IgG4-related disease. Arthritis Rheumatol. 2015;67(9):2476–81.
Kawa S, Kitahara K, Hamano H, Ozaki Y, Arakura N, Yoshizawa K, et al. A novel immunoglobulin-immunoglobulin interaction in autoimmunity. PLoS One. 2008;3(2):e1637.
van der Neut Kolfschoten M, Schuurman J, Losen M, Bleeker WK, Martinez-Martinez P, Vermeulen E, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317(5844):1554–7.
Anhalt GJ, Labib RS, Voorhees JJ, Beals TF, Diaz LA. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. N Engl J Med. 1982;306(20):1189–96.
Zack DJ, Stempniak M, Wong AL, Weisbart RH. Localization of an Fc-binding reactivity to the constant region of human IgG4. Implications for the pathogenesis of rheumatoid arthritis. J Immunol. 1995;155(10):5057–63.
Ferrari S, Mudde GC, Rieger M, Veyradier A, Kremer Hovinga JA, Scheiflinger F. IgG subclass distribution of anti-ADAMTS13 antibodies in patients with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2009;7(10):1703–10.
Cole RN, Reddel SW, Gervasio OL, Phillips WD. Anti-MuSK patient antibodies disrupt the mouse neuromuscular junction. Ann Neurol. 2008;63(6):782–9.
Robinson DS, Larche M, Durham SR. Tregs and allergic disease. J Clin Invest. 2004;114(10):1389–97.
Shiokawa M, Kodama Y, Kuriyama K, Yoshimura K, Tomono T, Morita T, et al. Pathogenicity of IgG in patients with IgG4-related disease. Gut. 2016;65(8):1322–32.
Ohara H, Okazaki K, Tsubouchi H, Inui K, Kawa S, Kamisawa T, et al. Clinical diagnostic criteria of IgG4-related sclerosing cholangitis 2012. J Hepatobiliary Pancreat Sci. 2012;19(5):536–42.
Ohara H, Nakazawa T, Kawa S, Kamisawa T, Shimosegawa T, Uchida K, et al. Establishment of a serum IgG4 cut-off value for the differential diagnosis of IgG4-related sclerosing cholangitis: a Japanese cohort. J Gastroenterol Hepatol. 2013;28(7):1247–51.
Boonstra K, Culver EL, de Buy Wenniger LM, van Heerde MJ, van Erpecum KJ, Poen AC, et al. Serum immunoglobulin G4 and immunoglobulin G1 for distinguishing immunoglobulin G4-associated cholangitis from primary sclerosing cholangitis. Hepatology. 2014;59(5):1954–63.
Oseini AM, Chaiteerakij R, Shire AM, Ghazale A, Kaiya J, Moser CD, et al. Utility of serum immunoglobulin G4 in distinguishing immunoglobulin G4-associated cholangitis from cholangiocarcinoma. Hepatology. 2011;54(3):940–8.
Nakazawa T, Ohara H, Sano H, Ando T, Joh T. Schematic classification of sclerosing cholangitis with autoimmune pancreatitis by cholangiography. Pancreas. 2006;32(2):229.
Mendes FD, Jorgensen R, Keach J, Katzmann JA, Smyrk T, Donlinger J, et al. Elevated serum IgG4 concentration in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2006;101(9):2070–5.
Vosskuhl K, Negm AA, Framke T, Weismuller T, Manns MP, Wedemeyer H, et al. Measurement of IgG4 in bile: a new approach for the diagnosis of IgG4-associated cholangiopathy. Endoscopy. 2012;44(1):48–52.
Navaneethan U, Gutierrez NG, Jegadeesan R, Venkatesh PG, Poptic E, Sanaka MR, et al. IgG4 levels in bile for distinguishing IgG4-associated cholangiopathy from other biliary disorders: a single blinded pilot study. Clin Endosc. 2014;47(6):555–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Ito, T., Watanabe, T., Muraki, T., Kawa, S. (2019). Serum IgG4. In: Kamisawa, T., Kim, MH. (eds) IgG4-Related Sclerosing Cholangitis. Springer, Singapore. https://doi.org/10.1007/978-981-10-4548-6_6
Download citation
DOI: https://doi.org/10.1007/978-981-10-4548-6_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4547-9
Online ISBN: 978-981-10-4548-6
eBook Packages: MedicineMedicine (R0)