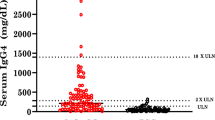
Abstract
IgG4-related sclerosing cholangitis (IgG4-SC) is a biliary manifestation of a systemic condition known as IgG4-related disease (IgG4-RD). IgG4-RD may involve various organs with type 1 autoimmune pancreatitis (AIP) being the leading manifestation. Older men are more affected by IgG4-SC than women (ratio of 4:1), and the most common clinical manifestation is obstructive jaundice. The pathogenic mechanism of IgG4-SC remains unclear. Steroid therapy can lead to clinical and radiological improvement when provided in the inflammatory phase of this disease. However, long-term outcome data in patients with IgG4-SC are lacking. In this chapter, we focus on the prognosis of IgG4-SC, including the natural course, treatment response, risk of malignancy, and long-term outcome, based on current evidence.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- IgG4-related Sclerosing Cholangitis (IgG4-SC)
- Autoimmune Pancreatitis (AIP)
- Biliary Manifestations
- Long-term Outcome Data
- Biliary Intraepithelial Neoplasia
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
IgG4-related sclerosing cholangitis (IgG4-SC) is a biliary manifestation of a systemic condition known as IgG4-related disease (IgG4-RD). IgG4-RD may involve various organs with type 1 autoimmune pancreatitis (AIP) being the leading manifestation. Older men are more affected by IgG4-SC than women (ratio of 4:1), and the most common clinical manifestation is obstructive jaundice. The pathogenic mechanism of IgG4-SC remains unclear [1]. Steroid therapy can lead to clinical and radiological improvement when provided in the inflammatory phase of this disease. However, long-term outcome data in patients with IgG4-SC are lacking. In this chapter, we focus on the prognosis of IgG4-SC, including the natural course, treatment response, risk of malignancy, and long-term outcome, based on current evidence.
Natural Course
The natural history of IgG4-SC has not been well defined. Nakazawa et al. reported a classification of cholangiographic changes in IgG4-SC as follows: stenosis is located only in the lower part of the common bile duct in type 1; stenosis is diffusely distributed in the intra- and extrahepatic bile ducts in type 2; stenosis is detected in hilar hepatic lesions and the lower part of the common bile duct in type 3; and strictures of the bile duct are detected only in hilar hepatic lesions in type 4 [2]. Substantial spontaneous improvement is sometimes observed in type 1 IgG4-SC, with improvement of strictures probably correlating with reduced pancreatic inflammation around the distal common bile duct. However, in IgG4-SC types 2–4, an improvement without treatment is unusual [3]. Most AIP cases that spontaneously improve do not have bile duct stenosis. Kamisawa et al. noted that, among 21 patients with AIP, spontaneous improvement was detected in two (10%) patients without jaundice [4]. Kubota et al. compared clinicopathological parameters in 8 patients with AIP and remission in the absence of steroid therapy and in 12 patients with remission after steroid therapy [5]. They found an association between spontaneous remission and the absence of obstructive jaundice. Based on these observations, spontaneous remission of bile duct stenosis in IgG4-SC appears to be rare, except for some type 1 cases.
Treatment Response and Relapse
The aims of treatment in IgG4-RD are to alleviate symptoms and to prevent disease-related complications and irreversible fibrosis. An international consensus of experts on disease management concluded that urgent treatment is appropriate in biliary disease, even when asymptomatic, to prevent infectious cholangitis and permanent fibrosis that might complicate untreated disease [6]. Despite an absence of randomized, placebo-controlled trials, the mainstay of treatment for IgG4-RD is systemic corticosteroids, extrapolated from findings in AIP. Kamisawa et al. reported that use of steroid-induced remission was quicker and more consistent and had a lower relapse rate compared with the conservative approach in 563 patients with AIP, 314 (55.8%) of whom had coexisting IgG4-SC (Table 19.1) [7].
Comparison among studies can be difficult because of heterogeneous patient groups, use of different diagnostic criteria, and definitions of response. Despite these limitations, most patients with IgG4-SC respond to steroid therapy. Ghazale et al. reported an illustrative series including 53 patients with IgG4-SC, 30 of whom were treated initially with prednisone (40 mg/day for 4 weeks followed by tapering of 5 mg per week for a total of 11 weeks) (Table 19.1) [8]. An initial response to glucocorticoids was observed in 29 (97%) of these patients, but only 18 (60%) had resolution of strictures and normalization of liver biochemical tests. Sandanayake et al. performed a prospective study that evaluated 28 patients with AIP, 23 of whom had coexisting IgG4-SC (Table 19.1) [9]. All of the patients responded within 6 weeks to glucocorticoid therapy (prednisolone 30 mg/day followed by tapering of 5 mg per every 2 weeks). A total of 23 (82%) patients achieved remission after a median of 5 months of treatment (range 1.5–17 months), whereas 5 (18%) patients could not be weaned because of a disease flare.
Patients with IgG4-SC are at high risk of relapse, the majority of which occurs within 6 months of discontinuing or tapering steroid treatment. Relapse rates have been reported in approximately 30–50% of patients after corticosteroid therapy (Table 19.1). Known risk factors for relapse include increased IgG4 levels and the presence of proximal bile duct strictures [7,8,9,10,11]. Relapsed disease develops either at the same site as the original disease or in a different portion of the biliary tree. New lesions may also appear in other organs. Additional high-dose steroids remain highly successful for reinduction of remission in patients with relapse. Other approaches include immunomodulators, such as azathioprine, 6-mercaptopurine, and mycophenolate mofetil. However, no reliable data are available in terms of how effective these drugs are in reinduction of remission in patients with relapsed IgG4-SC [11].
Rituximab, a monoclonal CD20 antibody leading to B-cell depletion, has been increasingly recognized as a promising treatment for IgG4-RD [12, 13]. The first reported patient with IgG4-RD who was administered rituximab had relapsed IgG4-SC and was refractory to steroids and 6-mercaptopurine [13]. This patient was treated with rituximab, and remission was successfully achieved. Based on the findings of subsequent studies, including a recent phase I/II study, rituximab appears to be effective for inducing and maintaining remission [11, 13, 14]. Therefore, rituximab may be worth considering for patients with previous intolerance to high-dose steroids and those at a high risk of relapse [12, 13]. Therefore, as is the case with IgG4-RD in other organs, the initial response to glucocorticoids is favorable, but treatment for steroid-refractory cases of IgG4-SC needs further study.
Risk of Malignancy
Some studies have suggested that the presence of IgG4-RD is associated with an increased risk of malignancy, which may involve a variety of organs and tissues. This risk may be particularly increased in the year after diagnosis of IgG4-RD. However, other studies have not found such a risk, and this issue remains controversial.
Huggett et al. reported the risk of type 1 AIP and IgG4-SC in patients in a UK cohort [10]. A total of 115 patients, 68 (59%) of whom had IgG4-SC, were included, with a median follow-up from diagnosis of 32.5 months (range: 0.8–107 months). At least 12-month follow-up data were available for 88 patients (77% of the cohort). Among 115 patients, 13 (11%) were diagnosed with a malignancy within 3 years before the diagnosis of IgG4-RD, concurrently, or during follow-up, including three hepatopancreaticobiliary cancers. The risk of any cancer at diagnosis or during follow-up compared with matched national statistics was increased (odds ratio, 2.25; 95% confidence interval [CI] 1.12–3.94). We also reported that patients with type 1 AIP were at high risk of having various cancers [15]. In a series of 108 Japanese patients with AIP, 57 (52.8%) of whom had coexisting IgG4-SC, 18 cancers were found in 15 (13.9%) patients; the median follow-up was 3.3 years. The standardized incidence ratio (SIR) of cancer was 2.7 (95% CI 1.4–3.9), which was stratified into the first year (6.1, 95% CI 2.3–9.9) and subsequent years (1.5, 95% CI 0.3–2.8) after diagnosis of AIP. The relative risk of cancer among patients with AIP at the time of diagnosis of AIP was 4.9 (95% CI 1.7–14.9). In six of eight patients whose cancer lesions could be assessed before corticosteroid therapy for AIP, abundant IgG4-positive plasma cellular infiltration was observed in the cancer stroma. These six patients experienced no relapse of AIP after successful treatment of cancer. These data suggest that a certain portion of AIPs can be categorized as a paraneoplastic syndrome.
However, Hart et al. reported that the incidence of malignancy in 116 patients with type 1 AIP was not significantly higher than that in 344 age- and sex-matched control subjects [16]. In their study, the median follow-up was 3.6 years for patients with AIP and 3.2 years for control subjects. The proportion of patients diagnosed with cancer at any point before diagnosis of AIP (10.3%) was lower than that in the matched control subjects (17.4%). The odds of having AIP were lower in patients with cancer before the index date compared with those without cancer (odds ratio, 0.5; 95% CI 0.23–1.08), but this was not significant (P = 0.08). Similarly, the risk of developing cancer after the index date was lower for patients with AIP compared with those without AIP, but this did not reach significance (hazard ratio, 0.64; 95% CI 0.272–1.51; P = 0.31).
An association of IgG4-SC with invasive carcinoma of the biliary tree has not been demonstrated, but some case reports have been published. Oh et al. described epithelial atypia in the common bile duct (suggestive of biliary intraepithelial neoplasia) in the presence of bile duct affection of AIP [17]. Straub et al. reported a case of intrahepatic cholangiocarcinoma arising in a patient with IgG4-SC [18].
Further observation and investigation are required to establish or rule out a possible relation between IgG4-SC and the risk of malignancy including cholangiocarcinoma.
Long-Term Outcome
With regard to long-term outcomes, whether IgG4-SC progresses to liver cirrhosis and how rapidly this could occur remain unclear. End-stage liver disease is an uncommon complication in patients with IgG4-SC [14]. A 2012 Japanese national survey of primary sclerosing cholangitis and IgG4-SC identified 43 patients with IgG4-SC who did not have pancreatic involvement (Table 19.2) [19]. The median follow-up period was 2.3 ± 1.8 years for patients with IgG4-SC. According to this survey, the 3-year survival rate was 90.0% for IgG4-SC, and liver transplantation was not performed in any of the patients with IgG4-SC (Table 19.2).
Hirano et al. reported long-term follow-up of IgG4-SC cases without steroid therapy [20]. They showed that two patients developed portal obstruction and liver atrophy, but there were no signs of liver cirrhosis or failure. Ghazale et al. reported that four of 53 patients with IgG4-SC showed portal hypertension and liver cirrhosis within 5 years of the onset of initial symptoms (Table 19.2) [8]. Three of these patients were treatment-naive at the time of diagnosis of cirrhosis and one patient was a treatment nonresponder. The time from onset of initial symptoms to the diagnosis of cirrhosis was 62, 36, and 9 months in the 3 treatment-naive patients and 26 months in the treatment nonresponder. With the exception of one patient who had a history of moderate alcohol intake, no coexisting etiologies of chronic liver disease were identified in these four patients. Death occurred in 7 of the 53 patients. The causes of death were complications of end-stage liver disease (n = 1), metastatic pancreatic cancer (n = 1), pneumonia and sepsis (n = 1), acute stroke (n = 1), congestive heart failure (n = 1), and unknown causes (n = 2) [8]. Huggett et al. reported 115 patients with type 1 AIP and IgG4-SC in a UK cohort and found that 68 (59%) had IgG4-SC and 6 (5%) developed liver cirrhosis during follow-up (Table 19.2) [10]. Cirrhosis was diagnosed on liver biopsy in two patients and clinically in four patients (patients with signs of synthetic dysfunction and/or hepatic decompensation with consistent radiology). One patient underwent a successful liver transplantation. Portal and/or splenic vein thrombosis developed in 9% of patients, but there was no evidence of variceal bleeding in any of the patients. Eleven (10%) patients died during follow-up. The causes of death were complications of end-stage liver disease (n = 1), cholangiocarcinoma (n = 1), transitional cell carcinoma of the bladder (n = 1), lung cancer (n = 1), cancer of an unknown primary site (n = 1), autoimmune encephalitis (n = 1), pulmonary fibrosis (n = 2), pulmonary embolism (n = 1), pneumonia (n = 1), and postoperative death (n = 1).
Collectively, IgG4-SC appears to have a favorable prognosis, probably due to the excellent response to corticosteroid therapy. However, importantly, a few cases of IgG4-SC can progress to liver cirrhosis. Further studies are required to determine the long-term outcome of IgG4-SC.
Conclusions
Recent studies have shown that patients with IgG4-SC have a favorable response to steroid treatment, but many patients experience relapse after therapy. However, because IgG4-SC is a relatively new concept, our knowledge regarding the risk of malignancy and long-term outcome is still limited. Additionally, various criteria for diagnosis, treatment response, remission, and relapse in previous studies make it difficult for comparison among studies. To resolve these problems and to obtain a better understanding about the pathophysiology IgG4-SC, further studies, including global case series and multicenter studies, are required using standardized criteria for diagnosis and treatment outcome.
References
Okazaki K, Uchida K, Koyabu M, Miyoshi H, Ikeura T, Takaoka M. IgG4 cholangiopathy: current concept, diagnosis, and pathogenesis. J Hepatol. 2014;61:690–5.
Nakazawa T, Ohara H, Sano H, Ando T, Joh T. Schematic classification of sclerosing cholangitis with autoimmune pancreatitis by cholangiography. Pancreas. 2006;32:229.
Culver EL, Chapman RW. IgG4-related hepatobiliary disease: an overview. Nat Rev Gastroenterol Hepatol. 2016;13:601–12.
Kamisawa T, Yoshiike M, Egawa N, Nakajima H, Tsuruta K, Okamoto A. Treating patients with autoimmune pancreatitis: results from a long-term follow-up study. Pancreatology. 2005;5:234–40.
Kubota K, Iida H, Fujisawa T, Yoneda M, Inamori M, Abe Y, et al. Clinical factors predictive of spontaneous remission or relapse in cases of autoimmune pancreatitis. Gastrointest Endosc. 2007;66:1142–51.
Khosroshahi A, Wallace ZS, Crowe JL, Akamizu T, Azumi A, Carruthers MN, et al. Second international symposium on IgG4-related disease. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol. 2015;67:1688–99.
Kamisawa T, Shimosegawa T, Okazaki K, Nishino T, Watanabe H, Kanno A, et al. Standard steroid treatment for autoimmune pancreatitis. Gut. 2009;58:1504–7.
Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, et al. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134:706–15.
Sandanayake NS, Church NI, Chapman MH, Johnson GJ, Dhar DK, Amin Z, et al. Presentation and management of post-treatment relapse in autoimmune pancreatitis/immunoglobulin G4-associated cholangitis. Clin Gastroenterol Hepatol. 2009;7:1089–96.
Huggett MT, Culver EL, Kumar M, Hurst JM, Rodriguez-Justo M, Chapman MH, et al. Type 1 autoimmune pancreatitis and IgG4-related sclerosing cholangitis is associated with extrapancreatic organ failure, malignancy, and mortality in a prospective UK cohort. Am J Gastroenterol. 2014;109:1675–83.
Hart PA, Kamisawa T, Brugge WR, Chung JB, Culver EL, Czakó L, et al. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut. 2013;62:1771–6.
Hart PA, Topazian MD, Witzig TE, Clain JE, Gleeson FC, Klebig RR, et al. Treatment of relapsing autoimmune pancreatitis with immunomodulators and rituximab: the Mayo Clinic experience. Gut. 2013;62:1607–15.
Topazian M, Witzig TE, Smyrk TC, Pulido JS, Levy MJ, Kamath PS, et al. Rituximab therapy for refractory biliary strictures in immunoglobulin G4-associated cholangitis. Clin Gastroenterol Hepatol. 2008;6:364–6.
Zen Y, Kawakami H, Kim JH. IgG4-related sclerosing cholangitis: all we need to know. J Gastroenterol. 2016;51(4):295–312.
Shiokawa M, Kodama Y, Yoshimura K, Kawanami C, Mimura J, Yamashita Y, et al. Risk of cancer in patients with autoimmune pancreatitis. Am J Gastroenterol. 2013;108:610–7.
Hart PA, Law RJ, Dierkhising RA, Smyrk TC, Takahashi N, Chari ST. Risk of cancer in autoimmune pancreatitis: a case-control study and review of the literature. Pancreas. 2014;43:417–21.
Oh HC, Kim JG, Kim JW, Lee KS, Kim MK, Chi KC, et al. Early bile duct cancer in a background of sclerosing cholangitis and autoimmune pancreatitis. Intern Med. 2008;47:2025–8.
Straub BK, Esposito I, Gotthardt D, Radeleff B, Antolovic D, Flechtenmacher C, et al. IgG4-associated cholangitis with cholangiocarcinoma. Virchows Arch. 2011;458:761–5.
Tanaka A, Tazuma S, Okazaki K, Tsubouchi H, Inui K, Takikawa H. Nationwide survey for primary sclerosing cholangitis and IgG4-related sclerosing cholangitis in Japan. J Hepatobiliary Pancreat Sci. 2014;21:43–50.
Hirano A, Nakazawa T, Ohara H, Ando T, Hayashi K, Tanaka H, et al. Liver atrophy and portal stenosis in two cases of sclerosing cholangitis associated with autoimmune pancreatitis. Intern Med. 2008;47:1689–94.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Kuwada, T., Shiokawa, M., Tomono, T., Uza, N., Kodama, Y. (2019). Prognosis. In: Kamisawa, T., Kim, MH. (eds) IgG4-Related Sclerosing Cholangitis. Springer, Singapore. https://doi.org/10.1007/978-981-10-4548-6_19
Download citation
DOI: https://doi.org/10.1007/978-981-10-4548-6_19
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4547-9
Online ISBN: 978-981-10-4548-6
eBook Packages: MedicineMedicine (R0)