Abstract
Epithelial ovarian tumors (EOTs) are associated with a variety of distinct morphological characteristics that include serous, endometrioid, clear cell, and mucinous features and have a spectrum of biological behavior that ranges from benign to malignant. Traditionally, EOTs were believed to originate from the ovarian surface epithelium (OSE), but the latest research supports the concept that some subtypes of EOTs originate from extra-ovarian sites. Although a couple of paradigms in regard to the morphological and molecular pathogenesis of EOTs have dramatically changed in recent years, the delineation between old and new concepts remains confused. This chapter summarizes those concepts and the morphological and molecular alterations associated with each major subtype of EOT, to improve our understanding of the pathogenesis of EOTs.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Epithelial ovarian tumor
- Serous tumor
- Endometrioid tumor
- Clear cell tumor
- Secondary Müllerian system
- Imported disease
- Two-tiered classification
- Type I
- Type II
3.1 Introduction
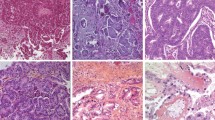
Epithelial ovarian tumors (EOTs) represent a complex family of neoplasms, each with different morphologies that do not necessarily reflect that of the ovary. The major morphological types of EOTs (serous, endometrioid, clear cell, and mucinous) may variously resemble the epithelia of the adnexal (fallopian tube) and uterine regions (proliferative endometrium, endometrium with Arias-Stella reaction, and endocervix) but also the intestinal epithelium (Fig. 3.1). In regard to clinical behavior, EOTs can be further subdivided into benign and malignant tumors, with intermediate tumors of borderline malignancy. Malignant EOTs are generally known as “ovarian carcinomas.”
The major morphological types of epithelial ovarian tumors and the mimic normal epithelia; (a and b) fallopian tubal epithelium (a) and serous carcinoma (b); (c and d) endometrium in proliferative phase (c) and endometrioid carcinoma (d); (e and f) gestational endometrium with Arias-Stella reaction (e) and clear cell carcinoma (f); (g and h) endocervical epithelium (g) and mucinous carcinoma which recently is classified in seromucinous carcinoma (h); (i and j) intestinal epithelium (i) and mucinous carcinoma (j) [(a–j) hematoxylin and eosin staining; (a–j) ×200]
The source of EOTs has been a recent topic of debate [1, 2]. The past and current paradigm is that EOTs arise from the ovarian surface epithelium (OSE) covering the ovary and lining inclusion cysts which are derived from surface invaginations. OSE originating developmentally from the coelomic epithelium is composed of flat, nondescript cells morphologically similar to the mesothelium lining of the peritoneal cavity. The OSE is thought to be capable of metaplasia to a Müllerian phenotype resembling oviductal, endometrial, or endocervical epithelia, known as a secondary Müllerian system [3,4,5]. Thus, the OSE is suspected to carry pluripotent cells, i.e., putative stem cells. The recent identification of such stem cells implicates the OSE in the pathogenesis of EOTs [6, 7].
Other recent studies have indicated that a considerable number of EOTs originate in the fallopian tube and the endometrium, before migrating to the ovary. This theory, one of “imported disease”, is thought to be an influential paradigm shift in the morphological theory of EOTs. According to this theory, serous tumors arise from the implantation of epithelium from the oviduct, and endometrioid and clear cell tumors are associated with endometriosis that mainly develops from retrograde menstruation [4].
Clinical, morphological, and molecular studies have provided a model for malignant EOTs, with two broad categories designated as Type I and Type II. Type I carcinomas progress in an indolent course, are usually confined to the ovary at diagnosis, and are relatively genetically stable. Type I carcinomas exhibit a shared lineage with their corresponding benign and borderline-malignant tumors, supporting the concept of a morphological sequence of tumor progression. In contrast, Type II carcinomas are highly aggressive, progress rapidly, and are usually in an advanced stage at diagnosis. Type II carcinomas do not exhibit the shared lineage and are genetically unstable [8].
In the first section of this chapter, these important theories of ovarian tumorigenesis and the two-tiered system of classification are introduced and organized. Finally, the morphological and molecular details of four representative malignant EOTs, namely, serous carcinoma (high grade, low grade), endometrioid carcinoma, clear cell carcinoma, and mucinous carcinoma, will be presented and discussed.
3.2 Representative Theories Related to the Morphological Pathogenesis of EOTs
3.2.1 The Theory of the Secondary Müllerian System
In the first half of the twentieth century, it was believed that the OSE, which was also referred to as the germinal epithelium, carried pluripotent stem cells which differentiate to germ cells and follicular cells [9, 10]. Even now, it is thought that the OSE is derived from a common embryonic origin in the pluripotent coelomic epithelium which gives rise to the Müllerian ducts, i.e., the epithelia of the fallopian tubes, endometrium, uterine cervix, and upper part of the vagina. According to this theory, a subset of pluripotent OSE cells and cells lining the inclusion cysts have the propensity to differentiate along the lineage of the Müllerian epithelium [11], with this therefore being referred to as a secondary Müllerian system [12,13,14]. For example, serous metaplasia of OSE inclusion cysts is characterized by a cuboidal epithelium with cilia, which mimics the endosalpingeal epithelium [15] (Fig. 3.2a). With this in mind, the morphological alteration of the OSE and its inclusion cysts has been suggested as a potential site of origin for the development of EOTs [5] (see also Sect. 5.3 in Chap. 5).
Putative or possible sources of the major types of epithelial ovarian tumors. (a) inclusion cyst (cuboidal epithelium partially with cilia); (b) endometriosis; (c) adenofibroma (clear cell type); (d) teratoma (squamous cell and mucinous epithelia); (e) transitional cell (Walthard) nests [(a–e) hematoxylin and eosin staining; (a–e) ×200]
In regard to EOT tumorigenesis, the “incessant ovulation” hypothesis for ovarian cancer, which postulates that follicular rupture [16] and repair trauma increases OSE cell proliferation and risk of transformation, was proposed more than 40 years ago [17]. Besides primary endocrinological functions, gonadotropin hormones, such as follicle-stimulating hormone (FSH), luteinizing hormone (LH), and human chorionic gonadotropin (hCG), are thought to be involved in OSE cell proliferation and the repair of OSE following ovulatory trauma [18, 19]. Invagination and inclusion cysts form in the ovarian cortex as a result of the repair, and exposure of the entrapped cyst-lined OSE cells to foreign micro-substances within the cystic lumen, which come from the outside environment via the fallopian tube, causes their transformation [5]. In this process, stemlike cells undergo metaplasia and transformation to acquire a highly complex morphology resembling either the Müllerian duct-derived fallopian tube (serous type), the endometrium (endometrioid type), the endometrium with Arias-Stella reaction (clear cell type), or the endocervix (seromucinous or previously mucinous type) [20] (Fig. 3.1a–h).
Initially, the “incessant ovulation” hypothesis proposed for the stemlike cells of OSE was not widely accepted. The first scientific evidence for the existence of putative stem cells on the ovarian surface came in 2008, with a subset of stemlike cells experimentally identified by a stemness assay [6]. Subsequently, a subset of OSE cells expressing a common hematopoietic stem cell marker (Ly6a+) were identified in adult mouse ovaries [21], and more recent in vivo studies have used fate-mapping methodologies to provide direct evidence for the existence and location of self-renewing epithelial stem cells in the ovary [7, 22]. Stemlike OSE cells that display high aldehyde dehydrogenase (ALDH) activity [22] and high ALDH activity with expression of LGR5 (leucine-rich repeat-containing G protein-coupled receptor 5) [7] have been located in both the murine and human ovary hilum [20]. In view of such findings, it has been suggested that OSE stem cells might participate in postovulatory wound closure, as well as the tumorigenesis of EOTs [18, 23, 24].
3.2.2 The Theory of Imported Disease
Recent investigations have revealed that high-grade serous carcinomas are derived from the fimbriae of the fallopian tube. The theory of tubal involvement in the tumorigenesis of high-grade serous carcinoma proposes that serous tubal intraepithelial carcinomas (STICs) (Fig. 3.3a, b), which are known to occur in the fimbriae, are ectopically implanted into the ovarian stroma as cortical inclusion cysts, and that the exposure of these cells to the ovarian stromal microenvironment, which produces abundant growth factors designed for folliculogenesis, leads to “ovarian cancer” consisting of high-grade serous carcinoma [4, 8].
In endometrioid and clear cell carcinoma, it has been well recognized that malignant transformation can occur at epithelial components of endometriosis in endometriotic cysts of the ovary. A follow-up program for endometriotic cysts confirmed the risk of malignant transformation resulting from endometriosis [25]. Endometriosis is thought to occur via retrograde menstruation, where endometrial epithelial cells and stromal cells move from the uterus through the fallopian tubes and subsequently become established as an endometriotic cyst [26, 27]. It is also known that retrograde stromal cells from the endometrium can implant to the ovary during menstruation, inducing a metaplastic change in the OSE and resulting in endometriosis [28]. This creates a unique microenvironment where menstruation-like blood are trapped within the cyst, resulting in high concentrations of iron in a confined space, subsequent oxidative stress, and a hypoxic environment that promotes DNA damage and the accumulation of mutations [29,30,31,32].
Such studies suggest that the fallopian tube epithelium (benign or malignant) can implant onto the ovary to give rise to both low-grade and high-grade serous carcinomas and that similarly, endometrial tissue can implant onto the ovary with resulting endometriosis, then undergoing malignant transformation into endometrioid and clear cell carcinoma. According to the theory of “imported disease”, these EOTs are not ovarian in origin therefore but rather represent “imported disease”, and it is logical to conclude that the only true primary ovarian neoplasms are germ cell and gonadal stromal tumors, analogous to the situation in the testis which does not have epithelial tumors [4] (see also Sect. 5.3 in Chap. 5).
3.3 Two-Tiered Classification for Clinical, Morphological, and Molecular Pathogenesis
EOTs can be classified into Types I and II, which correspond to two distinct models of clinical, morphological, and molecular pathogenesis [33]. Type I tumors develop slowly, in a stepwise manner, from premalignant conditions or borderline tumors, and include low-grade serous carcinomas, endometrioid carcinomas, clear cell carcinomas, and mucinous carcinomas. In contrast, Type II tumors grow rapidly and are typically found to have spread beyond the ovaries at presentation. The predominate Type II tumors are high-grade serous carcinomas, with the remainder being carcinosarcomas and undifferentiated carcinomas. It was originally thought, since these tumors are rarely associated with morphologically recognizable precursor lesions, that they develop de novo from ovarian inclusion cysts or the surface epithelium [34, 35]. More recently, however, it has been recognized that Type II tumors with pelvic dissemination include carcinomas arising from the epithelium of the fimbriae.
Molecular studies have revealed that distinct biological signatures, compatible with the Type I and Type II classification system, exist among EOT subtypes. Although Type I carcinomas lack mutations in the TP53 gene and have a stable genome, each morphological subtype exhibits a distinctive molecular profile. Moreover, Type I carcinomas typically exhibit a shared lineage with their corresponding benign and borderline-malignant tumors, supporting the concept of a morphological sequence of tumor progression. Type II carcinomas display TP53 mutations in 80% or more of cases and rarely harbor the mutations that are found in Type I carcinomas. Type II carcinomas are typically associated with chromosome aneuploidy or chromosomal copy number abnormality resulting from an inherent chromosomal instability [8].
3.4 Morphological and Molecular Pathogenesis in Four Representative Malignant EOTs
3.4.1 Serous Carcinoma
3.4.1.1 High-Grade Serous Carcinoma
High-grade serous carcinoma is the most common type of malignant EOT and is classified as Type II. Morphologically, the tumor cells of high-grade serous carcinoma resemble the secretory cells of three distinct cell types from the fallopian tube epithelium, namely, secretary cells, ciliated cells, and peg cells [36,37,38]. Almost all of these tumors express the transcription factor PAX8 that is a marker of the secretory cell lineage in the fallopian tube epithelium. Until recently, all high-grade serous carcinomas were presumed to arise de novo in ovarian inclusion cysts or the OSE, although identification of putative precursors in these tissues had previously been difficult. Since the discovery of the tumor suppressor genes BRCA1 [39] and BRCA2 [40] (BRCA1/2) in 1994 and 1995, respectively, the use of mutation analysis in healthy women with a family history of hereditary breast and ovarian cancer (HBOC) syndrome has increased rates of prophylactic bilateral salpingo-oophorectomy. These surgical specimens have revealed that a subset of the fimbrial epithelium of the fallopian tube have lesions of occult carcinoma and STIC without any lesions in the ovary [41, 42]. It has also been reported that STIC of the fimbriae is concomitant with high-grade serous carcinoma of the ovary in sporadic but not only germline types and that STIC lesions have the same TP53 mutations as the ovarian lesions. The TP53 mutation findings indicate that there is clonal expansion between STIC and high-grade serous carcinoma of the ovary [43]. In fact, p53 immunopositivity by TP53 mutation in STIC is occasionally found in cases with high-grade serous carcinoma (Fig. 3.3). Furthermore, it has been revealed that small foci of p53-immunoreactive cells exist in largely histologically normal fallopian tube epithelium [36]. These foci, which predominate in the distal portion of the fallopian tube, have been designated “p53 signatures”. These p53 signatures probably represent early clonal expansion [44] and are found at the same frequency in women with or without BRCA1/2 mutation [36]. TP53 mutation is thus one of the earliest events in the genesis of high-grade serous carcinoma and may occur first in the discrete foci that lead to STIC in the distal fallopian tube. Extensive investigations have now examined the role of the fallopian tube in pathogenesis of the serous type of EOTs [36, 43, 45,46,47], yet it is clear that at least a subset of ovarian high-grade serous carcinomas do not have STIC involvement. Therefore, it is still considered that OSE may be a candidate as the site of origin for high-grade serous carcinoma without STIC. The exact proportion of tumors of ovarian and tubal derivation in cases of high-grade serous carcinoma could be revealed with the widespread implementation of an established pathology protocol for sectioning and examination of the fimbriae [46].
In high-grade serous carcinomas of the ovary including sporadic and hereditary types, TP53 gene mutations are found in 95% or more of cases [48, 49]. Mutations in several other tumor suppressor genes and oncogenes, such as NF1, RB1, and CDK12, have been reported, but their mutation frequency is low [49,50,51]. As somatic mutations in BRCA1/2 are known to be uncommon in sporadic serous tumors, it is possible that these genes are inactivated by mechanisms (loss of heterozygosity and/or methylation) other than mutation [52,53,54]. The proposed model is that loss of p53 and BRCA1/2 are early events that lead to a deficiency in homologous recombination repair of DNA double-strand breaks, triggering chromosomal instability and widespread copy number changes [34, 44, 49, 55,56,57,58,59,60,61]. The most common amplifications affect the genes MYC, CCNE, and MECOM, each of which is highly amplified in more than 20% of high-grade serous carcinomas [49], but it is the MYC gene that is the most often amplified and overexpressed [62]. In regard to other cancer-associated pathways harboring mutations, copy number changes, or changes in gene expression, the RB and phosphoinositide 3-kinase (PI3K)/RAS pathways are deregulated in 67% and 45% of high-grade serous carcinomas, respectively [49]. Various experimental models using OSE cells or tubal epithelial cells have supported the concept that molecular pathways based on TP53 mutation play an important role for the carcinogenesis of high-grade serous carcinomas or Type II tumors [23, 63, 64] (Fig. 3.4a) (see also Sect. 7.2.2 in Chap. 7).
Schematic diagram of morphological and molecular pathogenesis for ovarian carcinogenesis (a) serous tumors; (b) endometrioid tumors; (c) clear cell tumors; (d) mucinous tumors. Solid lines, major pathways; broken lines, minor or putative pathways; blue frames, gene mutations; red frames, genomic status. OSE, ovarian surface epithelium; FTE, fallopian tubal epithelium; STIC, serous tubal intraepithelial carcinomas; CI, chromosomal instability; MI, microsatellite instability; +, positive; −, negative
3.4.1.2 Low-Grade Serous Carcinoma
Low-grade serous carcinoma is much less common than high-grade carcinoma and is classified as a Type I tumor. These carcinomas frequently have a component of serous borderline tumor (SBT) or micropapillary serous carcinoma [65] and are thought to evolve in a stepwise fashion from benign serous cystadenoma through to SBT and finally to carcinoma. Morphologically, low-grade serous carcinomas also resemble tubal secretary cells and show small papillae of tumor cells exhibiting uniform nuclei within variable amounts of hyalinized stroma, which often contains psammoma bodies [66]. Low-grade serous carcinomas, like high-grade serous carcinomas, typically express the transcription factors PAX8 [67,68,69].
Low-grade serous carcinomas arise via the transformation of benign and SBTs, thought to be derived either from inclusion cysts originating from the OSE or from tubal epithelium that is shed and implanted onto the ovary and gives rise to inclusion cysts and subsequent serous neoplasms (Fig. 3.2a). An immunohistochemical study has shown that 80% of ovarian cortical inclusions express PAX8, a Müllerian marker, but not calretinin, a mesothelial marker, findings that support the concept of a tubal phenotype [70]. Recently, it has also been suggested that papillary tubal hyperplasia may be a putative precursor lesion for SBTs [71, 72].
Low-grade serous carcinomas are not associated with BRCA1/2 germline mutation and rarely have TP53 mutations, in contrast to KRAS and BRAF mutations which are frequently present. KRAS and BRAF are the upstream regulators in the RAS/RAF/MEK/ERK/MAP signal transduction pathway, which plays a critical role in the transmission of growth signals to the nucleus [73]. Oncogenic mutations in BRAF and KRAS result in the constitutive activation of this pathway and thus contribute to neoplastic transformation. Several studies have demonstrated that activating mutations in codon 12 (and less commonly in codon 13) of KRAS or in codons 599 and 600 of BRAF occur in approximately two thirds of SBTs and low-grade serous carcinomas [74, 75]. Mutations in KRAS and BRAF are mutually exclusive, such that tumors with mutant KRAS do not have mutant BRAF and vice versa. It has been suggested that mutations of KRAS and BRAF are early events associated with the initiation of SBTs and low-grade serous carcinomas and that a small subset of serous cystadenomas that acquire KRAS or BRAF mutations may progress to SBTs. Low-grade serous carcinomas do not show chromosomal instability and thus lack the complex genomic abnormalities seen in high-grade serous carcinomas (Fig. 3.4a) (see also Sect. 7.6 in Chap. 7).
3.4.2 Endometrioid Carcinoma
In 1927, Sampson was the first to describe the malignant transformation of endometriosis to ovarian carcinoma [76]. Thereafter, many studies have provided supporting evidence that malignant transformation can occur in ovarian endometriosis or the endometriotic cyst [77, 78] (Fig. 3.2b). The observation of a morphological transition from endometriosis to carcinoma in over one third of endometrioid carcinomas has led to endometriosis being considered its likely cause. It has thus been accepted that atypical endometriosis at the transition site is the precursor lesion for endometrioid carcinoma associated with endometriosis, and common genetic alterations have been documented in adjacent endometriosis, atypical endometriosis, and carcinoma [79]. Besides endometriosis, the coexistence of benign endometrioid neoplasms, such as adenofibromas or borderline tumors, with endometrioid carcinomas has been also recognized [80].
Endometriosis is thought to occur via retrograde menstruation, whereby epithelial and stromal cells of the endometrium are carried from the uterus through the fallopian tubes and can establish as an endometriotic cyst within the ovary [81]. Recent investigations suggest that the endometriotic cyst, in which chocolate-like blood is trapped for long time, maintains high concentrations of iron in the cystic fluid and that the iron-rich environment causes oxidative stress and hypoxia leading to DNA damage and accumulation of mutations [30, 32].
Like endometrial cancers, ovarian endometrioid carcinoma is commonly encountered in patients with Lynch syndrome. Microsatellite instability has been also observed in 13–20% of endometrioid carcinomas. Mutations in the PTEN tumor suppressor gene, resulting in the activation of PI3K signaling and inhibition of apoptosis, have been reported in a fifth or less of endometrioid carcinomas and are rare in other types of malignant EOT [82, 83]. Mutations in PIK3CA, which encodes the p110 catalytic subunit of PI3K, have also been identified in a fifth of endometrioid carcinomas and similarly result in activation of PI3K signaling [84, 85]. PTEN and PIK3CA mutations co-occur in a subset of endometrioid carcinomas [86, 87]. The Wnt/β-catenin signaling pathway is involved in the regulation of several important cellular processes, including cell fate determination, proliferation, motility, and survival. Mutations in CTNNB1, which encodes β-catenin, are typically found in endometrioid carcinomas but are uncommon in the other types of ovarian carcinoma [88], and several studies have noted the association of CTNNB1 mutation with squamous differentiation.
The tumor suppressor gene ARID1A, which encodes BAF250a, plays a crucial role in chromatin remodeling as a member of the SWI/SNF chromatin remodeling complex. ARID1A mutation induces changes in the expression of multiple genes (CDKN1A, SMAD3, MLH1, and PIK3IP1) as the result of chromatin remodeling dysfunction and has been shown to contribute to molecular pathogenesis and cellular transformation in cooperation with the PI3K pathway [82, 83, 86, 89,90,91,92]. KRAS and BRAF mutations have been identified in endometrioid carcinomas, but the frequency of these mutations is rather low, being 7% or less [92,93,94,95,96]. The fact that PTEN, KRAS, and ARID1A mutations are also found in the epithelial components of endometriosis adjacent to endometrioid carcinomas provides additional evidence for the precursor role of endometriosis in the molecular pathogenesis of ovarian endometrioid carcinomas [29, 97, 98]. In regard to TP53, mutations have been reported in poorly differentiated or high-grade endometrioid carcinomas. TP53 mutations are uncommon in tumors with Wnt/β-catenin and/or PI3K/PTEN signaling defects [96].
Using genetically engineered mice, experimental models of endometrioid tumor have now been developed. In one approach, simultaneous activation of KRAS and inactivation of PTEN in the OSE resulted in the development of ovarian tumors resembling human endometrioid carcinomas associated with endometriosis [99]. In another approach, conditional bi-allelic inactivation of APC and PTEN in the OSE promoted ovarian endometrioid tumors harboring Wnt and PI3K pathway defects comparable to human endometrioid carcinomas [92]. Furthermore, conditional inactivation of one or both ARID1A alleles in the OSE concurrently with APC and PTEN inactivation in these mice induced endometrioid tumors with morphological features similar to those of human endometrioid carcinoma [100] (Fig. 3.4b) (see also Sect. 7.4.1 in Chap. 7).
3.4.3 Clear Cell Carcinoma
The morphological characteristics of clear cell carcinoma are multiple complex papillae, densely hyaline basement membrane material expanding the cores of these papillae, and hyaline bodies. Mitotic figures are less frequent than in other types of ovarian carcinoma. As is the case for endometrioid carcinoma, there is a close association between endometriosis and clear cell carcinoma [101, 102]. The coexistence of adenofibromas or borderline tumors, with clear cell carcinomas, has been also recognized, being distinct from those arising from endometriosis [103, 104] (Fig. 3.2c).
Hepatocyte nuclear factor-1β (HNF-1β) is upregulated in clear cell tumors, including benign tumors, borderline tumors, and carcinomas [105], and thus most clear cell carcinomas are positive for HNF1-β [106, 107]. This transcription factor is expressed in the mid-to-late secretory and gestational endometrium with Arias-Stella reaction, atypical and inflammatory endometriosis, and clear cell carcinoma [105]. HNF-1β regulates several genes such as dipeptidyl peptidase IV (involved in the control of glycogen synthesis [108]), glutathione peroxidase 3, and annexin A4 [109]. The fact that HNF-1β is important in controlling multiple genes involved in glucose and glycogen metabolism suggests that upregulation of this factor may be responsible for the characteristic morphological feature of clear cell carcinoma, namely, a glycogen-rich cytoplasm with clear appearance [108, 110].
Mutations involving PI3K/PTEN signaling are common in clear cell carcinomas, with PIK3CA mutations reported in 20–25% of tumors and PTEN mutations in 8% of tumors [84, 86, 98]. Recently, it has been found that nearly half of clear cell carcinomas carry ARID1A mutations and lack BAF250 protein [97]. The occurrence of somatic mutations of PTEN and ARID1A in a subset of ovarian endometriotic cysts, within both tumor tissue and adjacent endometriosis, but not in distant endometriosis sites, suggests shared molecular alterations between clear cell and endometrioid carcinomas of the ovary and their putative precursor lesion [98]. This finding also suggests that PTEN and ARID1A inactivation occurs early during the malignant transformation of endometriosis [97].
Clear cell carcinomas do not appear to share other genetic changes with endometrioid carcinomas. Wnt signaling pathway defects and microsatellite instability, for example, have not been observed with significant frequency in these tumors [86, 111, 112] (Fig. 3.4c) (see also Sects. 7.3.3 and 7.3.4 in Chap. 7).
3.4.4 Mucinous Carcinoma
Although mucinous tumors account for 10–15% of EOTs, almost all are benign, with the remainder being of borderline malignancy. The tumors usually show cystic and multilocular features; those that are large and unilateral are likely to be primary lesions, while metastatic tumors are typically smaller and bilateral. Primary ovarian mucinous carcinomas are usually confined to the ovary however, and if external metastases to the ovary, particularly from the gastrointestinal tract, are carefully excluded, only 3–4% of ovarian carcinomas are typically found to be of the mucinous type. The cells of mucinous tumors may resemble those of the gastric pylorus, intestine, or endocervix (Fig. 3.1g–j). Recently, mucinous tumors with cells resembling those of the endocervical epithelium have been classified as separate category of seromucinous tumor that is associated with endometriosis or low-grade serous carcinoma [113]. The origin of mucinous tumors, which includes inclusion cyst or OSE, is not well characterized, but the association of some mucinous tumors with teratoma indicates that some may be of germ cell origin [114] (Fig. 3.2d). More recent data suggest that transitional cell (Walthard) nests, which relate to Brenner tumors, present at the tubal-mesothelial junction may also be a possible origin for these tumors [72, 115] (Fig. 3.2e) (see also Sect. 5.3 in Chap. 5 and Sect. 7.5.1 in Chap. 7). Mucinous carcinomas are often heterogeneous. Benign, borderline, noninvasive, and invasive features may all coexist within an individual tumor, suggesting that tumor progression proceeds from benign to borderline and from borderline to carcinoma [116].
KRAS mutations are frequent in mucinous carcinomas and are considered to be an early tumorigenic event [117]. Ovarian mucinous tumors are generally immunoreactive for cytokeratin 7 (CK7), whereas metastatic tumors from colorectal adenocarcinoma sites are usually CK7 negative but positive for CK20 [118]. Mucinous tumors express several mucin genes (MUC2, MUC3, and MUC17) irrespective of their tissue origins, as well as additional genes that are markers of intestinal differentiation, such as the caudal-type homeobox transcription factors CDX1 and CDX2 and LGALS4. LGALS4 is an intestinal cell surface adhesion molecule overexpressed in a spectrum of mucinous tumors, including intestinal carcinomas, but is not detectable in normal OSE. It is overexpressed in all ovarian mucinous tumors, however, including benign, borderline, and malignant tumors, indicating that LGALS4 overexpression is associated with a very early step in the molecular pathogenesis of this cancer type [119].
KRAS, BRAF, and CDKN2A (which encodes p16/INK4a) are often mutated in mucinous tumors, with the RAS/RAF pathway and p16/INK4a thought to be important contributors to molecular pathogenesis [120,121,122]. A recent study has suggested that a high percentage of mucinous carcinomas may have a TP53 mutation (50–70%). While there is a similar, but lower (10–20%), frequency of TP53 mutation in benign and borderline tumors, the high prevalence of TP53 mutation in mucinous carcinomas suggests that aberrant p53 contributes to the invasive phenotype as a late event in the tumorigenic process [120, 123]. Interestingly, mucinous carcinomas do not share the widespread genomic instability seen in high-grade serous carcinomas that carry TP53 mutations, suggesting that the effect of p53 mutation is distinct in these two kinds of malignant EOTs [120] (Fig. 3.4d) (see also Sect. 7.5.2 in Chap. 7).
Conclusion
Historically, the early morphological and molecular alterations in ovarian tumorigenesis have been a black box. While it is relatively easy to biopsy early lesions in cervical and endometrial carcinomas, this is difficult in ovarian carcinomas because of the location within the pelvic cavity. Since the introduction in 1995 of prophylactic salpingo-oophorectomy for carriers of BRCA1/2 mutations, early lesions of high-grade serous carcinomas in HBOC have become readily identifiable. However, the identification of extra-ovarian STIC as a precursor lesion in such cases has led to a paradigm shift from the theory of the secondary Müllerian system of OSE to one of “imported disease”. Despite this, many secrets of the black box remain, including the cellular origins of especially high-grade serous carcinoma without STIC, low-grade serous carcinoma, and mucinous carcinoma and the biological basis for the observed morphological diversity in these diseases. Further molecular studies are required to answer these remaining questions in regard to the pathogenesis of EOTs.
References
Banet N, Kurman RJ. Two types of ovarian cortical inclusion cysts: proposed origin and possible role in ovarian serous carcinogenesis. Int J Gynecol Pathol. 2015;34:3–8.
Auersperg N. Article by Natalie Banet and Robert J. Kurman: Two types of ovarian cortical inclusion cysts: proposed origin and possible role in ovarian serous carcinogenesis; Int. J. Gynecol. Pathol. 2015;34:3-8. Int J Gynecol Pathol. 2015;34:303-4.
Auersperg N. Ovarian surface epithelium as a source of ovarian cancers: unwarranted speculation or evidence-based hypothesis? Gynecol Oncol. 2013;130:246–51.
Kuhn E, Kurman RJ, Shih IM. Ovarian cancer is an imported disease: fact or fiction? Curr Obstet Gynecol Rep. 2012;1:1–9.
Okamura H, Katabuchi H. Pathophysiological dynamics of human ovarian surface epithelial cells in epithelial ovarian carcinogenesis. Int Rev Cytol. 2005;242:1–54.
Szotek PP, Chang HL, Brennand K, Fujino A, Pieretti-Vanmarcke R, Lo Celso C, et al. Normal ovarian surface epithelial label-retaining cells exhibit stem/progenitor cell characteristics. Proc Natl Acad Sci U S A. 2008;105:12469–73.
Ng A, Tan S, Singh G, Rizk P, Swathi Y, Tan TZ, et al. Lgr5 marks stem/progenitor cells in ovary and tubal epithelia. Nat Cell Biol. 2014;16:745–57.
Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–43.
Dubeau L. The cell of origin of ovarian epithelial tumours. Lancet Oncol. 2008;9:1191–7.
Allen BM. The embrionic development of the ovary and testes of the mammals. Am J Anat. 1904;3:89–153.
Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001;22:255–88.
Lauchlan SC. The secondary Mullerian system. Obstet Gynecol Surv. 1972;27:133–46.
Okamura H, Katabuchi H. Detailed morphology of human ovarian surface epithelium focusing on its metaplastic and neoplastic capability. Ital J Anat Embryol. 2001;106:263–76.
Okamura H, Katabuchi H, Ohba T. What we have learned from isolated cells from human ovary? Mol Cell Endocrinol. 2003;202:37–45.
Katabuchi H, Okamura H. Cell biology of human ovarian surface epithelial cells and ovarian carcinogenesis. Med Electron Microsc. 2003;36:74–86.
Okamura H, Katabuchi H, Nagai R. Ultrastructure of human ovulation: histofunctional parameters. In: Motta PM, editor. Microscopy of reproduction and development: a dynamic approach. Rome: Antonio Delfino Editore; 1997. p. 155–61.
Fathalla MF. Incessant ovulation--a factor in ovarian neoplasia? Lancet. 1971;2:163.
Tashiro H, Katabuchi H, Begum M, Li X, Nitta M, Ohtake H, et al. Roles of luteinizing hormone/chorionic gonadotropin receptor in anchorage-dependent and -independent growth in human ovarian surface epithelial cell lines. Cancer Sci. 2003;94:953–9.
Ji Q, Liu PI, Chen PK, Aoyama C. Follicle stimulating hormone-induced growth promotion and gene expression profiles on ovarian surface epithelial cells. Int J Cancer. 2004;112:803–14.
Ng A, Barker N. Ovary and fimbrial stem cells: biology, niche and cancer origins. Nat Rev Mol Cell Biol. 2015;16:625–38.
Gamwell LF, Collins O, Vanderhyden BC. The mouse ovarian surface epithelium contains a population of LY6A (SCA-1) expressing progenitor cells that are regulated by ovulation-associated factors. Biol Reprod. 2012;87:80.
Flesken-Nikitin A, Hwang CI, Cheng CY, Michurina TV, Enikolopov G, Nikitin AY. Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature. 2013;495:241–5.
Motohara T, Masuko S, Ishimoto T, Yae T, Onishi N, Muraguchi T, et al. Transient depletion of p53 followed by transduction of c-Myc and K-Ras converts ovarian stem-like cells into tumor-initiating cells. Carcinogenesis. 2011;32:1597–606.
Bhartiya D, Singh J. FSH-FSHR3-stem cells in ovary surface epithelium: basis for adult ovarian biology, failure, aging, and cancer. Reproduction. 2015;149:R35–48.
Kobayashi H, Sumimoto K, Moniwa N, Imai M, Takakura K, Kuromaki T, et al. Risk of developing ovarian cancer among women with ovarian endometrioma: a cohort study in Shizuoka, Japan. Int J Gynecol Cancer. 2007;17:37–43.
Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389–98.
Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–99.
Ohtake H, Katabuchi H, Matsuura K, Okamura H. A novel in vitro experimental model for ovarian endometriosis: the three-dimensional culture of human ovarian surface epithelial cells in collagen gels. Fertil Steril. 1999;71:50–5.
McConechy MK, Ding J, Senz J, Yang W, Melnyk N, Tone AA, et al. Ovarian and endometrial endometrioid carcinomas have distinct CTNNB1 and PTEN mutation profiles. Mod Pathol. 2014;27:128–34.
Yamaguchi K, Mandai M, Toyokuni S, Hamanishi J, Higuchi T, Takakura K, et al. Contents of endometriotic cysts, especially the high concentration of free iron, are a possible cause of carcinogenesis in the cysts through the iron-induced persistent oxidative stress. Clin Cancer Res. 2008;14:32–40.
Van Langendonckt A, Casanas-Roux F, Dolmans MM, Donnez J. Potential involvement of hemoglobin and heme in the pathogenesis of peritoneal endometriosis. Fertil Steril. 2002;77:561–70.
Kobayashi H, Kajiwara H, Kanayama S, Yamada Y, Furukawa N, Noguchi T, et al. Molecular pathogenesis of endometriosis-associated clear cell carcinoma of the ovary (review). Oncol Rep. 2009;22:233–40.
Kurman RJ, Shih IM. Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. Int J Gynecol Pathol. 2008;27:151–60.
Cho KR, Shih IM. Ovarian cancer. Annu Rev Pathol. 2009;4:287–313.
Eddy JA, Hood L, Price ND, Geman D. Identifying tightly regulated and variably expressed networks by differential rank conservation (DIRAC). PLoS Comput Biol. 2010;6:e1000792.
Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35.
Jarboe E, Folkins A, Nucci MR, Kindelberger D, Drapkin R, Miron A, et al. Serous carcinogenesis in the fallopian tube: a descriptive classification. Int J Gynecol Pathol. 2008;27:1–9.
Paik DY, Janzen DM, Schafenacker AM, Velasco VS, Shung MS, Cheng D, et al. Stem-like epithelial cells are concentrated in the distal end of the fallopian tube: a site for injury and serous cancer initiation. Stem Cells. 2012;30:2487–97.
Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71.
Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789–92.
Paley PJ, Swisher EM, Garcia RL, Agoff SN, Greer BE, Peters KL, et al. Occult cancer of the fallopian tube in BRCA-1 germline mutation carriers at prophylactic oophorectomy: a case for recommending hysterectomy at surgical prophylaxis. Gynecol Oncol. 2001;80:176–80.
Carcangiu ML, Peissel B, Pasini B, Spatti G, Radice P, Manoukian S. Incidental carcinomas in prophylactic specimens in BRCA1 and BRCA2 germ-line mutation carriers, with emphasis on fallopian tube lesions: report of 6 cases and review of the literature. Am J Surg Pathol. 2006;30:1222–30.
Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–9.
Bowtell DD. The genesis and evolution of high-grade serous ovarian cancer. Nat Rev Cancer. 2010;10:803–8.
Piek JM, van Diest PJ, Zweemer RP, Jansen JW, Poort-Keesom RJ, Menko FH, et al. Dysplastic changes in prophylactically removed fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–6.
Medeiros F, Muto MG, Lee Y, Elvin JA, Callahan MJ, Feltmate C, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30:230–6.
Folkins AK, Jarboe EA, Saleemuddin A, Lee Y, Callahan MJ, Drapkin R, et al. A candidate precursor to pelvic serous cancer (p53 signature) and its prevalence in ovaries and fallopian tubes from women with BRCA mutations. Gynecol Oncol. 2008;109:168–73.
Salani R, Kurman RJ, Giuntoli R 2nd, Gardner G, Bristow R, Wang TL, et al. Assessment of TP53 mutation using purified tissue samples of ovarian serous carcinomas reveals a higher mutation rate than previously reported and does not correlate with drug resistance. Int J Gynecol Cancer. 2008;18:487–91.
Network CGAR. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15.
Merajver SD, Pham TM, Caduff RF, Chen M, Poy EL, Cooney KA, et al. Somatic mutations in the BRCA1 gene in sporadic ovarian tumours. Nat Genet. 1995;9:439–43.
Sangha N, Wu R, Kuick R, Powers S, Mu D, Fiander D, et al. Neurofibromin 1 (NF1) defects are common in human ovarian serous carcinomas and co-occur with TP53 mutations. Neoplasia. 2008;10:1362–72.
Cannistra SA. BRCA-1 in sporadic epithelial ovarian cancer: lessons learned from the genetics of hereditary disease. Clin Cancer Res. 2007;13:7225–7.
Wang C, Horiuchi A, Imai T, Ohira S, Itoh K, Nikaido T, et al. Expression of BRCA1 protein in benign, borderline, and malignant epithelial ovarian neoplasms and its relationship to methylation and allelic loss of the BRCA1 gene. J Pathol. 2004;202:215–23.
Baldwin RL, Nemeth E, Tran H, Shvartsman H, Cass I, Narod S, et al. BRCA1 promoter region hypermethylation in ovarian carcinoma: a population-based study. Cancer Res. 2000;60:5329–33.
Venkitaraman AR. Linking the cellular functions of BRCA genes to cancer pathogenesis and treatment. Annu Rev Pathol. 2009;4:461–87.
Pothuri B, Leitao MM, Levine DA, Viale A, Olshen AB, Arroyo C, et al. Genetic analysis of the early natural history of epithelial ovarian carcinoma. PLoS One. 2010;5:e10358.
Norquist BM, Garcia RL, Allison KH, Jokinen CH, Kernochan LE, Pizzi CC, et al. The molecular pathogenesis of hereditary ovarian carcinoma: alterations in the tubal epithelium of women with BRCA1 and BRCA2 mutations. Cancer. 2010;116:5261–71.
Schraml P, Schwerdtfeger G, Burkhalter F, Raggi A, Schmidt D, Ruffalo T, et al. Combined array comparative genomic hybridization and tissue microarray analysis suggest PAK1 at 11q13.5-q14 as a critical oncogene target in ovarian carcinoma. Am J Pathol. 2003;163:985–92.
Meinhold-Heerlein I, Bauerschlag D, Hilpert F, Dimitrov P, Sapinoso LM, Orlowska-Volk M, et al. Molecular and prognostic distinction between serous ovarian carcinomas of varying grade and malignant potential. Oncogene. 2005;24:1053–65.
Mayr D, Kanitz V, Anderegg B, Luthardt B, Engel J, Lohrs U, et al. Analysis of gene amplification and prognostic markers in ovarian cancer using comparative genomic hybridization for microarrays and immunohistochemical analysis for tissue microarrays. Am J Clin Pathol. 2006;126:101–9.
Nowee ME, Snijders AM, Rockx DA, de Wit RM, Kosma VM, Hamalainen K, et al. DNA profiling of primary serous ovarian and fallopian tube carcinomas with array comparative genomic hybridization and multiplex ligation-dependent probe amplification. J Pathol. 2007;213:46–55.
Tashiro H, Miyazaki K, Okamura H, Iwai A, Fukumoto M. C-myc over-expression in human primary ovarian tumours: its relevance to tumour progression. Int J Cancer. 1992;50:828–33.
Sasaki R, Narisawa-Saito M, Yugawa T, Fujita M, Tashiro H, Katabuchi H, et al. Oncogenic transformation of human ovarian surface epithelial cells with defined cellular oncogenes. Carcinogenesis. 2009;30:423–31.
Jazaeri AA, Bryant JL, Park H, Li H, Dahiya N, Stoler MH, et al. Molecular requirements for transformation of fallopian tube epithelial cells into serous carcinoma. Neoplasia. 2011;13:899–911.
Katabuchi H, Tashiro H, Cho KR, Kurman RJ, Hedrick EL. Micropapillary serous carcinoma of the ovary: an immunohistochemical and mutational analysis of p53. Int J Gynecol Pathol. 1998;17:54–60.
Motohara T, Tashiro H, Miyahara Y, Sakaguchi I, Ohtake H, Katabuchi H. Long-term oncological outcomes of ovarian serous carcinomas with psammoma bodies: a novel insight into the molecular pathogenesis of ovarian epithelial carcinoma. Cancer Sci. 2010;101:1550–6.
O'Neill CJ, Deavers MT, Malpica A, Foster H, McCluggage WG. An immunohistochemical comparison between low-grade and high-grade ovarian serous carcinomas: significantly higher expression of p53, MIB1, BCL2, HER-2/neu, and C-KIT in high-grade neoplasms. Am J Surg Pathol. 2005;29:1034–41.
Shimizu M, Toki T, Takagi Y, Konishi I, Fujii S. Immunohistochemical detection of the Wilms' tumor gene (WT1) in epithelial ovarian tumors. Int J Gynecol Pathol. 2000;19:158–63.
Liliac L, Carcangiu ML, Canevari S, Caruntu ID, Ciobanu Apostol DG, Danciu M, et al. The value of PAX8 and WT1 molecules in ovarian cancer diagnosis. Romanian J Morphol Embryol. 2013;54:17–27.
Li J, Abushahin N, Pang S, Xiang L, Chambers SK, Fadare O, et al. Tubal origin of 'ovarian' low-grade serous carcinoma. Mod Pathol. 2011;24:1488–99.
Kurman RJ, Vang R, Junge J, Hannibal CG, Kjaer SK, Shih IM. Papillary tubal hyperplasia: the putative precursor of ovarian atypical proliferative (borderline) serous tumors, noninvasive implants, and endosalpingiosis. Am J Surg Pathol. 2011;35:1605–14.
Lim D, Oliva E. Precursors and pathogenesis of ovarian carcinoma. Pathology. 2013;45:229–42.
Peyssonnaux C, Eychene A. The Raf/MEK/ERK pathway: new concepts of activation. Biol Cell. 2001;93:53–62.
Singer G, Oldt R 3rd, Cohen Y, Wang BG, Sidransky D, Kurman RJ, et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95:484–6.
Jones S, Wang TL, Kurman RJ, Nakayama K, Velculescu VE, Vogelstein B, et al. Low-grade serous carcinomas of the ovary contain very few point mutations. J Pathol. 2012;226:413–20.
Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927;3:93–110.43.
Scully RE, Richardson GS, Barlow JF. The development of malignancy in endometriosis. Clin Obstet Gynecol. 1966;9:384–411.
Sainz de la Cuesta R, Eichhorn JH, Rice LW, Fuller AF Jr, Nikrui N, Goff BA. Histologic transformation of benign endometriosis to early epithelial ovarian cancer. Gynecol Oncol. 1996;60:238–44.
Jiang X, Morland SJ, Hitchcock A, Thomas EJ, Campbell IG. Allelotyping of endometriosis with adjacent ovarian carcinoma reveals evidence of a common lineage. Cancer Res. 1998;58:1707–12.
Bell KA, Kurman RJ. A clinicopathologic analysis of atypical proliferative (borderline) tumors and well-differentiated endometrioid adenocarcinomas of the ovary. Am J Surg Pathol. 2000;24:1465–79.
Fukunaga M, Nomura K, Ishikawa E, Ushigome S. Ovarian atypical endometriosis: its close association with malignant epithelial tumours. Histopathology. 1997;30:249–55.
Obata K, Morland SJ, Watson RH, Hitchcock A, Chenevix-Trench G, Thomas EJ, et al. Frequent PTEN/MMAC mutations in endometrioid but not serous or mucinous epithelial ovarian tumors. Cancer Res. 1998;58:2095–7.
Catasus L, Bussaglia E, Rodrguez I, Gallardo A, Pons C, Irving JA, et al. Molecular genetic alterations in endometrioid carcinomas of the ovary: similar frequency of beta-catenin abnormalities but lower rate of microsatellite instability and PTEN alterations than in uterine endometrioid carcinomas. Hum Pathol. 2004;35:1360–8.
Campbell IG, Russell SE, Choong DY, Montgomery KG, Ciavarella ML, Hooi CS, et al. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004;64:7678–81.
Nakayama K, Nakayama N, Kurman RJ, Cope L, Pohl G, Samuels Y, et al. Sequence mutations and amplification of PIK3CA and AKT2 genes in purified ovarian serous neoplasms. Cancer Biol Ther. 2006;5:779–85.
Willner J, Wurz K, Allison KH, Galic V, Garcia RL, Goff BA, et al. Alternate molecular genetic pathways in ovarian carcinomas of common histological types. Hum Pathol. 2007;38:607–13.
Catasus L, Gallardo A, Cuatrecasas M, Prat J. PIK3CA mutations in the kinase domain (exon 20) of uterine endometrial adenocarcinomas are associated with adverse prognostic parameters. Mod Pathol. 2008;21:131–9.
Wright K, Wilson P, Morland S, Campbell I, Walsh M, Hurst T, et al. Beta-catenin mutation and expression analysis in ovarian cancer: exon 3 mutations and nuclear translocation in 16% of endometrioid tumours. Int J Cancer. 1999;82:625–9.
Palacios J, Gamallo C. Mutations in the beta-catenin gene (CTNNB1) in endometrioid ovarian carcinomas. Cancer Res. 1998;58:1344–7.
Gamallo C, Palacios J, Moreno G. Calvo de Mora J, Suarez a, Armas a. Beta-catenin expression pattern in stage I and II ovarian carcinomas : relationship with beta-catenin gene mutations, clinicopathological features, and clinical outcome. Am J Pathol. 1999;155:527–36.
Saegusa M, Okayasu I. Frequent nuclear beta-catenin accumulation and associated mutations in endometrioid-type endometrial and ovarian carcinomas with squamous differentiation. J Pathol. 2001;194:59–67.
Wu R, Hendrix-Lucas N, Kuick R, Zhai Y, Schwartz DR, Akyol A, et al. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/beta-catenin and PI3K/Pten signaling pathways. Cancer Cell. 2007;11:321–33.
Enomoto T, Weghorst CM, Inoue M, Tanizawa O, Rice JM. K-ras activation occurs frequently in mucinous adenocarcinomas and rarely in other common epithelial tumors of the human ovary. Am J Pathol. 1991;139:777–85.
Caduff RF, Svoboda-Newman SM, Bartos RE, Ferguson AW, Frank TS. Comparative analysis of histologic homologues of endometrial and ovarian carcinoma. Am J Surg Pathol. 1998;22:319–26.
Amemiya S, Sekizawa A, Otsuka J, Tachikawa T, Saito H, Okai T. Malignant transformation of endometriosis and genetic alterations of K-ras and microsatellite instability. Int J Gynaecol Obstet. 2004;86:371–6.
Mayr D, Hirschmann A, Lohrs U, Diebold J. KRAS and BRAF mutations in ovarian tumors: a comprehensive study of invasive carcinomas, borderline tumors and extraovarian implants. Gynecol Oncol. 2006;103:883–7.
Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–43.
Sato N, Tsunoda H, Nishida M, Morishita Y, Takimoto Y, Kubo T, et al. Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. 2000;60:7052–6.
Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med. 2005;11:63–70.
Zhai Y, Kuick R, Tipton C, Wu R, Sessine M, Wang Z, et al. Arid1a inactivation in an Apc- and Pten-defective mouse ovarian cancer model enhances epithelial differentiation and prolongs survival. J Pathol. 2016;238:21–30.
Komiyama S, Aoki D, Tominaga E, Susumu N, Udagawa Y, Nozawa S. Prognosis of Japanese patients with ovarian clear cell carcinoma associated with pelvic endometriosis: clinicopathologic evaluation. Gynecol Oncol. 1999;72:342–6.
Matsuo Y, Tashiro H, Yanai H, Moriya T, Katabuchi H. Clinicopathological heterogeneity in ovarian clear cell adenocarcinoma: a study on individual therapy practice. Med Mol Morphol. 2015;48:146–54.
Yamamoto S, Tsuda H, Yoshikawa T, Kudoh K, Kita T, Furuya K, et al. Clear cell adenocarcinoma associated with clear cell adenofibromatous components: a subgroup of ovarian clear cell adenocarcinoma with distinct clinicopathologic characteristics. Am J Surg Pathol. 2007;31:999–1006.
Veras E, Mao TL, Ayhan A, Ueda S, Lai H, Hayran M, et al. Cystic and adenofibromatous clear cell carcinomas of the ovary: distinctive tumors that differ in their pathogenesis and behavior: a clinicopathologic analysis of 122 cases. Am J Surg Pathol. 2009;33:844–53.
Kato N, Sasou S, Motoyama T. Expression of hepatocyte nuclear factor-1beta (HNF-1beta) in clear cell tumors and endometriosis of the ovary. Mod Pathol. 2006;19:83–9.
Kobel M, Kalloger SE, Carrick J, Huntsman D, Asad H, Oliva E, et al. A limited panel of immunomarkers can reliably distinguish between clear cell and high-grade serous carcinoma of the ovary. Am J Surg Pathol. 2009;33:14–21.
Kobel M, Kalloger SE, Boyd N, McKinney S, Mehl E, Palmer C, et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med. 2008;5:e232.
Senkel S, Lucas B, Klein-Hitpass L, Ryffel GU. Identification of target genes of the transcription factor HNF1beta and HNF1alpha in a human embryonic kidney cell line. Biochim Biophys Acta. 1731;2005:179–90.
Kobayashi H, Yamada Y, Kanayama S, Furukawa N, Noguchi T, Haruta S, et al. The role of hepatocyte nuclear factor-1beta in the pathogenesis of clear cell carcinoma of the ovary. Int J Gynecol Cancer. 2009;19:471–9.
Tanaka T, Tomaru Y, Nomura Y, Miura H, Suzuki M, Hayashizaki Y. Comprehensive search for HNF-1beta-regulated genes in mouse hepatoma cells perturbed by transcription regulatory factor-targeted RNAi. Nucleic Acids Res. 2004;32:2740–50.
Fujita M, Enomoto T, Yoshino K, Nomura T, Buzard GS, Inoue M, et al. Microsatellite instability and alterations in the hMSH2 gene in human ovarian cancer. Int J Cancer. 1995;64:361–6.
King BL, Carcangiu ML, Carter D, Kiechle M, Pfisterer J, Pfleiderer A, et al. Microsatellite instability in ovarian neoplasms. Br J Cancer. 1995;72:376–82.
Kobel M, Bell DA, Carcangiu ML, Oliva E, Prat J, Shih IM, et al. Seromucinous tumours. In: Kurman RJ, Carcangiu ML, Herrington CS, Young RH, editors. WHO classification of tumours of female reproductive organs. 4th ed. IARC: Lyon; 2014.
Vang R, Gown AM, Zhao C, Barry TS, Isacson C, Richardson MS, et al. Ovarian mucinous tumors associated with mature cystic teratomas: morphologic and immunohistochemical analysis identifies a subset of potential teratomatous origin that shares features of lower gastrointestinal tract mucinous tumors more commonly encountered as secondary tumors in the ovary. Am J Surg Pathol. 2007;31:854–69.
Seidman JD, Khedmati F. Exploring the histogenesis of ovarian mucinous and transitional cell (Brenner) neoplasms and their relationship with Walthard cell nests: a study of 120 tumors. Arch Pathol Lab Med. 2008;132:1753–60.
Rodriguez IM, Prat J. Mucinous tumors of the ovary: a clinicopathologic analysis of 75 borderline tumors (of intestinal type) and carcinomas. Am J Surg Pathol. 2002;26:139–52.
Cuatrecasas M, Villanueva A, Matias-Guiu X, Prat J. K-ras mutations in mucinous ovarian tumors: a clinicopathologic and molecular study of 95 cases. Cancer. 1997;79:1581–6.
Park SY, Kim HS, Hong EK, Kim WH. Expression of cytokeratins 7 and 20 in primary carcinomas of the stomach and colorectum and their value in the differential diagnosis of metastatic carcinomas to the ovary. Hum Pathol. 2002;33:1078–85.
Heinzelmann-Schwarz VA, Gardiner-Garden M, Henshall SM, Scurry JP, Scolyer RA, Smith AN, et al. A distinct molecular profile associated with mucinous epithelial ovarian cancer. Br J Cancer. 2006;94:904–13.
Ryland GL, Hunter SM, Doyle MA, Caramia F, Li J, Rowley SM, et al. Mutational landscape of mucinous ovarian carcinoma and its neoplastic precursors. Genome Med. 2015;7:87.
Anglesio MS, Kommoss S, Tolcher MC, Clarke B, Galletta L, Porter H, et al. Molecular characterization of mucinous ovarian tumours supports a stratified treatment approach with HER2 targeting in 19% of carcinomas. J Pathol. 2013;229:111–20.
Hunter SM, Gorringe KL, Christie M, Rowley SM, Bowtell DD, Campbell IG. Pre-invasive ovarian mucinous tumors are characterized by CDKN2A and RAS pathway aberrations. Clin Cancer Res. 2012;18:5267–77.
Mackenzie R, Kommoss S, Winterhoff BJ, Kipp BR, Garcia JJ, Voss J, et al. Targeted deep sequencing of mucinous ovarian tumors reveals multiple overlapping RAS-pathway activating mutations in borderline and cancerous neoplasms. BMC Cancer. 2015;15:415.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Tashiro, H., Imamura, Y., Motohara, T., Sakaguchi, I., Katabuchi, H. (2017). Morphological and Molecular Pathogenesis of Epithelial Ovarian Tumors. In: Katabuchi, H. (eds) Frontiers in Ovarian Cancer Science. Comprehensive Gynecology and Obstetrics. Springer, Singapore. https://doi.org/10.1007/978-981-10-4160-0_3
Download citation
DOI: https://doi.org/10.1007/978-981-10-4160-0_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4159-4
Online ISBN: 978-981-10-4160-0
eBook Packages: MedicineMedicine (R0)