Abstract
With a substantial success of immune checkpoint inhibitor such as anti-CTLA-4 antibodies and anti-PD-L1/PD-1 antibodies, cancer immunotherapy is now drawing a broad attention. In ovarian cancer, several trials have already shown a promising result of anti-PD-L1/PD-1 therapy. In addition, basic research using ovarian cancer cell line has demonstrated a rationale of immune checkpoint inhibition against ovarian cancer. Nevertheless, given the extraordinary cost of using these drugs and relatively low response rate, it is still unclear whether immunotherapy can be widely applied and used for the treatment of ovarian cancer. In order to promote immunotherapy, development of effective biomarkers that can predict response of immune checkpoint inhibitors is most important. At the same time, appropriate handling of immunotherapy-specific adverse effects, that has also been noted in clinical trials, is another important issue. If we could solve these problems, immunotherapy will serve as a major treatment modality for ovarian cancer in the future.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Introduction
Ovarian cancer is the leading cause of mortality from gynecological malignancies. Because ovarian cancer is generally diagnosed at late stages, it is commonly spread into the peritoneal cavity at the time of diagnosis. Therefore, treating advanced disease is the main focus of ovarian cancer therapies. During the past two decades, the standard medical treatment for ovarian cancer has been surgical cytoreduction and cytotoxic chemotherapy, especially the combination of carboplatin and paclitaxel. The effective combination of thorough debulking surgery and recent development of chemotherapies has significantly improved the outcomes of patients with ovarian cancer. Nevertheless, achieving a complete cure remains difficult. Recently, in addition to conventional cytotoxic chemotherapeutic reagents, novel molecular targeted drugs have been employed in many malignant tumors, including ovarian cancer. Prospective studies have demonstrated that bevacizumab, an antiangiogenic reagent that acts against vascular endothelial growth factor (VEGF), is clinically effective in ovarian cancer in both adjuvant and recurrent settings [1, 2]. The “Japan Society of Gynecologic Oncology guidelines 2015 for the treatment of ovarian cancer including primary peritoneal cancer and fallopian tube cancer” recommends bevacizumab as a molecular targeting drug to be considered to use in combination with chemotherapy in these settings [3]. Olaparib, an inhibitor of the enzyme poly ADP ribose polymerase (PARP), has also been reported to be promising in treating BRCA-positive ovarian cancer [4].
Cancer immunotherapy has been expected to be a promising modality for solid tumors. Since ovarian cancer biology is deeply associated with microenvironment in the abdominal cavity, altering the intraperitoneal environment is thought to be useful as a treatment strategy. As described below, the immune microenvironment in the abdominal cavity also significantly affects ovarian cancer progression. Therefore, several immunotherapy clinical trials for ovarian cancer have been conducted. However, the results were not as effective as expected [5]. Very recently, a novel type of immunotherapy that targets the CD28/CTLA-4 family, especially the programmed cell death ligand-1 (PD-L1)/programmed cell death-1 (PD-1) signaling pathway, has been introduced and was found to be surprisingly effective in many solid tumors, including malignant melanoma and lung cancer [6, 7]. This class of drugs is known as immune checkpoint inhibitors and is creating a new frontier in cancer treatment.
13.2 Before Immune Checkpoint Inhibition: Conventional Immunotherapies Against Ovarian Cancer
More than 50 clinical trials (including phase III trials) of immunotherapy for ovarian cancer have been conducted thus far. There are many immune therapies, which generally can be classified into four types (Table 13.1). One is to activate the host’s own anticancer immunity by some means (Table 13.2). The so-called cancer vaccine belongs to this category, which consists of therapies such as the peptide vaccine and dendritic cell therapies. The peptide vaccine is the most popular because it is relatively easy to produce. Cancer antigens including HER2/new, p53, MUC1, NY-ESO-1, and WT-1 have also been used to target ovarian cancer. While dendritic cell therapy theoretically has potent vaccine efficacy, the process of its ex vivo amplification and efficient antigen stimulation is technically difficult and not suitable for large-scale production. Another category of immunotherapy is “passive immunotherapy,” which primarily comprises antibody therapies (Table 13.3). Developing therapeutic antibodies is expensive and time consuming because of strict quality control requirements. However, once developed, these antibodies are suitable for large-scale production. Therefore, this category is now regarded as the most important among immunotherapies. Among the available immunotherapies, antibodies that target ovarian cancers include the anti-CA-125 antibody, anti-folate receptor antibody, and double antibodies against EpCAM and CD3.
13.2.1 Active Immunotherapy for Ovarian Cancer (Table 13.2)
13.2.1.1 Immunotherapy that Targets MUC1
MUC1 is highly expressed in many ovarian cancers and has been a primary target candidate for immunotherapy. Dobrzanski and colleagues conducted phase I and phase II studies by using a Th1 type of self-replenishing CD4+ T cells producing IL-10 and IFN-γ to combat recurrent ovarian cancer [8]. One of the four cases showed remission, and another showed a tumor-bearing survival of 16 weeks; however, the remaining two cases died of cancer within 3–5 months. T cells from long-term survivors showed IFN-γ production and an increase in the number of memory cells and TNF family ligands. Moreover, the therapy was likely to contribute to the survival of ovarian cancer patients by affecting the percentage of the regulatory T-cell subsets and by improving the number of memory CD4+ T cells.
13.2.1.2 Vaccine Therapy with p53 Peptide
Genetic aberrations of p53 and abnormal accumulation of p53 protein have been observed in the majority of serous ovarian cancers. In a cohort of stage III, stage IV, and recurrent ovarian cancer patients with no obvious disease, Rahma et al. compared one group directly administered with p53(264–272) peptide (group A) and another group administered with dendritic cells expressing the p53(264–272) peptide (group B) in a phase II study in the USA [10] and observed a tumor immune response in 69% of the group A patients and 83% of the group B patients. Progression-free survival (PFS) was 4.2 months and 8.7 months, respectively. Because there was no significant difference, they concluded that simple subcutaneous administration may be sufficient.
On the other hand, a Dutch research group conducted a phase II study for recurrent ovarian cancer by using a vaccine comprising a long-chain peptide of p53 (p53-synthetic long peptide, p53-SLP). Only two of the 20 cases showed stable disease, and they did not show a p53-specific immune response. The researchers concluded that there was no obvious effect of p53 peptide on improving the subsequent chemosensitivity or PFS [9, 10].
13.2.1.3 Immunotherapy Targeting HER2-Derived Peptide
Since HER2 is known to be highly expressed in many ovarian cancers, it is considered a good immunotherapy target. However, there was no significant clinical effect observed with by a vaccine using p369–p377 (Table 13.2). Although the trial or similar attempts using the DC vaccine are ongoing, a clinically useful vaccine has not been developed.
13.2.1.4 Vaccine Therapy Targeting WT-1
WT-1 is known to be expressed in more than half of serous ovarian cancers. A phase II trial use a WT-1 peptide as a vaccine against ovarian cancer has been performed in Japan. In 12 cases of treatment-resistant ovarian cancer, SD was noted in three cases, and the remaining nine cases were PD [11].
13.2.1.5 Active Immunotherapy Targeting CA-125
Because CA-125 is a specific protein that is expressed in a majority of ovarian cancers, it has been considered to be a good immunotherapy target. Aside from oregovomab (which will be discussed later), another potential immunotherapy for CA-125 includes the use of an anti-idiotypic antibody against the anti-CA-125 antibody ACA-125 (abagovomab). Since ACA-125 is structurally similar to CA-125, it was expected that abagovomab would elicit antitumor immunity against CA-125 if administered as a vaccine. Wagner et al. reported that in 42 patients with recurrent ovarian cancer, the immune response after administration of abagovomab (which was measured as the production of Ab3) was correlated with improved prognosis [12]. Reinartz et al. also reported that in 119 patients with ovarian cancer, individuals with a good immune response showed a significantly better outcome [13].
13.2.2 Passive Immunotherapy for Ovarian Cancer (Table 13.3)
13.2.2.1 Passive Immunotherapy with Anti-CA-125 Antibody
Large-scale development of the immunotherapy reagent oregovomab, a mouse monoclonal antibody B43.13 against CA-125, has been produced. The initial exploratory study showed that among the patients administered oregovomab, patients in whom anti-idiotypic antibodies (Ab2) and T-cell immunity have been induced showed a better tendency of prognosis and elicited the expected immunotherapeutic response to oregovomab (Table 13.3). Then, Berek et al. conducted a randomized phase II trial in which oregovomab was administered as a maintenance therapy to 145 patients with recurrent ovarian cancer after postoperative TC therapy. This study showed that the PFS of patients with increased Ab2 levels was 18.8 months, while that of the cases with a weak immune reaction was 6.1 months; the PFS of the placebo group was 10.3 months [14]. Unfortunately, a phase III trial with 373 cases failed to reproduce the results of the phase II study [15]. Thus, a single use of oregovomab did not show an apparent clinical effect. However, another research group indicated that based on the results of a randomized phase II study of 40 cases of ovarian cancer, the combination of paclitaxel/carboplatin chemotherapy with oregovomab may augment antitumor immunity [16].
13.2.2.2 Immunotherapy with an Antibody Against the Folate Receptor
Elevated expression of folate receptor α, which is thought to be involved in cancer growth, has been shown in ovarian cancer as well as in many other cancers. A treatment effect with anti-folate receptor antibodies against ovarian cancer has been reported in expiratory clinical trials (Table 13.3). In 2007, MORAb-003 (farletuzumab), a humanized antibody against folate receptor, was developed by Morphotek, Inc. In the phase I study for platinum-resistant ovarian cancer, 36% of patients maintained SD [17]. Furthermore, phase II trials for platinum-sensitive and platinum-resistant ovarian cancer have been reported. Currently, a phase III trial using the combination of chemotherapy and farletuzumab is underway [18].
13.2.2.3 Immunotherapy Using Antibodies Against EpCAM and CD3
Another promising passive immunotherapy for ovarian cancer targets epithelial cell surface antigen (EpCAM). Catumaxomab is a double antibody against both EpCAM and CD3. Heiss et al. examined the inhibitory effect of catumaxomab on cancerous ascites and showed that the period to next puncture was significantly extended in the administration group [19]. Furthermore, Baumann et al. reported the clinical effect of catumaxomab in ovarian cancer [20] (Table 13.3).
13.2.3 Problems Toward the Development and Clinical Application of Immunotherapy
As described above, there have been continuous attempts to develop immunotherapy for ovarian cancer, and recently, clinical efficacy has been shown in some instances. However, despite a long history of immunotherapy against solid cancers, the effect of cancer immunotherapy has been limited because there are several problems in its development. Every time new knowledge of tumor immunity was discovered in the basic fields, the new idea of immunotherapy was usually evaluated in preclinical trials with animal experiments similar to the process of evaluating other anticancer reagents. In this step, in vivo mouse models play an important role as an immunotherapy model, but there is inherent problem with the use of mouse models as the evaluation system. The use of established cancer cell lines along with pure mouse strains is thought to mimic the immune reaction of human immunity. This experimental system has been established using cancer cell lines from a wide variety of organs. The effect of the immunotherapies is validated in animal models as the preclinical phase and is eventually administered to actual cancer patients in clinical trials. However, in most cases, only a small percentage of the patients show efficacy despite a marked effect in the animal experiments. One of the reasons is that the immune reaction is a highly complex biological phenomenon compared to chemotherapy agents. In the case of chemotherapy agents, a mouse model system using transplanted tumor has much in common with human cancers, and the effectiveness of a therapy in animal experiments may predict clinical efficacy. In contrast, animal models of cancer immunotherapy are only a simplified model of the true complex tumor immunity in humans, and there is a large gap between them. For example, an established murine cell line often grows rapidly in vivo, but human cancers can maintain a state of dormancy for years. Such differences may influence the evaluation of tumor immunity.
Second, the evaluation method in clinical trials is also challenging regarding the clinical efficacy of immunotherapy. With chemotherapy agents, evaluating the tumor size may be associated with long-term efficacy. However, in case of immunotherapy, an initial antitumor effect does not necessarily correspond to the final clinical efficacy. Compared to conventional chemotherapies, immunotherapy requires a longer time interval to elicit an antitumor effect. However, we are unaware of the exact signaling mechanisms in the immune system, and we do not have a reliable method to predict the final effect of immunotherapy. Similar to other molecularly targeted drugs, it is important to develop effective predictive biomarkers in order to efficiently implement immunotherapy.
13.3 Immune Checkpoint Inhibition in Ovarian Cancer
Several years ago, a novel immunotherapy attracted a great deal of attention. A therapy using an anti-PD-1 antibody, which inhibits the binding of PD-L1 to PD-1, was used for malignant melanoma, renal cancer, and lung cancer. The first trial showed that the antibody had a high antitumor effect not only in melanoma and renal cancer (which have high immunogenicity) but also in lung cancer, which is not considered to be immunogenic [21, 22].
13.3.1 Basic Mechanism of Function of Immune Checkpoint Molecules
Generally, an immune reaction to antigens (including cancers) is initiated with antigen recognition by antigen-presenting cells (APCs) such as dendritic cells (cognitive phase). Following antigen recognition, dendritic cells migrate to lymph nodes and present specific antigens to T cells via MHC class II molecules. As a result, T cells, including CD8-positive cytotoxic T cells, recognize the existence of cancer and become activated in a tumor-specific manner via the T-cell receptor (TCR). This interaction between the MHCs on APCs and TCRs on T cells is called the “first signal.” At the same time, a “second signal” is sent via interaction of specific molecules known as immune checkpoint molecules. If this interaction occurs between B7 and CD28, active immunity is initiated. In contrast, if the interaction occurs between B7 and CTLA-4 (cytotoxic T lymphocyte-associated protein 4), there is inhibition of the immune response [23].
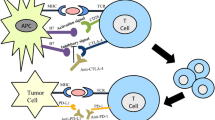
This type of pro-/anti-immune mechanism also exists in local immunity when T cells recognize their targets (effector phase). During this phase, the interaction between PD-1 on T cells and PD-L1 on target cells is thought to result in the attenuation of the immune response. Therefore, if tumor cells express PD-L1, there is a reduction in the immune attack by T cells [24] (Fig. 13.1).
Tumor immune escape hypothesis. This hypothesis is proposed to explain why systemic immunotherapy has not been successful. According to the hypothesis, by using unknown mechanism, e.g., expressing some immunoinhibitory molecules, tumor cells keep their local immune environment in immunosuppressive state. Therefore, even if we can successfully elicit potent systemic antitumor immunity, it does not effectively reach tumor cells
These immunoinhibitory molecules are considered to serve as cancer immune escape machinery; thus, inhibition of these signals is expected to be a target for potent immunotherapies (Fig. 13.1).
13.3.2 Immune Checkpoint Inhibition as a Cancer Immunotherapy
In 1999, clinical trials using the anti-CTLA-4 antibodies ipilimumab and tremelimumab were initiated [25, 26]. Ipilimumab is a humanized IgG1-type anti-CTLA-4 antibody and is currently used clinically to treat malignant melanoma. On the other hand, antibodies against PD-1/PD-L1 have been used in various cancers [6, 21, 22]. Nivolumab is a current treatment for malignant melanoma and lung cancer in Japan and the USA, and pembrolizumab has also been approved for use against these cancers in the USA. In addition, the anti-PD-L1 antibody atezolizumab is administered to treat urothelial cancer. At present, many immune checkpoint inhibitors are being developed and are expected to have clinical applications in the near future.
13.3.3 Rationale of Immune Checkpoint Inhibition in Ovarian Cancer
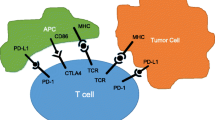
Failure of conventional cancer immunotherapies has brought forth the idea of an immune escape mechanism in tumors. According to this theory, cancer cells actively alter and attenuate their local micro-immune environment by expressing immunosuppressive molecules (Fig. 13.2a). Thus, simply strengthening the immunity of the whole body is insufficient to achieve a therapeutic effect due to the local immune escape mechanism (Fig. 13.1). We and other researchers have shown that the local immune environment, especially tumor infiltration of CD8+ T cells, is closely associated with outcome of patients with ovarian cancer [27,28,29]. Furthermore, we showed that PD-L1 expression in ovarian cancer is a prognostic factor for ovarian cancer, and its expression is negatively associated with CD8+ T-cell infiltration, suggesting that the expression of PD-L1 in tumor cells plays a major role in the suppression of local immunity [29].
Concept of immune checkpoint inhibition. (a) Some of the cancer cells express PD-L1 on their surface, and through PD-L1/PD-1 interaction, they send a signal to attenuate antitumor immunity of cytotoxic T cells. (b) If immune checkpoint inhibitors such as anti-PD-1 antibody can block PD-L1/PD-1 interaction, tumor immunity will be restored, and cytotoxic T cells will regain capability to attack cancer cells
In addition, we performed an in vivo study. When PD-L1 expression was knocked down in PD-L1-high mouse ovarian cancer cells, the cells became less immunogenic; moreover, in a mouse xenograft model, the tumor grew more rapidly, leading to shorter survival [30, 31].
Matsuzaki et al. reported that tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer [32]. Expression of LAG-3 and PD-1 on CD8+ T cells was upregulated by IL-10, IL-6, and tumor-derived antigen-presenting cells. Dual blockade of LAG-3 and PD-1 during T-cell priming efficiently augmented the proliferation of and cytokine production by NY-ESO-1-specific CD8+ T cells. Krempski et al. reported that tumor-infiltrating programmed death receptor-1+ dendritic cells mediate immunosuppression in ovarian cancer [33]. PD-1 blockade in mice bearing ovarian cancer cells substantially reduced the tumor burden and increased the effector Ag-specific T-cell responses. Thus, multiple basic/preclinical studies convinced us that inhibition of PD-L1/PD-1 signaling in ovarian cancer could be an effective treatment strategy (Fig. 13.2b).
13.3.4 Clinical Application of Immune Checkpoint Inhibitors in Ovarian Cancer (Table 13.4)
13.3.4.1 Clinical Trials Using Anti-PD-1 Antibodies
In 2010, we initiated the first clinical trial of nivolumab, a humanized anti-PD-1 antibody, for ovarian cancer. It was a principal investigator-initiated, phase II trial in patients with platinum-resistant refractory ovarian cancer [34]. A total of 20 patients were included, and they received one of two doses every 2 weeks up to 1 year: 1 mg/kg for ten patients and 3 mg/kg for ten patients. Among the 20 patients, the best overall response rate was 15% (2 CR and 1 PR), and the disease control rate was 45%. The median overall survival was 20.0 months, and the median PFS was 3.5 months at the end of trial. Two patients with CR showed no evidence of disease for over 1 year.
A phase Ib clinical trial of pembrolizumab, another humanized anti-PD-1 antibody, was conducted as part of the KEYNOTE-028 trial, which included 26 patients with recurrent ovarian cancer. In the interim analysis, response rate was 11.5% (1 CR and 2 PR), and the disease control rate was 34.6% [35].
13.3.4.2 Clinical Trials Using Anti-PD-L1 Antibodies
Avelumab is a human anti-PD-L1 antibody with naïve Fc receptor. A phase I trial for 75 patients with recurrent ovarian cancer was conducted [36]. The overall response rate was 10.7% (0 CR and 8 PR), and the disease control rate was 54.7%. Another phase I trial using a different human anti-PD-L1 antibody, BMS-936599, included 17 ovarian cancer patients, and the response rate was 6.9% (0 CR, 1 PR) [22].
In addition to these trials, many trials testing anti-PD-1 and anti-PD-L1 antibodies for ovarian cancer are ongoing, as shown in Table 13.1.
13.4 Problems with Current Trials of Immunotherapy
There is no doubt that immunotherapy has great potential and is a promising approach for future cancer treatments, including ovarian cancer, but there are also many issues to be addressed.
13.4.1 Biomarker to Predict Efficacy of Immune Checkpoint Inhibitors
Considering the extraordinarily high cost of immune checkpoint inhibitors, identifying the ideal patient who would most benefit from this treatment is mandatory. For this purpose, a search for effective biomarkers to identify patients who are expected to have favorable response is necessary. In ovarian cancer, clear cell histology is often associated with a chemoresistant phenotype. However, at least several cases in early trial have shown that a clear cell histology has a good response to immune checkpoint inhibitors [34]. Thus, the histology of ovarian cancers may not predict the response of these drugs.
The first biomarker candidate is the expression of PD-L1 on tumor cells. Studies have reported that PD-L1 expression on ovarian cancer cells is associated with worse prognosis [6, 29]. Moreover, several clinical trials studying melanoma and non-squamous cell lung cancer showed that PD-L1 expression was correlated with an antitumor response upon anti-PD-1 antibody treatment [6]. By contrast, PD-L1 expression was not shown to be predictive of a response in other trials, including a phase II ovarian cancer trial [34]. These conflicting data may be ascribed to the different anti-PD-L1 antibodies used to evaluate PD-L1 expression. However, it may also be possible that PD-L1 expression cannot serve as a predictive factor in some cancers. Further studies that either use multiple antibodies or standardize the evaluation methods are necessary.
Another promising candidate of predictive biomarkers is the so-called mutation burden of cancer cells. It is well known that the frequency of mutations is high in melanoma and lung cancer, in which immune checkpoint inhibition is effective. It was reported that in colorectal cancer patients, the treatment response was significantly better in patients with deficiencies in DNA mismatch repair [37]. There is currently no data on whether mutation burden could also serve as a predictive marker in ovarian cancer; however, BRCA gene mutations in ovarian cancer are associated with hypermutations within the tumors and clinically associated with a favorable outcome [38]. Therefore, BCRA may be a good candidate as a predictive biomarker of immune checkpoint inhibition.
13.4.2 How to Combine Novel Immunotherapies with Other Treatments
A realistic issue in the application of immunotherapies for clinical practice is how to combine them with other treatments, including conventional chemotherapy or molecularly targeted therapies. In ovarian cancer, the response rate of first-line chemotherapy is relatively high, and chemotherapy is thought to maintain its status as the primary treatment for ovarian cancer. Therefore, it is important to know whether a combination of chemotherapy and immunotherapy is more effective. By using a mouse model, we have shown that some chemotherapy reagents induce PD-L1 expression on cancer cells, and the combination of chemotherapy and anti-PD-L1 antibodies increases the efficacy of treatment, possibly by inducing a cytotoxic immune reaction after chemotherapy [39]. A phase II clinical trial of the combination of paclitaxel/carboplatin chemotherapy with pembrolizumab in ovarian cancer is ongoing (NCT02520154).
Other combinations of immunotherapy with radiation therapy, molecularly targeted reagents, and other cancer immunotherapies have also been considered. Preclinical studies indicated that radiotherapy can enhance the efficacy of the blockade of CTLA-4 and PD-1 [40, 41]. In addition, several clinical cases and retrospective studies suggest that radiotherapy may enhance the efficacy of the immune checkpoint blockade [42], and there are prospective trials underway to address this possibility. Molecularly targeted therapy is another emerging treatment for various cancers. A combination of this therapy with immune checkpoint inhibition is currently under investigation, but early reports indicate issues with adverse effects [43]. Several trials such as the combination of an anti-CTLA-4 antibody with a PARP inhibitor (NCT02571725) or an anti-PD-L1 antibody with bevacizumab (NCT02659384) are ongoing. Finally, one of the most promising combination strategies is the combination of two different immunotherapies. Concomitant CTLA-4 and PD-1 blockades in patients with melanoma resulted in a highly durable response rate and an impressive overall survival [44]. A phase II trial of the combination treatment of an anti-PD-L1 antibody and an anti-CTLA-4 antibody for ovarian cancer is underway (NCT02261220).
13.4.3 Handling Immune-Specific Adverse Events
With increasing data regarding immune checkpoint inhibition for various cancers, it is becoming clear that there are adverse effects specific to immunotherapy. Immune-specific adverse effects likely arise from general immunologic enhancement and thus include dermatological, gastrointestinal, hepatic, endocrine, and other less common inflammatory events [44, 45]. The most clinically relevant events are diarrhea/colitis, endocrinopathies affecting the pituitary, adrenal, and thyroid glands and pneumonitis. Treatments of these adverse effects generally involve temporary immunosuppression with corticosteroids, tumor necrosis factor-α antagonists, mycophenolate mofetil, or other agents [46]. However, no standard treatment strategy has been established. In addition, early detection and initiation of treatment against adverse reactions are believed to be important.
13.5 Conclusion: Future Directions
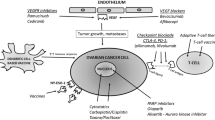
Immunotherapy, especially immune checkpoint inhibition targeting PD-1/PD-L1, has been shown to improve the outcome of patients with a variety of malignancies. Ovarian cancer is obviously another malignancy that should be examined to determine the efficacy of immune checkpoint inhibitors; presently, many clinical trials addressing this question are ongoing. One of the advantages of immunotherapies is their durability. Once the patient is responsive to immunotherapy, the patient is often cured instead of simply prolonging survival. However, there are still many obstacles to be solved in order to apply immunotherapy for the clinical management of ovarian cancer. First, considering the high cost of these drugs, we should attempt to find an effective biomarker (companion marker) to select patients. Second, we should become more familiar with immune-specific adverse effects, which are significantly different from those of chemotherapy. Third, we should pursue the best way to implement immunotherapies, especially regarding combinations with other treatments. To address these three problems, it is necessary to more thoroughly understand the biological consequences of immunotherapy in human cancers, including the use of basic research. Further understanding of the mechanisms involved in immunological manipulation may lead us to personalized and more finely tuned immunotherapy in the future (Fig. 13.3).
Future direction of the cancer immunotherapy. Firstly, to obtain effective cancer immunotherapy, improvement of both systemic and local immunities is mandatory. Then, further combination with other cancer treatments including chemotherapy should be optimized. Establishment of evaluation method of tumor immunity is also important, which should lead to optimization and personalization of immunotherapy. As a result, we can apply immunotherapy in future daily practice
References
Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE, Boente M, Birrer MJ, Liang SX, Gynecologic Oncology Group. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–83.
Poveda AM, Selle F, Hilpert F, Reuss A, Savarese A, Vergote I, Witteveen P, Bamias A, Scotto N, Mitchell L, Pujade-Lauraine E. Bevacizumab combined with weekly paclitaxel, pegylated liposomal doxorubicin, or topotecan in platinum-resistant recurrent ovarian cancer: analysis by chemotherapy cohort of the randomized phase III AURELIA trial. J Clin Oncol. 2015;33:3836–8.
Komiyama S, Katabuchi H, Mikami M, Nagase S, Okamoto A, Ito K, Morishige K, Nao Suzuki N, Kaneuchi M, Yaegashi N, Udagawa Y, Yoshikawa H, Japan Society of Gynecologic Oncology guidelines 2015 for the treatment of ovarian cancer including primary peritoneal cancer and fallopian tube cancer. Int J Clin Oncol 2016; 21:435–446.
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott CL, Meier W, Shapira-Frommer R, Safra T, Matei D, Fielding A, Spencer S, Dougherty B, Orr M, Hodgson D, Barrett JC, Matulonis U. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–61.
Fujii S, Takayama T, Asakura M, Aki K, Fujimoto K, Shimizu K. Dendritic cell-based cancer immunotherapies. Arch Immunol Ther Exp. 2009;57:189–98.
Hamanishi J, Mandai M, Matsumura N, Abiko K, Baba T, Konishi I. PD-1/PD-L1 blockade in cancer treatment: perspectives and issues. Int J Clin Oncol. 2016;21:462–73.
Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, Konishi I. Anti-PD-L1/PD-1 immune therapies in ovarian cancer: basic mechanism and future clinical application. Int J Clin Oncol. 2016;21:456–61.
Dobrzanski MJ, Rewers-Felkins KA, Samad KA, Quinlin IS, Phillips CA, Robinson W, Dobrzanski DJ, Wright SE. Immunotherapy with IL-10- and IFN-γ-producing CD4 effector cells modulate “natural” and “inducible” CD4 TReg cell subpopulation levels: observations in four cases of patients with ovarian cancer. Cancer Immunol Immunother. 2012;61:839–54.
Rahma OE, Ashtar E, Czystowska M, Szajnik ME, Wieckowski E, Bernstein S, Herrin VE, Shams MA, Steinberg SM, Merino M, Gooding W, Visus C, Deleo AB, Wolf JK, Bell JG, Berzofsky JA, Whiteside TL, Khleif SN. A gynecologic oncology group phase II trial of two p53 peptide vaccine approaches: subcutaneous injection and intravenous pulsed dendritic cells in high recurrence risk ovarian cancer patients. Cancer Immunol Immunother. 2012;61:373–84.
Leffers N, Lambeck AJ, Gooden MJ, Hoogeboom BN, Wolf R, Hamming IE, Hepkema BG, Willemse PH, Molmans BH, Hollema H, Drijfhout JW, Sluiter WJ, Valentijn AR, Fathers LM, Oostendorp J, van der Zee AG, Melief CJ, van der Burg SH, Daemen T, Nijman HW. Immunization with a P53 synthetic long peptide vaccine induces P53-specific immune responses in ovarian cancer patients, a phase II trial. Int J Cancer. 2009;125:2104–13.
Ohno S, Kyo S, Myojo S, Dohi S, Ishizaki J, Miyamoto K, Morita S, Sakamoto J, Enomoto T, Kimura T, Oka Y, Tsuboi A, Sugiyama H, Inoue M. Wilms’ tumor 1 (WT1) peptide immunotherapy for gynecological malignancy. Anticancer Res. 2009;29:4779–84.
Wagner U, Köhler S, Reinartz S, Giffels P, Huober J, Renke K, Schlebusch H, Biersack HJ, Möbus V, Kreienberg R, Bauknecht T, Krebs D, Wallwiener D. Immunological consolidation of ovarian carcinoma recurrences with monoclonal anti-idiotype antibody ACA125: immune responses and survival in palliative treatment. Clin Cancer Res. 2001;7:1154–62.
Reinartz S, Köhler S, Schlebusch H, Krista K, Giffels P, Renke K, Huober J, Möbus V, Kreienberg R, DuBois A, Sabbatini P, Wagner U. Vaccination of patients with advanced ovarian carcinoma with the anti-idiotype ACA125: immunological response and survival (phase Ib/II). Clin Cancer Res. 2004;10:1580–7.
Berek JS, Taylor PT, Gordon A, Cunningham MJ, Finkler N, Orr J Jr, Rivkin S, Schultes BC, Whiteside TL, Nicodemus CF. Randomized, placebo-controlled study of oregovomab for consolidation of clinical remission in patients with advanced ovarian cancer. J Clin Oncol. 2004;22:3507–16.
Berek J, Taylor P, McGuire W, Smith LM, Schultes B, Nicodemus CF. Oregovomab maintenance monoimmunotherapy does not improve outcomes in advanced ovarian cancer. J Clin Oncol. 2009;27:418–25.
Braly P, Nicodemus CF, Chu C, Collins Y, Edwards R, Gordon A, McGuire W, Schoonmaker C, Whiteside T, Smith LM, Method M. The immune adjuvant properties of front-line carboplatin-paclitaxel: a randomized phase 2 study of alternative schedules of intravenous oregovomab chemoimmunotherapy in advanced ovarian cancer. J Immunother. 2009;32:54–65.
Konner JA, Bell-McGuinn KM, Sabbatini P, Hensley ML, Tew WP, Pandit-Taskar N, Vander Els N, Phillips MD, Schweizer C, Weil SC, Larson SM, Old LJ. Farletuzumab, a humanized monoclonal antibody against folate receptor alpha, in epithelial ovarian cancer: a phase I study. Clin Cancer Res. 2010;16:5288–95.
Jelovac D, Armstrong DK. Role of farletuzumab in epithelial ovarian carcinoma. Curr Pharm Des. 2012;18:3812–5.
Heiss MM, Murawa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV, Dudnichenko AS, Aleknaviciene B, Razbadauskas A, Gore M, Ganea-Motan E, Ciuleanu T, Wimberger P, Schmittel A, Schmalfeldt B, Burges A, Bokemeyer C, Lindhofer H, Lahr A, Parsons SL. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int J Cancer. 2010;127:2209–21.
Baumann K, Pfisterer J, Wimberger P, Burchardi N, Kurzeder C, du Bois A, Loibl S, Sehouli J, Huober J, Schmalfeldt B, Vergote I, Lück HJ, Wagner U. Intraperitoneal treatment with the trifunctional bispecific antibody Catumaxomab in patients with platinum-resistant epithelial ovarian cancer: a phase IIa study of the AGO Study Group. Gynecol Oncol. 2011;123:27–32.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54.
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65.
Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–42.
Hamanishi J, Mandai M, Konishi I. Immune checkpoint inhibition in ovarian cancer. Int Immunol. 2016;28:339–48.
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23.
Calabrò L, Morra A, Fonsatti E, Cutaia O, Fazio C, Annesi D, Lenoci M, Amato G, Danielli R, Altomonte M, Giannarelli D, Di Giacomo AM, Maio M. Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy-resistant malignant mesothelioma: an open-label, single-arm, phase 2 study. Lancet Respir Med. 2015;3:301–9.
Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13.
Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–43.
Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–5.
Abiko K, Mandai M, Hamanishi J, Yoshioka Y, Matsumura N, Baba T, Yamaguchi K, Murakami R, Yamamoto A, Kharma B, Kosaka K, Konishi I. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin Cancer Res. 2013;19:1363–74.
Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, Baba T, Konishi I, Mandai M. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112:1501–9.
Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, Old LJ, Odunsi K. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–80.
Krempski J, Karyampudi L, Behrens MD, Erskine CL, Hartmann L, Dong H, Goode EL, Kalli KR, Knutson KL. Tumor-infiltrating programmed death receptor-1+ dendritic cells mediate immune suppression in ovarian cancer. J Immunol. 2011;186:6905–13.
Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S, Matsumura N, Abiko K, Baba T, Yamaguchi K, Ueda A, Hosoe Y, Morita S, Yokode M, Shimizu A, Honjo T, Konishi I. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33:4015–22.
Varga A, Piha-Paul SA, Ott PA, et al. Antitumor activity and safety of pembrolizumab in patients with PD-L1 positive advanced ovarian cancer: interim result from a phase Ib study. J Clin Oncol. 2015;33(Suppl.): Abstract no. 5510.
Dsis ML, Patel MR, Pant S et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patient with previously treated, recurrent or refractory ovarian cancer: a phase Ib, open-label expansion trial. J Clin Oncol. 2015;33(Suppl.): Abstract no. 5509.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20.
Birkbak NJ, Kochupurakkal B, Izarzugaza JM, Eklund AC, Li Y, Liu J, Szallasi Z, Matulonis UA, Richardson AL, Iglehart JD, Wang ZC. Tumor mutation burden forecasts outcome in ovarian cancer with BRCA1 or BRCA2 mutations. PLoS One. 2013;8:e80023.
Peng J, Hamanishi J, Matsumura N, Abiko K, Murat K, Baba T, Yamaguchi K, Horikawa N, Hosoe Y, Murphy SK, Konishi I, Mandai M. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-κB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. 2015;75:5034–45.
Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–34.
Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–95.
Grimaldi AM, Simeone E, Giannarelli D, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology. 2014;3:e28780.
Ribas A, Hodi FS, Callahan M, et al. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368:1365–6.
Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33.
Marrone KA, Ying W, Naidoo J. Immune-related adverse events from immune checkpoint inhibitors. Clin Pharmacol Ther. 2016;100:242–51.
Champiat S, Lambotte O, Barreau E, Belkhir R, Berdelou A, Carbonnel F, Cauquil C, Chanson P, Collins M, Durrbach A, Ederhy S, Feuillet S, François H, Lazarovici J, Le Pavec J, De Martin E, Mateus C, Michot JM, Samuel D, Soria JC, Robert C, Eggermont A, Marabelle A. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016;27:559–74.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mandai, M., Hamanishi, J., Abiko, K., Matsumura, N., Baba, T., Konishi, I. (2017). Immunology and Immunotherapy in Ovarian Cancer. In: Katabuchi, H. (eds) Frontiers in Ovarian Cancer Science. Comprehensive Gynecology and Obstetrics. Springer, Singapore. https://doi.org/10.1007/978-981-10-4160-0_13
Download citation
DOI: https://doi.org/10.1007/978-981-10-4160-0_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4159-4
Online ISBN: 978-981-10-4160-0
eBook Packages: MedicineMedicine (R0)