Abstract
Based on their morphological aspect, 45 strains of rhizobia isolated from root nodules of the wild forage legume Hedysarum flexuosum L. sampled from four soil regions of Morocco were tested for their physiological and biochemical characteristics. Their host plants were submitted to analysis of nodule intensity, dry matter yield, and nitrogen content. Moreover, soil samples from the sampling sites of nodulation surveys were collected and analyzed in order to assess the relationship between diversity of Hedysarum rhizobia and some soil properties. Even though many of the isolates were from the same plant, they exhibited a wide range of phenotypic diversity in relation to geographical origin. An overall increase in zinc and manganese was the main factor driving compositional differences among rhizobial populations. Their symbiotic efficiency appears to be sensitive to chlorine and aluminum. Although, high chromium in soil may have a positive effect on nodulation and subsequent nitrogen fixation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
8.1 Introduction
Given the great diversity revealed among nitrogen-fixing rhizobia isolated from different legumes, there is an increasing concern in getting knowledge on environmental factors influencing diversity and structure of soil microbial communities. This diversity has been reported to be linked to the large number of leguminous species and their wide geographical distribution (Wei et al. 2002). Many researches reputed that legumes can be responsible for variation in the soil bacterial community composition (Bakhoum et al. 2012; Rahi et al. 2012; Lorenzo et al. 2010; Silva et al. 2005), including changes in the communities of symbiotic nitrogen fixers (Rodríguez-Echeverría 2010). However, diversity of rhizobial strains toward their geographical origin remains scattered.
One of the most important forage legumes in the Mediterranean Basin is Hedysarum sp. It is a perennial plant, known for its good agronomical traits both in terms of high-quality forage and for soil nitrogen supply. Despite that the genus Hedysarum counts more than 100 species, however, only a few species of Hedysarum are recorded as being nodulated (Allen and Allen 1981; Sprent 2001). Among this species, Hedysarum flexuosum L., often known under the name of sulla, is an important forage legume in the northern parts of Morocco. It is reputed to be tolerant to the stress factors of drought, salinity, and alkaline soil which renders sulla well adapted to marginal areas. The ability of H. flexuosum L. to establish a strictly host-specific symbiosis with nitrogen-fixing rhizobia (Glatzle et al. 1986) makes them excellent candidates for use in sustainable agricultural systems.
Considering the potential value of H. flexuosum L., we decided to collect and characterize the rhizobia nodulating H. flexuosum L. in Northwestern Morocco from different environmental locations with the intent to study some soil properties which drive the phenotypic and efficiency diversity of the rhizobia associated. In the first place, plant samples were collected from each location and analyzed for nitrogen and dry matter content. On the other hand, the bacteria were evaluated in terms of their response of various physiological characters such as salinity stresses, extreme pH, high temperature, heavy metals, and antibiotics tolerance, with attention to select potentially useful strains of rhizobia strains which have to be highly effective in nitrogen fixation, highly competitive, and well adapted to the adverse conditions prevailing in these soils.
8.2 Materials and Methods
8.2.1 Root Nodule and Soil Sampling
The collection of spontaneous nodulated plants of H. flexuosum L. was conducted across Northwest part of Morocco during the spring of year 2014. Root nodules were collected from young and green plants at vegetative stage from four sites located in four different regions. From each site, ten plants were randomly collected. Healthy, unbroken, and pink nodules were randomly chosen from each plant. All the nodules were placed on cotton in screw cap plastic tubes containing silica gel as desiccant (Vincent 1970) and stored in 4 °C until isolation. Systematically, rhizosphere soil samples were randomly collected from a depth of 30 cm from the surface of three spots of each sampling site in which H. flexuosum L. has been grown naturally. They were mixed, air dried at room temperature, and screened through a 2 mm mesh for physical analysis at National Institute of Agronomic Research-Morocco-Rabat and for chemical analysis at the National Center of Scientific and Technical Research (CNRST) in Rabat, Morocco. Soil characteristics are presented in Tables 8.1 and 8.2. Moreover, plants samples were collected from each location and weighed instantly in the field for fresh weight determination. After transportation to the laboratory all samples were oven-dried at 70 °C until reaching a constant weight to determine dry matter.
8.2.2 Rhizobia Isolation
The method of isolating root-nodulating bacteria from nodules was as described by Vincent (1970). After incubation for 3 days at 28 °C, single colonies were picked and checked for purity by repeated streaking on to YEM plate containing Congo red (25 mg/ml) and Gram stain reaction. The pure isolates were stored in 25% (v⁄v) glycerol at −20 °C.
8.2.3 Cultural Characteristics
Isolates were subjected to different cultural and biochemical tests for identification, namely, Congo red test, growth on peptone glucose agar medium (Vincent 1970), and acid or alkali production in YEM medium containing bromothymol blue (0.025%). All plates were incubated at 28 °C for 6–7 days. Presence of growth was observed after 48 h according to Vincent (1970).
8.2.4 Response to Environmental Stress Factors
The isolates were examined for growth under different stress conditions of high temperature, high salinity, and extreme pH. In the case of temperature tolerance, isolates were kept at 28 (as a control), 35 or 40 °C on YEM plates for 4–5 days. The ability of the isolates to grow in different concentrations of salt was tested by streaking isolates on YEM medium containing 0.5%, 1%, and 2% (w/v) NaCl. Similarly, growth of rhizobial strains was compared at different pH (4.0, 5.0, 8.0, and 9.0) in YEM medium.
8.2.5 Utilization of Carbon and Nitrogen Sources
Isolates were tested for their ability to utilize some carbohydrate as a sole carbon source. For analysis of carbohydrate utilization, a modified YEM agar where yeast extract was reduced to 0.05 g/L (Somasegaran and Hoben 1994) and 0.01% NH4NO3 as a source of nitrogen was used. Mannitol was replaced by one of the following carbohydrates to a final concentration of (1%, w/v). Two control media were used for comparison; YEM containing mannitol was used as a positive control and the medium without any carbon source as a negative one. A modified mannitol medium, at which yeast extract was replaced by (0.1%, w/v) of the tested amino acid and mineral salts, was used to investigate the utilization of nitrogen compounds. N-free modified mannitol medium (devoid of any nitrogen source) was used as a control. All the plates were incubated at 28 °C for 2–7 days.
8.2.6 Antibiotic Sensitivity and Heavy Metal Tolerance
All isolates were tested for their sensitivity to eight heavy metals salts, namely, HgCl2, CuCl2, CdCI2, ZnCl2, MnCl2.4H2O, CoCl2.6H2O, AlCl3, and PbCl2, and to three antibiotics including kanamycin, erythromycin, and streptomycin. Sensitivity pattern was studied on YEM agar plate containing graded concentration of antibiotics or heavy metals. The stock solution of both antibiotics was prepared in distilled water, and solution was added to YEM medium after filtration through Millipore membrane (0.2 μm porosity). In all experiments growth was recorded after 3 days of incubation at 28 °C in triplicate.
8.2.7 Nodulation Assessment and Effectiveness Evaluation
Productivity and symbiotic efficiency was estimated at the vegetative stage on ten healthy plants collected from each field. A nodule scoring chart was applied to evaluate the infectivity of strains using the chart proposed by (Howieson and Dilworth 2016). Effectiveness of strains in nitrogen fixation was evaluated by scoring total dry matter, plant high and total nitrogen with the Kjeldahl method.
8.2.8 Numerical Analysis
The unweighted pair group method with arithmetic averages (UPGMA) was used for cluster analysis of phenotypic features. The similarity coefficient was computed, and the results are shown as a dendrogram using XLSTAT software (2014). Data obtained from will subject to statistical analysis using SAS software (2002) and followed by mean comparison by Duncan’s test. Values are means of three replicates.
8.3 Results and Discussion
8.3.1 Phenotypic Evaluation
A total of 45 bacteria were recovered from root nodules of H. flexuosum L. collected from different sites in the region of Tanger. The natural pastures of these plants are found primarily in calcareous clay soils except those in Ashakkar which grow in predominantly sandy soils (Table 8.1). The low level of calcareous found in Khandak Lihoudi soil could be related to shovel structure observed on their root system which acts as bioaccumulator of calcium salts resulting in a localized depletion of CaCO3 from the soil as already proved for Hedysarum coronarium L. (Tola et al. 2009). The pH of soils did not vary so much across the study sites confirming the adaptation of this crop to alkaline soils (Moore et al. 2006). Nominal values of soil nitrogen (N), phosphorus (P), and potassium (K) among the three sites varied considerably (Table 8.1), which ultimately affect the plant growth and nitrogen fixation as will be seen below.
The different rhizobial isolates were characterized by studying their presumptive morphological and the physiological characteristics. Generally, most rhizobia are developing a mature colony after days of incubation at 28 °C on YEMA plates. The colonies were characterized by a circular shape, white color, viscous, and differ slightly in their absorption of Congo red dye similarly to other bacteria hosted in the root nodules of the three Mediterranean wild legume species Hedysarum (Benhizia et al. 2004). Other interesting and useful characteristics of rhizobia are other growth reactions in the standard YM medium containing bromothymol blue (BTB) as the pH indicator. In our study, all colonies produce an acid reaction YMA-BTB plates and change to yellow after 3 days of incubation at 28 °C. These rhizobia can be qualified as fast-growing rhizobia according to Somasegaran and Hoben (1994). Unlike earlier belief that rhizobia have no ability to grow on glucose peptone agar medium (Somasegaran and Hoben 1994; Vincent 1970), in this study, some isolates grew on this medium and turn the medium to yellow. Finally, all retrieved strains were Gram negative. According to Vincent (1970) and Somasegaran and Hoben (1994), these characteristics are the first clues to the identification of rhizobia.
8.3.1.1 The Numbers Are the Number of Isolates Giving Positive Reaction
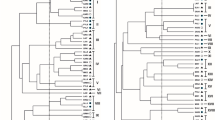
Regarding physiological properties of isolated strains (Table 8.3), they showed a large diversity among rhizobia and form heterogeneous group, based on phenotypic characteristics, such as tolerance to pH, salt, temperature and antibiotics, heavy metal, and carbon and nitrogen substrate assimilation tests depending on their geographic origin (Table 8.3). This geographic diversity in rhizobial species composition has been shown to be related to local environmental conditions (Yang et al. 2013; Li et al. 2012). The obtained UPGMA phenogram exhibited a few isolates clustering independently from their geographical origins (Fig. 8.1). All rhizobial strains were included in three distinctive clusters formed at 34% similarity level. Of the three clusters formed, one (C) was composed of rhizobia isolated from two different soil origins, namely, Boukhalef and Melloussa; their apparent consistent phenotype profile found among isolates could suggest some degree of genomic relatedness. Instead, the two other clusters (A and B) were composed of isolates originating only from one geographical site (Fig. 8.1). The presence of phenotypic clusters containing only isolates of one soil might indicate an evolution in the rhizobial population with the mutation and/or selection and proliferation of and particular subpopulations in relation to their soil characteristics in which they grow. This probability could explain the repetitive phenotypic profile for some isolates of Boukhalef site (HFB2, HFB4, HFB8, HFB9, HFB12, and HFB15) (Fig. 8.1), suggesting a lack of genetic diversity among isolates of this site. Notably, soils from Boukhalef and Melloussa were characterized by low heavy metal specially zinc and manganese (Table 8.2). This study suggests that metal-contaminated soils may preserve a higher diversity of rhizobia as the case of those isolated from Khandak Lihoudi and Ashakkar sites.
By the same token, isolates from different soil showed different resistance to the selected heavy metal (Table 8.3). Metal phenotypes varied within and between each group of isolates. Cluster (C) richer in isolates of Boukhalef and Melloussa showed higher metal tolerance especially to Mn and Zn, suggesting that both metal tolerances may be controlled by same mechanisms of tolerance.
Furthermore, this tolerance was not correlated with their soil origin (Table 8.2). Indeed, in spite of the presence of Mn or Zn in soil of Khandak Lihoudi and Ashakkar sites, their correspondent isolate shows a low tolerance, suggesting no such adaptation to this tows metal. In fact, metal tolerance of rhizobia was demonstrated to be linked to either slow or progressive increase of metal concentration in the soil. Slow metal increase favored the adaptation of more rhizobia to strive with the metal toxicity, contrary to rapid metal charge and long-term effects, and contributed to strong selection of rhizobia strains with high metal tolerance (Giller et al. 1998). This evidence could be ecologically important to investigate the degree of stress imposed by such metal.
8.3.2 Effectiveness Assessment
As well as the phenotypic results, the dry matter yield and nitrogen content of sulla varied from site to site and seemed to be related to the abundance of nodulation (Table 8.4). Thus, all plants assessed in field are either abundant or adequate in nodule. This could be explained by the relative size of the effective native rhizobial populations present in soil in relation to the plant cultivation history and the persistence of their root nodule bacteria in soil (Thami Alami and El Mzouri 2000). The abundance of nodulation found in Melloussa site could be due to abandoned sulla in the last years. Interestingly, the plants from Ashakkar site were only one with the least nodule abundance probably due to the low physical protection of native rhizobia in relation to the low proportion of clay in soil (Table 8.1). Consequently, Ashakkar soil samples did not promote nodule formation. This suggests that the potential of nodulation was not fully displayed in field due to unfavorable environmental conditions such as water availability and levels of nitrogen and phosphorus in soil (Zahran 1999). Accordingly, the low efficiency in terms of nitrogen content recorded in Ashakkar could be related to low level of phosphorus (3.50%) found in soil. Several studies found that nodulation and nitrogen fixation are directly linked to the phosphorus (P) supply. Although, strains of rhizobia differ markedly in tolerance to phosphorus deficiency (Beck and Munns 1985). Not only phosphorus but also mineral nitrogen levels in soil (0.061%) could have a negative effect on symbiotic efficiency. It is widely accepted that the nitrogen-fixing capacity of legumes is influenced by the presence of mineral nitrogen in the soil in which it is grown. Nevertheless, a low dose of nitrogen in the soil can stimulate plant growth until the starts of symbiotic nitrogen fixation (Muller and Pereira 1995). In other hand, adequate potassium (K) fertility proved not only to have positive effect on nodulation and subsequent nitrogen fixation but also alleviate the effects of water shortage (1.40% in Ashakkar site) on symbiotic nitrogen fixation (Sangakkara et al. 1996). However, the absorption by plants of this macronutrients (N, P, K) in addition to others micronutrients such as zinc (Zn), copper (Cu), iron (Fe), and manganese (Mn) could be limited by the presence or absence of native arbuscular mycorrhizal fungi (AMF) even on a calcareous soil (Labidi et al. 2012, 2015; Smith and Read 2008; Azaizeh et al. 1995; Li et al. 1991).
Outstandingly, the nodulation in Boukhalef site was relatively high, but nitrogen content remained limited, probably indicating the low efficiency of the nodulating rhizobia or could be related to high level of chlorine in soil (Table 8.2). In fact, several environmental factors such as physicochemical composition of the soil including heavy metals and water scarcity can affect the infection process and symbiotic nitrogen fixation by Rhizobium (Zahran 1999; Ahmad et al. 2012; Arora et al. 2010; Kinkema et al. 2006; Collavino et al. 2005). Soils from Boukhalef were characterized by high aluminum (10.7%) compared to the other sites (Table 8.2). Consequently, rhizobia populations seem to be sensitive to this metal. Studies reported that aluminum is extremely toxic to growth and enzyme activity of both fast- and slow-growing rhizobial species (Arora et al. 2010; Paudyal et al. 2007). Comparatively, the plasmid profiles of ineffective isolates surviving at high concentrations of heavy metals were all very similar (Giller et al. 1989), confirming the observations made above.
In this study the highest nitrogen content (3.75%) were found in Melloussa site (Table 8.4) conjointly with abundant pink nodules, typical of healthy and effective nodules. This result is relatively high comparatively with those obtained by Fitouri et al. (2012a)) for H. coronarium L. (max 2.94% in Tunis site). In fact inoculation of H. coronarium L. by different rhizobial strains significantly improved air-dry biomass production and the crude protein content. However, this improvement depends all times of the strain used (Fitouri et al. 2012b; Ben Taâmallah 1998). Therefore, testing the ability of the single isolate to induce root nodules on their host plant is primary. As a matter of fact, the high symbiotic efficiency recorded in this site may be as a result of the high level of chromium (Cr) in soil (Table 8.2) as already been demonstrated (Casella et al. 1988).
Conclusion
As has been noted, symbiotic effectiveness of nitrogen-fixing rhizobia varies according to their soil properties in which the plant grown naturally. In the field these factors could be operated interdependently and/or synergistically, affecting ultimately plant growth and symbioses. As a result, identifying the most prevailing factors affecting legume-Rhizobium symbiosis remains imperative in order to achieve optimum level of efficiency by culturing sulla in suitable environment conditions.
References
Ahmad E, Zaidi A, Khan MS, Oves M (2012) Heavy metal toxicity to symbiotic nitrogen-fixing microorganism and host legumes. In: Zaidi A et al (eds) Toxicity of heavy metals to legumes and bioremediation. Springer, Wien, pp 29–44
Allen ON, Allen EK (1981) The Leguminosae. Univerdity of Wisconsin Press, Madison. 812 p
Arora NK, Khare E, Singh S, Maheshwari DK (2010) Effect of Al and heavy metals on enzymes of nitrogen metabolism of fast and slow growing rhizobia under explant conditions. World J Microbiol Biotechnol 26:811–816. doi:10.1007/s11274-009-0237-6
Azaizeh HA, Marschner H, Romheld V, Wittenmayer L (1995) Effects of a vesicular-arbuscular mycorrhizal fungus and other soil microorganisms on growth, mineral nutrient acquisition and root exudation of soil-grown maize plants. Mycorrhiza 5:321–327
Bakhoum N, Ndoye F, Kane A, Assigbetse K, Fall D, Sylla SN (2012) Impact of rhizobial inoculation on Acacia senegal (L.) Willd. growth in greenhouse and soil functioning in relation to seed provenance and soil origin. World J Microbiol Biotechnol 28:2567–2579
Beck DP, Munns DN (1985) Effect of calcium on the phosphorus nutrition of Rhizobium meliloti. Soil Sci Soc Am J 49:334–337
Ben Taâmallah S (1998) Effets de la disponibilité en eau et de différentes souches de Rhizobium sur la productivité en herbe du sulla (Hedysarum coronarium) en Tunisie. Fourrages 154:191–196
Benhizia Y, Benhizia H, Benguedouar A, Muresu R, Giacomini A, Squartini A (2004) Gamma proteobacteria can nodulate legumes of the genus Hedysarum. Syst Appl Microbiol 27:462–468
Casella S, Frassinetti S, Lupi F, Squartini A (1988) Effect of cadmium, chromium and copper on symbiotic and free-living Rhizobium leguminosarum biovar trifolii. FEMS Microbiol Lett 49:343–347
Collavino M, Riccillo PM, Grasso DH, Crespi M, Aguilar OM (2005) GuaB activity is required in Rhizobium tropici during the early stages of nodulation of determinate nodules but is dispensable for the Sinorhizobium meliloti-alfalfa symbiotic interaction. Mol Plant Microbe Interact 18:742–750
Fitouri SD, Trabelsi D, Saïdi S, Zribi K, Ben Jeddi F, Mhamdi R (2012a) Diversity of rhizobia nodulating sulla (Hedysarum coronarium L.) and selection of inoculant strains for semi-arid Tunisia. Ann Microbiol 62:77–84. doi:10.1007/s13213-011-0229-2
Fitouri SD, Faysal BJ, Kais Z, Salah R, Ridha M (2012b) Effet de l’inoculation par une souche osmotolerante de Rhizobium sullae sur la croissance et la production en proteine du sulla (Sulla coronarium L.) sous déficit hydrique. J Appl Biosci 51:3642–3651
Giller KE, McGrath SP, Hirsch PR (1989) Absence of nitrogen fixation in clover grown on soil subject to long-term contamination with heavy metals is due to survival of only ineffective rhizobium. Soil Biol Biochem 21:841–848
Giller KE, Witter E, McGrath SP (1998) Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: a review. Soil Biol Biochem 30:1389–1414
Glatzle A, Schulte-Batenbrock T, Brockwell J (1986) Symbiotic incompatibility between two forage species of Hedysarum, grown in Morocco, and their homologous rhizobia. FEMS Microbiol Lett 37:39–43
Howieson JG, Dilworth MJ (2016) Australian Centre for International Agricultural Research, 2016. Working with rhizobia
Kinkema M, Scott PT, Gresshoff PM (2006) Legume nodulation: successful symbiosis through short and long-distance signalling. Funct Plant Biol 33:1–15. doi:10.1071/FP06056.
Labidi S, Ben Jeddi F, Tisserant B, Debiane D, Rezgui S, Grandmougin-Ferjani A, Lounès-Hadj Sahraoui A (2012) Role of arbuscular mycorrhizal symbiosis in root mineral uptake under CaCO3 stress. Mycorrhiza 22:337–345
Labidi S, Jeddi FB, Tisserant B, Yousfi M, Sanaa M, Dalpé Y, Sahraoui ALH (2015) Field application of mycorrhizal bio-inoculants affects the mineral uptake of a forage legume (Hedysarum coronarium L.) on a highly calcareous soil. Mycorrhiza 25:297–309. doi:10.1007/s00572-014-0609-0
Li XL, Marschner H, George E (1991) Acquisition of phosphorus and copper by VA- mycorrhizal hyphae and root-to-shoot transport in white clover. Plant Soil 136:49–57
Li M, Li Y, Chen WF, Sui XH, Li Y, Li Y, Wang ET, Chen WX (2012) Genetic diversity, community structure and distribution of rhizobia in the root nodules of Caragana spp. from arid and semi-arid alkaline deserts, in the north of China. Syst Appl Microbiol 35:239–245
Lorenzo P, Rodríguez-Echeverría S, González L, Freitas H (2010) Effect of invasive Acacia dealbata Link on soil microorganisms as determined by PCR-DGGE. Appl Soil Ecol 44:245
Moore GA, Sanford P, Wiley T (2006) Perennial pastures for Western Australia
Muller SH, Pereira PAA (1995) Nitrogen fixation of common bean (Phaseolus vulgaris L.) as affected by mineral nitrogen supply at different growth stages. Plant Soil 177:55–61
Paudyal SP, Aryal RR, Chauhan SVS, Maheshwari DK (2007) Effect of heavy metals on growth of Rhizobium strains and symbiotic efficiency of two species of tropical legumes. Sci World 5:27–32
Rahi P, Kapoor R, Young JPW, Gulati A (2012) A genetic discontinuity in root-610 nodulating bacteria of cultivated pea in the Indian trans-Himalayas. Mol Ecol 611(21):145–159
Rodríguez-Echeverría S (2010) Rhizobial hitchhikers from down under: invasional meltdown in a plant-bacteria mutualism? J Biogeogr 37:1611–1622
Sangakkara UR, Hartwig UA, Noesberger J (1996) Soil moisture and potassium affect the performance of symbiotic nitrogen fixation in faba bean and common bean. Plant Soil 184:123–130
Silva C, Vinuesa P, Eguiarte LE, Souza V, Martínez-Romero E (2005) Evolutionary 633 genetics and biogeographic structure of Rhizobium gallicum sensu lato, a widely 634 distributed bacterial symbiont of diverse legumes. Mol Ecol 14:4033–4050
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic, London
Somasegaran P, Hoben HJ (1994) Handbook for Rhizobia: methods in Legume-Rhizobium Technology. Springer, New York. 450 pp
Sprent JI (2001) Nodulation in legumes. Royal Botanic Gardens, Kew
Thami Alami I, El Mzouri E (2000) Etude de l'efficacité et de la persistance des souches de Rhizobium de sulla. In: Sulas L (ed) Legumes for Mediterranean forage crops, pastures and alternative uses. CIHEAM, Zaragoza, pp 321–325
Tola E, Henriquez-Sabà JL, Polone E, Dazzo FB, Concheri G, Casella S, Squartini A (2009) Shovel roots: a unique stress-avoiding developmental strategy of the legume plant Hedysarum coronarium L. Plant Soil 322:25–37. doi:10.1007/s11104-008-9861-4
Vincent JM (1970) A manual for the practical study of root nodule bacteria. In: IBP Handbook, No 15. Blackwell Scientific Publications Ltd., Oxford
Wei GH, Wang ET, Tan ZY, Zhu ME, Chen WX (2002) Rhizobium indigoferae sp. nov. and Sinorhizobium kummerowiae sp. nov. respectively isolated from Indigofera spp. and Kummerowia stipulacea. Int J Syst Evol Microbiol 52:2231–2239
Yang W, Kong Z, Chen W, Wei G (2013) Genetic diversity and symbiotic evolution of rhizobia from root nodules of Coronilla varia. Syst Appl Microbiol 36:49–55
Zahran HH (1999) Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol Mol Biol Rev 63:968–989
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Elyemlahi, A., Arakrak, A., Laglaoui, A., Bakkali, M. (2017). Diversity and Efficiency of Rhizobia Nodulating Hedysarum flexuosum L. in Northwestern of Morocco in Relation to Soil Properties. In: Kumar, V., Kumar, M., Sharma, S., Prasad, R. (eds) Probiotics and Plant Health. Springer, Singapore. https://doi.org/10.1007/978-981-10-3473-2_8
Download citation
DOI: https://doi.org/10.1007/978-981-10-3473-2_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-3472-5
Online ISBN: 978-981-10-3473-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)