Abstract
Annexin V, a human protein with nanomolar affinity for cell membrane-bound phosphatidylserine (PS), is the most widely used conjugate for the detection of apoptosis by using the imaging modalities such as SPECT, PET, MRI, and optical imaging. This chapter will initially focus on the most recent reports on annexin V-conjugated imaging agents in both animals and humans, followed by conclusions and the possible future directions of annexin V imaging.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Positron Emission Tomography
- Annexin Versus
- Fluorescence Molecular Tomography
- Label Annexin Versus
- Hepatic Apoptosis
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Annexin V, a human protein with nanomolar affinity for cell membrane-bound phosphatidylserine (PS), is the most widely used conjugate for the detection of apoptosis by using the imaging modalities such as SPECT, PET, MRI, and optical imaging. This chapter will initially focus on the most recent reports on annexin V-conjugated imaging agents in both animals and humans, followed by conclusions and the possible future directions of annexin V imaging.
1 Introduction
Apoptosis or programmed cell death plays a critical role in normal physiology and pathology of numerous disease states [1]. Therefore, the in vivo visualization of apoptosis would allow for both early detection of therapy efficiency and evaluation of disease progression. Several agents have been developed and investigated for apoptosis imaging by using different imaging modalities such as single-photon emission computed tomography (SPECT), positron emission tomography (PET), optical imaging (OI), and magnetic resonance imaging (MRI). Each imaging modality has certain advantages as well as limitations. The choice of the right imaging modality or hybrid scanner depends on the parameter of interest under consideration (i.e., anatomical structure, functional metabolism, etc.).
2 Annexin V-Phospholipid Complex
The externalization of the phosphatidylserine (PS) on the cell membrane has been identified as a major biochemical marker of apoptosis and could in principle be exploited for the detection of apoptosis [2]. Annexin V (36 kDa), which interacts strongly and specifically with phosphatidylserine residues, has been the most studied imaging probe for apoptosis [3] (Table 14.1 ).
3 SPECT and PET Imaging
3.1 Single-Photon Emission Computed Tomography (SPECT) Imaging
Since the first apoptotic imaging reported in 1999 [4, 5], in vivo imaging of cell death with radiolabeled annexin V has been widely used in animal studies and clinical trials [6]. The unique advantages of radiotracers include their high sensitivity and the translational potential. Among various SPECT radionuclides, technetium-99 m (99mTc) is the most prominent isotope for the nuclear imaging because of its ideal nuclear properties and easy availability at low cost [7, 8].
Based on the previous study [9, 10], Blankenberg et al. reported an improved 99mTc-annexin V radioprobe using the bifunctional agent hydrazino nicotinamide (HYNIC) [5]. 99mTc-HYNIC-annexin V showed the greatest uptake in the kidneys, liver, and urinary bladder; however, it was devoid of any bowel excretion, resulting in excellent signal to background ratio in the abdominal region. With the modified procedure of preparation, 99mTc-HYNIC-annexin V could be synthesized efficiently with high yield. By far, 99mTc-HYNIC-annexin V has been extensively investigated in animal models [11–15]. Multiple clinical trials have confirmed the clinical utility of 99mTc-HYNIC-annexin V in determining the efficacy of chemotherapy in the patients of non-small cell lung cancer for the detection of apoptotic regions [16, 17].
In 2000, Tait et al. reported 99mTc-HYNIC-cys-annexin V117, which revealed site-specific labeling; however, it showed similar apoptosis avidity when compared to the previous version of 99mTc-HYNIC-annexin V [18]. Similar radiolabeled annexin V probes such as 99mTc(CO)3-HIS-cys-AnxV [19, 20] and 99mTc-His10-annexin V [21] demonstrated improved sensitivity for detecting dead or dying cells.
Yang et al. reported the use of 99mTc-EC-annexin using ethylenedicysteine (EC) as a chelator to assess the level of apoptosis of tumor cell [26]. The preclinical data of breast cancer patients showed the total effective dose equivalent for 99mTc-EC-annexin V of 6.80–7.89 mSv could be reasonable and allow it for clinical use and it could be a predictor for evaluating the treatment-related apoptosis after induction of chemotherapy [27].
Recently, 99mTc-C3(BHam)2-annexin V was developed using a bis(hydroxamide) derivative [C3(BHam)2] as a bifunctional chelating agent [28]. In vivo evaluation of 99mTc-C3(BHam)2-annexin V showed decreased uptake and retention in nonspecific tissues and much lower kidney accumulation of radioactivity when compared to 99mTc-HYNIC-annexin V. Their findings also indicated that 99mTc-C3(BHam)2-annexin V could be a potential candidate as a predictor for response to chemotherapy.
Additional to 99mTc, 67Ga [22, 23] and 111In [24, 25] were also used to label annexin V or its mutants for site-specific detection of apoptosis.
3.2 Positron Emission Tomography (PET) Imaging
The major advantages of PET imaging over SPECT are its much higher sensitivity, spatial resolution, and quantitative imaging; therefore, annexin V has been radiolabeled with fluorine 18 (18F) and many other isotopes for positron emission tomography.
18F-labeled annexin V with N-succinimidy-4-18F-fluorobenzoate (18F-SFB) has been investigated by several groups [29, 30] [31]. These studies of 18F-SFB annexin V demonstrated comparable apoptotic imaging feasibility to 99mTc-labeled annexin V and a fast clearance [31]. Moreover, 18F-SFB annexin V showed a significant higher accumulation in the mice treated with doxorubicin when compared to the control group [30].
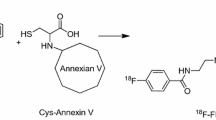
Annexin V can also be labeled with thiol-reactive agents such as N-substituted maleimides, and iodoacetamide can be used to modify proteins at cysteines at specific sites [32].18F-N-[2-(4-fluorobenzamido)ethyl]maleimide (18F-FBEM) was used to label thiol-containing proteins as a novel site-specific labeling prosthetic group [33, 34]. Compared to the previous generation of 18F-SFB-labeled annexin V, the novel 18F-FBEM-cys-annexin V showed faster renal and a lesser extent of hepatobiliary excretion in normal mice and more sensitivity of site-specific detection in the rats of hepatic apoptosis model [35].
4 MRI Imaging
One of the main differences between magnetic resonance imaging (MRI) scan and other imaging modalities like PET is that MRI scan which reveals high spatial resolution allows scientists to navigate through the entire living organism, down to the cellular level. Several annexin V-based contrast agents have been developed. However, due to the fundamentally low sensitivity of MRI, how to deliver sufficient contrast agents safely and acquire sufficient imaging signals in vivo is definitely the concern.
4.1 T-Positive Images: Gadolinium-Labeled Annexin V
To access the redistribution of phosphatidylserine in the event of apoptosis, annexin V was linked to gadolinium diethylenetriamine pentaacetate (Gd-DTPA)-coated liposomes [36]. A significant increase in signal intensity was visible in those regions containing cardiomyocytes in the early stage of apoptosis. The in vivo Gd-DTPA-annexin V MRI imaging provided a rapid targeting of apoptotic cells in the ischemic and reperfused myocardium. Moreover, van Tilborg and his colleagues reported Gd-DTPA-bis(stearylamide) (Gd-DTPA-BSA)-labeled annexin V, the multiple functions of lipid-based bimodal contrast agent, enables the detection of apoptotic cells with both MRI and optical techniques [37]. Gd-DTPA-BSA was covalently coupled multiple human recombinant annexin V to introduce specificity for apoptotic cells. The imaging results showed a significant increase of the relaxation rates of apoptotic cell pellets when compared to the untreated control cells, which may have applications for the in vivo detection of apoptosis. In 2010, the same group developed a small micellar annexin A5-functionalized nanoparticle for noninvasive MRI and fluorescent imaging of PS exposing cells in atherosclerotic lesions [38]. In vivo MRI images of the abdominal aorta in atherosclerotic ApoE(−/−) mice revealed enhanced uptake of the annexin A5-micelles as compared to control micelles, which was corroborated with ex vivo near-infrared fluorescence images of excised whole aortas.
4.2 T2-Negative Images: Iron Oxide-Labeled Annexin V
Compared to T1 agents, superparamagnetic iron oxide nanoparticle-based T2 agents are assumed to be the preferred MRI contrast agents for evaluating apoptosis due to their high sensitivity. Up to date, the common labeling approach for apoptotic imaging is based on cross-linked derivative of monocrystalline iron oxide (MION), also known as cross-linked iron oxide (CLIO) [39].
Annexin V-CLIO allowed the identification of cell suspensions containing apoptotic cells by MRI even at very low concentrations of magnetic substrate [40]. Van Tilborg et al. investigated the internalization of, when co-exposed to apoptotic stimuli, annexin A5 was shown to internalize into endocytic vesicles by using annexin A5-functionalized iron oxide particles [41].
Recently, our group present annexin V conjugated with superparamagnetic iron oxide nanoparticles (USPIO-annexin V) to the mice with Fas-induced hepatic apoptosis (data unpublished). The results showed that USPIO-annexin V accumulated in the region of hepatic apoptosis significantly decreased in comparison with control group (p< 0.05) (Fig. 14.1). USPIO-annexin V MRI may provide useful properties such as quantitative pharmacologic hepatic apoptosis that can be used as an indicator for hepatitis or liver injury induced by chemotherapy or after radiation exposure.
5 Optical Imaging
Petrovsky et al. first demonstrated that annexin V-labeled fluorophore Cy5.5 could be used as a nonradioactive probe for apoptosis [42]. Later in 2004, the modified annexin V-Cy5.5 conjugate was used to measure the tumor response to chemotherapy by fluorescence molecular tomography (FMT). This probe provided higher quantification accuracy validated by histology when compared to the traditional planar illumination methods [43]. The quantitative results also showed tenfold increase of fluorochrome intensity in cyclophosphamide-sensitive tumors and a sevenfold increase of resistant tumors compared with controls. Smith et al. developed a fluorescent imaging probe conjugating zinc(II)-dipicolylamine (Zn-DPA) with annexin V [44]. In vivo studies demonstrated that the fluorescent Zn-DPA targeting ligand selectively targeted to the apoptotic tumor cells was consistent with ex vivo biodistribution and histological analyses [45].
6 Multiple Imaging Modality
Multiple imaging modalities generate more informative and effective imaging in the diagnosis and treatment of a large number of diseases, particularly if the machine combines both functional and anatomical imaging modalities. By using multiple imaging instruments, researchers can track multiple molecular targets simultaneously and obtain more accurate localization and precise expression of biomarkers [46].
AnxCLIO-Cy5.5, the first magneto-optical nanoparticle, can be used as a bifunctional tracer in MRI and fluorescence imaging [47]. The in vivo images demonstrated that myocardial T2 signals of AnxCLIO-Cy5.5 were significantly lower in the mice receiving transient coronary artery (LAD) occlusion, and fluorescence target to background ratio was significantly higher when compared to the controls [48]. In addition, annexin V-conjugated quantum dots with a paramagnetic lipidic coating (Gd-DTPA) for MRI and fluorescent imaging showed high specificity for detecting apoptotic cells [38, 49].
As an alternative to MRI/optical imaging, nuclear/optical imaging was also developed for the detection of apoptosis. Zhang et al. evaluated 111In-labeled annexin A5-conjugated core-cross-linked polymeric micelles (CCPM) for micro-single-photon emission tomography/computed tomography (μSPECT/CT) and fluorescence molecular tomography (FMT) imaging in various disease models including tumor apoptosis, hepatic apoptosis, and inflammation. [50] [51]. Zhang et al. provided the clue that multiple imaging techniques should be advantageous in assessing and validating early diagnosis and therapeutic responses in diseases associated with apoptosis.
7 Conclusions and Perspectives
Over the past two decades, there have been many tracers proposed by using different modalities for apoptosis imaging, but none of them yet has achieved fully the validation for the differential localization or biochemical cellar progression of apoptosis. In this review, we focus on imaging agents conjugated with annexin V by using different imaging modalities such as single-photon emission computed tomography (SPECT), positron emission tomography (PET), optical imaging (OI), and magnetic resonance imaging (MRI). Each modality allows for the in vivo noninvasive detection of apoptotic cells and cell products. Not surprisingly, multimodal imaging, combining two or more of these techniques (PET/MRI or SPECT/CT or optical/CT), will become a key player for basic and translational medicine in humans and animals in the future, despite the challenges when considering acquiring and combining nonredundant images as well as imaging time, throughput, and cost of technology.
However, for the development of apoptosis-detecting imaging agents, there are several concerns that should be taken in mind such as the pharmacokinetic/pharmacodynamics of new agents, signal to background ratio in the abdominal region, and differentiation of apoptosis and necrosis. Consequently, we believe that all of these factors will be integrated and clear obstacles to introduce a successful apoptosis imaging agent.
References
Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–57.
Thiagarajan P, Tait JF. Binding of annexin V/placental anticoagulant protein I to platelets. Evidence for phosphatidylserine exposure in the procoagulant response of activated platelets. J Biol Chem. 1990;265(29):17420–3.
Arur S et al. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev Cell. 2003;4(4):587–98.
Blankenberg FG et al. Imaging of apoptosis (programmed cell death) with 99mTc annexin V. J Nucl Med. 1999;40(1):184–91.
Blankenberg F, Narula J, Strauss HW. In vivo detection of apoptotic cell death: a necessary measurement for evaluating therapy for myocarditis, ischemia, and heart failure. J Nucl Cardiol. 1999;6(5):531–9.
Lu C et al. Preliminary biological evaluation of novel (99 m)Tc-Cys-annexin A5 as a apoptosis imaging agent. Molecules. 2013;18(6):6908–18.
Zeng W et al. Molecular imaging of apoptosis: from micro to macro. Theranostics. 2015;5(6):559–82.
Ogawa K, Aoki M. Radiolabeled apoptosis imaging agents for early detection of response to therapy. ScientificWorldJournal. 2014;2014:732603.
Abrams MJ, J.M. tenKate CI, Schwartz DA, Hauser MM, Gaul FE. Technetium-99 m-human polyclonal IgG radiolabeled via the hydrazino nicotinamide derivative for imaging focal sites of infection in rats. J Nucl Med. 1990;31:2022–8.
Stratton JR et al. Selective uptake of radiolabeled annexin V on acute porcine left atrial thrombi. Circulation. 1995;92(10):3113–21.
Guo MF et al. In vivo99mTc-HYNIC-annexin V imaging of early tumor apoptosis in mice after single dose irradiation. J Exp Clin Cancer Res. 2009;28:136.
Ohtsuki K et al. Technetium-99 m HYNIC-annexin V: a potential radiopharmaceutical for the in-vivo detection of apoptosis. Eur J Nucl Med. 1999;26(10):1251–8.
Takei T et al. Time course of apoptotic tumor response after a single dose of chemotherapy: comparison with 99mTc-annexin V uptake and histologic findings in an experimental model. J Nucl Med. 2004;45(12):2083–7.
Kemerink GJ et al. Safety, biodistribution, and dosimetry of 99mTc-HYNIC-annexin V, a novel human recombinant annexin V for human application. J Nucl Med. 2003;44(6):947–52.
Rottey S et al. 99mTc-HYNIC Annexin-V imaging of tumors and its relationship to response to radiotherapy and/or chemotherapy. Q J Nucl Med Mol Imaging. 2007;51(2):182–8.
Kartachova M et al. Prognostic significance of 99mTc Hynic-rh-annexin V scintigraphy during platinum-based chemotherapy in advanced lung cancer. J Clin Oncol. 2007;25(18):2534–9.
Rottey S et al. Sequential 99mTc-hydrazinonicotinamide-annexin V imaging for predicting response to chemotherapy. J Nucl Med. 2006;47(11):1813–8.
Tait JF et al. Development and characterization of annexin V mutants with endogenous chelation sites for (99 m)Tc. Bioconjug Chem. 2000;11(6):918–25.
de Saint-Hubert M et al. Site-specific labeling of ‘second generation’ annexin V with 99mTc(CO)3 for improved imaging of apoptosis in vivo. Bioorg Med Chem. 2010;18(3):1356–63.
de Saint-Hubert M et al. Preclinical imaging of therapy response using metabolic and apoptosis molecular imaging. Mol Imaging Biol. 2011;13(5):995–1002.
Ye F et al. Evaluation of adenosine preconditioning with 99mTc-His10-annexin V in a porcine model of myocardium ischemia and reperfusion injury: preliminary study. Nucl Med Biol. 2011;38(4):567–74.
Smith-Jones PM, A.A Zanzonico P, Tait J, Larson SM, Strauss HW. 68Ga labelling of annexin-V: comparison to 99mTc-annexin-V and 67Ga-Annexin. J Nucl Med Biol. 2003;44(Suppl 1):S49–50.
Wangler C et al. A universally applicable 68Ga-labeling technique for proteins. J Nucl Med. 2011;52(4):586–91.
Wen X et al. Improved radiolabeling of PEGylated protein: PEGylated annexin V for noninvasive imaging of tumor apoptosis. Cancer Biother Radiopharm. 2003;18(5):819–27.
Ke S et al. Imaging taxane-induced tumor apoptosis using PEGylated, 111In-labeled annexin V. J Nucl Med. 2004;45(1):108–15.
Yang DJ et al. In vivo and in vitro measurement of apoptosis in breast cancer cells using 99mTc-EC-annexin V. Cancer Biother Radiopharm. 2001;16(1):73–83.
Kurihara H et al. Imaging and dosimetry of 99mTc EC annexin V: preliminary clinical study targeting apoptosis in breast tumors. Appl Radiat Isot. 2008;66(9):1175–82.
Ogawa K et al. Development and evaluation of a novel (99 m)tc-labeled annexin A5 for early detection of response to chemotherapy. PLoS One. 2013;8(12):e81191.
Zijlstra S, Gunawan J, Burchert W. Synthesis and evaluation of a 18F-labelled recombinant annexin-V derivative, for identification and quantification of apoptotic cells with PET. Appl Radiat Isot. 2003;58(2):201–7.
Hu S et al. Longitudinal PET imaging of doxorubicin-induced cell death with 18F-Annexin V. Mol Imaging Biol. 2012;14(6):762–70.
Murakami Y et al. 18F-labelled annexin V: a PET tracer for apoptosis imaging. Eur J Nucl Med Mol Imaging. 2004;31(4):469–74.
de Bruin B et al. 1-[3-(2-[18F]fluoropyridin-3-yloxy)propyl]pyrrole-2,5-dione: design, synthesis, and radiosynthesis of a new [18F]fluoropyridine-based maleimide reagent for the labeling of peptides and proteins. Bioconjug Chem. 2005;16(2):406–20.
Gao H et al. PET of insulinoma using (1)(8)F-FBEM-EM3106B, a new GLP-1 analogue. Mol Pharm. 2011;8(5):1775–82.
Kiesewetter DO et al. Automated radiochemical synthesis of [18F]FBEM: a thiol reactive synthon for radiofluorination of peptides and proteins. Appl Radiat Isot. 2011;69(2):410–4.
Lu C et al. Preliminary biological evaluation of (1)(8)F-FBEM-Cys-Annexin V a novel apoptosis imaging agent. Molecules. 2015;20(3):4902–14.
Hiller KH et al. Assessment of cardiovascular apoptosis in the isolated rat heart by magnetic resonance molecular imaging. Mol Imaging. 2006;5(2):115–21.
van Tilborg GA et al. Annexin A5-functionalized bimodal lipid-based contrast agents for the detection of apoptosis. Bioconjug Chem. 2006;17(3):741–9.
van Tilborg GA et al. Annexin A5-functionalized bimodal nanoparticles for MRI and fluorescence imaging of atherosclerotic plaques. Bioconjug Chem. 2010;21(10):1794–803.
Tassa C, Shaw SY, Weissleder R. Dextran-coated iron oxide nanoparticles: a versatile platform for targeted molecular imaging, molecular diagnostics, and therapy. Acc Chem Res. 2011;44(10):842–52.
Schellenberger EA et al. Annexin V-CLIO: a nanoparticle for detecting apoptosis by MRI. Acad Radiol. 2002;9(Suppl 2):S310–1.
van Tilborg GA et al. Internalization of annexin A5-functionalized iron oxide particles by apoptotic Jurkat cells. Contrast Media Mol Imaging. 2009;4(1):24–32.
Petrovsky A et al. Near-infrared fluorescent imaging of tumor apoptosis. Cancer Res. 2003;63(8):1936–42.
Ntziachristos V et al. Visualization of antitumor treatment by means of fluorescence molecular tomography with an annexin V-Cy5.5 conjugate. Proc Natl Acad Sci U S A. 2004;101(33):12294–9.
Smith BA et al. Optical imaging of mammary and prostate tumors in living animals using a synthetic near infrared zinc(II)-dipicolylamine probe for anionic cell surfaces. J Am Chem Soc. 2010;132(1):67–9.
Smith BA et al. In vivo optical imaging of acute cell death using a near-infrared fluorescent zinc-dipicolylamine probe. Mol Pharm. 2011;8(2):583–90.
Louie A. Multimodality imaging probes: design and challenges. Chem Rev. 2010;110(5):3146–95.
Schellenberger EA et al. Magneto/optical annexin V, a multimodal protein. Bioconjug Chem. 2004;15(5):1062–7.
Sosnovik DE et al. Molecular MRI of cardiomyocyte apoptosis with simultaneous delayed-enhancement MRI distinguishes apoptotic and necrotic myocytes in vivo: potential for midmyocardial salvage in acute ischemia. Circ Cardiovasc Imaging. 2009;2(6):460–7.
van Tilborg GA et al. Annexin A5-conjugated quantum dots with a paramagnetic lipidic coating for the multimodal detection of apoptotic cells. Bioconjug Chem. 2006;17(4):865–8.
Zhang R et al. Annexin A5-conjugated polymeric micelles for dual SPECT and optical detection of apoptosis. J Nucl Med. 2011;52(6):958–64.
Zhang R et al. Annexin A5-functionalized nanoparticle for multimodal imaging of cell death. Mol Imaging. 2013;12(3):182–90.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Yeh, S.HH., Kong, FL., Lin, MH. (2017). Visualization of Apoptosis: Annexin V Imaging. In: Inoue, T., Yang, D., Huang, G. (eds) Personalized Pathway-Activated Systems Imaging in Oncology. Springer, Singapore. https://doi.org/10.1007/978-981-10-3349-0_14
Download citation
DOI: https://doi.org/10.1007/978-981-10-3349-0_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-3348-3
Online ISBN: 978-981-10-3349-0
eBook Packages: MedicineMedicine (R0)