Abstract
Serine proteases and their inhibitors are being extensively studied in the past few decades, accentuating their pivotal role in diverse biological processes. In this chapter, we have discussed about their role as drug targets, associated pathologies and therapeutic interventions. Fine tune equilibrium between proteolytic enzymes and their respective inhibitors enables normal functions of the body. The upregulation or downregulation of this class of molecules is deleterious and results in various diseased conditions like inflammation, cancer, skin diseases, atherosclerosis, immunological disorders, coagulation abnormalities, pulmonary and neuronal disorders, and other pathologies. Several approaches to illustrate this relationship are comprehended with consequent stress on how these findings apply to pathologies that are the outcome of malfunction of serine proteases or their inhibitors. We have outlined the history and classification of proteases and their inhibitors as therapeutics and drug targets. Also an overview of their current clinical applications and approaches to improve and expand their use is discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Cellular life is orchestrated by equilibrium between proteolytic activity and their respective inhibitors as well as the concentration and state of the enzymes. Proteases, the enzymes that hydrolyse proteins, are therefore critical elements of the genome. Proteolytic enzymes are encoded by 2–4% of genes in a typical genome [1]. In some of the physiological processes like complement activation, phagocytosis, immune responses, tissue reorganization, blood clotting, fibrinolysis, blood pressure regulation, food digestion, defence mechanisms, etc. proteases serve a promising role. The human degradome consists of at least 1449 proteases and homologues, of which 399 are serine proteases (MEROPS release 10.0) [2]. The cascade of activation and deactivation mechanisms of proteases needs to be controlled at every point of regulation, for example at mRNA translation, transcription of protease gene, activation of zymogen, etc. Undesired proteolysis leading to various disease states is prevented by plethora of protease inhibitors with diverse specificities. Increase in proteolytic activity in certain instances mediates damage of cartilage in diarthrodial joints [3]. In certain diseases like tumor invasion, gingivitis, emphysema and other inflammatory ailments it is thought that proteases are the cause for tissue injury [3]. Fibrous proteins such as elastin and collagen can be solubilized by some serine proteases like cathepsin G, collagenase and elastase that are the culprits in extracellular matrix damage [4].
If the specificity of proteases is known with respect to their amino acid residues, there is every probability to inhibit those enzymes that are involved in various pathologies. Inhibitors with prospective inhibitory potential can be developed as new therapeutic agents. It is known that there are specific protease inhibitors against serine proteases of mammals, which are isolated from various plants and animals. This provides an excellent opportunity for developing novel medicines. Hence, the extracts of PIs from various sources are key in developing non-toxic drugs [3]. The future studies will mostly be concentrated on testing the specificity of protease inhibitors at clinical level against inflammation, cancer, dermatitis and emphysema. A good number of proteases are potential drug targets or are versatile molecules of consideration as diagnostic and prognostic biomarkers [5].
2 Serine Proteases
2.1 Introduction and Classification
Nature has provided abundant sources of serine proteases which are distributed among all living cells. The presences of nucleophilic Serine residue in the active site that attacks the carbonyl moiety of substrate, derives its name as serine protease. Ser proteases are endoproteases and catalyse polypeptide bond hydrolysis in the middle of the chain. A similar feature can be observed in several other exoprotease families.
Barrett and Rawlings classified all known proteolytic enzymes based on sequence similarity and structure into clans and families and termed this database as MEROPS [6].
Three useful methods of grouping peptidases are currently in use as shown in Table 1.
-
1.
Based on catalytic mechanism
-
2.
Based on reaction catalysed and
-
3.
Based on molecular structure and homology.
2.1.1 Based on Catalytic Mechanism
Enzymes exhibiting proteolytic activity are classified as cysteine, glutamic, serine, threonine, aspartic, asparagine or metalloproteases. Depending upon the catalytic type, the clans and families of proteolytic enzymes are named as C, G, S, T, A, M, N and U in the MEROPS database. Plus, P is given to proteases which are of mixed type [7].
2.1.2 Based on Reaction Catalysed
Though peptidases are known to catalyse peptide bond, no single type of enzyme catalyses all peptide bonds. They are specific to certain polypeptide chains of the substrate. Based on the polypeptide chain specificity they are classified into exopeptidases, endopeptidases, aminopeptidases, carboxypeptidases, omega-peptidases, dipeptidases, dipeptidyl-peptidases, peptidyl-dipeptidases and tripeptidyl-peptidases [8].
2.1.3 Based on Homology and Molecular Structure
With the advent of high throughput technologies, classification based on homology and molecular structure is the latest. Classification by Rawlings and Barrett assigns individual peptidases into families and further into clans. In order to develop the MEROPS database, such scheme was designed which was further extended to store the information about proteins that inhibit peptidases [8].
2.2 Serine Proteases as Therapeutics
Despite many studies on proteases, their physiologically specific substrates are unknown. Their activity in a particular tissue in the human body also differs. The characteristics manifested by most proteases also differ in disease. Dysregulation of hydrolysis of proteins is a common trait identified in different diseases and in inflammatory responses. Tumor metastasis, invasion and growth in different types of cancer are commonly associated with upregulation of proteolysis [7]. In using proteases as therapeutics, one needs to understand the regulatory mode of their activity in both intra and extra cellular regions [8].
2.2.1 Thrombolytic Therapeutic u-PA (Urokinase-Type Plasminogen Activator)
Thrombolytic therapy based on enzymes is one of the best alternatives for surgical treatment and the first protease drug approved by FDA is u-PA (urokinase). u-PA is in vogue since 1978 for its efficacy in reviving patency of clogged blood vessels and catheters clearing. u-PA is obtained from cultures of primary neonatal kidney cells and known to possess three domains. u-PA exhibits lower affinity for fibrin relative to other molecules known for fibrinolytic activity. Localized administration of u-PA to the site of thrombosis minimizes the adverse side effects and enhances focused activity. u-PA is a potential drug for both cancer treatment and diagnosis due to its association with the degeneration of matrix proteins in cancer cell proliferation [9]. Though newer agents with similar properties are coming up u-PA continues to be the choice for catheters cleaning due to its low cost [10].
t-PA is the second protease to be marketed as drug for treatment of thrombotic disease [11]. t-PA also hydrolyses plasminogen to form active enzyme plasmin. Plasmin exhibits property of digestion of blood clots by degrading fibrin mesh. The difference between u-PA and t-PA is their specificity towards fibrin [12]. Of the five domains of t-PA, the EGF2 and fibronectin finger domains, binds explicitly to fibrin. Characteristically t-PA acts locally, i.e. at the site of fibrin mesh formation contrary to streptokinase and u-PA. Systemic fibrinolysis is prevented by preferential activation of plasminogen by t-PA. First generation product of t-PA is alteplase marketed by Genentech as Activase®. In addition to alteplase, reteplase (Retavase®; Boehringer Mannheim) and tenecteplase (TNKase®, TNK-tPA; Genentech) are the bi-variants of t-PA which are in current usage.
The t-PA’s truncated form reteplase is also an approved drug for treatment of thrombolytic AMI (Acute Myocardial Infarction). Protease affinity is reduced on fibrin due to deletion of EGF, Kringle and N-terminal fibronectin, which increases the clearance rate. Thus, half-life of reteplase is increased in plasma. Rather than infusing reteplase, it can be directed by double bolus, which is cost-effective and lowers time of administration. Genetic engineering methods like manipulation of carbohydrate content and site-directed mutagenesis are employed to improve tenecteplase half-life [13].
Thrombolytic therapy is the frontline method for treatment of stroke and AMI. There is still a significant need in development for improved pharmacodynamics and pharmacokinetics. Continuous efforts are made for developing next generation t-PAs and simultaneously for developing formulation of plasmin, the serine protease formed in vivo by t-PA activity. Genetic engineering techniques are in vogue for increasing the life span of therapeutic proteases [14].
2.2.2 Coagulation Factors
Many of the bleeding disorders are nowadays treated by employing proteases and proteins of coagulation cascade, as well as in combination also. FVII, FVIII and FIX are employed in treating haemophilia A, B and C, respectively. FVII and FIX are serine protease zymogens whereas FVIII is a bulky protein which on activation figures in as the cofactor for FIXa. Replacement of missing coagulation factors extracted from plasma is the mode of treatment for haemophilia in earlier days [15]. But in the recent past numerous plasma-derived biologicals having proteases are accepted. Safe recombinant versions of coagulation factors are the need of the day especially in AIDS and hepatitis C cases. Risk of contamination by prions and human viruses is a major challenge in plasma-derived biologicals. However, viral inactivation and screening can be done to remove contaminants. Genetic engineering is the favoured method for the production of proteases [16].
2.2.2.1 Thrombin
Thrombin is the heart of coagulation cascade converting fibrinogen to fibrin [17]. It also activates factor V, VIII, PAR-1 and even PAR-4 in severe injury [18]. It also exhibits anticoagulant activity by complexing with thrombomodulin there by activating protein-C. This dual nature facilitated the production of anticoagulant molecules that lack procoagulant functionality [19].
Prothrombin an inactive thrombin consists of a serine protease domain, N-terminal Gla domain and two kringle domains [20]. Removal of calcium- and membrane-binding Gla domain by FXa bound to FVa allows thrombin to diffuse locally to the site of its action. This leads to the development of Recothrom® (Zymogenetics) a topical recombinant human thrombin which helps in stopping small blood vessels bleeding after surgery. It was approved by FDA in 2008 and it is less immunogenic compared to bovine thrombin [21].
2.2.2.2 Activated Protein C (APC)
APC a serine protease is associated with both inflammation and blood clotting; this property augments its use as a therapeutic molecule [22]. APC removes cofactors function from FVa and FVIIIa in the presence of the cofactor Protein-S and down regulates the coagulation response [23]. The pleiotropic effects exhibited by APC limits its use clinically through its interaction with endothelial Protein-C receptor. APC functions as anti-apoptotic and anti-inflammatory agent [24]. Riewald and his co-workers elucidated the cytoprotective effect of APC [25]. The gene expression profile towards the anti-inflammatory and anti-apoptotic pathways is due to downstream signalling through PAR-1 [26]. Activation by thrombin is in paradox to that of PAR-1 [27]. Downstream signalling via APC is independent of PAR-1 but is through apolipoprotein-E receptor [28] and integrins β3 and β1 [29]. Severe sepsis is treated by recombinant human APC and it is marketed as Xigris® [drotrecogin alfa, (EliLilly) and was approved in 2001].
2.2.3 In Dermatology—Penzyme
Proteinases from lysosomes are under active consideration for the treatment of scar tissue and for restoration of healthy tissue. A combination of chymotrypsin and trypsin, the Penzyme digests the outer layer of skin for the treatment of psoriasis, in addition to treatment of dermatological conditions. Penzyme is isolated from the alimentary canal of North Atlantic Cod and shows promise [8].
Owing to the multifaceted physiological roles and undesired emanations, therapeutic usage of proteases should be meticulously executed. For example, Lanetoplase an isoform of t-PA was developed for reduced dosage regime and extended half-life, but was found to exhibit adverse effects like intracranial haemorrhage [30]. The pharmacological and physiological role at biological level is needed to be understood for use of proteases as therapeutics. Development of proteins via engineering with site specific protease activity is inevitable for future course of research and development.
2.3 Serine Proteases as Drug Targets
Proteases play a vital role in development of drugs which are articulated to act upon the proteases and proteasome involved in dysregulation and tumor suppression. Irregular protease signalling pathways lead to cancer, cardiovascular, neurodegenerative and pulmonary diseases. Substrates that are specific to upregulated proteases are used as prodrugs and the prodrug is activated into therapeutic by substrate catalysation. At present, a number of proteases are recognized as diagnostic and prognostic biomarkers. Activation of pro-MMPs is initiated by urokinase plasminogen activator (u-PA), membrane-type matrix metalloproteinase (MMP) and cathepsin B. Extracellular serine proteases u-PA, urokinase plasminogen activator receptor (u-PAR), plasminogen and MMPs activate extracellular matrix (ECM) degradation and also initiate, invasiveness, cellular motility and tumor growth factors cascade [31, 32].
2.3.1 Kallikreins in Relation to Cancer
Kallikreins are serine protease secretions of epithelial cells from skin, brain, breast, pancreas and prostate. They are also found in saliva, cerebrospinal fluid, sweat and seminal plasma milk. These are conventionally linked to clinical prognosis of human carcinoma. Human kallikrein 3 (hK3) is the most often used diagnostic biomarker for prostate cancer and hk3 thereby is also known as prostate specific antigen. Amino acids serine, histidine and aspartic acid present in serine proteases in proximity to hKs and also to one another bring about substrate cleavage. Sex-steroid hormones regulate the expression of tissue kallikrein gene (KLK) [31], like androgen regulation of KLK2 and KLK3 [33]. In some diseased conditions like cancer, dysregulation of hKs occur. Upregulation of 12 KLK genes take place in ovarian cancer [31]. Based on the type of tissue and hormone balance, tumor progression is either inhibited or promoted by hKs [34]. hK3, hK8, hK9, hK10, hK13 and hK14 play role in tumor suppression [35]. hKs are overexpressed in various cancers and are the choice for drug delivery.
Various hKs are involved in cancer progression, with its interaction through other serine proteases like u-PA and u-PAR. hK3 endorses prostate tumor growth by instigating growth factors and proteolytic surge to vitiate the extracellular matrix. Binding protein proteases hK3 and hK2 are recognized as insulin-like growth factors (IGF) [36]. Bioavailability of IGF is increased, when binding proteases degrade the binding protein in the tumor fuelling proliferation of prostate cancer. Moreover, the inactive plasminogen activator inhibitor-1 form active u-PA proteolytic cascade by hK2 and hK4 [37]. In the absence of inhibitor, activation of plasminogen to plasmin takes place via u-PA and its receptor. Plasmin further activates pro-MMPs and causes the discharge of growth factors like EGF, augment angiogenesis and ECM degradation [38]. hK3 influences the activity of TFGβ (tumor growth factor β) and can also block FGF-2 (fibroblast growth factor-2) [39]. In addition, ECM degrades MMPs like type IV collagenases through activation of hKs [40]. u-PA–u-PAR pathway offer potential drug targets such as PSA, hK2, u-PA, plasmin and MMPs (proteases) for therapeutic applications in cancer [41]. Upregulation of u-PA and other serine proteases was reported in different cancers like gastric [42], prostate [43], cervical [9] and colorectal [44]. PAR (Protease-activated receptor) G-protein coupled receptor is expressed both in cancer and tumor cells. PAR signalling is another pathway that has been implicated in different cancers. Trypsin and thrombin activate PAR by cleaving its extracellular domain and mediate signals in the cell that trigger cancer cell proliferation [45].
2.3.2 Epidermal Serine Proteases
2.3.2.1 Matriptase and Prostasin
Profilaggrin plays a significant role in epidermal barrier function of skin. It is converted into filaggrin monomers at stratum granulosum/stratum corneum (SG/SC) interphase. Filaggrins form macro fibrils by crosslinking and maintain hydration of SC and act as natural moisturizing factors (NMFs) [46]. Human genetic studies have underscored profilaggrin proteolysis in maintaining epidermal architecture and hydration. It was also reported that ichthyosis vulgaris, asthma and atopic dermatitis are caused due to loss-of-functional mutations in profilaggrin, which disturb epidermal barrier and allow the free entry of infection causing agents and allergens [47].
Serine proteases prostasin, a membrane anchored glycosyl-phosphatidyl-inositol, and type II transmembrane matriptase are essential for initiation of profilaggrin processing. Arnett et al., has reported that in cascade of zymogen activation, the auto-activated protease matriptase acts upstream to that of prostasin. Autoactivating protease matriptase regulates terminal epidermal differentiation and is required for prostasin zymogen activation [48]. Epidermal appendages, incomplete terminal differentiation of epidermis and oral epithelium are associated with reduced expression of matriptase [49]. Recent reports also suggest that mutations in matriptase gene cause icthyosis [50]. Matriptase is present in the skin and when exposed to acidic pH it gets activated immediately, which suggests that its activation is a response of direct exposure to proton [51]. The proteolytic cascade of matriptase-prostasin, is regulated either by the hepatocyte growth factor activator inhibitor-1 (HAI-1) or by prostasin activation. These mechanisms rapidly inhibit both prostasin and matriptase, which provides the opportunity for matriptase and prostasins to act on their respective substrates [52]. The role of these membrane-bound proteins was thought to be limited only for skin homeostasis. However, recent studies suggest that matriptase has ability to activate kallikrein-related proteases which play a significant role in conditions causing skin inflammation [53].
2.3.2.2 Kallikreins
One of the major family in tryptic–chymotryptic serine protease cluster is kallikrein-related proteases (KLKs) encoded by 15 different genes located on chromosome 19q13.4. Keratinocytes of the stratum granulosum (SG) present in skin produces KLKs and are liberated into upper SG and lower SC. The present knowledge on serine protease activity in SC is ascribed to KLKs of human tissues [54]. In healthy skin tissues around eight different KLKs are known to be expressed, among those KLK14, KLK8, KLK7 and KLK5 are observed to be most important [55]. These KLKs are extensively studied and their putative functions were determined [55, 56]. There are a number of reports which suggest that KLK5 and KLK7 have proteolytic function in SC. Previously KLK5 and KLK7 proteases were termed as ‘stratum corneum tryptic enzyme (SCTE)’ and ‘stratum corneum chymotryptic enzyme (SCCE)’ respectively. In ex vivo conditions it is known that serine protease inhibitors are capable of inhibiting shedding of corneocytes, hence KLK5 and KLK7 are very important in desquamation process [57]. They are most commonly found to be expressed in SG, and located in SC interstices. Both KLK5 and KLK7 are thought to have self-activation capabilities to form a proteolytic cascade [46]. It is believed that these two enzymes are capable of hydrolysing DSG-1, corneodesmosin and desmocollin-1 when they are active as suggested in in vitro studies. Recent studies have also shown certain evidences that other KLKs also have role in desquamation, as about half of the total proteolytic activity in SC is carried out by KLK14. The reason behind this may be that KLK14 can catalyse and also can be triggered by KLK5.
KLK8 is also known for its role in the cascade of proteolytic activity controlling desquamation. KLK8 is synthesized abundantly and is co-localized with other KLKs of sweat glands and human epidermis. KLKs play an important role in barrier function of SC and are transported and exocytosed into the SG/SC interface by lamellar bodies. The recombinant KLK8 plays an important part in the upper SG where in the pH is normal and the optimum activity is at pH 8.5 [56]. KLK8 activity was also identified in extracts of SC and sweat where, kininase II and KLK1 were known to be active. Invariably, this suggests its prospective role in desquamation of skin, although there is lot to know about its physiological substrates.
2.3.2.3 Neutrophil Serine Proteases
The primary cell infiltrates are neutrophils, during skin infection causing ‘neutrophilic dermatoses’. Pustule formation can be observed in the epidermis when there is massive infiltrate. At the time of infection, microbes are phagocytosed by neutrophils within the phagolysosome. This is done by the α-defensins, ROS generating systems, as well as by proteases and is liberated from 1° to 2° granules [58]. High levels of cathepsin-G, protease-3 and human leukocyte elastase are found only in primary granules. Phagocytosis alone is not responsible for the release of these enzyme, also ‘frustrating phagocytosis’ and development of NETs (neutrophil extracellular traps) comprising neutrophil-derived DNA [59]. In patients with psoriasis (neutrophilic dermatosis) the HLE activity is observed on the surface of skin lesion [60]. Hence, neutrophil serine proteases are said to be important in innate immune regulation [61].
2.3.3 Serine Protease in Synaptic Function and Behaviour
A large number of studies suggest that the activity of proteases, the corresponding receptors and inhibitors are co-opted by brain to regulate various synaptic activities.
2.3.3.1 Thrombin
The normal functioning of brain is associated with synthesis of thrombin from prothrombin in brain [62, 63]. Thrombin has a profound impact on both glial cells and neurons. The neurite outgrowth inhibition with growth cone collapse [64] was observed in cultured neurons and neuroblastoma cells when thrombin was applied [65]. A number of responses in Astrocytes like reversal of stellate morphology and stimulating proliferation are induced by thrombin [66]. Administration of high amounts of thrombin for therapy exhibits programmed cell death in astrocytes and neurons [67]. These observations emphasise a significant role of thrombin in neuronal development and maintenance. The deleterious effects on cognitive function of brain are most likely associated with high levels of thrombin. It was observed that when rats were treated with thrombin via intra cerebroventricular infusion, it resulted in higher memory errors and task completion potency in 8 arm radial maze [68]. Numerous neuropathological changes can be observed with such behavioural deficits which include increased astrogliosis, cell death and expanded cerebral ventricles. Even increase in levels of apolipoprotein-E (ApoE) and phosphorylated neurofilament proteins were also observed. These findings also predict the role of thrombin in cognitive decline and neuropathology related to Alzheimer’s disease [62, 63, 66, 68].
2.3.3.2 Tissue Plasminogen Activator (t-PA)
Tissue plasminogen activator (t-PA) is one of the most widely studied serine protease associated with CNS. Endothelial cell, Neurons and Glial cells synthesize and release t-PA in brain, and are expressed highly in hippocampus, amygdala cerebellum and cortex [69]. Literature available on t-PA suggests it to be a synaptic activity modulator, but this mechanism is under debate [70]. It is also reported that NMDAR (N-Methyl-d-Aspartate receptor) signalling is enhanced by cleavage of the GluN1 subunit and is associated with proteolytic activity of t-PA [71].
2.3.4 Serine Proteases in Human Immune System
The human immune system consists of cells known as endosomal vesicles which are responsible for expression of chymase and tryptase in mast cells, proteases in granulocytes and granzymes in lymphocytes. Serine proteases from endosomal vesicles are associated with inflammation, tissue remodelling, apoptosis and phagocytosis. Increase in serine protease activity contributes to pathology in allergy, auto-immune disorders and in cancer proliferation.
2.3.4.1 Granzymes
Apoptosis via granule associated enzymes (granzymes) and by death receptor pathway are the two mechanisms employed by CTLs (cytotoxic T lymphocytes) and NK cells (natural killer cells) to efficiently combat virus-infected cells and tumor cells [67]. Apoptosis induced by granzyme relies on a pore-forming protein called perforin. Perforins are localized in the same granules and helps in delivering granzymes to target [72]. Granzymes are efficient in activating the apoptotic pathways in cytoplasm in two ways, by targeting proteins responsible for integrity of mitochondria and DNA, secondly by cleavage of caspases.
In humans, five variants of granzymes are known which include Gzm A, B, H, K and M. All the variants have exclusive pattern of expression and possess substrate specificity, despite structural similarities among them. The best-studied and most abundant members among human granzyme family are GzmA and GzmB. Both GzmA and GzmB granzymes are expressed in effector CD8+ T cells and CD56dim NK cells. Additionally, with GzmA expression GzmK is also expressed simultaneously within the granules of memory T cells [73]. Expression of GzmA and GzmB is higher with activation of T and NK cells but GzmK levels are unaltered [74]. After activation Regulatory T cells expresses only granzymes. GzmA are most prominently expressed by activated natural Treg cells whereas GzmB levels are highly expressed by adaptive Treg cells. Perforin-dependent apoptosis can be induced by both the Treg subtypes in autologous target cells. This property suggests that granzymes are utilized by Treg cells to exert their anti-proliferative effects [75]. Non-cytolytic cell types like basophils, mast cells and chondrocytes lack perforin but expresses GzmB supporting alternative function of granzymes i.e. inducing cell death [76]. Several studies suggest that granzymes are important in immuno-pathological processes. Increased numbers of positive granzyme lymphocytes was observed during various immune-mediated disorders which include transplant rejection, systemic lupus erythematosus and rheumatoid arthritis [77].
2.3.4.2 Proteases from Neutrophils
Neutrophils are the first cells from bodies defence mechanism that turn up in the vicinity of inflammation. Neutrophils through acquiescent action of proteases, free radicals and antimicrobial peptides successfully mortify microorganisms. Three serine proteases are identified in neutrophil granules: (1) Neutrophil elastase (NE), (2) Proteinase-3 (PR3) and (3) Cat-G. They exhibit intra and extracellular activities. Cat-C cleaves them into active forms as they are synthesized as zymogens [78]. G-CSF, C5a and TNF trigger neutrophil degranulation and remarkably augment inflammation by interacting with cellular surfaces and extracellular matrix [79]. Neutrophil proteases inside the phagolysosome are implicated in phagocytosis and tissue injury. Neutrophils are meant for first line of defence and are essential during infections. However, in some cases imbalance in its activity contribute to certain diseases. The reason for imbalance of neutrophils may be due to lack of control on neutrophil proteases or may be because of over-exposure to proteolytic activity in the vicinity of inflamed region. Inflammatory bowel disease (IBD), chronic obstructive pulmonary disease (COPD) and inflammation of genital tract exhibit increased levels of neutrophil elastase [80]. This strong parallel correspondence between neutrophil elastase and activation has attracted scientists to study the usage of neutrophil elastase as a biomarker. This will be a breakthrough in diagnosing and monitoring inflammation in episodes like COPD [81]. However, lack of specificity dissuades elastases from neutrophils and like serine proteases as biomarkers for diagnosis in the clinic. More or less, same NE levels are observed in sputum of healthy smokers and COPD patients. But serine protease can be of use in monitoring disease progression and reflects the standing of immune cells. Neutrophil proteases in blood can be utilized for diagnosis of the origin of the disease. Neutropenia is caused by massive neutrophil activation. Low levels of neutrophil elastase combined with neutropenia indicate defective production and survival of neutrophils.
Another example of imbalance of neutrophil proteases leading to disease is cystic fibrosis (CF), which is characterized by gentle and persistent breakdown of airway architecture. Viscosity of the mucus increases paving way for bacteria to invade small airways and colonize. The colonized bacteria trapped in the mucus layer draw good number of neutrophils that eventually undergoes necrosis. The release of pro-inflammatory compounds and toxic substances initiates epithelial damage; reduce mucus clearance and inflammatory cell infiltration [82]. This is a vicious cycle of events and the activity of protease from neutrophils in CF makes serine proteases as potential drug targets (Fig. 1).
NE induces Chemokine IL-8 production in epithelial cells of lungs and can inactivate the innate defence system by inhibiting surfactant D molecule which thereby enhances the colonization of bacteria and hence NE are said to be fundamental mediators of inflammatory responses [83]. The expression of CD40, CD80 and CD86 is downregulated when neutrophil elastase from sputum of COPD patients or purified neutrophil elastase is incubated with dendritic cells (DC). DC antigen-presenting function and maturation also is impaired by NE. Collectively, this outcome advocates that NE disables DC and restricts ample immune responses towards microbial infections. There is reduced expression of CD8 and CD4 on CD3+ T cells when incubated with purified NE or Cat-G which renders these cells less cytotoxic [84]. Hence it is clear that, sputum from patients suffering from CF exhibit lower CD4+ and CD8+ T cells and increased Cat-G and NE levels. Neutrophil proteases induce T-cell dysfunction need to be targeted to combat CF.
Wegener’s granulomatosis is another example of imbalance of neutrophil proteases. The disease characterized by granulomatous inflammation affects several organs like kidneys and lungs. PR3 combating antibodies known as anti-neutrophils are present in most of the patients. As observed in the literature such antibodies can activate neutrophils [85].
2.3.4.3 Proteases from Mastocytes
Mastocytes (Mast cells) is synonymous with reactions to allergens. Mast cells are loaded with granules containing cytokines, histamine, proteases and proteoglycans, chymase and tryptase as zymogens. Stimulation of mastocytes leads to degranulation [86]. Degranulation releases granular contents initiating inflammation, vasoconstriction, blood coagulation and extracellular matrix degradation [87]. Expression of GzmB and Cat-G depends on the activation status and/or localization of mastocytes [88]. Pathogen clearance is efficiently enabled by the action of mastocyte proteases.
Mastocytes are associated with a host of IMID (Immune-Mediated Inflammatory Diseases); e.g. allergic diseases, IBD (Inflammatory Bowel disease) and atherothrombosis allied with the activity of proteases from mastocytes [89]. Mast cell degranulation is evidenced by increased levels of tryptase in vivo and suggests IgE-mediated response [90]. Mastocytes present in the joints of patients suffering from rheumatoid arthritis and tumor invasion sites account for degradation of neighbouring connective tissue [91]. This phenomenon points to role of serine proteases and are yet to be elucidated for their role in induction and perpetuation of disease. IgA nephropathy and lupus nephritis are inflammatory kidney diseases due to protease activity of mast cells [92]. Tryptase plays a vital role through activated receptors and formation of type I collagen in the genesis of connective tissue fibrosis [93]. Increase of tryptase levels in blood represents mastocyte incitement and is associated with itching in patients undergoing hemodialysis. Mastocytes release various pruritogens and influence of tryptase on subsequent itch development in vivo is difficult to separate [94]. In a nut shell Table 2 depicts serine proteases as potential drug targets.
3 Serine Protease Inhibitors
3.1 Introduction
Fermi and Pernossi in 1894, for the first time, reported the presence and availability of protease inhibitors in nature [95]. They are very important as they regulate the activity of proteolytic enzymes thereby maintaining homeostasis [96]. Proteins proficient in impeding the catalytic activity of proteolytic enzymes, stoichiometrically, competitively and reversibly are categorized as protease inhibitors. Uncontrolled proteolysis by endogenous and exogenous proteases is prevented by these inhibitors. Protease inhibitors specificity is very helpful in targeting some of the proteases which are known for pathogenesis in humans, viz. hepatitis, pancreatitis, cancer, arthritis, AIDS, emphysema, high blood pressure, thrombosis, muscular dystrophy, etc. Having such potential, the new era of drugs and diagnostics associated to protease inhibitors are being emerged [97]. The most abundant and extensively distributed protease inhibitors are from serine protease family [98]. Around 17,451 serine protease inhibitors account from genomes of all five kingdoms [99, 100]. In order to inhibit the targets, serine proteases use specific changes in the conformation [96]. The molecular weight of serine proteases is about 40–60 kDa, having 330–500 amino acid residue and are monomeric protein molecules [101]. These are homologous proteins exhibiting sundry functions that carry out many physiological and biological activities like fibrinolysis, clot formation, apoptosis, cell growth, inflammation, angiogenesis and tumor suppression [96, 102]. Table 3 shows function and dysfunction of serpins.
3.2 Classification
SERPINs represent a superfamily of proteins deriving its terminology from serine protease inhibitors [103]. MEROPS database currently holds 71 different families accounting for about 17,451 inhibitors based on sequence homology of proteins. Serpins are segregated into 38 clans depending on tertiary structure and this classification is regularly updated [99]. The term proteinase was replaced with peptidase in human gene in 2005. Serpins are classified based on
-
1.
Clade
-
2.
Group
3.2.1 Based on Clade
Serpins are divided into clades, known as clade based classification system. Serpins are categorized into 16 clades as A-P, among them A to I include first nine clades which are human serpins [104]. The sequence similarity and specific phylogenetic relationships are grouped and determined as clade where as those which cannot be grouped are known as orphans, around 10 orphan sequences are found. Based on the function and inhibitory activity they are classified into inhibitory and non-inhibitory groups [98]. Based on the amino acid sites and gene structure clades further consist of six sub-groups [105]. To understand the nomenclature, for example in SERPINA1, letter A denotes the clade and number 1 denotes the gene number within clade. In eukaryotes, serpins are omnipresent. About 36 functional protein coding human genes are identified, clade A representing extracellular serpins whereas clade B with intracellular serpins. In humans, SERPIN-A the α1 proteinase inhibitor anti-trypsin accounts as the largest group followed by SERPIN-B the ov-like serpins. SERPIN-C and SERPIN-D involve orphan heparin cofactor II (HCF-II) and anti-thrombin (ATIII) [106].
3.2.2 Based on Group
In vertebrates, serpin genes are categorized based on “group-based classification”. The criteria of classification is based on gene structure and comprises of 6 groups namely V1–V6 [107]. Serpins are very specific and target mostly serine proteases, in some cases even target caspases known as cathepsins [108], papain-like cysteine proteases [109] and some proteases involved in hormone transport and blood pressure regulation [106]. Serpins play a significant role in corticosteroid binding, blood pressure regulation, coagulation and hormone transport. And in seldom, a non-inhibitory function is also significant, for example, they function as molecular chaperones [110], as hormone transporters [111] and some as tumor suppressors [112]. Inhibitors play a vital role in protease characterization and are used in the pharmaceutical industry [99].
3.3 Serpins Mechanism
Serpins structurally comprise 3-β-sheets viz., A, B and C, 8-9 α-helices and a Reactive Centre Loop (RCL) (Fig. 2). RCL plays an important role in targeting proteases [113]. Based on RCL these protein inhibitors exist in different variants viz., active, latent, cleaved, delta and polymeric. The amino acid terminus of the RCL inserts into the β-A sheet forming a fourth strand, this progression is called stressed (S) to relaxed (R) shift and results eventually into a cleaved form. Increase of potential in inhibition is observed for serpins which bind to cofactors. Serpins are capable of interchanging from active form to latent form and vice versa. However, such shift is not observed in all serpins. The inhibitory activity is not seen in latent form, but through refolding and denaturation it can translate into active form. The secondary structures of serpins have noticeable RCL, which target the protease active site and inhibit its activity. The inhibitory activities of protease indispensably rely on the conformational change of tertiary structure [103]. Ser195, His57 and Asp102 are catalytic triad residues in serpins liable for hydrolysis of amide bond (Fig. 3). Serpins are grouped into three major classes which are trypsin-like, elastase-like and chymotrypsin-like [114].
The five-stranded A-sheet is in red, the six-stranded B-sheet in green, and the four-stranded B-sheet in yellow. α-helices are shown in cyan. The RCL is at the top of the molecule in magenta. Two functionally important regions of the serpin, the breach and the shutter, are labelled. a Structure of human native anti-trypsin. b Mechanism of protease inhibition by serpins
With all these potential functions this class of protease inhibitors are the emerging therapeutics, which are used in treating infections pertaining to fungal—Candida albicans, viral—HIV, Hepatitis, Herpes and parasitic—schistosomiasis, malaria and diseases of respiratory, cardiovascular, inflammatory, immunological and neurodegenerative disorders [115].
Sometimes the change in conformation and deficiency of serpins leads to different diseases like emphysema, thrombosis and angioedema. Increase in the levels of serpins in endoplasmic reticulum is defined in diseases like chronic fatigue, hypothyroidism, hypertension, cirrhosis, tumor progression and familial dementia [116].
3.4 Serpins as Therapeutics
In human plasma, next to albumin and immunoglobulins, the third largest functional groups of proteins are plasma protease inhibitors based on the weight of molecule. These inhibitors account for about 10% of plasma proteins which are capable of controlling many critical processes like complement activation, coagulation, connective tissue turnover, inflammatory reactions and fibrinolysis.
α1PI-neutrophil elastase; α2-AP-plasmin; α1Achy-cathepsin G and AT III-thrombin are present in human proteome which are specific of pairing with inhibitor-target. Clr and Cls are the complement proteases which are controlled by CI-Inh, yet this mechanism needs to be completely understood and that in regulation of mast cell chymase α1Achy are involved. Human α2M, functions as fast and effective clearing agent due to its ability to inactivate proteases, whenever they are freely found in circulation. Till date there are no specific reports about the functional role of IαI, β1AC and αCPI [117].
3.4.1 In Diabetes
Protease inhibitors inhibit the enzymatic degradation of insulin and hence are widely used along with insulin to increase absorption. There are several reports that suggest that in clinical trials, when protease inhibitors and insulin are administered together it had showed better hypoglycemic effect when compared to patients who were administered only with insulin. Contradictory to this even there are some reports, which suggest that concomitant administration of protease inhibitors and insulin has shown no improvement in absorption of insulin. This may be due to short period of exposure time of aprotinin, i.e. 24 h only and even, dose of aprotinin may be low. Nevertheless, their role in insulin therapy still remains uncertain. To understand their positive effect in insulin therapy more clinical studies in larger settings is required [118].
In diabetic nephropathy, mesangial matrix accumulation is associated with reduction in activity of plasmin, MMP-2 (matrix metalloproteinase) and MMP-9. Megsin is the recently identified protein belonging to serpin family and is overexpressed in mesangial cells that progressively induce mesangial proliferation in mice. Hyperglycemia upregulates megsin at translational level in both in vivo model of type II diabetic nephropathy and in vitro model of cultured mesangial cells. The decreased degradation of mesangial matrix was observed when MMP-2 and MMP-9 were inhibited by megsin. Anti-megsin neutralizing antibodies specific to MMPs reinstated MMP activity. Hyperglycemia in diabetes upregulates megsin which in turn inhibits plasmin and MMP activities suggesting the accumulation of mesangial matrix [119].
3.4.2 In Obesity
Vaspin a serine protease inhibitor is recognized in visceral adipose tissue. Vaspin exerts an insulin-sensitizing effect targeted toward visceral white adipose tissues (WATs) in states of obesity. These findings drive the search for identification of potential protease substrate that helps in developing anti-protease inhibitor therapy. This would enhance insulin sensitivity and reverse altered expression of insulin resistance in subjects suffering from diabetes [120].
3.4.3 In Cancer
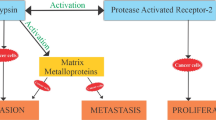
Human Serpins associated with cancers and role of proteases in ECM degradation, invasion and metastasis are depicted in Table 4 and Fig. 4.
3.4.4 In Central Nervous System
The role of serine proteases in glial and neuronal function is suggestive for their involvement in health and disease of nervous system. Deranged proteolytic balance is identified in many pathological conditions of nervous system. It is known that in cognitive function, the synaptic proteolysis plays a very important role. Further understanding of the association of neurotrypsin in mental retardation is of prime importance. Extrication of molecular basis of other neurological disorders which are not associated with the mutation of neuroserpin or neurotrypsin may involve different components which are associated with the extracellular proteolytic signalling pathway. Understanding such phenotypes associated neurological disorders will help in elucidating novel targets. Identification of novel targets will optimistically contribute to proper management of these disorders [121].
3.4.5 In Skin Diseases
In desquamation and terminal differentiation process, serine proteases play a very crucial role. Stratified epithelium is formed by a complex differentiation mechanism without disturbing the barrier function. Corneocytes are separated from one another by the timely and specially orchestrated proteolytic system. In recent times, it is also known that the proteolytic homeostasis is crucial not only for physical barrier but also for immunological responses. Most of the recent research was focused on epidermal proteases their inhibitors and their role in pathogenesis (Fig. 4). Augmented desquamation, atopy, dry skin and abnormalities of hair, e.g. bamboo hair are the characteristic feature of ichthyosiform skin disease. The symptoms are decreased levels of functional LEKTI (Lympho Epithelial Kazal-Type Related Inhibitor) or the synthesis of abnormal LEKTI forms that are devoid of enzyme inhibiting domains. It is also demonstrated that decreased levels of LEKTIs are inversely correlated to activity of serine protease in Statum Corneum (SC). Specific expression of another Kazal-type inhibitor in hand and foot is an interesting speculation attributed to be a significant factor in eczema of hand and foot. This Kazal-type inhibitor named as LEKTI2/SPINK9 specifically inhibits KLK5. Meyer-Hoffert and Wiedow in 2011 identified SPINK6 in skin which is a selective inhibitor of KLKs [61]. SPINK6 was identified in skin appendages like sweat glands and sebaceous glands in SG of healthy individuals. On the contrary, downregulated expression of SPINK6 is identified in lesions of patients suffering from atopic dermatitis.
Other than LEKTIs a large number of other protease inhibitors are produced by keratinocytes. Of which, one group is termed as ‘trappins’ (transglutaminase substrate, WAP-domain-containing proteins) [122]. Elafin and secretory leukocyte protease inhibitor (SLPI) are two human epidermal protease inhibitors. Inhibition of elastase and protease-3 by elafin, cathepsin G and elastase by SLPI is suggestive of the efficacy of these two inhibitors against neutrophil serine proteases. All these findings demonstrate the protective role of neutrophil serine proteases during inflammation. In psoriasis, a highly inflammatory disease elafin is upregulated, whereas SLPI gene is a housekeeping gene expressed continuously in the epidermis [123]. Elafin is stored as a proenzyme in lamellar bodies and is secreted at the junction of SG and SC and via transglutamination is crosslinked to the proteinaceous molecules of cornified envelopes. It is reported that in human skin few members of SERPIN family are expressed which include SERPINB13 (headpin/hurpin), SERPINB4 (squamous cell carcinoma antigen-2) and SERPINB3 (squamous cell carcinoma antigen-1). Subtilisin A is inhibited by SERPINB8 and SERPINB9 which suggest that SERPINs has a protective role from tissue proteolysis caused by bacterial proteases.
Dandruff or Seborrheic Dermatitis (SD) is most common affliction of human scalp. Generally, dandruff or SD is caused by dermatophytic fungi, most commonly Malassezia furfur and other species of Malassezia, which are opportunistic pathogens on human scalp and skin. SD is often characterized by scaling on the scalp and causes inflammation [124]. Malassezia degrade sebum and release multiple free fatty acids from available triglycerides. Fungal colonies consume specific saturated fatty acids to proliferate leaving unsaturated fatty acids (USFAs) behind. These USFAs alters the combination of sebaceous secretions and penetrates into stratum corneum, the outermost layer of epidermis, disrupting the skin barrier function, resulting in inflammation, irritation and leads to scalp flaking. Studies found that SNTI, a Soap Nut Trypsin Inhibitor, was effective against dandruff causing fungi compared to the antifungal chemical drugs—fluconazole and ketoconazole [125].
3.4.6 In Human Airway Inflammation
Bronchial asthma, fever and COPD are the symptoms in response to viral replication and release of inflammatory cytokines in influenza. Viral entry and replication is facilitated by serine proteases secreted by epithelial cells of the airway. Surface epithelial cells of the human nasal mucosa, trachea, distal airways, lung and human alveolar epithelial cell line A549 expressed TMPRSS-1 (Transmembrane protease serine S-1), TMPRSS-2, TMPRSS-4 and TMPRSS-11D which belong to serine protease family. Protease inhibitors like aprotinin and camostat are found to reduce replication of influenza virus and release TNF-α (Tumor Necrosis Factor) and IL-6 (InterLeukin) into cell supernatants. Conversion of HA0 the precursor protein into subunit HA1 in influenza virus is retarded by camostat. These findings authenticate the role of serine proteases in the proteolytic activation of influenza virus in human tracheal epithelial cells. Thus, serpins are coveted therapeutics candidates in treating viral influenza [126].
3.4.7 In Blood Clotting Abnormalities
To curb excessive thrombin activity a few anticoagulant strategies are in vogue which in turn control hyper-coagulation. Warfarin, agonist of vitamin K is being used since 1950, but its administration requires careful monitoring because of its interactions with different drugs and food and also because of its narrow therapeutic range. Heparin and LMWH (Low Molecular Weight Heparin) are used to enhance anti-thrombin activity and are incapable to inhibit clot-bound thrombin. Based on these elements, over the last twenty years’ enormous efforts are put forward to design low molecular weight, orally bioavailable and selective thrombin inhibitors [115]. Table 5 portrays the clinical status of serine protease inhibitors as therapeutics.
4 Conclusion
Serine proteases account to about one-third of all the known proteases. Large and diversified clusters of serine proteases and their inhibitors are encoded by human genome. Search for low molecular weight inhibitors to regulate proteases and their activity is attracting pharmaceutical industry. However, it is an uphill task in view of the expression of closely related proteins in the genome. Active site recognition enables the regulation of multiple protease targets and focuses on active site-directed therapies. But now other regions are also being targeted. Macromolecular recognition is envisaged by exosites and allosteric communication between these regions and the active site resulting in conformational changes. This interplay is crucial in biological system as exemplified by proteases in coagulation and immune system. Disturbance in the delicate balance between serine protease and serpins is the reason for a wide range of pathologies. Early reports of such imbalance are identified in blood coagulation in which there was deficiency of factor IX. On the contrary, overexpression of immune system serine proteases culminates in inflammatory states. So also, imbalance in serine protease inhibition affects multiple biological systems. Emphysema, cirrhosis and thrombosis result from aberrant conformations and belong to proteinopathies. Unravelling the molecular interactions in the regulatory pathways of proteolysis in vivo continues to be a puzzling and intuitive venture to alleviate human well-being. Pharmaceutical, biotechnological industries, academic researchers and their financial backers consider serine proteases and serpins as future medicine worthy for clinical trials in human applications.
This is a humble endeavour to elucidate the progress of protease and protease inhibitors, predict their future and some of the hurdles overcome till date. They remain to be the challenging molecules that are to be expounded as a promising class of new drug targets and therapeutic agents.
References
Puente XS, Sánchez LM, Gutiérrez-Fernández A et al (2005) A genomic view of the complexity of mammalian proteolytic systems. Biochem Soc Trans 33:331–334
Rawlings ND, Barrett AJ, Finn R (2015) Twenty years of the MEROPS database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res 44:343–350
Royston D (1996) Preventing the inflammatory response to open-heart surgery: the role of aprotinin and other protease inhibitors. Int J Cardiol 53:S11–S37
Berquin IM, Sloane BF (1996) Cathepsin B expression in human tumors. Adv Exp Med Biol 389:281–294
Turk B (2006) Targeting proteases: successes, failures and future prospects. Nat Rev Drug Discov 5:785–799
Rawlings ND, Morton FR, Kok CY, Kong JBA (2007) MEROPS: the peptidase database. Nucleic Acids Res 36:D320–D325
Duffy M, McGowan P, Gallagher W (2008) Cancer invasion and metastasis: changing views. J Pathol 214:283–293
Craik CS, Page MJ, Madison EL (2011) Proteases as therapeutics. Biochem J 435:1–16
Dass K, Ahmad A, Azmi AS et al (2008) Evolving role of uPA/uPAR system in human cancers. Cancer Treat Rev 34:122–136
Dillon PW, Jones GR, Bagnall-Reeb HA et al (2004) Prophylactic urokinase in the management of long-term venous access devices in children: a Children’s Oncology Group study. J Clin Oncol 22:2718–2723
Rijken DC, Lijnen HR (2009) New insights into the molecular mechanisms of the fibrinolytic system. J Thromb Haemost 7:4–13
Hoylaerts M, Rijken DC, Lijnen HR, Collen D (1982) Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem 257:2912–2919
Semba CP, Sugimoto K, Razavi MK, Society of Cardiovascular and Interventional Radiology (SCVIR) (2001) Alteplase and tenecteplase: applications in the peripheral circulation. Tech Vasc Interv Radiol 4:99–106
Ambrus JL, Ambrus CM, Back N et al (1957) Clinical and experimental studies on fibrinolytic enzymes. Ann N Y Acad Sci 68:97–137
Eley RC, Green AA, McKhann CF et al (1936) The use of a blood coagulant extract from the human placenta in the treatment of hemophilia. J Pediatr 8:135–147
Pipe SW, Kaufman RJ (2000) A chamber of hope for hemophilia. Nat Biotechnol 18:264–265
Di Cera E (2008) Thrombin. Mol Aspects Med 29:203–254
Coughlin SR (2005) Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost 3:1800–1814
DI Cera E (2007) Thrombin as procoagulant and anticoagulant. J Thromb Haemost 5:196–202
Van De Locht A, Stubbs MT, Bauer M, Bode W (1996) Crystallographic evidence that the F2 kringle catalytic domain linker of prothrombin does not cover the fibrinogen recognition exosite. J Biol Chem 271(7):3413–3416
Bowman LJ, Anderson CD, Chapman WC (2010) Topical recombinant human thrombin in surgical hemostasis. Semin Thromb Hemost 36:477–484
Esmon CT (2006) The endothelial protein C receptor. Curr Opin Hematol 13:382–385
Kisiel W (1979) Human plasma protein C: isolation, characterization, and mechanism of activation by alpha-thrombin. J Clin Invest 64:761–769
Esmon CT (2006) Inflammation and the activated protein C anticoagulant pathway. Semin Thromb Hemost 32(Suppl 1):49–60
Riewald M, Petrovan RJ, Donner A et al (2002) Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science 296:1880–1882
Joyce DE, Gelbert L, Ciaccia A et al (2001) Gene expression profile of antithrombotic protein c defines new mechanisms modulating inflammation and apoptosis. J Biol Chem 276:11199–11203
Nakanishi-Matsui M, Zheng YW, Sulciner DJ et al (2000) PAR3 is a cofactor for PAR4 activation by thrombin. Nature 404:609–613
Yang XV, Banerjee Y, Fernández JA et al (2009) Activated protein C ligation of ApoER2 (LRP8) causes Dab1-dependent signaling in U937 cells. Proc Natl Acad Sci U S A 106:274–279
Elphick GF, Sarangi PP, Hyun Y-M et al (2009) Recombinant human activated protein C inhibits integrin-mediated neutrophil migration. Blood 113:4078–4085
Bhana N, Spencer CM (2000) Lanoteplase. BioDrugs 13:217–224
Borgoño CA, Diamandis EP (2004) The emerging roles of human tissue kallikreins in cancer. Nat Rev Cancer 4:876–890
Gabriel D, Zuluaga MF, van den Bergh H et al (2011) It is all about proteases: from drug delivery to in vivo imaging and photomedicine. Curr Med Chem 18:1785–1805
Cleutjens KB, van Eekelen CC, van der Korput HA et al (1996) Two androgen response regions cooperate in steroid hormone regulated activity of the prostate-specific antigen promoter. J Biol Chem 271:6379–6388
Yousef GM, Diamandis EP (2001) The new human tissue kallikrein gene family: structure, function, and association to disease. Endocr Rev 22:184–204
López-Otín C, Matrisian LM (2007) Emerging roles of proteases in tumor suppression. Nat Rev Cancer 7:800–808
Réhault S, Monget P, Mazerbourg S et al (2001) Insulin-like growth factor binding proteins (IGFBPs) as potential physiological substrates for human kallikreins hK2 and hK3. Eur J Biochem 268:2960–2968
Takayama TK, McMullen BA, Nelson PS et al (2001) Characterization of hK4 (prostase), a prostate-specific serine protease: activation of the precursor of prostate specific antigen (pro-PSA) and single-chain urokinase-type plasminogen activator and degradation of prostatic acid phosphatase. Biochemistry 40:15341–15348
Pepper MS (2001) Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol 21:1104–1117
Fortier AH, Nelson BJ, Grella DK, Holaday JW (1999) Antiangiogenic activity of prostate-specific antigen. J Natl Cancer Inst 91:1635–1640
Desrivières S, Lu H, Peyri N et al (1993) Activation of the 92 kDa type IV collagenase by tissue kallikrein. J Cell Physiol 157:587–593
Foekens JA, Peters HA, Look MP et al (2000) The urokinase system of plasminogen activation and prognosis in 2780 breast cancer patients. Cancer Res 60:636–643
Duffy MJ, Maguire TM, McDermott EW, O’Higgins N (1999) Urokinase plasminogen activator: a prognostic marker in multiple types of cancer. J Surg Oncol 71:130–135
Miyake H, Hara I, Yamanaka K et al (1999) Elevation of serum levels of urokinase-type plasminogen activator and its receptor is associated with disease progression and prognosis in patients with prostate cancer. Prostate 39:123–129
Stephens RW, Nielsen HJ, Christensen IJ et al (1999) Plasma urokinase receptor levels in patients with colorectal cancer: relationship to prognosis. J Natl Cancer Inst 91:869–874
Morris DR, Ding Y, Ricks TK et al (2006) Protease-activated receptor-2 is essential for factor VIIa and Xa-induced signaling, migration, and invasion of breast cancer cells. Cancer Res 66:307–314
Ovaere P, Lippens S, Vandenabeele P, Declercq W (2009) The emerging roles of serine protease cascades in the epidermis. Trends Biochem Sci 34:453–463
Sandilands A, O’Regan GM, Liao H, Zhao Y, Terron-Kwiatkowski A, Watson RM, Cassidy AJ, Goudie DR, Smith FJ, McLean WH, Irvine AD (2006) Prevalent and rare mutations in the gene encoding filaggrin cause ichthyosis vulgaris and predispose individuals to atopic dermatitis. J Invest Dermatol 126:1770–1775
Netzel-Arnett S, Currie BM, Szabo R et al (2006) Evidence for a matriptase-prostasin proteolytic cascade regulating terminal epidermal differentiation. J Biol Chem 281:32941–32945
Bugge TH, List K, Szabo R (2007) Matriptase-dependent cell surface proteolysis in epithelial development and pathogenesis. Front Biosci 12:5060–5070
Alef T, Torres S, Hausser I et al (2009) Ichthyosis, follicular atrophoderma, and hypotrichosis caused by mutations in ST14 is associated with impaired profilaggrin processing. J Invest Dermatol 129:862–869
Tseng I-C, Xu H, Chou F-P et al (2010) Matriptase activation, an early cellular response to acidosis. J Biol Chem 285:3261–3270. doi:10.1074/jbc.M109.055640
Chen Y-W, Wang J-K, Chou F-P et al (2010) Regulation of the matriptase-prostasin cell surface proteolytic cascade by hepatocyte growth factor activator inhibitor-1 during epidermal differentiation. J Biol Chem 285:31755–31762. doi:10.1074/jbc.M110.150367
Sales KU, Masedunskas A, Bey AL et al (2010) Matriptase initiates activation of epidermal pro-kallikrein and disease onset in a mouse model of Netherton syndrome. Nat Genet 42:676–683
Borgoño CA, Michael IP, Komatsu N et al (2007) A potential role for multiple tissue kallikrein serine proteases in epidermal desquamation. J Biol Chem 282:3640–3652
Lundwall A, Brattsand M (2008) Kallikrein-related peptidases. Cell Mol Life Sci 65:2019–2038
Eissa A, Amodeo V, Smith CR, Diamandis EP (2011) Kallikrein-related peptidase-8 (KLK8) is an active serine protease in human epidermis and sweat and is involved in a skin barrier proteolytic cascade. J Biol Chem 286:687–706
Lundström A, Egelrud T (1988) Cell shedding from human plantar skin in vitro: evidence of its dependence on endogenous proteolysis. J Invest Dermatol 91:340–343
Faurschou M, Borregaard N (2003) Neutrophil granules and secretory vesicles in inflammation. Microbes Infect 5:1317–1327
Brinkmann V, Reichard U, Goosmann C et al (2004) Neutrophil extracellular traps kill bacteria. Science 303:1532–1535
Wiedow O, Wiese F, Streit V, Kalm CCE (1992) Lesional elastase activity in psoriasis, contact dermatitis, and atopic dermatitis. J Invest Dermatol 99:306–309
Meyer-Hoffert U, Wiedow O (2011) Neutrophil serine proteases: mediators of innate immune responses. Curr Opin Hematol 18:19–24
Turgeon VL, Houenou LJ (1997) The role of thrombin-like (serine) proteases in the development, plasticity and pathology of the nervous system. Brain Res Rev 25:85–95
Turgeon VL, Salman N, Houenou LJ et al (2000) Thrombin. Thromb Res 99:417–427
de La Houssaye BA, Mikule K, Nikolic D, Pfenninger KH (1999) Thrombin-induced growth cone collapse: involvement of phospholipase A(2) and eicosanoid generation. J Neurosci 19:10843–10855
Gill JS, Pitts K, Rusnak FM et al (1998) Thrombin induced inhibition of neurite outgrowth from dorsal root ganglion neurons. Brain Res 797:321–327
Rohatgi T, Sedehizade F, Reymann KG, Reiser G (2004) Protease-activated receptors in neuronal development, neurodegeneration, and neuroprotection: thrombin as signaling molecule in the brain. Neuroscientist 10:501–512
Chávez-Galán L, Arenas-Del Angel MC, Zenteno E et al (2009) Cell death mechanisms induced by cytotoxic lymphocytes. Cell Mol Immunol 6:15–25
Mhatre M, Nguyen A, Kashani S et al (2004) Thrombin, a mediator of neurotoxicity and memory impairment. Neurobiol Aging 25:783–793
Lochner JE, Honigman LS, Grant WF et al (2006) Activity-dependent release of tissue plasminogen activator from the dendritic spines of hippocampal neurons revealed by live-cell imaging. J Neurobiol 66:564–577
Samson AL, Medcalf RL, Baranes D et al (2006) Tissue-type plasminogen activator: a multifaceted modulator of neurotransmission and synaptic plasticity. Neuron 50:673–678
Samson AL, Nevin ST, Croucher D et al (2008) Tissue-type plasminogen activator requires a co-receptor to enhance NMDA receptor function. J Neurochem 107:1091–1101
Cullen SP, Martin SJ (2008) Mechanisms of granule-dependent killing. Cell Death Differ 15:251–262
Bratke K, Kuepper M, Bade B et al (2005) Differential expression of human granzymes A, B, and K in natural killer cells and during CD8+ T cell differentiation in peripheral blood. Eur J Immunol 35:2608–2616
Bade B, Lohrmann J, ten Brinke A et al (2005) Detection of soluble human granzyme K in vitro and in vivo. Eur J Immunol 35:2940–2948
Grossman WJ, Verbsky JW, Barchet W et al (2004) Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity 21:589–601
Romero V, Andrade F (2008) Non-apoptotic functions of granzymes. Tissue Antigens 71:409–416
Grassi M, Capello F, Bertolino L et al (2009) Identification of granzyme B-expressing CD-8-positive T cells in lymphocytic inflammatory infiltrate in cutaneous lupus erythematosus and in dermatomyositis. Clin Exp Dermatol 34:910–914
Méthot N, Rubin J, Guay D et al (2007) Inhibition of the activation of multiple serine proteases with a cathepsin C inhibitor requires sustained exposure to prevent pro-enzyme processing. J Biol Chem 282:20836–20846
Nathan C (2006) Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 6:173–182
Langhorst J, Elsenbruch S, Koelzer J et al (2008) Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol 103:162–169
Tzortzaki EG, Lambiri I, Vlachaki E, Siafakas NM (2007) Biomarkers in COPD. Curr Med Chem 14:1037–1048
Roghanian A, Sallenave J-M (2008) Neutrophil elastase (NE) and NE inhibitors: canonical and noncanonical functions in lung chronic inflammatory diseases (cystic fibrosis and chronic obstructive pulmonary disease). J Aerosol Med Pulm Drug Deliv 21:125–144
Cooley J, McDonald B, Accurso FJ et al (2008) Patterns of neutrophil serine protease-dependent cleavage of surfactant protein D in inflammatory lung disease. J Leukoc Biol 83:946–955
Döring G, Frank F, Boudier C et al (1995) Cleavage of lymphocyte surface antigens CD2, CD4, and CD8 by polymorphonuclear leukocyte elastase and cathepsin G in patients with cystic fibrosis. J Immunol 154:4842–4850
Kallenberg CG (2008) Pathogenesis of PR3-ANCA associated vasculitis. J Autoimmun 30:29–36
Pejler G, Åbrink M, Ringvall M, Wernersson S (2007) Mast cell proteases. Adv Immunol 95:167–255
Sakai K, Ren S, Schwartz LB (1996) A novel heparin-dependent processing pathway for human tryptase. Autocatalysis followed by activation with dipeptidyl peptidase I. J Clin Invest 97:988–995
Strik MCM, de Koning PJA, Kleijmeer MJ et al (2007) Human mast cells produce and release the cytotoxic lymphocyte associated protease granzyme B upon activation. Mol Immunol 44:3462–3472
Kovanen PT (2007) Mast cells: multipotent local effector cells in atherothrombosis. Immunol Rev 217:105–122
Schwartz LB, Bradford TR, Rouse C et al (1994) Development of a new, more sensitive immunoassay for human tryptase: use in systemic anaphylaxis. J Clin Immunol 14:190–204
Hartveit F, Thoresen S, Tangen M, Maartmann-Moe H (1984) Mast cell changes and tumor dissemination in human breast carcinoma. Invasion Metastasis 4:146–155
Blank U, Essig M, Scandiuzzi L et al (2007) Mast cells and inflammatory kidney disease. Immunol Rev 217:79–95
Frungieri MB, Weidinger S, Meineke V et al (2002) Proliferative action of mast-cell tryptase is mediated by PAR2, COX2, prostaglandins, and PPAR gamma: possible relevance to human fibrotic disorders. Proc Natl Acad Sci U S A 99:15072–15077
Erin EM, Leaker BR, Zacharasiewicz A et al (2006) Effects of a reversible beta-tryptase and trypsin inhibitor (RWJ-58643) on nasal allergic responses. Clin Exp Allergy 36:458–464
Fermi C, Pernossi L (1894) Ueber die Enzyme. Zeitschrift für Hyg und Infekt 18:83–127
Huntington JA, Read RJ, Carrell RW (2000) Structure of a serpin-protease complex shows inhibition by deformation. Nature 407:923–926
Vijaya Rachel K, Sirisha Gandreddi V D (2014) A review of protease inhibitors from different sources. Int J Appl Phys Bio-Chem Res 4:1–18
Law RHP, Zhang Q, McGowan S et al (2006) An overview of the serpin superfamily. Genome Biol 7:216
Rawlings ND (2010) Peptidase inhibitors in the MEROPS database. Biochimie 92:1463–1483
Potempa J, Korzus E, Travis J (1994) The serpin superfamily of proteinase inhibitors: structure, function, and regulation. J Biol Chem 269:15957–15960
van Gent D, Sharp P, Morgan K, Kalsheker N (2003) Serpins: structure, function and molecular evolution. Int J Biochem Cell Biol 35:1536–1547
Carrell RW, Pemberton PA, Boswell DR (1987) The serpins: evolution and adaptation in a family of protease inhibitors. Cold Spring Harb Symp Quant Biol 52:527–535
Heit C, Jackson BC, McAndrews M et al (2013) Update of the human and mouse serpin gene superfamily. Hum Genomics 7:22
Irving JA, Pike RN, Lesk AM, Whisstock JC (2000) Phylogeny of the serpin superfamily: implications of patterns of amino acid conservation for structure and function. Genome Res 10:1845–1864
Ragg H, Lokot T, Kamp PB et al (2001) Vertebrate serpins: construction of a conflict-free phylogeny by combining exon-intron and diagnostic site analyses. Mol Biol Evol 18:577–584
Silverman GA, Bird PI, Carrell RW et al (2001) The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem 276:33293–33296
Kumar A, Ragg H, Silverman G et al (2008) Ancestry and evolution of a secretory pathway serpin. BMC Evol Biol 8:250
Ray CA, Black RA, Kronheim SR et al (1992) Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1β converting enzyme. Cell 69:597–604
Irving JA, Pike RN, Dai W et al (2002) Evidence that serpin architecture intrinsically supports papain-like cysteine protease inhibition: engineering alpha(1)-antitrypsin to inhibit cathepsin proteases. Biochemistry 41:4998–5004
Nagata K (1996) Hsp47: a collagen-specific molecular chaperone. Trends Biochem Sci 21:22–26
Pemberton PA, Stein PE, Pepys MB et al (1988) Hormone binding globulins undergo serpin conformational change in inflammation. Nature 336:257–258
Zou Z, Anisowicz A, Hendrix MJ et al (1994) Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science 263:526–529
Nakashima T, Pak SC, Silverman GA et al (2000) Genomic cloning, mapping, structure and promoter analysis of HEADPIN, a serpin which is down-regulated in head and neck cancer cells. Biochim Biophys Acta 1492:441–446
Hedstrom L (2002) Serine protease mechanism and specificity. Chem Rev 102:4501–4524
Abbenante G, Fairlie DP (2005) Protease inhibitors in the clinic. Med Chem (Shāriqah (United Arab Emirates)) 1:71–104
Page MJ, Cera E Di, Page MJ, Di Cera E (2008) Serine proteases and serine protease inhibitors. Wiley Encycl Chem Biol
Travis J, Salvesen GS (1983) Human plasma proteinase inhibitors. Annu Rev Biochem 52:655–709
Ansari MJ (2015) Role of protease inhibitors in Insulin therapy of Diabetes: are these beneficial? Bull Environ Pharmacol Life Sci 4:01–08
Ohtomo S, Nangaku M, Izuhara Y et al (2008) The role of megsin, a serine protease inhibitor, in diabetic mesangial matrix accumulation. Kidney Int 74:768–774
Hida K, Wada J, Eguchi J et al (2005) Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci 102:10610–10615
Molinari F, Meskanaite V, Munnich A et al (2003) Extracellular proteases and their inhibitors in genetic diseases of the central nervous system. Hum Mol Genet R195–R200
Schalkwijk J, Wiedow O, Hirose S (1999) The trappin gene family: proteins defined by an N-terminal transglutaminase substrate domain and a C-terminal four-disulphide core. Biochem J 340:569–77. doi:10.1042/bj3400569
Wiedow O, Schröder JM, Gregory H et al (1990) Elafin: an elastase-specific inhibitor of human skin. Purification, characterization, and complete amino acid sequence. J Biol Chem 265:14791–14795
Ranganathan S, Mukhopadhyay T (2010) Dandruff: the most commercially exploited skin disease. Indian J Dermatol 55:130
Rachel KV, Vimala Y, Apta Chaitanya D (2013) A trypsin inhibitor-SNTI with antidandruff activity from Sapindus trifoliatus. Indian J Appl Res 3:3–5
Yamaya M, Shimotai Y, Hatachi Y, Morio H, Nishimura H (2016) Serine proteases and their inhibitors in human airway epithelial cells: effects on influenza virus replication and airway serine proteases and their inhibitors in human airway epithelial cells: effects on influenza virus replication and airway inflammation. Clin Microbiol 5:1–10
Zheng D, Chen H, Davids J et al (2013) Serpins for diagnosis and therapy in cancer. Cardiovasc Hematol Disord Drug Targets 13:123–132
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Rachel, K.V., Sirisha, G.V.D. (2017). Serine Proteases and Their Inhibitors in Human Health and Disease. In: Chakraborti, S., Chakraborti, T., Dhalla, N. (eds) Proteases in Human Diseases. Springer, Singapore. https://doi.org/10.1007/978-981-10-3162-5_10
Download citation
DOI: https://doi.org/10.1007/978-981-10-3162-5_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-3161-8
Online ISBN: 978-981-10-3162-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)