Abstract
The mortality rate of TBI currently remains as high as 30 %. To properly understand and apply minimally invasive TBI surgery is of great importance. Our present study encapsulates the foundation for decompressive craniectomy (DC) in diffuse, non-penetrating TBI, with the effects and complications of craniectomy in TBI. We introduced the information following: 1. why choose DC for TBI patients (including surgical technique); 2.outcomes after DC in TBI; 3. complications of DC. DC can save lives for patients with severe TBI, but many questions still remain for its application, and the outcome is highly related to the severity of the initial TBI. DC is a potential therapeutic option in a variety of situations, and neurosur-geons should be aware of all the complications of DC.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The mortality rate of TBI currently remains as high as 30 %. Therefore, neurosurgeons and trauma physicians are devoted to reducing this mortality rate with its associated disabilities. To properly understand and apply minimally invasive TBI surgery is of great importance. The concept of minimal invasion refers to a set of medical behaviors (especially surgeries), performed to achieve the best therapeutic effect with minimal tissue damage. Since different tissues have different functions in the human body, physicians must focus on protecting the important tissues and perform a full assessment of their functions and injury levels before surgery. In neurosurgery, the importance of tissue protection should be from the inside to the outside (e.g. brain → dura → skull → scalp). Currently some intracranial lesions can be primarily treated using specific modern technologies, such as intraoperative navigation, ultrasound, intervention, and endoscopy, to achieve the best excision effect through minimally invasive approach to the scalp (i.e. small incision).
However, with regards to TBI surgery, simply applying the above-mentioned methods through a small bone window or small incision is clearly unfitting and cannot protect brain tissues due to the diversity and complication that come with TBI.
Our present study encapsulates the foundation for DC in diffuse, non-penetrating TBI, with the effects and complications of craniectomy in TBI.
1.1 Why Choose DC for TBI Patients
If TBI surgery were a battle, choosing an appropriate surgical approach would be the crucial strategy that determines its success or failure. The decisions regarding which surgical approach to choose, whether to use a large/small craniectomy or drilling alone, not only evaluates a physician’s general understanding of the disease, it also tests his or her surgical skills. Inappropriate surgical approaches are bound to greatly reduce the surgical efficacy and can lead to serious adverse complications on the patient as well.
Large craniectomy was proposed and applied by American physicians in the 1970s. The incised bone flap area by this technique is nearly twice as large as that by conventional approach. It was introduced in China during the 1990s. After decades of practice, it is being widely used to treat severe TBI and has achieved acceptable effects.
Standard treatments, such as mannitol and hypertonic solution, can reduce the ICP; however, aggressive intracranial hypertension need further management procedures. We know that the concept of “widely opening the skull to decompress the brain in order to decrease the pressure” has been in existence for more than 100 years [1]. DC was used for the patients who presented with a progressive increase in intracranial pressure (ICP) after traumatic brain injury . DC has been proven to decrease intracranial pressure where patients sometimes get good outcomes from this procedure, but there is a debatable effect of DC with its association on having a better clinical outcome.
Cranial cavity is fixed, if brain edema will cause intracranial hypertension and cerebral herniation. Thus, pieces of skull are under resection, so an increase in the cranial cavity will be the most effective treatment. DC, which can increase the cranial cavity, is a useful and invasive procedure which can reduce ICP immediately after TBI [2]. Severe TBI is usually a diffuse injury; DC can expand our operation horizons and avoid missing the original traumatic lesions.
1.2 Surgical Technique
DC was usually chosen to eliminate the contusion and evacuate the hematoma in order to reduce the ICP in TBI, stroke or other central diseases causing a mass effect [3–5]. The traditional process is that we confirm the place where DC has to be done and do operation a little maximal around that. However, for diffuse brain edema, we need to combine operations with neurocritical care, such as hypothermia [6].
For children, Mhann et al. [7] retrospectively analyzed the medical records of children with severe TBI who were treated with DC in one center reported that early DC improves functional outcomes in pediatric patients with severe TBI, but does not improve mortality rates which were similar to Taylor et al. and Prasad et al. [8, 9] reports. They used DC in a randomized trial of very early DC in children with TBI and sustained intracranial hypertension, reported that a benefit in mortality and improved functional outcomes.
Previously described procedures about DC in adults were unilateral craniectomy [10] and bilateral craniectomy [11]. Liu et al.’s [3] method comprised of the unilateral craniectomy or bilateral craniectomy opening to maximize the reduction in ICP. For bilateral craniectomy, the dura is then opened in a cutting that resembles an “X”, (like that of a “fish mouth”) with the sagittal sinus ligated and anteriorly cut. The size of dura which is being opened is close to the cranial window. The sort of craniectomy performed (unilateral/bilateral) is based on the computed chromatography (CT) scan results. Unilateral craniectomy is performed in patients with unilateral swelling; however, in cases with general swelling, bilateral craniectomy is chosen. The area covered in unilateral craniectomy is approximately 12 cm × 15 cm, and that in the bilateral craniectomy is about 10 cm × 25 cm. Larger craniectomies may reduce the risks of herniation that leads to contusions at the skull edge [12]. This procedure is similar to the description Kjellberg [11] and Polin et al. [13]. Other neurosurgeons avoid sectioning the sagittal sinus or parting a strip of skull over the sagittal sinus when choosing craniotomies [9, 10, 14–16]. Leaving a strip of skull over the sagittal sinus may be easy to do crainoplasty in the future. However, the first thing is to reduce the ICP maximal after TBI. It is lack of evidence that whether leaving a strip of skull can affect the outcome of patients with severe TBI. Although most of these patients require to remove bone flap, it should be clear that we must know large craniotomy is not equal to decompressive craniotomy. Depending on the state of brain after the operation, we can choose whether we can remove the bone flap.
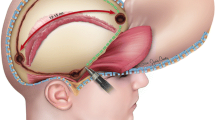
The illustration of large craniectomy:
The principle of surgical approach: “trapezia approach by layer” from skin to bone to dura to brain in turn.
The Surgical indications:
-
1.
Severe widespread cerebral contusion or intracerebral hematoma
-
2.
Cerebral hernia caused by acute subdural hematoma
-
3.
Diffuse brain edema/swelling
-
4.
Dilated double pupils caused by TBI.
Steps:
-
1.
The unilateral frontotemporoparietal large craniectomy
-
(a)
Position: supine, head to the lateral about 45°, shoulder padded high 15° in surgery side.
-
(b)
Scalp incision: starting from the zygomatic arch up—1.5 cm beyond tragus—the auricle- parietal tuber—to the midpoint of the line along the midline sagittal forward-to former hair line—large “?” flap (Fig. 1).
-
(c)
Cranial window: the size is equivalent to two-thirds of the area of one side, and the average size is 12 × 15 cm2 (Fig. 2a, b).
-
(d)
Dura: It is easy to suturedura such as cutting like “H”. The size is close to the cranial window (Fig. 3).
-
(e)
Intracranial check: Check carefully and completely remove the hematoma/contusion.
-
(f)
Acute encephalocele during operation.
-
(g)
Check the airway carefully to confirm whether the airway is obstruction, using mannitol to reduce ICP at the same time.
-
(h)
According to CT, it is to confirm the cause of acute intracranial hypertension, such as acute diffuse brain swelling (Fig. 4).
-
(i)
Suture of dura: After the operation completed, the expanded suture of dura should be to expand the volume of the cranial cavity (Fig. 5).
-
(j)
Superficial temporal fascia interrupted gashed (instead of the temporalis muscle resection). Height of the drainage bag is generally the same level with the head (Fig. 6).
-
(a)
-
2.
Bifrontal larger decompressive craniectomy
-
(a)
Position: supine, and shoulder padded 5 cm in both sides.
-
(b)
Scalp incision: Coronal Suture—Pterional-Two sides Zygomatic Arch.
-
(c)
Cranial window: Down to eyebrow, up to skin line, two sides to zygomatic arch by pterional avoiding opening frontal sinus (Fig. 7a, b).
-
(d)
Dura: It is easy to suturedura such as cutting like “X”. The sagittal sinus is ligated. The size is close to the cranial window (Fig. 8).
-
(e)
Suture of dura: After the operation completed, the expanded suture of dura should be to expand the volume of the cranial cavity (Fig. 9).
-
(f)
Superficial temporal fascia interrupted gashed (instead of the temporalis muscle resection). Height of the drainage bag is generally the same level with the head (Fig. 10).
-
(a)
2 Outcomes After DC in TBI
TBI is known to be a highly varied disease and the factors that govern the outcomes are partly implicit. These concerns complicate clarification of all trials in TBI, and the case series and trials involving DC in TBI are no exception. Recognition of this knowledge gap has driven recent efforts to systematize data collection in the TBI literature.
A 2015 meta-analysis on the outcomes of early DC after severe TBI included 8 articles and 256 cases [17]. The results of this meta-analysis demonstrated that the benefits of DC in cases of TBI were not significant enough for DC to be recommended over conventional medical management. However, the limitation of results was the lack of the number of high-quality studies.
A 2012 meta-analysis on the relation between DC and ICP, CPP for TBI comprised of 20 articles and 479 cases [2]. The meta-analysis showed that ICP was decreased immediately after DC and CPP increased. In our study about the effect of DC for severe TBI patients with fixed dilated pupils [3], the 6 month follow up showed: 39.76 % of patients (DC group) and 87.80 % of patients (CC group) died, and 34.33 % of patients (DC group) and 0 % of patients (CC group) had mild or no disability.
Andrews et al. [6] showed that therapeutic hypothermia with standard care could reduce ICP but outcomes were not better than those with standard care alone. During this clinical trial, DC was not used more often, stage 3 treatments (barbiturates and decompressive craniectomy) were used if all stage 2 treatments failed to control intracranial pressure. In that stage, the brain may be under irreversible edema. DC can reduce the ICP immediately, but the outcomes were related to lots of factors. In the future, in our experience, when ICP was more than 20 mm Hg, we need to take DC for patients with severe TBI.
Mhanna et al. [7] disclosed that there was no significant differences in survival between patients with DC and controls for children (71 % [12/17] vs. 82 % [14/17], respectively; p = 0.34). However, among survivors, at 4 years (IQR 1–6 years) after the TBI, 42 % (5/12) of the DC patients had mild disability or a Glasgow Outcome Scale score of 5 vs. none (0/14) of the controls (p = 0.012). In a study of 23 patients younger than 19 years of age, Jagannathan et al. [18.] reported that DC group had a favorable outcome, with a mean GOS score of 4.2 at follow-up. The outcomes was similar to the Pérez Suárez et al. [19] report.
3 Complications of DC
Complications happen during all surgical procedures, particularly in critical medicine; as such DC has been associated with a high rate of complications. The most common complications are hydrocephalus, subdural hygroma and infection.
A 2015 review on the complications associated with DC included a total of 142 eligible records. Kurland et al. [20] showed that complications were of three major types: hemorrhagic, infectious/inflammatory, and disturbances of the CSF compartment.
However, Mhann et al. [7], in comparison with their controls, patients who had a DC had a higher percentage of extradural hemorrhage, skull fractures, cerebral herniations, and cerebral edema underlying a more severe TBI on their admitting CT scans. However, the group of patients who had a DC had fewer CSF drainage devices in place in comparison with the control group. Although DC is known to be a simple surgical technique, complications commonly occur, sometimes with significant clinical impacts on patient outcome.
4 Conclusion
Decompressive craniectomy can save lives for patients with severe TBI, but many questions still remain for its application, and the outcome is highly related to the severity of the initial TBI. DC is a potential therapeutic option in a variety of situations, and neurosurgeons should be aware of all the complications of DC.
References
T K. Die Therapie des Hirndruckes. In: Holder A, editor. Hirnerschutterung, Hirndruck und chirurgische Eingriffe bei Hirnkrankheiten 1901.
Bor-Seng-Shu E, Figueiredo EG, Amorim RL, Teixeira MJ, Valbuza JS, de Oliveira MM, et al. Decompressive craniectomy: a meta-analysis of influences on intracranial pressure and cerebral perfusion pressure in the treatment of traumatic brain injury. J Neurosurg. 2012;117(3):589–96.
Mao X, Miao G, Hao S, Tao X, Hou Z, Li H, et al. Decompressive craniectomy for severe traumatic brain injury patients with fixed dilated pupils. Ther Clin Risk Manage. 2015;11:1627–33.
Yuan Q, Liu H, Wu X, Sun Y, Hu J. Comparative study of decompressive craniectomy in traumatic brain injury with or without mass lesion. Br J Neurosurg. 2013;27(4):483–8.
Servadei F. Clinical value of decompressive craniectomy. New Engl J Med. 2011;364(16):1558–9.
Andrews PJ, Sinclair HL, Rodriguez A, Harris BA, Battison CG, Rhodes JK, et al. Hypothermia for intracranial hypertension after traumatic brain injury. New Engl J Med. 2015;373(25):2403–12.
Mhanna MJ, Mallah WE, Verrees M, Shah R, Super DM. Outcome of children with severe traumatic brain injury who are treated with decompressive craniectomy. J Neurosur Pediatr. 2015:1–7.
Taylor A, Butt W, Rosenfeld J, Shann F, Ditchfield M, Lewis E, et al. A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Child’s Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg. 2001;17(3):154–62.
Prasad GL, Gupta DK, Mahapatra AK, Sharma BS. Surgical results of decompressive craniectomy in very young children: A level one trauma centre experience from India. Brain Inj. 2015;29(13–14):1717–24.
Guerra WK, Gaab MR, Dietz H, Mueller JU, Piek J, Fritsch MJ. Surgical decompression for traumatic brain swelling: indications and results. J Neurosurg. 1999;90(2):187–96.
Kjellberg RN, Prieto A Jr. Bifrontal decompressive craniotomy for massive cerebral edema. J Neurosurg. 1971;34(4):488–93.
Li X, von Holst H, Kleiven S. Decompressive craniectomy causes a significant strain increase in axonal fiber tracts. J Clin Neurosci Off J Neurosurg Soc Australas. 2013;20(4):509–13.
Polin RS, Shaffrey ME, Bogaev CA, Tisdale N, Germanson T, Bocchicchio B, et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997;41(1):84–92; discussion—4.
Bao YH, Liang YM, Gao GY, Pan YH, Luo QZ, Jiang JY. Bilateral decompressive craniectomy for patients with malignant diffuse brain swelling after severe traumatic brain injury: a 37-case study. J Neurotrauma. 2010;27(2):341–7.
Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P, et al. Decompressive craniectomy in diffuse traumatic brain injury. New Engl J Med. 2011;364(16):1493–502.
Akyuz M, Ucar T, Acikbas C, Kazan S, Yilmaz M, Tuncer R. Effect of early bilateral decompressive craniectomy on outcome for severe traumatic brain injury. Turk Neurosurg. 2010;20(3):382–9.
Wang R, Li M, Gao WW, Guo Y, Chen J, Tian HL. Outcomes of early decompressive craniectomy versus conventional medical management after severe traumatic brain injury: a systematic review and meta-analysis. Medicine. 2015;94(43):e1733.
Jagannathan J, Okonkwo DO, Dumont AS, Ahmed H, Bahari A, Prevedello DM, et al. Outcome following decompressive craniectomy in children with severe traumatic brain injury: a 10-year single-center experience with long-term follow up. J Neurosurg. 2007;106(4 Suppl):268–75.
Perez Suarez E, Serrano Gonzalez A, Perez Diaz C, Garcia Salido A, Martinez de Azagra Garde A, Casado Flores J. Decompressive craniectomy in 14 children with severe head injury: clinical results with long-term follow-up and review of the literature. J Trauma. 2011;71(1):133–40.
Kurland DB, Khaladj-Ghom A, Stokum JA, Carusillo B, Karimy JK, Gerzanich V, et al. Complications associated with decompressive craniectomy: a systematic review. Neurocrit Care. 2015;23(2):292–304.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Liu, B., Mao, X. (2017). The Normalization and Promotion of Large Craniotomy Treatment for Severe Traumatic Brain Injury. In: Fu, X., Liu, L. (eds) Advanced Trauma and Surgery. Springer, Singapore. https://doi.org/10.1007/978-981-10-2425-2_4
Download citation
DOI: https://doi.org/10.1007/978-981-10-2425-2_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-2424-5
Online ISBN: 978-981-10-2425-2
eBook Packages: MedicineMedicine (R0)