Abstract
Autosomal Dominant Polycystic Kidney Disease (ADPKD) is one of the most common inherited disorders. It is the fourth leading cause of renal replacement and renal failure worldwide. Mutations in PKD1 or PKD2 cause ADPKD. Patients with ADPKD show progressive growth of renal cysts filled with cystic fluid, leading to end-stage renal disease (ESRD) and renal failure by their sixth decade of life. Currently, there are no curative treatments for ADPKD. Therefore, patients require dialysis or kidney transplantation. To date, researchers have elucidated many of the mechanisms that cause ADPKD and developed many methods to diagnose the disease. ADPKD is related to growth factors, signaling pathways, cell proliferation, apoptosis, inflammation, the immune system, structural abnormalities, epigenetic mechanisms, microRNAs, and so on. Various therapies have been reported to slow the progression of ADPKD and alleviate its symptoms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Autosomal Dominant Polycystic Kidney Disease
1.1 Pathogenesis of ADPKD
Three inherited cystic diseases of the kidney are known. These are autosomal dominant polycystic liver disease (ADPLD), autosomal recessive polycystic kidney disease (ARPKD), and autosomal dominant polycystic kidney disease (ADPKD). PRKCSH and SEC63 genes are involved in ADPLD, and it causes bile duct cystic dilations. Abnormal expression of the PKHD1 gene results in ARPKD, which manifests as fusiform collecting duct dilatations, congenital hepatic fibrosis, and liver cysts.
Polycystic kidney disease 1 and 2 (PKD1 and PKD2) are two causative genes of ADPKD. Patients with ADPKD have kidney and bile duct cysts. Germline mutation of PKD1 or PKD2 leads to cyst formation. Monogenic disorders of these two genes have an incidence of 1 in 600 to 1 in 1000 individuals, meaning that ADPKD is one of the most common hereditary disorders. Also, it is the fourth leading cause of renal replacement and renal failure worldwide (Fedeles et al. 2014). Most cases of ADPKD are inherited, while about 10 % are due to de novo mutation (Rossetti et al. 2001).
1.2 Manifestations of ADPKD
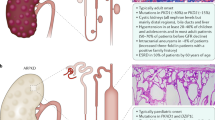
Patients with ADPKD have symptoms as follows: They have fluid-filled renal cysts in their kidneys and other epithelial organs. Usually, both of their kidneys are extremely enlarged and filled with cystic fluid. Because of the formation of cysts, patients have renal enlargement and it eventually causes end-stage renal failure (ESRD ) (Hou et al. 2002). Approximately 10 % of ESRD cases are caused by ADPKD. In normal adult humans, the kidneys comprise about 0.5 % of body weight. The kidneys of ADPKD patients weigh about 22 kg, comprising about 20 % of body weight (Ekser and Rigotti 2010). There are also various manifestations of ADPKD that are unrelated to the kidneys. One of the most common manifestations in ADPKD patients is hypertension (Martinez and Grantham 1995). Connective tissue defects such as cardiac defects , intracranial aneurysms , and hepatocystic disease also occur in ADPKD patients (Wu et al. 2000; Hughes et al. 1995). When these symptoms become aggravated, patients have to undergo dialysis and transplantation .
One of the most pronounced phenotypes of ADPKD is cyst formation in the kidneys. When the disease becomes severe, patients with enlarged cysts can even resemble pregnant women. Then, how does cyst formation progress in the kidney?
First, it starts at the normal renal tubule. Germline mutation of PKD1 or PKD2 causes the loss of one allele, and a somatic second hit causes the loss of the other normal allele (explained in Chap. 2). Then, one or more additional steps lead to cystogenesis as a ‘third hit ’, which may include nephrotoxic injury and/or ischemia. The third hit leads to cell proliferation in the renal tubules and causes small dilations that subsequently expand and form fluid-filled cysts of various sizes (Weimbs 2007; Bell et al. 2011). As cell proliferation persists, the dilation also progresses. Then, the dilated regions are separated from their original tubules, becoming a distinct cyst (Weimbs 2011). Once cysts are formed, they grow increasingly larger as the disease progresses. Therefore, cystogenesis is the most remarkable feature of ADPKD.
In addition, a variety of mechanisms can evoke ADPKD disease progression and cystogenesis, including somatic mutation, germ line mutation, modifying genes, increased cell proliferation, apoptosis, defective planar cell polarity, extracellular matrix abnormalities, fluid secretion, inflammation, and environmental factors (Paul and Vanden Heuvel 2014; Zhou 2009). To date, researchers have elucidated a variety of mechanisms that can cause ADPKD, methods to diagnose the disease, and therapies to slow and alleviate the symptoms of ADPKD.
2 Studies on ADPKD
As many studies on other diseases have investigated their early diagnosis and treatments, those on ADPKD have also sought to identify the mechanisms of disease and to develop methods to cure ADPKD. For several decades, researchers have been working towards these goals. Some of most remarkable approaches that have been employed to study ADPKD are as follows.
2.1 Growth Factors, Signaling Pathways, and Cell Proliferation
Cyst expansion in the kidneys of ADPKD patients is the most remarkable feature of the disease. The tubular epithelial cells that surround the cysts proliferate and drive their enlargement. Therefore, inhibiting cell proliferation is an important target for easing the symptoms of ADPKD (LaRiviere et al. 2015). In normal kidneys, a homeostatic balance is maintained between cell proliferation and apoptosis. However, in the kidneys of ADPKD patients, cell proliferation is more frequent than apoptosis. This imbalance eventually leads to cyst formation (Gregoire et al. 1987). Many studies have suggested a variety of mechanisms that may cause or contribute to cell proliferation and cyst enlargement in cystic kidney epithelia.
A wide range of growth factors are involved in cystogenesis. One of the main growth factors is epidermal growth factor (EGF), which along with EGF receptor, and other members of the EGF family such as transforming growth factor (TGF)-α, heparin-binding EGT, and amphiregulin, plays an important role in regulating cell proliferation of the cystic epithelia (Du and Wilson 1995). TGF-α is overexpressed in human polycystic kidney. Also, TGF-β is a major growth factor in ADPKD. Upregulation of TGF-β is related to cyst expansion during disease progression, but it is less involved in cyst initiation (Hassane et al. 2010; Wilson et al. 1996). Other growth factors such as hepatocyte growth factor (HGF), insulin-like growth factor 1 (IGF-1), and tyrosine kinase receptor of HGF and IGF-1 are also related to cyst formation in ADPKD.
In addition to growth factors, many signaling pathways are involved in ADPKD. First of all, the second messenger adenosine 3ʹ, 5ʹ cyclic monophosphate (cAMP ) is crucial in cystic kidney. cAMP, an intracellular mediator of adenylyl cyclase signaling, promotes cell proliferation of cystic epithelia. ADPKD patients and various animal models of polycystic kidney disease show an elevated cAMP level in kidney. Even in normal human kidney, stimulation by cAMP drives a cystic phenotype. Upregulation of cAMP mainly influences calcium signaling. cAMP induces cyst expansion and fluid secretion in intact cysts (Ye and Grantham 1993). When the level of intracellular calcium is decreased, cAMP/PKA signaling activates the Src/Ras/Raf/MEK/ERK pathways in ADPKD patients. ERK signaling, which is induced by PKA, also leads to the activation of mTOR signaling (Spirli et al. 2010; Distefano et al. 2009). Signal transducer and activator of transcription 3 (STAT3 ) responses to cAMP. STAT3 plays key roles in the development and maintenance of proinflammatory conditions in cystic kidneys (Martinez and Grantham 1995; Talbot et al. 2014).
Following the identification of clear mechanisms driving cell proliferation in the cysts of ADPKD, there have been many clinical trials to alleviate disease progression. Some representative drugs are vasopressin V2 receptor (V2R) antagonist, somatostatin analogs, mTOR inhibitors (rapamycin , sirolimus , and everolimus ), Raf kinase inhibitors (PLX5568 and sorafenib ), Src/Abl inhibitor SKI-606 (bosutinib ), and MEK inhibitors (PD184653 and UO126) (Renken et al. 2011; Sweeney et al. 2008; Shillingford et al. 2006; Elliott et al. 2011; Omori et al. 2006; Tao et al. 2005; Buchholz et al. 2011; Shibazaki et al. 2008; Yamaguchi et al. 2010).
2.2 Inflammation and Immune System
Recently, activation of macrophages was detected in the cyst lining epithelia in several mouse models of ADPKD. This find means that activated macrophages are involved in the proliferation of tubular epithelial cells (Karihaloo et al. 2011; Rae et al. 2007; Swenson-Fields et al. 2013). In addition, some inflammatory responses and gene expression patterns associated with the immune system were determined to be involved in ADPKD through computational analysis (Song et al. 2009). An increased concentration of monocyte chemotactic protein-1 (MCP-1) induces an increased number of mononuclear cells. We can detect MCP-1 in urine samples from ADPKD patients, which indicates an impairment of the innate immune system because of disease progression (Swenson-Fields et al. 2013). Macrophages play a major role in early developmental stages by removing apoptotic cells after the differentiation of organs. An increased number of macrophages results in the up-regulation of cell proliferation, down-regulation of apoptosis, and finally enlargement of cysts in the kidneys. Several studies have suggested that the downregulation of macrophages might be helpful for curing polycystic kidney diseases.
2.3 Structural Abnormality
The progressive accumulation of extracellular matrix is one of the notable hallmarks of fibrosis in ADPKD. Some animal models of polycystic kidney disease exhibit a thickened and laminated basement membrane and express high levels of α1 type IV collagen and laminins β1 and β2 (Katz et al. 1989). Polycystin-1, the protein encoded by PKD1, is involved in interactions between cells and the extracellular matrix. An excessive accumulation of fibroblasts results in cyst expansion in ADPKD kidney. Recently, in studies using zebrafish, researchers have found that polycystin proteins might be engaged in producing collagen. Therefore, we can infer that the accumulation of collagen is due to malfunctions of PKD1 and PKD2 (Mangos et al. 2010).
Extracellular matrix maintains its structure through continual turnover. The rate of the degradation of extracellular matrix is mediated by the matrix metalloproteinases (MMPs) , and tissue inhibitors of metalloproteinases (TIMPs) (Catania et al. 2007). In the kidneys of a mouse model with a mutation in Pkd1, the levels of MMP-2 and MMP-14 were upregulated (Hassane et al. 2010). Moreover, the levels of MMP-1, MMP-9, and TIMP-1 in serum were increased in the kidneys of ADPKD patients (Nakamura et al. 2000). Taken together, most MMPs and TIMPs were elevated in cystic conditions. In addition, the extracellular matrix interacts with cells. This interaction controls cell proliferation, differentiation, and other cellular functions through specific matrix receptor proteins. Typical examples of these matrix receptor proteins are integrins and proteoglycan-containing syndecans (Geiger et al. 2001). To summarize many studies, researchers found that several integrins and syndecans were increased in ADPKD patients, particularly α2β1 integrin, integrin α8, integrin αv, integrin β4, and syndecan-4 (Wilson and Burrow 1999; Wallace et al. 2008; Wilson et al. 1999; Zeltner et al. 2008).
The main physiological function of the kidney is filtration , and it is essential for homeostasis . Components that are over-accumulated or unnecessary are secreted, while other essential factors remain in the circulation. This process occurs when body fluid passes through the kidney, especially in the renal tubules. A sensory organelle called the cilium can detect physical and chemical stimuli such as the flow of fluid (Hildebrandt and Otto 2005). Cilia protrude from the epithelial cells towards the lumen of renal tubules. Cilia are microtubule-based structures and they originate from the basal body or centrosome (Paul and Vanden Heuvel 2014). Alongside many other factors, ciliary defects can also cause polycystic kidney disease. Recent studies have demonstrated that malfunctions of the cilia could influence cyst development (Garcia-Gonzalo and Reiter 2012).
2.4 Epigenetic Changes and microRNA
Another biological mechanism that could explain the symptoms and pathogenesis of ADPKD is epigenetic regulation. Epigenetic changes including histone modifications such as acetylation, methylation, and phosphorylation are also related to the mechanisms of ADPKD (Li 2011). The mechanism that can control DNA methylation was revealed—an increased level of TGF-β evokes DNA methylation in ADPKD tissue, and it might result in fibrosis in kidney (Bechtel et al. 2010).
Furthermore, microRNAs can also evoke ADPKD by directly binding to their target genes. The importance of microRNAs in ADPKD has been emphasized. microRNAs can regulate the expression level of their target mRNA(s) and are related to cell proliferation, differentiation, apoptosis, and many other cellular processes. Individual microRNAs might be increased or decreased in the kidneys of ADPKD patients. Some microRNAs target PKD1 or PKD2 directly, or they can target other genes related to the phenotype of ADPKD. For example, the miR-17 ~ 92 microRNA cluster, miRNA-21, miR-15a, and miRNA-199a have been identified as candidate ADPKD-involved microRNAs that can regulate cell proliferation and the pathogenesis of ADPKD (Patel et al. 2013; Sun et al. 2015; Lakhia et al. 2015; Lee et al. 2008).
Targeting microRNAs that bind to PKD1 or PKD2 might be a viable method to regulate the clinical manifestations of ADPKD, but most previous research about microRNAs and ADPKD has suggested that microRNAs alone are not sufficient for the treatment of ADPKD. Another factor is needed to cure the disease, so many studies have offered microRNAs and their target genes as new therapeutic targets. As a variety of microRNAs involved in cystogenesis have begun to be revealed by microRNA microarray data, research on microRNAs in ADPKD is predicted to become increasingly active in the future (Tan et al. 2011).
3 What Is Coming Next in ADPKD Research?
Until now, research on ADPKD has been conducted using various approaches such as those at the molecular and structural levels, as well as clinical trials. Although all of these approaches can yield useful results, we need to increasingly focus on identifying methods for the diagnosis and treatment of ADPKD. All researchers should seek to develop a framework for integrating studies across disciplines, and then apply that framework to deciphering an effective treatment modality for curing ADPKD. As many exciting studies are currently underway, it is possible that we may be able to discover a new therapeutic method in the near future.
References
Bechtel W, McGoohan S, Zeisberg EM, Muller GA, Kalbacher H, Salant DJ, Muller CA, Kalluri R, Zeisberg M (2010) Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat Med 16(5):544–550. doi:10.1038/nm.2135
Bell PD, Fitzgibbon W, Sas K, Stenbit AE, Amria M, Houston A, Reichert R, Gilley S, Siegal GP, Bissler J, Bilgen M, Chou PC, Guay-Woodford L, Yoder B, Haycraft CJ, Siroky B (2011) Loss of primary cilia upregulates renal hypertrophic signaling and promotes cystogenesis. J Am Soc Nephrol 22(5):839–848. doi:10.1681/ASN.2010050526
Buchholz B, Klanke B, Schley G, Bollag G, Tsai J, Kroening S, Yoshihara D, Wallace DP, Kraenzlin B, Gretz N, Hirth P, Eckardt KU, Bernhardt WM (2011) The Raf kinase inhibitor PLX5568 slows cyst proliferation in rat polycystic kidney disease but promotes renal and hepatic fibrosis. Nephrol Dial Transplant 26(11):3458–3465. doi:10.1093/ndt/gfr432
Catania JM, Chen G, Parrish AR (2007) Role of matrix metalloproteinases in renal pathophysiologies. Am J Physiol Renal Physiol 292(3):F905–F911. doi:10.1152/ajprenal.00421.2006
Distefano G, Boca M, Rowe I, Wodarczyk C, Ma L, Piontek KB, Germino GG, Pandolfi PP, Boletta A (2009) Polycystin-1 regulates extracellular signal-regulated kinase-dependent phosphorylation of tuberin to control cell size through mTOR and its downstream effectors S6K and 4EBP1. Mol Cell Biol 29(9):2359–2371. doi:10.1128/MCB.01259-08
Du J, Wilson PD (1995) Abnormal polarization of EGF receptors and autocrine stimulation of cyst epithelial growth in human ADPKD. Am J Physiol 269(2 Pt 1):C487–C495
Ekser B, Rigotti P (2010) Images in clinical medicine. Autosomal dominant polycystic kidney disease. N Engl J Med 363(1):71. doi:10.1056/NEJMicm0905399
Elliott J, Zheleznova NN, Wilson PD (2011) c-Src inactivation reduces renal epithelial cell-matrix adhesion, proliferation, and cyst formation. Am J Physiol Cell Physiol 301(2):C522–C529. doi:10.1152/ajpcell.00163.2010
Fedeles SV, Gallagher AR, Somlo S (2014) Polycystin-1: a master regulator of intersecting cystic pathways. Trends Mol Med 20(5):251–260. doi:10.1016/j.molmed.2014.01.004
Garcia-Gonzalo FR, Reiter JF (2012) Scoring a backstage pass: mechanisms of ciliogenesis and ciliary access. J Cell Biol 197(6):697–709. doi:10.1083/jcb.201111146
Geiger B, Bershadsky A, Pankov R, Yamada KM (2001) Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat Rev Mol Cell Biol 2(11):793–805. doi:10.1038/35099066
Gregoire JR, Torres VE, Holley KE, Farrow GM (1987) Renal epithelial hyperplastic and neoplastic proliferation in autosomal dominant polycystic kidney disease. Am J Kidney Dis 9(1):27–38
Hassane S, Leonhard WN, van der Wal A, Hawinkels LJ, Lantinga-van Leeuwen IS, ten Dijke P, Breuning MH, de Heer E, Peters DJ (2010) Elevated TGFbeta-Smad signalling in experimental Pkd1 models and human patients with polycystic kidney disease. J Pathol 222(1):21–31. doi:10.1002/path.2734
Hildebrandt F, Otto E (2005) Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat Rev Genet 6(12):928–940. doi:10.1038/nrg1727
Hou X, Mrug M, Yoder BK, Lefkowitz EJ, Kremmidiotis G, D’Eustachio P, Beier DR, Guay-Woodford LM (2002) Cystin, a novel cilia-associated protein, is disrupted in the cpk mouse model of polycystic kidney disease. J Clin Invest 109(4):533–540. doi:10.1172/JCI14099
Hughes J, Ward CJ, Peral B, Aspinwall R, Clark K, San Millan JL, Gamble V, Harris PC (1995) The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet 10(2):151–160. doi:10.1038/ng0695-151
Karihaloo A, Koraishy F, Huen SC, Lee Y, Merrick D, Caplan MJ, Somlo S, Cantley LG (2011) Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol 22(10):1809–1814. doi:10.1681/ASN.2011010084
Katz SK, Hakki A, Miller AS, Finkelstein SD (1989) Ultrastructural tubular basement membrane lesions in adult polycystic kidney disease. Ann Clin Lab Sci 19(5):352–359
Lakhia R, Hajarnis S, Williams D, Aboudehen K, Yheskel M, Xing C, Hatley ME, Torres VE, Wallace DP, Patel V (2015) MicroRNA-21 aggravates cyst growth in a model of polycystic kidney disease. J Am Soc Nephrol. doi:10.1681/ASN.2015060634
LaRiviere WB, Irazabal MV, Torres VE (2015) Novel therapeutic approaches to autosomal dominant polycystic kidney disease. Transl Res 165(4):488–498. doi:10.1016/j.trsl.2014.11.003
Lee SO, Masyuk T, Splinter P, Banales JM, Masyuk A, Stroope A, Larusso N (2008) MicroRNA15a modulates expression of the cell-cycle regulator Cdc25A and affects hepatic cystogenesis in a rat model of polycystic kidney disease. J Clin Invest 118(11):3714–3724. doi:10.1172/JCI34922
Li X (2011) Epigenetics and autosomal dominant polycystic kidney disease. Biochim Biophys Acta 1812(10):1213–1218. doi:10.1016/j.bbadis.2010.10.008
Mangos S, Lam PY, Zhao A, Liu Y, Mudumana S, Vasilyev A, Liu A, Drummond IA (2010) The ADPKD genes pkd1a/b and pkd2 regulate extracellular matrix formation. Dis Model Mech 3(5–6):354–365. doi:10.1242/dmm.003194
Martinez JR, Grantham JJ (1995) Polycystic kidney disease: etiology, pathogenesis, and treatment. Dis Mon 41(11):693–765
Nakamura T, Ushiyama C, Suzuki S, Ebihara I, Shimada N, Koide H (2000) Elevation of serum levels of metalloproteinase-1, tissue inhibitor of metalloproteinase-1 and type IV collagen, and plasma levels of metalloproteinase-9 in polycystic kidney disease. Am J Nephrol 20(1):32–36. doi:13552
Omori S, Hida M, Fujita H, Takahashi H, Tanimura S, Kohno M, Awazu M (2006) Extracellular signal-regulated kinase inhibition slows disease progression in mice with polycystic kidney disease. J Am Soc Nephrol 17(6):1604–1614. doi:10.1681/ASN.2004090800
Patel V, Williams D, Hajarnis S, Hunter R, Pontoglio M, Somlo S, Igarashi P (2013) miR-17 ~ 92 miRNA cluster promotes kidney cyst growth in polycystic kidney disease. Proc Natl Acad Sci U S A 110(26):10765–10770. doi:10.1073/pnas.1301693110
Paul BM, Vanden Heuvel GB (2014) Kidney: polycystic kidney disease. Wiley Interdiscip Rev Dev Biol 3(6):465–487. doi:10.1002/wdev.152
Rae F, Woods K, Sasmono T, Campanale N, Taylor D, Ovchinnikov DA, Grimmond SM, Hume DA, Ricardo SD, Little MH (2007) Characterisation and trophic functions of murine embryonic macrophages based upon the use of a Csf1r-EGFP transgene reporter. Dev Biol 308(1):232–246. doi:10.1016/j.ydbio.2007.05.027
Renken C, Fischer DC, Kundt G, Gretz N, Haffner D (2011) Inhibition of mTOR with sirolimus does not attenuate progression of liver and kidney disease in PCK rats. Nephrol Dial Transplant 26(1):92–100. doi:10.1093/ndt/gfq384
Rossetti S, Strmecki L, Gamble V, Burton S, Sneddon V, Peral B, Roy S, Bakkaloglu A, Komel R, Winearls CG, Harris PC (2001) Mutation analysis of the entire PKD1 gene: genetic and diagnostic implications. Am J Hum Genet 68(1):46–63. doi:10.1086/316939
Shibazaki S, Yu Z, Nishio S, Tian X, Thomson RB, Mitobe M, Louvi A, Velazquez H, Ishibe S, Cantley LG, Igarashi P, Somlo S (2008) Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum Mol Genet 17(11):1505–1516. doi:10.1093/hmg/ddn039
Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T (2006) The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A 103(14):5466–5471. doi:10.1073/pnas.0509694103
Song X, Di Giovanni V, He N, Wang K, Ingram A, Rosenblum ND, Pei Y (2009) Systems biology of autosomal dominant polycystic kidney disease (ADPKD): computational identification of gene expression pathways and integrated regulatory networks. Hum Mol Genet 18(13):2328–2343. doi:10.1093/hmg/ddp165
Spirli C, Okolicsanyi S, Fiorotto R, Fabris L, Cadamuro M, Lecchi S, Tian X, Somlo S, Strazzabosco M (2010) ERK1/2-dependent vascular endothelial growth factor signaling sustains cyst growth in polycystin-2 defective mice. Gastroenterology 138(1):360–371. e367. 10.1053/j.gastro.2009.09.005
Sun L, Zhu J, Wu M, Sun H, Zhou C, Fu L, Xu C, Mei C (2015) Inhibition of MiR-199a-5p reduced cell proliferation in autosomal dominant polycystic kidney disease through targeting CDKN1C. Med Sci Monit 21:195–200. doi:10.12659/MSM.892141
Sweeney WE Jr, von Vigier RO, Frost P, Avner ED (2008) Src inhibition ameliorates polycystic kidney disease. J Am Soc Nephrol 19(7):1331–1341. doi:10.1681/ASN.2007060665
Swenson-Fields KI, Vivian CJ, Salah SM, Peda JD, Davis BM, van Rooijen N, Wallace DP, Fields TA (2013) Macrophages promote polycystic kidney disease progression. Kidney Int 83(5):855–864. doi:10.1038/ki.2012.446
Talbot JJ, Song X, Wang X, Rinschen MM, Doerr N, LaRiviere WB, Schermer B, Pei YP, Torres VE, Weimbs T (2014) The cleaved cytoplasmic tail of polycystin-1 regulates Src-dependent STAT3 activation. J Am Soc Nephrol 25(8):1737–1748. doi:10.1681/ASN.2013091026
Tan YC, Blumenfeld J, Rennert H (2011) Autosomal dominant polycystic kidney disease: genetics, mutations and microRNAs. Biochim Biophys Acta 1812(10):1202–1212. doi:10.1016/j.bbadis.2011.03.002
Tao Y, Kim J, Schrier RW, Edelstein CL (2005) Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J Am Soc Nephrol 16(1):46–51. doi:10.1681/ASN.2004080660
Wallace DP, Quante MT, Reif GA, Nivens E, Ahmed F, Hempson SJ, Blanco G, Yamaguchi T (2008) Periostin induces proliferation of human autosomal dominant polycystic kidney cells through alphaV-integrin receptor. Am J Physiol Renal Physiol 295(5):F1463–F1471. doi:10.1152/ajprenal.90266.2008
Weimbs T (2007) Polycystic kidney disease and renal injury repair: common pathways, fluid flow, and the function of polycystin-1. Am J Physiol Renal Physiol 293(5):F1423–F1432. doi:10.1152/ajprenal.00275.2007
Weimbs T (2011) Third-hit signaling in renal cyst formation. J Am Soc Nephrol 22(5):793–795. doi:10.1681/ASN.2011030284
Wilson PD, Burrow CR (1999) Cystic diseases of the kidney: role of adhesion molecules in normal and abnormal tubulogenesis. Exp Nephrol 7(2):114–124. doi:20592
Wilson PD, Norman JT, Kuo NT, Burrow CR (1996) Abnormalities in extracellular matrix regulation in autosomal dominant polycystic kidney disease. Contrib Nephrol 118:126–134
Wilson PD, Geng L, Li X, Burrow CR (1999) The PKD1 gene product, “polycystin-1,” is a tyrosine-phosphorylated protein that colocalizes with alpha2beta1-integrin in focal clusters in adherent renal epithelia. Lab Invest 79(10):1311–1323
Wu G, Markowitz GS, Li L, D’Agati VD, Factor SM, Geng L, Tibara S, Tuchman J, Cai Y, Park JH, van Adelsberg J, Hou H Jr, Kucherlapati R, Edelmann W, Somlo S (2000) Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat Genet 24(1):75–78. doi:10.1038/71724
Yamaguchi T, Reif GA, Calvet JP, Wallace DP (2010) Sorafenib inhibits cAMP-dependent ERK activation, cell proliferation, and in vitro cyst growth of human ADPKD cyst epithelial cells. Am J Physiol Renal Physiol 299(5):F944–F951. doi:10.1152/ajprenal.00387.2010
Ye M, Grantham JJ (1993) The secretion of fluid by renal cysts from patients with autosomal dominant polycystic kidney disease. N Engl J Med 329(5):310–313. doi:10.1056/NEJM199307293290503
Zeltner R, Hilgers KF, Schmieder RE, Porst M, Schulze BD, Hartner A (2008) A promoter polymorphism of the alpha 8 integrin gene and the progression of autosomal-dominant polycystic kidney disease. Nephron Clin Pract 108(3):c169–c175. doi:10.1159/000116887
Zhou J (2009) Polycystins and primary cilia: primers for cell cycle progression. Annu Rev Physiol 71:83–113. doi:10.1146/annurev.physiol.70.113006.100621
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Shin, Y.B., Park, J.H. (2016). Recent Trends in ADPKD Research. In: Park, J., Ahn, C. (eds) Cystogenesis. Advances in Experimental Medicine and Biology, vol 933. Springer, Singapore. https://doi.org/10.1007/978-981-10-2041-4_1
Download citation
DOI: https://doi.org/10.1007/978-981-10-2041-4_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-2040-7
Online ISBN: 978-981-10-2041-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)