Abstract
In this work, a computational fluid dynamics (CFD) is used to analyze the effect of different opening between two valves of a normal popliteal vein. This study was focusing on the velocity and also vorticity of blood along the popliteal vein distribution. Based on the observation and analysis of the data, the different size of orifice between the first valve and second valve influencing the velocity and vorticity of the blood flow. The rotational motion of blood particle at the same spot will increasing the probability of blood accumulating which helping in development of thrombus. The effect of the irregularities in the blood flow will cause valve insufficiency that will lead to various disease related to venous vessel such as heart attack, deep vein thrombosis (DVT), pulmonary embolism (PE)and the worst is death. A series of experiment has been conducted by changing the size of valve orifice for the first and second valve along the vein distribution. The result of the CFD simulation shows a significant result of blood flow in term of velocity and vorticity.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
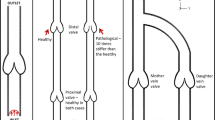
In the circulatory framework, veins is defined as any blood vessel that convey blood to the heart. Most veins carry deoxygenated blood from tissue back to the heart except pulmonary veins which carry oxygenated blood to the heart. Veins contain valves to prevent the backflow of blood toward the capillary beds. Without the valve system, it is nearly impossible for the blood to ever back to the heart whether in standing or sitting position. Dysfunctional of valve will lead to obstruction of blood circulation thus, can lead to downstream tissue dysfunction to all over the body. Coagulation of blood in vessel mainly can lead to heart attack or shock such as coronary thrombosis, deep vein thrombosis (DVT), pulmonary embolism (PE) and the worst is death [1]. It is for the most part considered that there are three foremost variables which are in charge of the thrombus development in the blood vessel: (1) hemocompatibility of pump materials, (2) morphology of blood contact with the vessel surface, (3) blood stream pattern inside blood vessel [2]. However, in this study, the condition was only focusing to the popliteal vein. Previous in vitro study demonstrate that the valve geometry, material properties of the vein and venous valve the blood progress of antegrade stream [1, 3]. Other than that, Lurie and Kistner [4] stated that the dramatic change of the three dimensional velocity profile of blood was due to the shape and size of the orifice. Since there are no studies that investigate the valve aperture orientation in relation to blood flow dynamic thus, this study was performed to address the problem of asynchronization between two valves in a popliteal vein.
The late advancement of computational fluid dynamics innovation now permits complex numerical reenactment of the cardiovascular framework. Computational fluid dynamics (CFD) investigations have been utilized to assess particular parameters in body such as wall shear stress, velocity of blood flow in vessel stream and pressure of the blood against the wall of the vessel which are very hard to obtain from in vivo experiment [5]. Using the advance technology of CFD, a three-dimensional simulation of blood flow in a venous vessel was performed. Hence, the model of the vein structure was implemented into ANSYS’s CFX software package.
2 Methodology
2.1 Vein’s Structure
To study the blood behavior, a geometrical structure of the popliteal vein have been constructed in SolidWorks. This is to show the three-dimensional view of the vein to replicate the real vein structure.
Figure 1 shows the structure of the vein design with a pair of leaflets attached at a point of vein wall and the red arrow shows the direction of blood flow. From the previous study, length of the vein was set to 10 cm. Since valve are reliably situated at particular area in the veins, they are normally present in pairs 3–5 cm separated from one another [4]. The previous studies stated that the leaflets will not go all the way to the wall of vein, therefore a will represent the size of leaflets opening where in this study it will vary from 30 to 70 % since the maximum leaflets opening is only 60–70 % [4] of the vein diameter. Since this study only focusing on the size of leaflets aperture, therefore the structure of the vein was assumed to be in a cylindrical shape.
2.2 CFD Implementation
Generally, before starting the simulation, the boundary condition of the model had to be defined including flow rate, velocity and pressure of the inlet and outlet of the vessel model [6]. Based on the geometrical structure of the vein, a triangle meshes were generated to obtain the optimum grid size for the model. The meshes consist of 65274 nodes and 332503 tetrahedral elements. For the purpose of this study, the blood was assumed as a non-Newtonian fluid flow at isothermal conditions [7]. While the vessel walls were considered to be rigid with no-slip condition of the wall. The list of the parameter used was shown on Table 1.
Table 2 shows the types of cases that will be investigated throughout the simulation. The first valve was set to 70 % while for the second valve, the orifice size was set to 50 and 30 %. This is to investigate, how the size of orifice will affect the flow pattern of the blood.
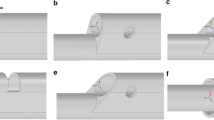
Figure 2 shows the designated valve according to the studied cases. The first valve was located right after the inlet while the following valve indicated as the second valve. The inlet and outlet is where the blood going in and out from the blood vessel.
2.3 Velocity and Vorticity of the Fluid
In this paper, the aim is to investigate the blood flow movement in the velocity and vorticity context. Theoretically, the previous studies shows that the flow of blood changes according to the velocity and vorticity of the blood. Two closely located valves may have combined effect on the flow if the relative synchronization of the leaflets opening has reasonable consistency between individuals. After blood passing through the first valve, the flow of blood remain pulsatile, however the velocity undergoes significant change [8]. If the second valve was not in synch with the first valve, then the blood will reflux and pooling at the same place in a specific time. The pooling of blood at the same place in a specific time will increase the blood particle contact with another, thus will lead to the accumulation of blood at the area. One importance effect of this mechanism is to prevent the blood stenosis at the back of the leaflets.
Vorticity is a measure of the rotation of fluid elements and is defined as 2 ɷ. An irrational fluid has a zero vorticity. However, in biofluid mechanics, due to the pressure and velocity, vorticity can be generated which means the fluid would be rotational. With the existence of vorticity a small parcel of blood particles will be rotated at the same place thus will disturbed the real flow of the blood.
3 Result
As previously mention, the simulation was consist of two cases which is A and B. Therefore, in this section the result of the two cases will be discuss briefly.
3.1 Velocity of the Blood
As the blood passing through the valve, a consistent pattern of blood dynamics can be observe during rhythmic cardiovascular pump [6]. The blood velocity in and out of the valve contain a specific pattern of the flow variation. To prove the specific velocity pattern of blood movement, therefore the velocity profile of the blood has been observe throughout the simulation.
Figure 3 shows the velocity profile along the vein distribution. The vein was divided into six segments line. Which is three segments for the first valve (a–c) and three segments for the second valve (d–f). The function of velocity profile is to monitor the pattern of the pressure flow at every segment. Every line segment was separated by 8 mm. Though from the figure it can be observe that the velocity profile had shown the bell-shaped figure, but the range value of the blood flow velocity in the vein is different. The red in colour represent the higher value of velocity, while blue colour represent the lower value of velocity. From the observation, the value of velocity obviously decreasing after the blood passing through the second valve. From the Fig. 3 it can be seen at the b segment, the colour contour is green which show the increasing of velocity as it passing through the leaflets as it following the previous clinical study [7].
From the Fig. 4, it can be seen that the pattern of the velocity profile is literally same with the first case. However, it can be seen from the colour contour that, the velocity range for the Case A and the Case B is significantly different to one another. This is due to the different of the second valve opening for the both cases. As for the second cases it can be observe that the colour for the both valves significantly different as well. The average velocity between the two valves for both cases has been shown in the Fig. 5.
Figure 5 shows the average of velocity that distributes along the vein distribution according to the designated segment. It can be observe that case B has higher velocity compared to the case A. Both case shows there are slightly increasing in velocity at the c segment. This is because the blood was passing through the valve and this was proven by the clinical study [7]. Other than that, the value of the velocity was consistently decreasing after going through the second valve. Since the case B, have smaller cross sectional area between the leaflets, therefore the flow of blood can be seen accelerated in this stenotic area. Thus resulting in proximally directed flow jet. On the other hand, case A have large cross sectional area between the leaflets thus, producing much slower velocity, resulting in reflected stream which against the normal blood flow.
3.2 Vorticity of the Blood
Vorticity is a condition where the fluid element has a rotational function. Human body contain blood particles which is extremely sensitive due to the existence of pressure as well as velocity which can generate rotational fluid along the blood stream. The properties of vorticity in a blood flow might help in determining the blood particles movement in the body. However, since it is difficult to conduct an in vivo experiment, therefore this method will be one of the mathematical approach to study the mechanism of the blood particle.
Figure 6a and b show the colour contour of the vorticity. The blue colour indicate the blood particles which in the clockwise rotation, while red colour indicates the anticlockwise rotation. Even though the size of the orifice for the first valve is the same for both case, however the velocity contour shows a significant different in size of the contour from one another. This is due the size of the orifice for the second valve which affecting the blood particle movement at that particular area. Vorticity contour in the Fig. 6b is slightly bigger than the Fig. 6a. This shows that, there are more rotational particles in case B comparing to case A. From the information obtain, case B would be one of the factor that will influence the accumulating of blood, thus, could lead to the formation of thrombus. Other than that, the blood particle can be observe rotating majorly at the valve area since the valve was obstructing the flow of the blood. Once the blood passing through the valve, some of the particles will be entering the valve cusps. That particular particles will be rotating at the same spot thus, will increasing the contact time of the particles of blood with the vessel wall and increasing the probability of blood clot formation.
4 Conclusion
The data for the 3D structure of vein have been provided by ANSYS-CFX package. Based on the observation and analysis of the data, the different size of orifice between the first valve and second valve influencing the velocity and vorticity of the blood flow. The biomechanics event of the fluid was depends on the mechanical properties of the valve orifice. In fact, to prevent the reflux of the blood, the valve functioning as flow modulator in helping the blood movement in the venous vessel. The existence of vorticity at the cusps of valve, showing that the blood particle was moving in rotational motion. The rotational motion of blood particle at the same spot will increasing the probability of blood accumulating which helping in development of thrombus.
References
Hossler FE, West RF (1988) Venous valve anatomy and morphometry: Studies on the duckling using vascular corrosion casting. Am J Anat 181:425–432
Wang H-S (1990) Thrombus formation and blood contacting surface in the pneumatic diaphragm blood pump [J]. In: Bioengineering, Proceedings of the Northeast Conference, pp 21–22
Raju S, Hudson CA, Fredericks R, Neglen P, Greene AB, Meydrech EF (1999) Studies in calf venous pump function utilizing a two- valve experimental model. Eur J Vasc Endovasc Surg 17:521–532
Lurie F, Kistner RL (2012) The relative position of paired valves at ve-nous junctions suggests their role in modulating three-dimensional flow pat-tern in veins. Eur J Vasc Endovasc Surg 44(3):337–340. doi:10.1016/j.ejvs.2012.06.018
Tokuda Y, Song M-H, Ueda Y, Usui A, Akita T, Yoneyama S, Maruyama S (2008) Three-dimensional numerical simulation of blood flow in the aortic arch during cardiopulmonary by-pass. Eur J Cardiothorac Surg Official J Eur Assoc Cardiothorac Surg 33:164–167. doi:10.1016/j.ejcts.2007.11.021
Uus A, Liatsis P, Rajani R, Man-dic L (2014) The impact of boundary conditions in patient-specific coronary blood flow simulation (May), pp 35–38
Wijeratne NS, Hoo KA (2008) Numerical studies on the hemodynamics in the human vein and venous valve, pp 147–152
Lurie F, Kistner RL, Eklof B (n.d.) The mechanism of venous valve closure in normal physiologic conditions, pp 713–717. doi:10.1067/mva.2002.121123
Acknowledgments
The authors like to thank Fundamental Research Grant Scheme (FRGS 1486) for the sponsorship doing this project. They also like to thank Universiti Tun Hussein Onn Malaysia (UTHM) for having the space of Medical Imaging laboratory and also MiNT SRC laboratory doing the experiment.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media Singapore
About this paper
Cite this paper
bt Aziz, N.S., bt Ibrahim, N., Abdullah, K., bt Mat Harun, N.H.I. (2017). Computational Fluid Dynamics Simulation on Blood Velocity and Vorticity of Venous Valve Behaviour. In: Ibrahim, H., Iqbal, S., Teoh, S., Mustaffa, M. (eds) 9th International Conference on Robotic, Vision, Signal Processing and Power Applications. Lecture Notes in Electrical Engineering, vol 398. Springer, Singapore. https://doi.org/10.1007/978-981-10-1721-6_67
Download citation
DOI: https://doi.org/10.1007/978-981-10-1721-6_67
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-1719-3
Online ISBN: 978-981-10-1721-6
eBook Packages: EngineeringEngineering (R0)