Abstract
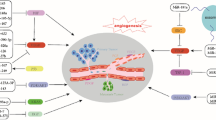
Solid tumors require angiogenesis to grow beyond 2 mm in size. In most cases, tumor cells undergo angiogenic switch and secrete substances that are required for generation of new capillary sprouting from existing blood vessels. Tumor angiogenesis is driven by a complex interplay between pro-angiogenic (VEGF/VEGFR, PDGF/PDGFR) and anti-angiogenic factors (TSP-1/TSP-2) within the tumor microenvironment. In addition, control of tissue remodeling and degradation by matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs) contribute to tumor angiogenesis. Furthermore, tumor suppressors or oncogenes that control cellular motility and maintain or promote hypoxia (HIFs and MYC) are also actively playing roles in tumor angiogenesis. Noncoding RNAs (ncRNAs), including microRNAs, are a novel class of regulatory molecules that control the gene expression in a posttranscriptional manner. MicroRNAs regulate important physiological processes, such as proliferation, apoptosis, and differentiation, as well as pathological conditions including oncogenesis. Accumulating evidence suggests that microRNAs directly modulate the process of angiogenesis by targeting important angiogenic factors and signaling molecules. Understanding the molecular mechanism behind the regulation of angiogenesis by microRNAs is important due to their therapeutic potential which may lead to improving outcome for cancer patients. Besides, ncRNAs with a regulatory role in angiogenesis, such as long noncoding RNAs (lncRNAs), have been identified in the genome. However, the mechanisms of the vast majority of lncRNAs are currently unknown. For the few lncRNAs characterized at the functional level, accumulating evidence shows that they play important roles in malignant diseases. The function and mechanism in angiogenesis will be described in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Angiogenesis occurs through a highly organized series of events by which new blood vessels form through the growth of existing blood vessels. During angiogenesis, quiescent endothelial cells, which cover the luminal side of all blood vessels, are activated in response to environmental triggers and start to proliferate, migrate, and organize themselves in tubular structures. The term angiogenesis can be distinguished from vasculogenesis, which refers to de novo production of endothelial cells from endothelial precursor cells (angioblast) during embryonic development. Vasculogenesis is typically followed by classical angiogenesis during prenatal development which leads to the growth and remodeling of the primitive vascular network into a complex network. In the adult body, the vascular endothelium acquires essentially a quiescent, non-angiogenic state and serves mainly as a nonthrombogenic surface to conduct nutritive blood flow to organs. These cells, however, retain considerable growth potential and are responsive to pro- and anti-angiogenic factors, which is essential for vascular remodeling during physiological conditions, like wound healing, inflammation, and pregnancy [1]. The same angiogenic pathways have also been adapted during pathological conditions such as disease as diverse as cancer, macular degeneration, psoriasis, diabetic retinopathy, and inflammatory disorders like arthritis and atherosclerosis [2]. Therefore, the control of reversing dormant endothelial cells and restoring their proliferative state must be regulated by a complex milieu of stimulatory and inhibitory signals and requires a number of molecular and cellular events to be temporarily and spatially orchestrated [3].
8.2 Tumor Angiogenesis
During tumorigenesis, malignant cells acquire multiple characteristics that provide a growth advantage over normal cells. Tumor angiogenesis is one of the hallmarks of cancer that drive tumor growth beyond a diffusion limit size and enhance metastasis [4]. In response to hypoxia, tumor cells tilt the balance toward stimulatory angiogenic factors, like vascular endothelial growth factor (VEGF) and angiopoietin, to facilitate neovascularization [5–7]. This process is called “angiogenic switch” and leads to activation of endothelial cells in nearby vessels. Subsequently, degradation of the extracellular matrix in activated endothelial cells by different proteolytic enzymes results in migration of endothelial cells toward chemotactic clues that come from the tumor tissue and the formation of vessel-like structures [8]. These newly formed vessels are premature and fragile. Subsequent inhibition of endothelial cell growth and the recruitment of pericytes and smooth muscle cells to form capillary tubes lead to maturation of vessels. Even though these vessels are disorganized and irregular in structure, they can still provide the growing tumor mass with required nutrients and metabolites [9].
Despite the wealth of data on pathological angiogenesis, it is still not clear what molecular mechanisms govern angiogenic switch in tumor angiogenesis. Recently, new opportunities for better understanding of tumor biology have come with the discovery of noncoding RNAs as a novel class of gene regulatory molecules [10]. These noncoding RNAs exert their gene regulatory function at many different levels, including posttranscriptional and posttranslational level. Here, we will review the functions and mechanisms of noncoding RNAs, mainly microRNAs and long noncoding RNAs, in angiogenesis and vasculature remodeling in cancer, as well as their significance in cancer development.
8.3 Importance of MicroRNAs in Regulation of Tumor Angiogenesis
MicroRNAs (miRNAs) are a class of small noncoding RNAs (~22 nucleotides) which play an important role in all biological pathways in multicellular organisms including mammals [11, 12]. MiRNAs regulate gene expression by binding to a target messenger RNA (mRNA), leading to either degradation or translational repression [13]. MiRNAs are generated by the act of two RNAse III endonucleases, Dicer and Drosha, in a two-step processing pathway [14]. The first evidence showing the role of microRNAs in the regulation of vascular development and angiogenesis came from studies on Dicer-deficient homozygous mice. Knockout mice die between 12.5 and 14.5 days of gestation due to lack of angiogenesis [15]. In addition, hypomorphic Dicer1 allele (Dicer d/d) mouse models are found to be infertile due to corpus luteum insufficiency resulted from impaired vascular formation in the ovary [16]. Similarly, Dicer mutants of zebra fish show disrupted blood circulations [17]. These data have been confirmed in vitro using short interfering RNA (siRNA) against Dicer in endothelial cells. Genetic silencing of Dicer in endothelial cells leads to downregulation of several key regulators of angiogenic phenotype, including reduced endothelial cell migration, capillary sprouting, and tube formation in vitro and in vivo. Experiments with cultured endothelial cells have revealed the important role of Dicer in several angiogenic pathways, including EC migration, proliferation, and capillary tube formation [18–20].
In contrast to Dicer, knockdown of Drosha does not lead to any major problem in angiogenesis in vivo. Even though knockdown of Drosha in cultured endothelial cells with siRNA results in significant reduction in tube formation and capillary sprouting, no significant blockade of angiogenesis has been observed in vivo [19]. This difference might be due to the presence of an alternative Drosha-independent miRNA processing pathway that compensates for the lack of Drosha [21–23] or the involvement of Dicer in other cellular pathways including the regulation of heterochromatin formation [24, 25].
8.3.1 Important miRNAs in Angiogenesis
Expression of miRNAs is strictly regulated in a tissue- and organ- specific manner. Three studies have been performed in an attempt to identify miRNAs involved in control of endothelial cell functions. Eight miRNAs, including let-7b, miR-16, miR-21, miR-23a, miR-29, miR-100, and miR-221, and miR-222, are shown to be highly expressed in human umbilical cord endothelial cells by all three sets of data. Meanwhile, only two out of the three studies find that let-7a, let-7d, miR-20, miR-99a, miR-126, miR-181a, and miR-320 are highly expressed in endothelial cells [18, 19, 26]. Only a few highly expressed miRNAs in endothelial cells have been functionally characterized in angiogenesis (see Table 8.1), which we will discuss in this section.
8.3.1.1 MicroRNA-17 Gene Family
The miR-17 ~ 92 cluster, also named oncomiR-1, is the first identified tumor-promoting miRNA [27]. This cluster consists of seven miRNAs, including miR-17, miR-5p/miR-3p, miR-18a, miR-19a, miR-20a, miR-19b-1, and miR-92a-1 that originate from the intronic region of c13orf25 on chromosome 13. Two paralogs of miR-17 ~ 92, miR-106a ~ 363 and miR-106b ~ 25, also exist in mammals. It has been shown that a higher degree of tumor vascularization is observed in vivo after overexpression of miR-17 ~ 92 cluster in ras-positive tumor cells, which provides the first clue to the importance of the member of this family in tumor angiogenesis [28], while inhibition of miR-17 ~ 92 in vitro represses EC sprouting and tube formation in Matrigel [29]. The angiogenic role of miR-17 confers this miRNA to play an important role in tumorigenesis [30–34]. One mechanism by which this cluster controls neovascularization is through modulating the production of angiogenic factors. For example, miR-18 and miR-19 preferentially suppress the expression of connective tissue growth factor (CTGF) and thrombospondin-1 (TSP-1), respectively, both of which inhibit angiogenesis. Meanwhile, knockdown of miR-17 ~ 92 cluster can partially restore the expression of TSP-1 and CTGF. Another member of this family, miR-17, targets tissue inhibitor of metalloproteinase 3 (TIMP-3) to modulate migration and proliferation of endothelial cells [35]. In fact, lack of angiogenesis in the corpus luteum in the hypomorphic Dicer allele (Dicer d/d) mice is attributed to the lack of miR-17-5p and let-7b [16].
Another microRNA in the microRNA-17 gene family with pro-angiogenic activity is miR-93 which belongs to the miR-106b ~ 25 cluster. The miRNA-106b ~ 25 cluster is composed of the highly conserved miRNA-106b, miRNA-93, and miRNA-25. Different studies have shown different molecular pathways through which miR-93 modulates angiogenesis. It has been demonstrated that overexpression of miR-93 in U87 glioblastoma cell line increases proliferation, migration, and tube formation of cocultured endothelial cells in vitro and enhances angiogenesis in vivo by modulating integrin signaling pathway through downregulation of integrin beta 8 (ITGB-8) [36]. Similarly, miR-93 increases tumor angiogenesis and metastasis in MT-1 breast carcinoma cell line by targeting large tumor suppressor, homology 2 (LATS2), which is involved in Hippo tumor suppressor pathway [37]. Hazarika et al. have reported enhanced proliferation and tube formation in ECs following miR-93 overexpression, which is caused by the downregulation of P21 and E2F1, the regulators of cell cycle pathway [38]. In addition, overexpression of miR-93 in non-small cell lung cancer H1299 cell line favors tube formation in endothelial cells in coculture studies. It is suggested that miR-93 can modulate angiogenesis in lung cancer cells by targeting beta-transducin repeat containing protein 2 (β-TRCP2) in ubiquitin proteasome pathway [39].
MiR-20b, a member of the miR-106a ~ 363 cluster, appears to have anti-angiogenic activity by modulating signals within tumor microenvironment. Mir-20b targets signal transducer and activator of transcription 3 (STAT3) and hypoxia-inducible factor-1 alpha (HIF-1α), leading to reduction in vascular endothelial growth factor A (VEGF-A) expression [40].
8.3.1.2 MiR-378
Mir-378 is an oncogene that enhances tumor growth, survival, and angiogenesis through targeting tumor suppressor genes, suppressor of fused (Sufu), and tumor suppressor candidate 2 (TUSC2), Sox2, fibronectin, and Nodal [41–45]. Sufu is a negative regulator of Sonic hedgehog (Shh) signaling, whose level of expression is inversely related to miR-378 expression in many cell lines tested. It has been known that Shh induces large blood vessel formation by promoting the expression of angiogenic cytokines including VEGF and angiopoietin-1 (Ang-1) and angiopoietin-2 (Ang-2) [46]. Thus, miR-378 enhances tumor angiogenesis by targeting Sufu and TUSC2 which are repressors of angiogenic cytokine production. Injection of miR-378 overexpressing cancer cells to nude mice results in much larger tumors with more blood vessels compared to control cells [41]. This data is consistent with a report that miR-378 enhances VEGF expression by competing with miR-125a for the same seed region in the 3’-UTR of the VEGF gene [41, 47]. Conversely, when cells are transfected with a construct expressing an antisense sequence against miR-378, the function of miR-378 in cell survival and angiogenesis will be reversed [41].
8.3.1.3 MiR-98
MiR-98 belongs to the let-7 family and has been shown to have anti-angiogenic function. Overexpression of miR-98 in highly invasive breast carcinoma cell lines inhibits tumor angiogenesis and invasion in vitro and in vivo by targeting active receptor-like kinase 4 (ALK4) and matrix metalloproteinase-11 (MMP11). Repressed ALK4 and MMP11 expression affect endothelial cell activity and prevent them from proliferation, spreading, and tube formation [48]. These results are consistent with a study that shows ectopic expression of miR-98 inhibits B16-F1 cell migration as well as in vivo metastasis and tumor angiogenesis by reducing interleukin-6 (IL-6) level [49].
8.3.1.4 MiR-126
MiR-126 is one of the best studied microRNAs in angiogenesis which is highly and exclusively expressed in endothelial cells (ECs) [50]. In both mouse and zebra fish, miR-126 is enriched in organs with high density of vascular component, like the heart and lung. It is encoded by intron 7 of the epidermal growth factor-like domain 7 (Egfl7), which encodes an EC-specific secreted peptide as an inhibitor and chemoattractant of smooth muscle cells [51]. MiR-126 regulates many aspects of EC biology, including, migration, sprouting, cytoskeleton organization, and capillary network stability. Even though reduction of miR-126 in zebra fish does not affect vascular patterning, it compromises the integrity of blood vessels as is shown by increased hemorrhage and vessel collapse [52]. Similarly, disruption of miR-126 in mice causes leaky vessels and hemorrhage leading to 50 % embryonic lethality [53]. Of the mutant embryos that survive birth, impaired angiogenesis is displayed during both physiological and pathologic process, indicating the unique role of miR-126 in neoangiogenesis of adult tissues in response to injury [53]. In line with these data, mice treated with high dose of antagomir against miR-126 have shown significant impaired angiogenic responses [54]. Different groups have proposed different mechanisms underlying the angiogenic activity of miR-126. For instance, Fish et al. have identified and validated three targets for miR-126 with respect to endothelial biology, including Sprouty-related EVH domain-containing protein (SPRED1), PI3 kinase regulatory subunit 2 (PIK3R2), and vascular cell adhesion molecule 1 (VCAM1). The first two mRNAs are negative regulators of VEGF signaling, and the latter gene helps recruit leukocyte to the vessel walls. VCAM1 and SPRED1 have been validated by Wang’s research team through their microarray analysis [50], while PIK3R2 has been described as a target for miR-126 by another team [53, 55]. However, the role of miR-126 in tumor angiogenesis is somewhat controversial. It has been observed that downregulation of miR-126 inversely correlates with an increased microvessel density (MVD) and vascular endothelial growth factor A (VEGF-A) expression in gastric cancer tissues [56]. Similarly, miR-126 has been reported to be downregulated in oral cancer which induces angiogenesis and lymphangiogenesis by restoration of VEGF-A level [55]. Nevertheless, others have found that miR-126 significantly enhances lung tumor angiogenesis, including increased EC proliferation and migration, by targeting VEGF-A (restoration of miR-126 downregulates VEGF and inhibits the growth of lung cancer cell lines).
8.3.1.5 MiR-221 and MiR-222
MiR-221 and miR-222 are located in close proximity on chromosome X11.3 which has been detected in endothelial cell by many miRNA profiling studies [18]. However, their expressions are not restricted to the endothelium. These two microRNAs belong to the same family and have common targets. Prediction algorithm suggests that miR-221 and miR-222 target c-kit mRNA in ECs. C-kit is a tyrosine kinase receptor for stem cell factor (SCF), which is a growth factor shown to be involved in angiogenesis by promoting survival, proliferation, migration, and tube formation in human umbilical vein cells (HUVECs). Interestingly, overexpression of miR-221 and miR-222 in HUVECs decreases cell proliferation, migration, and wound healing in response to SCF. In addition, high glucose treatment of HUVECs reduces c-kit expression by inducing the expression of miR-221, which impairs cell migratory response to SCF. These finding suggests that miR-221 can be an important regulator of diabetes-associated vascular dysfunction. It has also been shown that miR-221 and miR-222 overexpression in Dicer knockdown ECs restores the elevated level of endothelial nitric oxide synthase (eNOS), which is essential for endothelial cell function and vascular integrity. Since the 3’-UTR of eNOS has no target site for miR-221 and miR-222, it is proposed that the regulation of eNOS protein level by these miRNAs is likely to be indirect. All in all, miR-221 and miR-222 appear to function as anti-angiogenic factors in endothelial cells [57, 58].
Even though the overexpression of miR-221 and miR-222 has been shown to inhibit proliferation of endothelial cells, it promotes proliferation in cancer cells by targeting p27, a member of the cyclin-dependent kinase inhibitor, indicating cell type-specific function of these miRNAs.
8.3.1.6 MiR-15b and MiR-16
MiR-15b and miR-16 are located in the same cluster on chromosome 3. Although the role of these two miRNAs has not been investigated in endothelial cells, they might be involved in angiogenesis. It has been shown that hypoxia represses the expression of miR-15b and miR-16 in CNE cells from human nasopharyngeal carcinoma cell line [47]. Moreover, transfection of cells with miR-15b and miR-16 results in reduced VEGF protein expression. Therefore, hypoxia-induced downregulation of miR-15b and miR-16 contributes to VEGF expression which is a fundamental regulator of normal and abnormal angiogenesis. Meanwhile, miR-15b and miR-16 can target antiapoptotic protein, Bcl-2, to induce apoptosis in leukemic cells. Thus, overexpression of miR-15b and miR-16 can be a fascinating therapeutic approach to target tumor cell death and block VEGF-mediated angiogenesis [59].
8.3.1.7 MiR-130a
MiR-130a is one of the microRNAs frequently detected in ECs but has limited available data on its function. Upon exposure of ECs to serum, the level of miR-130 is found to rapidly increase [60]. MiR-130 has been shown to target two anti-angiogenic proteins, growth arrest-specific homeobox (GAX) and homeobox A5 (HOXA5). GAX is an important regulator of EC phenotype in response to pro- or anti-angiogenic factors and is expressed both in ECs and smooth muscle cells. Therefore, miR-130a is a pro-angiogenic microRNA, whose overexpression can antagonize the inhibitory effect of GAX on EC proliferation, migration, and tube formation and the inhibitory effect of HOXA5 on tube formation [60].
8.3.1.8 MiR-210
Hypoxia occurs during pathological condition, where cancer triggers an adaptive response to low oxygen by upregulation of genes that are essential for new blood vessel formation. It has been shown that the expression of miR-210 is induced under low-oxygen environment which drives the angiogenic response in endothelial cells. It is believed that miR-210 stimulates migration, proliferation, and the formation of capillary-like structure in ECs, whereas downregulation of miR-210 blocks cell migration and tube formation in response to VEGF [61]. In fact, upregulation of miR-210 is an essential element in response to hypoxia in ECs, affecting migration, survival, and differentiation. MiR-210 regulates angiogenesis mainly by targeting hypoxia-induced factor-1 alpha (HIF-1α) and ephrin-A3 (Eph-A3). Ephrin molecules have been known for their essential roles in vasculature and lymphatic vessel remodeling as well as EC, pericyte, and smooth muscle cell function [62]. It has been shown that HIF-1α induces the expression of miR-210 which leads to downregulation of Eph-A3 [63]. Repression of Eph-A3 is necessary and sufficient to induce tubelike structure and chemotactic migration of ECs in response to VEGF [64], while expression of Eph-A3 allele that is not targeted by miR-210 blocks the pro-angiogenic effect of miR-210 in ECs.
In addition to Eph-A3, miR-210 can target protein-tyrosine phosphatase 1B (PTP1B) [65, 66] which is a negative regulator of VEGF signaling. PTP1B can dephosphorylate VEGF receptor 2 (VEGFR2) in endothelial cells. Downregulation of PTP1B by miR-210 allows for activation of VEGF signaling under hypoxic condition [67].
8.3.1.9 Let-7 Family
Let-7 and its family members are highly conserved microRNAs across species which have been found highly expressed in HUVECs [18, 19]. The role of the let-7 family in angiogenesis was first revealed by the observation that let-7a, let-7b, let-7c, let-7f, and let-7g were reduced by more than 30 % after Drosha and Dicer knockdown [16, 18, 19]. This inhibition of let-7 family members leads to significant sprout formation in vitro [68]. Many angiogenesis-related genes are predicted to be the targets of let-7 family members, including thrombospondin-1 [16, 18], thrombospondin-2 [69], TIMP-1 [16], Nrp-2 and c-Met [26], TEK/Tie-2, KDR/VEGFR2, and Tie-1 [18]. One study has showed that let-7 can be involved in hypoxia-inducible factor-1α (HIF-1α)/let-7/argonaute 1(AGO1)/VEGF signal pathway in hypoxia-induced angiogenesis. HIF-1α, as a transcription factor, upregulates let-7 expression which in turn decreases the expression of AGO1. This will lead to a desupression of VEGF translation and an increase in angiogenesis [70].
8.3.1.10 MiR-296
MiR-296, also called angiomiR, is one of the important regulators of the angiogenic process [71, 72]. The knockdown and the overexpression of miR-296 inhibit and promote morphologic characteristics associated with angiogenesis of human ECs, respectively. Possible role for miR-296 in tumor angiogenesis is supported by the experiments showing reduced angiogenesis in tumor xenograft after inhibition of miR-296 using antagomirs. MiR-296 functions as a pro-angiogenic factor by inducing the expression of VEGF receptor (VEGFR2) and platelet-derived growth factor receptor (PDGFR) in angiogenic blood vessels. It also targets the hepatocyte growth factor-regulated tyrosine kinase substrate (HGS) which is involved in the degradative sorting of PDGFR, EGFR, and VEGFR. An expression analysis also shows that when HUVECs are cocultured with U87 glioma cells, the expression of miR-296 is upregulated. Moreover, miR-296 upregulation has been detected in ECs isolated from human brain tumors compared to ECs isolated from normal brain. Consistently, the expression of HGS is downregulated, while VEGFR2 and PDGFR are upregulated in these glioma blood vessel samples [73]. Altogether, these findings support a pro-angiogenic role for miR-296 in tumors.
8.3.2 Other miRNAs Related to Angiogenesis
It has been shown that miR-9 can regulate tumor angiogenesis by targeting VEGF-A. In breast cancer cells, MYC and MYCN transcription factors induce the expression of miR-9, which targets E-cadherin and hence increases cell motility and invasiveness. Downregulation of E-cadherin will activate β-catenin signaling, which in turn upregulates VEGF-A expression and increases in tumor angiogenesis [74]. The miR-143-145 cluster is highly expressed in smooth muscle cells (SMCs). Not only can this cluster regulate the vascular homeostasis but also play a role in neighboring endothelial cells. It has been shown that miR-143/miR-145 improves the angiogenic and vessel stabilization properties of ECs by regulating angiotensin-converting enzyme (ACE) and tropomyosin 4 (Tpm4) [75]. Another microRNA, miR-132, is highly expressed in human tumors and hemangiomas, which promote angiogenesis in endothelial cells by suppressing p120RasGAP, a molecular brake for RAS [71, 76]. MiR-29 is another functionally characterized microRNA with the role in regulating cell cycle and angiogenic phenotype of endothelial cells. This microRNA is upregulated in response to hypoxic stimuli in HUVECs. It has been shown that miR-29 promotes the proliferation and tube formation of HUVECs by targeting HBP1, a suppressor transcription factor [77]. Another study shows that miR-29 is regulated by TGF-β/Smad4 signaling in human and mice endothelial cells. Overexpression of miR-29 by TGF-β leads to downregulation of the phosphatase and tensin homolog (PTEN) in endothelial cells, which is a target of miR-29, and activates the AKT pathway, which will eventually lead to enhancement of angiogenesis [78].
8.3.3 Tumor-Specific MicroRNAs
Another area which has not been thoroughly looked into is tumor-specific expression of microRNAs [79]. This particular area can uncover the mechanisms and functions of different microRNAs, especially those that behave differently in different cell types. Below we will discuss some microRNAs that have been shown to have specific and differential expressions in certain cancer cell types.
8.3.3.1 In Colorectal Cancer Cells
Analysis of microRNAs in colorectal cancer has showed a significant decrease of three specific microRNAs, which include miR-145 [80, 81], miR-22 [82], and miR-126 [80, 82, 83]. All of these miRNAs share the same target protein p70S6K1 kinase, which activates HIF-1α and VEGF downstream, both strong promoters of angiogenesis [80]. The decrease in these microRNAs allows for an increase in the target mRNAs, which are sp70S6K1, HIF-1α, and VEGF, and increases angiogenesis in colorectal cancer cells.
8.3.3.2 In Glioblastoma Cells
The levels of miR-218 are significantly decreased in necrotic mesenchymal glioblastoma cells [80, 84]. MicroRNA-218 targets several mRNAs that are involved in the receptor tyrosine kinase (RTK) pathway. Thus, a decrease in miR-218 causes an increase in target RTK pathway-associated mRNA, which then leads to an increase in downstream targets, mainly HIF-2α. HIF-2α is responsible for promoting cell survival and tumor angiogenesis [80, 84].
8.3.3.3 In Human Gastric Cells
In human gastric cells, there is a significant upregulation of miR-382 and miR-18a [80, 85, 86]. Both of these microRNAs act to inhibit tumor angiogenesis, albeit through different mechanisms. MiR-382 has sequences matching to the 3’-UTR of PTEN mRNA, and this similarity leads to the inhibition of tumor angiogenesis [85]. On the other side, miR-18a binds to and inactivates targets in the mTOR signaling pathway [86]. Both of these miRNAs work to inhibit angiogenesis and stunt tube formation.
8.3.3.4 In Prostatic Cancer Cells
In prostatic cancer cells, it has been reported that there is a significant upregulation of miR-21, which targets PTEN mRNA. The increase in miR-21 leads to an increase in the AKT and ERK1/ERK2 signaling pathways, which increases the levels of HIF-1α and VEGF expression downstream. This cascade leads to tumor progression and angiogenesis [87].
8.3.4 Conclusion
More than 700 miRNAs have been identified in the human genome so far. However, the functions of a few specific miRNAs have been validated in regulating the functions of endothelial cells and angiogenesis. Moreover, miRNAs that have so far been studied are those highly expressed in endothelial cells. However, miRNAs that are expressed in smaller amounts under physiological conditions might have equally important functions in maintenance of the physiological state of endothelial cells. A single miRNA can target multiple mRNAs, whereas a single gene may be regulated by multiple miRNAs. Understanding the complex interaction between miRNAs and their targets will be an important area of investigation for the future, since it will lead us toward the development of miRNA drugs designed against specific molecular targets for clinical application.
8.4 Role of Long Noncoding RNAs in Regulation of Tumor Angiogenesis
Long noncoding RNAs (lncRNAs) are a class of noncoding RNAs that are over 200 nucleotides in size [88]. They are generally found in both the nucleus and the cytoplasm and have an array of effects on the cells. Studies have shown that lncRNA are involved in a plethora of cellular events, which include chromatin remodeling, protein scaffolding, translational control, splicing regulation, and microRNA sponges. However, recent studies have indicated that lncRNAs also have a noticeable impact on tumor progression through angiogenesis – a hallmark of cancer. LncRNAs can be found as natural antisense transcripts (NATs) that regulate their sense proteins, or they can be found between protein-coding genes [89, 90]. Evidence has shown that the downregulation of different lncRNAs leads to an abnormal gene expression that will promote tumor progression in various types of cancers [91]. However, the most convincing data shows that lncRNA interacts with critical angiogenesis regulators such as the VEGF pathway [92–97]. They also interact with other angiogenesis regulators such as phosphoglycerate kinase 1 (PGK1) [93]. Below, we will look further into some lncRNAs that have been associated with tumor angiogenesis.
8.4.1 Long Noncoding RNA MALAT1
Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is an lncRNA that has been associated with tumor angiogenesis [88]. The function of this lncRNA is to sustain endothelial cell proliferation. It is interesting to know that it is one of the few lncRNAs that are relatively well conserved between mice and humans [90]. MALAT1 is found in high amounts in the nucleus of the cell whose expression has been shown to increase under hypoxic conditions (in vitro). It has also been shown that MALAT1 is upregulated in many human tumors and promotes tumor cell invasion and metastasis [89, 91–95].
Recent studies have examined the effect of knocking down MALAT1 in cancer cell lines. It is found that decreased MALAT1 leads to a decrease in cell number with increased apoptosis, while MALAT1-deficient cells show increased sprouting and migration of endothelial cells. However, these sprouts do not have complete extensions, which show an ability defect of the cells to form new vascular networks [88, 89]. Additionally, when these cells have been treated with VEGF, no improvements in the outgrowth of the sprouts are observed [89]. Such indicates that MALAT1-deficient cells have a decreased ability to react to VEGF and undergo angiogenesis. Furthermore, MALAT1 knockout models have been tested in the developing mouse retina, where MALAT1-deficient cells show decreased vascular proliferation that leads to a reduced vascular network compared to wild-type mice retinas. Further in vivo experiments have shown decreased neovascularization and blood flow recovery in MALAT1-deficient mice [89]. In general, the loss of MALAT1 decreases the proliferative potential of a cell and increases its migratory behavior, which in turn decreases the cell’s ability to undergo angiogenesis. The mechanism of how MALAT1 works is through the deregulation of cell cycle-related factors. This lncRNA lowers the expression of endothelial cyclins CCNA2 and CCNB1/CCNB2, which are important factors in S-phase of the cell cycle, while the inhibitory factors of S-phase including the kinase p21 and kinase inhibitor p27Kip1 are upregulated [89].
Studies with pancreatic cancer cells have also found another mechanism for the function of MALAT1, which is inducing angiogenesis. Increased levels of MALAT1 promote cells to undergo epithelial to mesenchymal transitions (EMT), which may cause cancer cells to obtain stem cell-like properties. Since MALAT1 is overexpressed in many tumors, it has been shown to increase the number of cancer stem cells (CSCs), which very closely interact with angiogenesis [95]. Other findings show that upregulated MALAT1 causes an increased endothelial tube formation, an increased cell migration, and an increased amount of VEGF. These all lead to an enhanced amount of vascularization occurring. Another increased factor is the amount of CD31, which is another important indicator of tumor angiogenesis [49]. It has been hypothesized that the CSCs express many pro-angiogenic factors such as VEGF, which lead to the increased amount of angiogenesis in tumor cells. An increase in angiogenesis allows for further growth of CSCs, thus forming its positive feedback loop. There is also evidence that sex-determining region Y-box 2 (SOX2) may play a role in this mechanism because in MALAT1 knockdown studies, there is a large decrease in SOX2, which is important for cells to maintain their stemness. So by extension, if cells lose their stemness due to the loss of SOX2, we will also see a decrease in the amount of angiogenesis because of the decrease in cancer stem cells releasing pro-angiogenic factors [95].
Overall, decreasing MALAT1 in endothelial cells disrupts mechanisms linked to endothelial cell cycle progression and increased migratory behavior, which in turn negatively impacts the ability for these cells to form new vasculature. Moreover, there is a decrease in the number of CSCs. However, more studies are needed to clarify the function of MALAT1. Inhibition of MALAT1 is proposed as a treatment to prevent tumor growth and metastasis due to its pro-angiogenic properties.
8.4.2 Long Noncoding RNA MVIH
Hepatocellular carcinoma (HCC) is the fifth most common solid cancer in the world and the most common form of liver cancer. Unfortunately, it only has a 50 % survival rate after 5 years in for groups aging from 17 to 69 [91–93]. It is clearly a very deadly cancer mainly for its rapid growth caused in part by very active angiogenesis, which unavoidably leads to metastasis.
The cause of the increased angiogenesis has been linked to the long noncoding RNA associated with microvascular invasion in HCC (lncRNA MVIH). This lncRNA is located within the intron of the RPS24 gene, which is a ribosomal protein [90, 91, 94]. Using tissue samples from patients with HCC, it has been determined that lncRNA MVIH is overexpressed in HCC tumor cells, compared to non-tumor cells. Using RNA pull-down methods, it has been found that lncRNA MVIH is associated with phosphoglycerate kinase 1 (PGK1) [91]. PGK1 is an enzyme encoded by the PGK1 gene, which can be secreted by tumor cells. However, PGK1 acts to suppress angiogenesis. Therefore, it is imperative for a tumor cell to inhibit this anti-angiogenic factor in order to grow. LncRNA MVIH overexpressed by tumor cells will bind to PGK1, affectively reducing its function. Without PGK1’s presence to prevent angiogenesis, tumor cells in HCC will gain increased microvessel density, leading to rapid growth of tumors [91, 93, 98]. This downward spiral continues because the increased angiogenesis leads to increased microvascular invasion or metastasis – in particular intrahepatic metastasis [91]. This makes HCC very deadly.
In all, the lncRNA MVIH plays a crucial role in the tumorigenesis of hepatocellular carcinomas. These tumor cells upregulate lncRNA MVIH in order to increase angiogenesis and to eventually achieve metastasis. With this knowledge, the focus can be shifted to create a novel medicine that down regulates lncRNA MVIH, which may be able to reduce angiogenesis, thus decreasing cancer growth and metastasis.
8.4.3 The Long Noncoding RNA HOXD-AS1
LncRNA HOXD-AS1 is encoded in the HOXD gene cluster and can be found equally in the nucleus and cytoplasm. It has recently been shown that lncRNA HOXD-AS1 is a marker of neuroblastoma (NB) progression. Many lncRNAs have been studied to determine their differential expression in aggressive NB vs. noncancerous tissues, when treated with retinoic acid (RA). Of the many noncoding RNAs tested, lncRNA HOXD-AS1 is the only one upregulated substantially. RA is the first-line drug used to battle NB and works as a differentiating agent that typically arrests the growth of NB cells, making them more vulnerable to chemotherapeutic drugs [92, 93, 98]. This lncRNA is located between the HOXD1 and the HOXD3 genes, but it is antisense to both of these (hence being called the AS1). It is also highly conserved within hominids, but not so much with other primates [92].
Studies have indicated that the expression level of lncRNA HOXD-AS1 increases with progressing stage/aggressiveness of neuroblastoma, thus possibly playing a factor in its increased tumorigenesis [92]. The function of HOXD-AS1 has been assessed through knockdown experiments via siRNA. Many genes have been observed and found differential expressions involved with inflammation and angiogenesis. To be specific, the increased expressions of many cytokines, such as, CX311, CCL20, TNF, and GD15, have been found to be important for extracellular matrix communication. There is also a change in the expression of matrix remodeling genes LOX and ADAMTS3 and key regulators of angiogenesis and lymphangiogenesis ANG and PROX1 [92]. This shows that lncRNA HOXD-AS1 affects in some way angiogenesis in tumor cells, adding to the aggressiveness of the cancer. There is a significant increase in the JAK/STAT pathway, which is related to inflammation and angiogenesis. However, the PI3K/AKT pathway is found to be the main regulator of expression of HOXD-AS1. Furthermore, the expression of HOXD-AS1 is correlated to the expression of HOXD1 and HOXD2 genes, which implies common regulatory mechanisms. This is also another indicator of malignancy because the aberrant expression of HOX genes in tumor cells has been linked to malignancy [92].
Overall, the lncRNA HOXD-AS1 has many implications in the tumorigenicity of neuroblastoma. Although the mechanisms are not completely clear, it is evident that this lncRNA affects many regulators of angiogenesis. Thus, the increased amount of lncRNA HOXD-AS1 in increasing aggressiveness of tumors can be caused due to the increased expression of angiogenic factors, leading increased growth and metastasis of cancers. More studies need to be done in order to uncover the exact mechanisms of action of this lncRNA, which may enlighten its use as a target of a therapeutic drug. For now, lncRNA HOXD-AS1 remains a reliable biomarker of neuroblastoma.
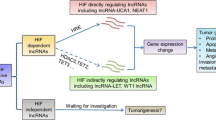
8.4.4 The Long Noncoding RNA HIF-1A-AS2
Hypoxia-inducible factor-1 alpha subunit antisense RNA 2 (lncRNA HIF-1A-AS1) is an lncRNA that is involved in tumor angiogenesis. LncRNA HIF-1A-AS2 is upregulated in non-papillary clear cell renal carcinomas and is a marker of poor prognosis in breast cancer [4, 94–97].
This lncRNA has been shown to be involved in angiogenic regulatory pathways, which may be the reason for its impact on tumor progression. LncRNA HIF-1A-AS2 has been shown to negatively regulate hypoxia-inducible factor-1 alpha (HIF-1α), which is a critical regulator of angiogenesis [4]. HIF-1α is increased in response to hypoxia and activates many genes that increase the amount of angiogenesis, which is vital for growing tumors to obtain sufficient nutrients and oxygen, while also excreting waste. However, in some cancers, the level of lncRNA HIF-1A-AS2 also increases and works by binding and causing the degradation of HIF-1α mRNA [4]. The lncRNA acts in a negative feedback manner to decrease the amount of angiogenesis. This is a prime example of a NAT regulating the expression of its sense protein.
In general, lncRNA HIF-1A-AS2 is involved in angiogenic pathways. However, the exact mechanisms and its true purpose have not yet been uncovered. It is overexpressed in some cancers, yet seems to act to counter angiogenesis by downregulating HIF-1α. This could be a possible reaction to the increase in angiogenesis caused by HIF-1α. More studies are needed to look into this particular lncRNA.
8.4.5 The Long Noncoding RNA MEG3
Maternally expressed gene 3 (MEG3) is an lncRNA that is expressed in many cells and tissues. MEG3 expression is lost in many different tumors, whether it is through gene deletion or hypermethylation of the promoter or other regions of the gene [4, 96]. Studies have shown that the re-expression of MEG3 in tumors causes inhibition of tumor cell proliferation through the accumulation of p53 and downstream activation of p53 genes. P53 acts as a transcription factor for many tumor suppressor genes. Therefore, when MEG3 function is lost, cells also lose the function of p53, leading to aggressive cancers. The lncRNA MEG3 may function as a novel tumor suppressor since its downregulation and/or deletion is largely associated with aggressive cancers [4].
Furthermore, the loss of MEG3 coincides with an increase in the expression of pro-angiogenic genes, which may be a main cause in the increased aggressiveness of these tumors. Studies using the mouse ortholog Meg3 have shown that several genes affecting angiogenesis are upregulated when Meg3 is knocked out. It has also been observed that VEGF-A and its receptor VEGFR1, which are bona fide primary regulators of angiogenesis, are significantly increased. Thus, when Meg3 is lost, angiogenesis increases dramatically [4, 96]. Since new blood vessels are vital for tumor growth, the inactivation of MEG3 is one way by which tumors can continue to develop.
In addition to VEGF pathway genes, there is also an increase in genes encoding for adherens junctions, which are critical for endothelial cell-to-cell interactions and interactions with the cell matrix. These allow for stable vessel formation [4, 97]. In addition, there is an increase in hemophilic cell adhesion, GTPase activator activity, and actin cytoskeleton organization and biogenesis, all of which relate to an increased vessel formation and angiogenesis. Moreover, there is an increase in Notch signaling, which also aids in vessel stability [4, 97].
All in all, the lncRNA MEG3 is a tumor suppressor, as its presence greatly decreases tumor cell proliferation and its downregulation is a fundamental step in tumor growth. MEG3 is heavily linked to angiogenesis, and in its absence, angiogenesis occurs undeterred. Consequently, in normal cells, it may be that MEG3 acts to suppress aberrant angiogenesis from occurring. There are many possibilities in using MEG3 in therapeutic settings to suppress tumor growth. More studies are needed to determine how MEG3 is exactly downregulated or deleted and how it can be used to battle tumor growth.
8.4.6 Other Long Noncoding RNAs
Besides the lncRNAs previously mentioned, there are a few other lncRNAs that may show some promise in uncovering more information about lncRNA association with angiogenesis.
One such lncRNA is sONE or NOS3AS, which happens to be another natural antisense transcript (NAT), much like lncRNA HIF-1A-AS2. This lncRNA regulates the expression of endothelial nitric oxide synthase (eNOS), under normal oxygen conditions and hypoxic conditions. Not much else is known about this molecule, and it has not been completely determined whether it acts as RNA or a protein, despite a possible protein product has been discovered [52]. However, there is potential link to angiogenesis due to its effect on endothelial cells involved in blood vessels. More studies are needed to determine whether this molecule acts as RNA or if it in fact codes for a functional protein and then to test its function on angiogenesis. Another NAT lncRNA is Tie-1-AS, which is the antisense transcript for tyrosine kinase containing immunoglobulin and epidermal growth factor homology domain 1 (tie-1). This lncRNA is highly conserved in humans, mice, and zebra fish [52]. It acts by binding to tie-1 mRNA transcripts and decreasing its levels. The decrease in tie-1 causes defects in endothelial cell junctions, which lead to poor vessel formation and angiogenesis [4, 99, 100]. This lncRNA is a very good candidate for further studies involving tumor angiogenesis. One large class of noncoding RNAs is pseudogenes. Pseudogenes can play important roles in angiogenesis and tumorigenesis [106, 107]. In some mRNAs, there are long fragments of 3’-untranslated regions (3’-UTRs), which may interact with miRNAs and function similarly to the long noncoding RNAs [101–105, 108].
8.5 Interactions Between MicroRNAs and Long Noncoding RNAs
Up to this point, we have discussed how different miRNAs and lncRNAs affect tumor angiogenesis. However, the interplay between noncoding RNAs and angiogenesis are more complicated when we realize the large amount of interactions that exist between different noncoding RNAs. These interactions add another layer of complexity, where the activity of a specific miRNA can alter the effect of an lncRNA and vice versa. This allows for certain noncoding RNA to affect tumor angiogenesis indirectly. There are many ways in which miRNA and lncRNA can affect each other. For instance, (1) miRNA can trigger the decay of lncRNA, (2) lncRNA can act as miRNA sponges, (3) miRNA and lncRNA can compete for the same mRNA, and (4) some lncRNA can generate miRNA. Regardless of the mechanism used, it is clear that there are significant interactions between these noncoding RNAs, thus offering a greater potential for control of tumor angiogenesis. Although this area is relatively new, there are some interactions discovered that are relevant to tumor angiogenesis.
8.5.1 MicroRNA Interactions with LncRNA MALAT1
Long noncoding RNA MALAT1 has been previously discussed and determined to be a pro-angiogenic factor. An increase of lncRNA MALAT1 causes a significant increase in the number of CSCs which induce pro-angiogenic effects, whereas MALAT1 deficiency leads to reduced levels of angiogenesis. Studies have shown that MALAT1 may also function as an miRNA sponge for miR-200c and miR-145. By decreasing the effects of the miRNAs (miR-200c and miR-145), their target gene Sox2 is upregulated, leading to an increase in stem cell-like properties. This is another mechanism for MALAT1 to elicit pro-angiogenic effects through miRNA interactions. Furthermore, other studies have shown that the overexpression of miR-9 decreases the levels of MALAT1, through which miRNA triggers lncRNA decay. More specifically, this occurs through miR-9 binding to MALAT1 and targeting it for AGO2-mediated degradation, which has been demonstrated in the Hodgkin lymphoma cell line L428 and glioblastoma cell line U87MG [87, 109]. Moreover, miR-9 also affects angiogenesis through other mechanisms, such as targeting VEGF-A, which has been discussed earlier. This example shows clear cross talk between two noncoding RNAs that have both been implicated in affecting tumor angiogenesis.
8.5.2 MicroRNA Let-7 Interactions with LncRNA
MicroRNAs in the let-7 family have been shown to be important in regulating angiogenesis with their anti-angiogenic effects. Let-7 miRNA targets many angiogenic genes, and their inhibition may promote tumor progression and angiogenesis. In addition, let-7 miRNA also interacts with many different lncRNAs which may affect the overall activity of let-7, thus affecting its function to regulate tumor angiogenesis.
Moreover, lncRNA-p21 inhibits translation, unlike most known lncRNAs. It is activated by the tumor suppressor protein p53. It has been shown to be negatively regulated in human cervical cancer cells by HuR, AGO2, and microRNA let-7b. Let-7b overexpression causes lncRNA-p21 degradation [87, 109, 110], and lncRNA HOTAIR is also impacted by let-7 miRNA in a similar fashion to lncRNA-p21 [87, 109–111].
Furthermore, long noncoding Nras functional RNA (ncNRFR) is an lncRNA that promotes tumorigenesis. Studies have shown that this lncRNA has a 22 nt sequence that perfectly matches the sequence of miRNA let-7a. Moreover, this sequence only differs from other members of the let-7 family by a few nucleotides, which includes let-7b to -7 g, let-7i, and miR-98. Studies have showed that increasing lncRNA ncNRFR leads to a decrease in let-7 miRNA function, which consequently increases let-7 target mRNAs [112]. Overall, this interaction leads to tumor promotion through suppression of let-7 microRNA, which is important in reducing tumor angiogenesis.
8.5.3 MicroRNA Interactions with LncRNA-RoR
The long noncoding RNA regulator of reprogramming (lncRNA-RoR) is found to be in high concentrations in embryonic stem cells. It is interesting to note that many miRNAs that are involved in angiogenesis interact with this particular lncRNA by binding and decreasing its function. MicroRNA-145 is known to interact with lncRNA-RoR, and previous studies have shown that this miRNA is involved in the stabilization of vessels in endothelial cells [113, 114]. Furthermore, miR-99 and miR-181 have also been shown to be important in angiogenesis and are both implicated in interacting with lncRNA-RoR [114].
The above examples show the complexity and many possible interactions that can occur between different noncoding RNAs. The involvement of microRNA and long noncoding RNA in tumor angiogenesis becomes much more complicated when they readily interact with each other. This means that some miRNAs that are very important in tumor angiogenesis may be influenced significantly by lncRNA and vice versa. These interactions need to be delved into for a discovery of new mechanisms and potential novel ways to combat tumor progression.
8.6 Conclusion
So far, researches into lncRNAs and their association to tumor angiogenesis have been promising. LncRNAs such as MALAT1, MVIH, HIF-A-AS2, and MEG3 have all shown large involvement in tumor progression through affecting angiogenesis. There are many other lncRNAs that have been indicated in affecting tumor progression, and more researches are justified to uncover novel lncRNAs and the mechanisms of their action. The study of lncRNA in tumor progression and specifically angiogenesis is still relatively new, which gives a potential for a wealth of new information. One area that can be further explored is the interactions between lncRNAs and microRNAs. LncRNAs generally function as microRNA sponges, and there are many microRNAs found affecting tumor angiogenesis [115–117]. Thus, there may be lncRNAs that affect angiogenesis by extension through microRNAs. Given that lncRNA interactions are complex and cover many biological pathways, it offers great potential for further therapeutic discoveries.
References
Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438(7070):932–6.
Carmeliet P, Peter C. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–60.
Yancopoulos GD, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407(6801):242–8.
Hanahan D, Douglas H, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70.
Folkman J, Judah F. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1(1):27–30.
Zheng PS, Wen J, Ang LC, et al. Versican/PG-M G3 domain promotes tumor growth and angiogenesis. FASEB J. 2004;18(6):754–6.
Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86(3):353–64.
Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6(4):389–95.
Folkman J, Judah F. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29(6Q):15–8.
Dalmay T, Edwards DR. MicroRNAs and the hallmarks of cancer. Oncogene. 2006;25(46):6170–5.
Garzon R, Calin GA, Croce CM. MicroRNAs in cancer. Annu Rev Med. 2009;60(1):167–79.
Lee YS, Anindya D. MicroRNAs in cancer. Annu Rev Pathol. 2009;4(1):199–227.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97.
Graves P, Zeng Y. Biogenesis of mammalian microRNAs: a global view. Genomics Proteomics Bioinformatics. 2012;10(5):239–45.
Yang WJ, Yang DD, Songqing N, et al. Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem. 2005;280(10):9330–5.
Otsuka M, Zheng M, Hayashi M, et al. Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J Clin Invest. 2008;118(5):1944–54.
Giraldez AJ, Cinalli RM, Glasner ME, et al. MicroRNAs regulate brain morphogenesis in zebrafish. Science. 2005;308(5723):833–8.
Suárez Y, Fernández-Hernando C, Pober JS, et al. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100(8):1164–73.
Kuehbacher A, Urbich C, Zeiher AM, et al. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101(1):59–68.
Shilo S, Roy S, Khanna S, et al. Evidence for the involvement of miRNA in redox regulated angiogenic response of human microvascular endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28(3):471–7.
Graham RJ, Jan CH, Bartel DP, et al. Intronic microRNA precursors that bypass Drosha processing. Nature. 2007;448(7149):83–6.
Barik S, Sailen B, Titus B. Intronic microRNA: creation, evolution and regulation. In: Gusev Y, editor. MicroRNA profiling in cancer: a bioinformatics perspective. Singapore: Pan Stanford; 2009. p. 117–31.
Okamura K, Hagen JW, Hong D, et al. The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell. 2007;130(1):89–100.
Pushpavalli SN, Bag I, Pal-Bhadra M, et al. Drosophila Argonaute-1 is critical for transcriptional cosuppression and heterochromatin formation. Chromosome Res. 2012;20(3):333–51.
Fukagawa T, Nogami M, Yoshikawa M, et al. Dicer is essential for formation of the heterochromatin structure in vertebrate cells. Nat Cell Biol. 2004;6(8):784–91.
Poliseno L, Tuccoli A, Mariani L, et al. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108(9):3068–71.
He L, Thomson JM, Hemann MT, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435(7043):828–33.
Dews M, Homayouni A, Yu D, et al. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006;38(9):1060–5.
Suárez Y, Fernandez-Hernando C, Yu J, et al. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc Natl Acad Sci U S A. 2008;105(37):14082–7.
Khorshidi A, Dhaliwal P, Yang BB. Anti-tumor activity of miR-17 in melanoma. Cell Cycle. 2015;14(16):2549–50.
Li H, Gupta S, Du WW, et al. MicroRNA-17 inhibits tumor growth by stimulating T-cell mediated host immune response. Oncoscience. 2014;1(7):531–9.
Fang L, Li H, Wang L, et al. MicroRNA-17-5p promotes chemotherapeutic drug resistance and tumour metastasis of colorectal cancer by repressing PTEN expression. Oncotarget. 2014;5(10):2974–87.
Li H, Yang BB. MicroRNA-regulated stress response in cancer and its clinical implications. Cell Cycle. 2013;12(13):1983–4.
Shan SW, Fang L, Shatseva T, et al. Mature miR-17-5p and passenger miR-17-3p induce hepatocellular carcinoma by targeting PTEN, GalNT7 and vimentin in different signal pathways. J Cell Sci. 2013;126(Pt 6):1517–30.
Yang X, Du WW, Li H, et al. Both mature miR-17-5p and passenger strand miR-17-3p target TIMP3 and induce prostate tumor growth and invasion. Nucleic Acids Res. 2013;41(21):9688–704.
Fang L, Deng Z, Shatseva T, et al. MicroRNA miR-93 promotes tumor growth and angiogenesis by targeting integrin-β8. Oncogene. 2011;30(7):806–21.
Fang L, Du WW, Yang W, et al. MiR-93 enhances angiogenesis and metastasis by targeting LATS2. Cell Cycle. 2012;11(23):4352–65.
Hazarika S, Farber CR, Dokun AO, et al. MicroRNA-93 controls perfusion recovery after hindlimb ischemia by modulating expression of multiple genes in the cell cycle pathway. Circulation. 2013;127(17):1818–28.
Savita U, Karunagaran D. MicroRNA-106b-25 cluster targets β-TRCP2, increases the expression of Snail and enhances cell migration and invasion in H1299 (non small cell lung cancer) cells. Biochem Biophys Res Commun. 2013;434(4):841–7.
Cascio S, D’Andrea A, Ferla R, et al. MiR-20b modulates VEGF expression by targeting HIF-1 alpha and STAT3 in MCF-7 breast cancer cells. J Cell Physiol. 2010;224(1):242–9.
Lee DY, Deng Z, Wang CH, et al. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc Natl Acad Sci U S A. 2007;104(51):20350–5.
Deng Z, Yang X, Fang L, et al. Misprocessing and functional arrest of microRNAs by miR-Pirate: roles of miR-378 and miR-17. Biochem J. 2013;450(2):375–86.
Deng Z, Du WW, Fang L, et al. The intermediate filament vimentin mediates microRNA miR-378 function in cellular self-renewal by regulating the expression of the Sox2 transcription factor. J Biol Chem. 2013;288:319–31.
Liu F, Lv Q, Du WW, et al. Specificity of miR-378a-5p targeting rodent fibronectin. Biochim Biophys Acta. 2013;1833(12):3272–85.
Luo L, Ye G, Nadeem L, et al. MicroRNA-378a-5p promotes trophoblast cell survival, migration and invasion by targeting Nodal. J Cell Sci. 2012;125(Pt 13):3124–32.
Pola R, Ling LE, Silver M, et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7(6):706–11.
Hua Z, Lv Q, Ye W, et al. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS ONE. 2006;1, e116.
Siragam V, Rutnam ZJ, Yang W, et al. MicroRNA miR-98 inhibits tumor angiogenesis and invasion by targeting activin receptor-like kinase-4 and matrix metalloproteinase-11. Oncotarget. 2012;3(11):1370–85.
Li F, Li XJ, Qiao L, et al. MiR-98 suppresses melanoma metastasis through a negative feedback loop with its target gene IL-6. Exp Mol Med. 2014;46:e116.
Wang S, Aurora AB, Johnson BA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15(2):261–71.
Soncin F, Mattot V, Lionneton F, et al. VE-statin, an endothelial repressor of smooth muscle cell migration. EMBO J. 2003;22(21):5700–11.
Fish JE, Santoro MM, Morton SU, et al. MiR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15(2):272–84.
Kuhnert F, Mancuso MR, Hampton J, et al. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development. 2008;135(24):3989–93.
Van Solingen C, Seghers L, Bijkerk R, et al. Antagomir-mediated silencing of endothelial cell specific microRNA-126 impairs ischemia-induced angiogenesis. J Cell Mol Med. 2009;13(8A):1577–85.
Sasahira T, Kurihara M, Bhawal UK, et al. Downregulation of miR-126 induces angiogenesis and lymphangiogenesis by activation of VEGF-A in oral cancer. Br J Cancer. 2012;107(4):700–6.
Chen H, Li L, Wang S, et al. Reduced miR-126 expression facilitates angiogenesis of gastric cancer through its regulation on VEGF-A. Oncotarget. 2014;5(23):11873–85.
Li Y, Song YH, Li F, et al. MicroRNA-221 regulates high glucose-induced endothelial dysfunction. Biochem Biophys Res Commun. 2009;381(1):81–3.
Minami Y, Satoh M, Maesawa C, et al. Effect of atorvastatin on microRNA 221 / 222 expression in endothelial progenitor cells obtained from patients with coronary artery disease. Eur J Clin Investig. 2009;39(5):359–67.
Guo CJ, Pan Q, Li DG, et al. MiR-15b and miR-16 are implicated in activation of the rat hepatic stellate cell: an essential role for apoptosis. J Hepatol. 2009;50(4):766–78.
Chen Y, Gorski DH. Regulation of angiogenesis through a microRNA (miR-130a) that down-regulates antiangiogenic homeobox genes GAX and HOXA5. Blood. 2008;111(3):1217–26.
He J, Wu J, Xu NH. MiR-210 disturbs mitotic progression through regulating a group of mitosis-related genes. Nucleic Acids Res. 2013;41(1):498–508.
Kuijper S, Turner CJ, Adams RH. Regulation of angiogenesis by Eph–ephrin interactions. Trends Cardiovasc Med. 2007;17(5):145–51.
Ivan M, Harris AL, Martelli F, et al. Hypoxia response and microRNAs: no longer two separate worlds. J Cell Mol Med. 2008;12(5A):1426–31.
Fasanaro P, D’alessandra Y, Stefanoet VD, et al. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand ephrin-A3. J Biol Chem. 2008;283(23):15878–83.
Fasanaro P, Greco S, Lorenzi M, et al. An integrated approach for experimental target identification of hypoxia-induced miR-210. J Biol Chem. 2009;284(50):35134–43.
Hu S, Huang M, Li Z, et al. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation. 2010;122(11 Suppl):S124–31.
Nakamura Y, Patrushev N, Inomata N, et al. Role of protein tyrosine phosphatase 1B in vascular endothelial growth factor signaling and cell-cell adhesions in endothelial cells. Circ Res. 2008;102(10):1182–91.
Yang X, Rutnam ZJ, Jiao CW, et al. An anti-let-7 sponge decoys and decays endogenous let-7 functions. Cell Cycle. 2012;11(16):3097–108.
Bae ON, Wang JM, Baek SH, et al. Oxidative stress-mediated thrombospondin-2 upregulation impairs bone marrow-derived angiogenic cell function in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2013;33(8):1920–7.
Chen Z, Lai TC, Jan YH, et al. Hypoxia-responsive miRNAs target argonaute 1 to promote angiogenesis. J Clin Invest. 2013;123(3):1057–67.
Anand S, Cheresh DA. MicroRNA-mediated regulation of the angiogenic switch. Curr Opin Hematol. 2011;18(3):171–6.
Wang S, Olson EN. AngiomiRs-key regulators of angiogenesis. Curr Opin Genet Dev. 2009;19(3):205–11.
Würdinger T, Tannous BA, Saydam O. MiR-296 regulates growth factor receptor overexpression in angiogenic endothelial cells. Cancer Cell. 2008;14(5):382–93.
Ma L, Young J, Prabhala H, et al. MiR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12(3):247–56.
Parmacek MS. MicroRNA-modulated targeting of vascular smooth muscle cells. J Clin Invest. 2009;119(9):2526–8.
Anand S, Majeti BK, Acevedo LM. MicroRNA-132–mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat Med. 2010;16(8):909–14.
Yang Z, Wu L, Zhu X, et al. MiR-29a modulates the angiogenic properties of human endothelial cells. Biochem Biophys Res Commun. 2013;434(1):143–9.
Wang J, Wang Y, Wang Y. Transforming growth factor β-regulated microRNA-29a promotes angiogenesis through targeting the phosphatase and tensin homolog in endothelium. J Biol Chem. 2013;288(15):10418–26.
Rutnam ZJ, Wight TN, Yang BB. miRNAs regulate expression and function of extracellular matrix molecules. Matrix Biol. 2013;32(2):74–85.
Wang W, Zhang E, Lin C. MicroRNAs in tumor angiogenesis. Life Sci. 2015;136:28–35.
Xu Q, Liu LZ, Qian X, et al. MiR-145 directly targets p70S6K1 in cancer cells to inhibit tumor growth and angiogenesis. Nucleic Acids Res. 2012;40(2):761–74.
Watanabe HS. Horizons in cancer research. Sunrise: Nova Science Publisher; 2014.
Zhang Y, Wang XY, Xu BH, et al. Epigenetic silencing of miR-126 contributes to tumor invasion and angiogenesis in colorectal cancer. Oncol Rep. 2013;30(4):1976–84.
Mathew LK, Skuli N, Mucaj V, et al. MiR-218 opposes a critical RTK-HIF pathway in mesenchymal glioblastoma. Proc Natl Acad Sci U S A. 2014;111(1):291–6.
Seok JK, Skuli N, Mucaj V, et al. MicroRNA-382 induced by HIF-1α is an angiogenic miR targeting the tumor suppressor phosphatase and tensin homolog. Nucleic Acids Res. 2014;42(12):8062–72.
Zheng Y, Li S, Ding Y, et al. The role of miR-18a in gastric cancer angiogenesis. Hepato-Gastroenterology. 2013;60(127):1809–13.
Liu LZ, Li C, Chen Q, et al. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1α expression. PLoS ONE. 2011;6(4), e19139.
Thum T, Fiedler J. LINCing MALAT1 and angiogenesis. Circ Res. 2014;114(9):1366–8.
Michalik KM, You X, Manavski Y, et al. Long non-coding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res. 2014;114(9):1389–97.
Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 2014;1839(11):1097–109.
Yuan SX, Yang F, Yang Y, et al. Long non-coding RNA associated with microvascular invasion in hepatocellular carcinoma promotes angiogenesis and serves as a predictor for hepatocellular carcinoma patients’ poor recurrence-free survival after hepatectomy. Hepatology. 2012;56(6):2231–41.
Yarmishyn AA, Batagov AO, Tan JZ, et al. HOXD-AS1 is a novel lncRNA encoded in HOXD cluster and a marker of neuroblastoma progression revealed via integrative analysis of non-coding transcriptome. BMC Genomics. 2014;15 Suppl 9:S7.
Huang JL, Zheng L, Hu YW, et al. Characteristics of long non-coding RNA and its relation to hepatocellular carcinoma. Carcinogenesis. 2014;35(3):507–14.
Kunej T, Obsteter J, Pogacar Z, et al. The decalog of long non-coding RNA involvement in cancer diagnosis and monitoring. Crit Rev Clin Lab Sci. 2014;51(6):344–57.
Jiao F, Hu H, Ha T, et al. Long non-coding RNA MALAT-1 enhances stem cell-like phenotypes in pancreatic cancer cells. Int J Mol Sci. 2015;16(4):6677–93.
Zhou Y, Zhang X, Klibanski A. MEG3 non-coding RNA: a tumor suppressor. J Mol Endocrinol. 2012;48(3):R45–53.
Gordon FE, Nutt CL, Cheunsuchon P, et al. Increased expression of angiogenic genes in the brains of mouse Meg3Null embryos. Endocrinology. 2010;151(6):2443–52.
Im JH, Muschel RJ. New evidence of lncRNA role in tumor progression and metastasis. Hepatobiliary Surg Nutr. 2012;1(1):55–6.
Iaconetti C, Gareri C, Polimeni A, et al. Non-coding RNAs: the “Dark Matter” of cardiovascular pathophysiology. Int J Mol Sci. 2013;4(10):19987–20018.
Derrien T, Guigó R, Johnson R, et al. The long non-coding RNAs: a new (P)layer in the “Dark Matter”. Front Genet. 2012;2:107. doi:10.3389/fgene.2011.00107. eCollection 2011.
Rutnam ZJ, Yang BB. The non-coding 3’ UTR of CD44 induces metastasis by regulating extracellular matrix functions. J Cell Sci. 2012;125(Pt 8):2075–85.
Fang L, Du WW, Yang X, et al. Versican 3’-untranslated region (3’-UTR) functions as a ceRNA in inducing the development of hepatocellular carcinoma by regulating miRNA activity. FASEB J. 2013;27(3):907–19.
Lee SC, Fang L, Wanget CH, et al. A non-coding transcript of nephronectin promotes osteoblast differentiation by modulating microRNA functions. FEBS Lett. 2011;585(16):2610–6.
Jeyapalan Z, Deng ZQ, Shatseva T, et al. Expression of CD44 3’-untranslated region regulates endogenous microRNA functions in tumorigenesis and angiogenesis. Nucleic Acids Res. 2011;39(8):3026–41.
Kahai S, Lee SC, Lee DY, et al. MicroRNA miR-378 regulates nephronectin expression modulating osteoblast differentiation by targeting GalNT-7. PLoS ONE. 2009;4(10):e7535.
Rutnam ZJ, Du WW, Yang W, et al. The pseudogene TUSC2P promotes TUSC2 function by binding multiple microRNAs. Nat Commun. 2014;5:2914.
Poliseno L, Salmena L, Zhang JW, et al. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465(7301):1033–8.
Lee DY, Shatseva T, Jeyapalan Z, et al. A 3’-untranslated region (3’UTR) induces organ adhesion by regulating miR-199a* functions. PLoS ONE. 2009;4(2):e4527.
Leucci E, Patella F, Waageet J, et al. MicroRNA-9 targets the long non-coding RNA MALAT1 for degradation in the nucleus. Sci Rep. 2013;3:2535. doi:10.1038/srep02535.
Yoon JH, Abdelmohsen K, Srikantan S, et al. LincRNA-p21 suppresses target mRNA translation. Mol Cell. 2012;47(4):648–55.
Yoon JH, Abdelmohsen K, Kim J, et al. Scaffold function of long non-coding RNA HOTAIR in protein ubiquitination. Nat Commun. 2013;4:2939.
Franklin JL, Rankinb CR, Levyet S, et al. Malignant transformation of colonic epithelial cells by a colon-derived long non-coding RNA. Biochem Biophys Res Commun. 2013;440(1):99–104.
Franco-Zorrilla JM, Valli A, Todesco M, et al. Target mimicry provides a new mechanism for regulation of microRNA activity. Nat Genet. 2007;39(8):1033–7.
Wang Y, Xu Z, Jiang J, et al. Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self-renewal. Dev Cell. 2013;25(1):69–80.
Yoon JH, Abdelmohsen K, Gorospe M. Functional interactions among microRNAs and long non-coding RNAs. Semin Cell Dev Biol. 2014;34:9–14.
Thum T, Condorelli G. Long non-coding RNAs and microRNAs in cardiovascular pathophysiology. Circ Res. 2015;116(4):751–62.
Paraskevopoulou MD, Georgakilas G, Kostoulas N, et al. DIANA-LncBase: experimentally verified and computationally predicted microRNA targets on long non-coding RNAs. Nucleic Acids Res. 2013;41(Database issue):D239–45.
Acknowledgments
This work was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (NSERC; 227937–2012) to BBY, who is the recipient of a Career Investigator Award (CI 7418) from the Heart and Stroke Foundation of Ontario.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Khorshidi, A., Dhaliwal, P., Yang, B.B. (2016). Noncoding RNAs in Tumor Angiogenesis. In: Song, E. (eds) The Long and Short Non-coding RNAs in Cancer Biology. Advances in Experimental Medicine and Biology, vol 927. Springer, Singapore. https://doi.org/10.1007/978-981-10-1498-7_8
Download citation
DOI: https://doi.org/10.1007/978-981-10-1498-7_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-1496-3
Online ISBN: 978-981-10-1498-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)