Abstract
Subcortical vascular cognitive impairment (SVCI) refers to the cognitive impairment associated with small vessel diseases (SVD), which encompasses both subcortical vascular mild cognitive impairment (svMCI) and subcortical ischemic vascular dementia (SVaD). The recent development of modern MRI techniques such as diffusion tensor imaging (DTI) or resting-state functional MRI (rs-fMRI) has allowed researchers to investigate neuroimaging characteristics related to the mechanisms that underlie cognitive dysfunctions in SVCI. Although ischemia is regarded as the primary underlying pathology of SVCI, one of the major concerns regarding diagnosis and research for SVCI is that the large proportion of clinically diagnosed SVCI patients often reveals concomitant Alzheimer’s disease pathology. With the recent availability of amyloid positron emission tomography (PET), the identification of pure SVCI has provided further insights into neuroimaging characteristics of pure SVCI. Here, this section will introduce an overview of the structural and functional neuroimaging characteristics of SVCI based on recent neuroimaging studies with MRI, especially focused on pure SVCI.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Subcortical vascular cognitive impairment (SVCI) refers to the cognitive impairment associated with small vessel diseases (SVD) such as lacunes, white matter hyperintensities (WMH), and cerebral microbleeds (CMB) in the subcortical areas [1], which encompasses both subcortical vascular mild cognitive impairment (svMCI) and subcortical ischemic vascular dementia (SVaD) [2, 3]. Clinically, it is important to detect SVCI as early as possible since early intervention for SVCI can delay the progression of the disease. Therefore, magnetic resonance imaging (MRI) is the most commonly used tool to diagnose SVCI which can detect SVD including lacunes, WMH, and CMB in the subcortical area. In addition, recent methodological advances in MR imaging analysis have enabled further image-based analyses to investigate neuroimaging characteristics related to the mechanisms that underlie cognitive dysfunctions in SVCI.
Although ischemia is regarded as the primary underlying pathology of SVCI, one of the major concerns regarding diagnosis and research for SVCI is that the large proportion of clinically diagnosed SVCI patients often reveal concomitant Alzheimer’s disease (AD) pathology [4]. The overlapping clinical manifestations and neuroimaging findings between pure SVCI and mixed AD concomitant with SVD (mixed dementia) have made it difficult to differentiate between the two. With the recent availability of amyloid positron emission tomography (PET) that allows for in vivo detection of cerebral amyloidosis [5], however, it becomes possible to discriminate patients with relatively pure SVCI defined as not having concomitant amyloid (negative amyloid PET scan) from those with mixed pathology (positive amyloid PET scan) [6]. Indeed, a recent study (AMPETIS study) suggests that pure SVCI is more common than expected, which shows that 68.9% of patients with severe WMH compatible with SVCI revealed negative for amyloid PET scan and they were slightly younger and had greater number of lacunes but less severe hippocampal atrophy on MRI than amyloid positive SVaD [6]. Therefore, more recent neuroimaging studies with amyloid PET scan in SVCI have shown that pure SVCI also has distinctive neuroimaging features compared to those in patients with AD or mixed pathology.
Here, this section aims to provide an overview of the structural and functional neuroimaging characteristics of SVCI based on the recent neuroimaging studies with MRI, with a special focus on pure SVCI.
10.1 Imaging Criteria for the Diagnosis of Pure SVCI
Criteria for brain imaging requirements by Erkinjuntti et al. were commonly used for diagnosing SVCI [7] (Table 10.1). This brain imaging criteria for SVaD not only cover cases having predominantly WMHs (“the white matter type,” Binswanger’s disease) but also is applicable for those with predominantly lacunar infarcts (“the lacunar state type”). It is common for both lacunar state and the white matter type to occur together as the underlying pathology involves the lenticulostriate and the penetrating subcortical arterioles of the hemispheric white matter simultaneously [8]. However, these criteria cannot discriminate pure SVCI from mixed dementia.
Recently, a new criterion that could help characterize pure SVaD from mixed dementia has been proposed using a combination of clinical and MRI findings, based on the data from SVaD patients with amyloid PET imaging [9] (Table 10.2). According to these criteria, among patients who met the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV) criteria for vascular dementia [10] and also had severe WMH with a cap or band ≥10 mm and a deep white matter lesion ≥25 mm, the cutoff values of clinical and MRI variables to discriminate pure SVaD from mixed dementia were age ≤75, ≥5 lacunes, and visual rating scale of medial temporal atrophy ≤3, which in total yielded an accuracy of 67.5% [9].
10.2 Characteristics of Structural Neuroimaging in SVCI
10.2.1 Diffusion Tensor Imaging
The subtle change of microstructural integrity of white matters is one of the earliest manifestations of SVCI, although the most prominent manifestations of SVCI on MRI include WMH and lacunes in subcortical areas [11]. Such early changes could not be detected by conventional MRI but can be noticed by diffusion tensor imaging (DTI) [12], and abnormal DTI parameters can identify axonal or myelin disruption in the white matter tracts [13].
Previous studies using DTI analysis indicate that patients with SVCI display reduced fractional anisotropy (FA) in the anterior corpus callosum, frontal and parietal white matter regions [14], and mean FA values of the whole brain [15, 16] compared to controls. This finding suggests that SVCI may have extensive microstructural abnormalities of white matters, although it often appears to be normal on conventional MRI [3].
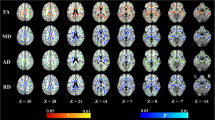
One of the recent methods for DTI analyses is tract-based spatial statistics (TBSS) that can localize microstructural alterations by mapping diffusion parameters onto a white matter skeleton [17]. This method confirmed decreased FA in the bilateral frontal, temporal, and parietal white matters of AD patients with positive [11C] Pittsburgh compound B (PiB) PET scan compared to the healthy controls (Fig. 10.1a). On the other hand, microstructural changes were seen in all of the white matters in the brain of patients with pure SVaD (characterized as PiB PET negative) when compared to normal controls [18] (Fig. 10.1b). Furthermore, direct comparison between patients with PiB(+) AD and PiB(−) SVaD also showed decreased FA in the anterior and posterior white matter regions of PiB(−) SVaD groups (Fig. 10.1c), unlike the PiB(+) AD group. Such results among the clinically diagnosed SVCI patients may stem from a combination of AD pathology [3]. However, this study based on PiB PET demonstrated that even pure SVCI patients also showed microstructural alterations in posterior white matter regions without amyloid pathology in the brain [18], although the pathophysiology of posterior involvement of white matters in SVCI patients remains unclear.
Tract-based spatial statistics results of FA images. Green represents the MNI152 standard brain and the skeleton image. Red-yellow color represents decreased FA (a) in PiB(+) AD patients compared with normal controls, (b) in PiB(−) SVaD patients compared with normal controls, and (c) in PiB(−) SVaD patients compared with PiB(+) AD patients. P < 0.01, FWE corrected for multiple comparisons. AD Alzheimer’s disease, PiB Pittsburgh compound-B, SVaD subcortical vascular dementia. (Adapted from European Journal of Neurology [18])
A more recent study using tract-specific statistical analysis (TSSA) based on 14 major white matter tracts also found that SVCI patients showed focal deficits in the bilateral anterior thalamic radiation, cingulum, superior longitudinal fasciculus, uncinate fasciculus, corticospinal tract, and the left inferior longitudinal fasciculus relative to normal controls, which suggests that SVCI patients might have significant deficits in the tracts that traverse frontal and parietal white matter regions [19]. This finding could support the idea that ischemia in SVCI may preferentially affect the white matter in frontal and parietal regions rather than in the temporal and occipital regions [20,21,22].
It is well known that cognitive impairments in SVCI are related to ischemic interruption of frontal cortical circuits or disruption of cholinergic pathways that traverse the subcortical white matter [23, 24]. DTI measurement can also be used as one of the markers that correlate with cognitive functions in SVCI once age and other SVD markers are adjusted [25]. It has been suggested that white matter integrity at specific locations in SVCI was related to specific cognitive performance [25]. For example, the low FA and high mean diffusivity (MD) in the genu and splenium of the corpus callosum were associated with lower scores in the global cognitive function and executive functions, whereas those in the cingulum bundle correlated with poorer verbal memory performance [25]. An approach with TSSA also indicated that FA values in the cingulum were associated with the scores in language, visuospatial, memory, and frontal functions, while those in the anterior thalamic radiation were associated with scores in attention, language, memory, and frontal functions [19].
It is also noteworthy that microstructural changes in SVCI were also correlated with motor symptoms such as gait disturbances [3, 26, 27]. A previous study showed that gait disturbance in SVCI was associated with decreased FA and increased MD values in the frontal and parietal white matter including anterior thalamic radiation, superior longitudinal fasciculus, cingulum, inferior fronto-occipital fasciculus, corticospinal tract, and corpus callosum [27].
10.2.2 Cortical Thickness
The term “subcortical” vascular cognitive impairment is used by researchers due to prominent WMH and lacunes within white and deep gray matter and their characteristic clinical features associated with this particular anatomic location [1, 28, 29]. Increasing evidence has highlighted that the impacts of SVD on subcortical areas may extend into the cerebral cortex, manifesting both as microscopic vascular lesions and cortical atrophy [30,31,32]. Therefore, cortical changes have now been considered as clinically relevant characteristics of SVCI [11, 30, 33].
Relative to normal controls, recent cortical thickness measurement revealed widespread cortical thinning in patients with SVCI, especially in the frontal areas including dorsolateral prefrontal cortex, superior medial frontal region, and orbitofrontal gyrus, which are known to be associated with the frontosubcortical circuits [34]. This is contrary to the previous findings showing that patients with AD demonstrated cortical thinning in temporoparietal association cortices including the medial temporal lobe [34,35,36]. Interestingly, more recent studies based on PiB PET showed that compared to controls, cortical thinning in PiB(−) SVCI was most profound in the perisylvian area, medial prefrontal area, and posterior cingulate gyri, while the precuneus and medial temporal lobes were relatively spared (Fig. 10.2b) [37, 38]. When the cortical thickness of AD and PiB(−) SVaD was directly compared, PiB(−) SVaD demonstrated significant cortical thinning in the bilateral inferior frontal, superior temporal gyri, and right medial frontal and orbitofrontal lobes, while AD showed significant cortical thinning in the right medial temporal region (Fig. 10.2c) [37]. Although the exact mechanisms underlying secondary cortical atrophy are poorly elucidated, it has been believed that cognitive impairments in SVCI arise when the subcortical ischemic vascular lesions demonstrated by WMH and lacunes disrupt the subcortical axonal damage and interrupt the white matter circuits that connect the various cortical regions and subcortical structures, especially the frontal subcortical circuits and the long association fibers, through which the cholinergic pathways pass [23, 39,40,41]. These lesions also result in a cascade of secondary neuronal degeneration in connected cortical regions via processes known as “dying back” and Wallerian degeneration [42, 43], manifesting as cortical atrophy which may also contribute toward cognitive impairment. In addition, impaired blood flow causes ischemia, and infarction in the gray matter such as cortical microinfarcts may lead to direct cortical neuronal damage and atrophy [37, 44].
Cortical thickness in PiB(−) SVaD. (a) Relative to NC, AD patients showed significant cortical thinning in widespread regions of the temporoparietal and frontal cortices, most prominently in the medial temporal lobes and the inferior parietal lobules. (b) PiB(−) SVaD patients showed cortical atrophy in the frontal, temporoparietal, medial frontal, and posterior cingulate cortices and the lingual gyri, while relative sparing in the medial temporal lobe and precuneus. (c) The bottom row shows a general trend of cortical thinning between AD (in blue) and PiB(−) SVaD (in red), in particular, statistically meaningful thinning was noted in right temporal lobe in AD and bilateral perisylvian, right medial frontal, and orbitofrontal regions in PiB(−) SVaD. NC normal controls, AD Alzheimer’s disease, PiB Pittsburgh compound-B, SVaD subcortical vascular dementia. (Adapted from Journal of Alzheimer’s Disease [37])
Interestingly, the perisylvian cortical area was one of the characteristic areas that was primarily affected in PiB(−) SVaD. It is plausible that cholinergic circuits may participate in cortical thinning in the perisylvian region. Cholinergic pathways penetrate through the anterior cap and into the perisylvian division of the lateral cholinergic pathway which are joined by the opercula and insular cortex [45]. Hence, the cholinergic pathway by the ischemic lesions in SVaD may contribute toward cortical thinning in the perisylvian area. A study which found impaired subinsular cholinergic fibers in dementia with SVD is in support of the cortical thinning in the perisylvian region [46]. Another possible interpretation could be that subcortical WMH or lacunes might affect the arcuate fasciculus, which consecutively results in cortical thinning in the adjacent perisylvian region, if there was a strong interrelation between perisylvian cortical thickness and fractional anisotropy of the arcuate fasciculus according to the previous study [47].
10.2.3 Hippocampal Volume and Shape
Although hippocampal atrophy is a key hallmark of AD patients [48, 49], previous studies reported that hippocampal atrophy is also present in patients with SVCI. Hippocampal atrophy in SVCI has been suggested whether it stems from the accumulating burden of ischemia or combined AD pathology [50,51,52].
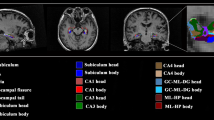
Recent studies have demonstrated that pure SVaD defined as the absence of amyloid deposition in PiB PET also had significant hippocampal atrophy compared to normal controls [38, 53] but less than that of AD [53]. Not only this, but hippocampal shape analysis from the same study also showed that both PiB(+) AD (Fig. 10.3a) and PiB(−) SVaD (Fig. 10.3b) patients displayed deflated shape of the cornus ammonis (CA) 1 and subiculum compared with normal controls but more inward deformity in the subiculum of the left hippocampus in PiB(+) AD compared to PiB(−) SVaD (Fig. 10.3c) [53]. Such results all support the idea that cumulative ischemia without amyloid pathology could lead to hippocampal atrophy and shape changes in SVaD.
Hippocampal shape comparison. (a) Compared with NC, PiB(+) AD patients demonstrate inward deformity in the lateral zones (LZ) containing CA 1 and inferior medial zones (IMZ) approximating subiculum. (b) In PiB(−) SVaD patients, the regional inward deformity appears most evident in the LZ and IMZ. (c) Compared with PiB(−) SVaD, PiB(+) AD patients show more inward deformity especially in left IMZ. Nonsignificant differences are colored in white. (Adapted from Neurobiology of Aging [53])
Another recent study using an incremental learning method has shown notable results on discriminating mixed dementia from pure SVaD by hippocampus and amygdala shape, with the sensitivity of 95.7%, specificity of 75.6%, and accuracy of 82.4% [54], with pure SVaD highlighted by nonexistence of amyloid deposition on brain confirmed with negative PiB PET scan.
10.3 Structural and Functional Network Analyses in SVCI
A myriad of evidence has suggested that the human brain is a large-scale network with “small-world” topology [55,56,57], which represents an optimal balance between the integration and segregation of information for efficient use [58]. Therefore, it is known that human cognitive functions depend on the efficient function of the brain networks that consist of cortical gray matter (hubs), subcortical brain regions, and white matter tracts [11, 59].
It has been noted that SVD pathologies in SVCI patients may disrupt the balance in structures and functions of brain network [11]. Undoubtedly, recent neuroimaging studies have shown that the effects of vascular lesions on cognitive functions are mediated through alterations in structural connectivity, which have consistently found an association between the burden of SVD-related brain lesions and reduced network efficiency [59, 60]. Some studies have investigated regional structural connectivity and cognitive functions in SVCI patients [61, 62], which found that reduced network efficiency mediates the effects of SVD-related lesions on frontal gray matter loss, as well as on executive dysfunction [62]. Given that the lower network efficiency predicts conversion to dementia along with older age and lower hippocampal volume [63], structural network disruptions in SVCI may play a pivotal role in the development of cognitive dysfunctions and dementia. In hindsight, these findings emphasize the importance of the network analysis of the structural connectivity as a potential predictive marker in SVCI [63].
Recently, resting-state functional MRI (rs-fMRI) has emerged as an effective, noninvasive imaging technique that is used to study the intrinsic functional architecture of the human brain when subjects are not engaged in external tasks. The rs-fMRI can measure the correlated spontaneous activity within cortical and subcortical regions that are functionally related as well as intrinsic functional organization of the human brain [64]. Although functional connectivity has been less well explored in SVCI, there is some evidence showing that the effects of vascular lesions on SVCI are influenced by altered functional connectivity to some degree [65,66,67,68], especially in the frontal brain regions [69, 70].
Recent analysis of resting-state default mode network (DMN) or central executive network (CEN) highlights some distinctive patterns among patients with PiB(−) SVaD, PiB(+) AD, and mixed dementia [70]. When the resting-state DMN of PiB(+) AD and PiB(−) SVaD patients were compared, the PiB(+) AD patients displayed lower functional connectivity particularly in the inferior parietal lobule, whereas the PiB(−) SVaD patients show lower functional connectivity in the medial frontal and superior frontal gyri. Mixed dementia patients, on the other hand, exhibits lower functional connectivity within the DMN specifically in the posterior cingulate gyrus compared to those in the PiB(−) SVaD or PiB(+) AD. In addition, when the resting-state CEN connectivity of PiB(+) AD and PiB(−) SVaD patients were compared (Fig. 10.4), the PiB(−) SVaD patients displayed lower functional connectivity especially in the anterior insular region compared to normal controls. Furthermore, it should be noted that the mixed dementia patients displayed lower functional connectivity within the CEN especially in the inferior frontal gyrus, compared to the PiB(−) SVaD or PiB(+) AD patients. Such accumulative findings point toward the fact that the DMN disruption occurs in both PiB(+) AD and PiB(−) SVaD patients in a disease-specific pattern, while the CEN disruption is more unique in PiB(−) SVaD. Additionally, more profound disruption of DMN and CEN is seen in patients with mixed dementia that combined AD and SVD burdens. All in all, a growing body of evidence suggests that disturbances in large-scale functional brain networks play a decisive role in cognitive decline [11].
Brain regions with significant differences in resting-state functional connectivity within the central executive network (CEN). Colored areas indicate significantly lower connectivity in the latter group in each comparison. >120 voxel size after post hoc tests with age, gender, and education adjusted. (a) Compared to the normal controls, the PiB(−) SVaD patients displayed lower functional connectivity within the CEN in the left insular area. (b) In the direct comparison between the PiB(+) AD and PiB(−) SVaD patients, the PiB(−) SVaD patients displayed lower functional connectivity within the CEN in the left insular area, while there was no region where the PiB(+) AD patients displayed lower functional connectivity. (c) Compared to the PiB(−) SVaD patients, the mixed dementia patients displayed lower functional connectivity within the CEN in the left inferior frontal gyrus, while there was no region where the PiB(−) SVaD patients displayed lower functional connectivity compared to the mixed dementia patients. (d) Compared to the PiB(+) AD patients, the mixed dementia patients displayed lower functional connectivity within the CEN in the left inferior frontal gyrus, while there was no region where the PiB(+) AD patients displayed lower functional connectivity. (Adapted from Journal of Alzheimer’s Disease [70])
10.4 Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT) Findings in SVCI
Functional neuroimaging techniques such as positron emission tomography (PET) and single photon emission computed tomography (SPECT) techniques are known to provide quantitative information on the possible functional changes which can occur in the early course of disease [71, 72]. SPECT, in particular, is advantageous to investigate cerebral blood flow (CBF), whereas PET can measure regional oxygen (15O isotope) and glucose (18F isotope) metabolism besides CBF and cerebral blood volume [73].
It is well known that the variations in CBF can be compensated with autoregulatory mechanism under normal condition, which means that blood vessels can dilate to maintain regional CBF when cerebral perfusion pressure decreases. With further decline of regional CBF, oxygen extraction fraction (OEF) increases to sustain regional cerebral oxygen metabolism (CMRO2) [1]. Therefore, increased OEF is a characteristic of ongoing ischemia and impending infarction [1].
The compromised autoregulatory reserve in patients with SVCI can increase the risk of ischemia if blood pressure should be abruptly lowered (e.g., orthostatic hypotension, aggressive antihypertensive treatment or cardiac failure, etc.) [1]. Several studies have shown that decreased CBF in SVCI may be directly linked to the compromised vascular reserve [74, 75]. A study using 15O PET discovered that resting CBF in patients with Alzheimer’s and Binswanger’s diseases both decrease within a similar range, with only the latter having a vasoreactive response to hypercapnia [74]. Such adjustment in vasoreactivity in response to acetazolamide is found only in SVaD, not in multi-infarct dementia [75]. Other 15O PET studies also support the above results showing that CBF and CMRO2 were notably reduced in both the white matter and parietal, frontal, and temporal cortices in patients with Binswanger’s disease [76, 77], which suggests patients with SVaD had misery perfusion and impaired cerebral vascular reserves.
In patients with Binswanger’s disease, the pattern of cerebral perfusion measured by SPECT using N-isopropyl-p-123I-iodoamphetamine (IMP) as a tracer revealed that regional perfusion was decreased in the thalamus, basal ganglia, and frontal areas, whereas perfusion in AD dementia patients was lowered in the parietal and temporal areas compared to normal controls [78]. Another SPECT study using the same tracer also demonstrated similar results showing that patients with Binswanger’s disease had greater CBF reduction in frontal and anterior cingulate cortices, whereas patients with AD dementia had greater CBF reduction in temporoparietal and posterior cingulate cortices compared to normal controls [79]. Similarly, statistical parametric mapping analysis of SPECT using 99mTc-ethyl-cysteinate dimer(99mTCD) as a tracer showed that reduced regional CBF was noted in the right thalamus, left caudate nucleus, and cingulate, bilateral superior temporal, and left ventral subcallosal gyri in patients with SVaD compared to normal controls [80] (Fig. 10.5). A more recent SPECT study showed that there were no differences in the regional patterns of CBF between the Binswanger’s and the lacunar type [81].
SPM analysis of SPECT results in SVaD. Areas with decreased regional cerebral blood flow in patients with SVaD in comparison to the healthy controls are presented in red and yellow colors (corrected P < 0.05). In patients with SVaD, reduced regional CBF was noted in the right thalamus, left caudate nucleus, and cingulate, bilateral superior temporal, and left ventral subcallosal gyri compared to normal controls. (Adapted from Journal of the Neurological Sciences [80])
In addition to oxygen metabolism, the pattern of glucose metabolism using [18F]-2-fluoro-2-deoxy-d-glucose (FDG) is also commonly applied to differentiate various types of dementia, which is one of well-established methods to reveal impaired function that usually precedes atrophy [71, 82]. With FDG-PET, SVaD patients showed decreased glucose metabolism in the frontal lobe and deep nuclei, whereas AD dementia patients revealed decreased metabolism in temporoparietal regions [83,84,85]. Even in patients with svMCI, FDG-PET shows hypometabolism in the subcortical areas especially in the thalamus, cerebellum, and brain stem, which is a distinctive feature compared to that of amnestic mild cognitive impairment [86] (Fig. 10.6). Frontal executive dysfunction in SVaD can also be explained by the FDG-PET study, which revealed that white matter lesions lead to impaired glucose metabolism in the frontal lobe regardless of their location [87].
Cerebral glucose metabolism in subcortical vascular MCI. The areas in red represent the brain regions that were more hypometabolic in subcortical vascular mild cognitive impairment (svMCI) than in normal controls (NC), and those in blue represent brain regions that were more hypometabolic in amnestic MCI (aMCI) than in NC. (Adapted from Journal of Neuroimaging [86])
10.5 Conclusions
MRI has a great potential for diagnosing and differentiating SVCI from various diseases, by providing disease-specific features in cortical, subcortical, and white matters that may occur before the onset of cognitive decline in SVCI. Modern MRI techniques such as DTI or rs-fMRI provide a good opportunity to investigate ischemic vascular lesion as well as detect early changes that are predictive of individual’s risk for developing dementia or progression of disease [88] in SVCI as a preventative measure. In addition, SPECT and PET can also be considered as a complement to characterize SVCI from other types of dementia, although there is little consensus on a specific brain perfusion pattern in SVCI. Furthermore, with the aid of amyloid PET, the identification of pure SVCI has provided further insights into the understanding of neuroimaging characteristics of pure SVCI, which may help choose proper management for SVCI according to the presence of amyloid deposition [71]. In the near future, the advancement of neuroimaging will in all likelihood broaden our knowledge of the development of cognitive decline in patients with SVCI.
References
Román GC, Erkinjuntti T, Wallin A, et al. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1(7):426–36.
Hong YJ, Kim CM, Kim JE, et al. Regional amyloid burden and lacune in pure subcortical vascular cognitive impairment. Neurobiol Aging. 2017;55:20–6.
Kim SH, Park JS, Ahn HJ, et al. Voxel-based analysis of diffusion tensor imaging in patients with subcortical vascular cognitive impairment: correlates with cognitive and motor deficits. J Neuroimaging. 2011;21(4):317–24.
Jellinger KA, Attems J. Neuropathological evaluation of mixed dementia. J Neurol Sci. 2007;257(1):80–7.
Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–19.
Lee JH, Kim SH, Kim GH, et al. Identification of pure subcortical vascular dementia using 11C-Pittsburgh compound B. Neurology. 2011;77(1):18–25.
Erkinjuntti T, Inzitari D, Pantoni L, et al. Limitations of clinical criteria for the diagnosis of vascular dementia in clinical trials. Is a focus on subcortical vascular dementia a solution? Ann N Y Acad Sci. 2000;903:262–72.
Jeong JH, Kim EJ, Seo SW, et al. Cognitive and behavioral abnormalities of vascular cognitive impairment. In: Miller BL, Boeve BF, editors. The behavioral neurology of dementia. Cambridge: Cambridge University Press; 2016. p. 301–30.
Kim GH, Lee JH, Seo SW, et al. Seoul criteria for PiB(−) subcortical vascular dementia based on clinical and MRI variables. Neurology. 2014;82(17):1529–35.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text revision ed. American Psychiatric Association: Washington, DC; 1994.
Dichgans M, Leys D. Vascular cognitive impairment. Circ Res. 2017;120(3):573–91.
Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–38.
Aung WY, Mar S, Benzinger TL. Diffusion tensor MRI as a biomarker in axonal and myelin damage. Imaging Med. 2013;5(5):427–40.
Zarei M, Damoiseaux JS, Morgese C, et al. Regional white matter integrity differentiates between vascular dementia and Alzheimer disease. Stroke. 2009;40(3):773–9.
Zhou Y, Qun X, Qin LD, et al. A primary study of diffusion tensor imaging-based histogram analysis in vascular cognitive impairment with no dementia. Clin Neurol Neurosurg. 2011;113(2):92–7.
Zhou Y, Lin FC, Zhu J, et al. Whole brain diffusion tensor imaging histogram analysis in vascular cognitive impairment. J Neurol Sci. 2008;268(1–2):60–4.
Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–505.
Kim Y, Kwon H, Lee JM, et al. White matter microstructural changes in pure Alzheimer’s disease and subcortical vascular dementia. Eur J Neurol. 2015;22(4):709–16.
Jung NY, Han CE, Kim HJ, et al. Tract-specific correlates of neuropsychological deficits in patients with subcortical vascular cognitive impairment. J Alzheimer Dis. 2016;50(4):1125–35.
Moody DM, Bell MA, Challa VR. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: an anatomic study. AJNR Am J Neuroradiol. 1990;11(3):431–9.
Gootjes L, Teipel SJ, Zebuhr Y, et al. Regional distribution of white matter hyperintensities in vascular dementia, Alzheimer’s disease and healthy aging. Dement Geriatr Cogn Disord. 2004;18(2):180–8.
Wen W, Sachdev PS. Extent and distribution of white matter hyperintensities in stroke patients: the Sydney Stroke Study. Stroke. 2004;35(12):2813–9.
Bocti C, Swartz RH, Gao FQ, et al. A new visual rating scale to assess strategic white matter hyperintensities within cholinergic pathways in dementia. Stroke. 2005;36(10):2126–31.
Jung WB, Mun CW, Kim YH, et al. Cortical atrophy, reduced integrity of white matter and cognitive impairment in subcortical vascular dementia of Binswanger type. Psychiatry Clin Neurosci. 2014;68(12):821–32.
Tuladhar AM, van Norden AG, de Laat KF, et al. White matter integrity in small vessel disease is related to cognition. NeuroImage Clin. 2015;7:518–24.
de Laat KF, Tuladhar AM, van Norden AG, et al. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain. 2011;134(Pt 1):73–83.
Kim YJ, Kwon HK, Lee JM, et al. Gray and white matter changes linking cerebral small vessel disease to gait disturbances. Neurology. 2016;86(13):1199–207.
Erkinjuntti T, Inzitari D, Pantoni L, et al. Research criteria for subcortical vascular dementia in clinical trials. J Neural Transm Supp. 2000;59:23–30.
Mungas D, Jagust WJ, Reed BR, et al. MRI predictors of cognition in subcortical ischemic vascular disease and Alzheimer’s disease. Neurology. 2001;57(12):2229–35.
Peres R, De Guio F, Chabriat H, et al. Alterations of the cerebral cortex in sporadic small vessel disease: a systematic review of in vivo MRI data. J Cereb Blood Flow Metab. 2016;36(4):681–95.
Gouw AA, Seewann A, van der Flier WM, et al. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry. 2011;82(2):126–35.
Smith EE, Schneider JA, Wardlaw JM, et al. Cerebral microinfarcts: the invisible lesions. Lancet Neurol. 2012;11(3):272–82.
Duering M, Righart R, Wollenweber FA, et al. Acute infarcts cause focal thinning in remote cortex via degeneration of connecting fiber tracts. Neurology. 2015;84(16):1685–92.
Seo SW, Ahn J, Yoon U, et al. Cortical thinning in vascular mild cognitive impairment and vascular dementia of subcortical type. J Neuroimaging. 2010;20(1):37–45.
Lerch JP, Pruessner JC, Zijdenbos A, et al. Focal decline of cortical thickness in Alzheimer’s disease identified by computational neuroanatomy. Cereb Cortex. 2005;15(7):995–1001.
Singh V, Chertkow H, Lerch JP, et al. Spatial patterns of cortical thinning in mild cognitive impairment and Alzheimer’s disease. Brain. 2006;129(11):2885–93.
Kim CH, Seo SW, Kim GH, et al. Cortical thinning in subcortical vascular dementia with negative 11C-PiB PET. J Alzheimer Dis. 2012;31(2):315–23.
Kim HJ, Ye BS, Yoon CW, et al. Cortical thickness and hippocampal shape in pure vascular mild cognitive impairment and dementia of subcortical type. Eur J Neurol. 2014;21(5):744–51.
Chui HC. Subcortical ischemic vascular dementia. Neurol Clin. 2007;25(3):717–40.
Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50(8):873–80.
Thong JY, Du J, Ratnarajah N, et al. Abnormalities of cortical thickness, subcortical shapes, and white matter integrity in subcortical vascular cognitive impairment. Hum Brain Mapp. 2014;35(5):2320–32.
Guimaraes JS, Freire MA, Lima RR, et al. Mechanisms of secondary degeneration in the central nervous system during acute neural disorders and white matter damage. Rev Neurol. 2009;48(6):304–10.
Wang JT, Medress ZA, Barres BA. Axon degeneration: molecular mechanisms of a self-destruction pathway. J Cell Biol. 2012;196(1):7–18.
Kovari E, Gold G, Herrmann FR, et al. Cortical microinfarcts and demyelination affect cognition in cases at high risk for dementia. Neurology. 2007;68(12):927–31.
Selden NR, Gitelman DR, Salamon-Murayama N, et al. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain. 1998;121(Pt 12):2249–57.
Tomimoto H, Ohtani R, Shibata M, et al. Loss of cholinergic pathways in vascular dementia of the Binswanger type. Dement Geriatr Cogn Disord. 2005;19(5–6):282–8.
Phillips OR, Clark KA, Woods RP, et al. Topographical relationships between arcuate fasciculus connectivity and cortical thickness. Hum Brain Mapp. 2011;32(11):1788–801.
Burton EJ, Barber R, Mukaetova-Ladinska EB, et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain. 2009;132(1):195–203.
Gosche KM, Mortimer JA, Smith CD, et al. Hippocampal volume as an index of Alzheimer neuropathology: findings from the Nun Study. Neurology. 2002;58(10):1476–82.
Du AT, Schuff N, Laakso MP, et al. Effects of subcortical ischemic vascular dementia and AD on entorhinal cortex and hippocampus. Neurology. 2002;58(11):1635–41.
Laakso MP, Partanen K, Riekkinen P, et al. Hippocampal volumes in Alzheimer’s disease, Parkinson’s disease with and without dementia, and in vascular dementia: an MRI study. Neurology. 1996;46(3):678–81.
Zarow C, Vinters HV, Ellis WG, et al. Correlates of hippocampal neuron number in Alzheimer’s disease and ischemic vascular dementia. Ann Neurol. 2005;57(6):896–903.
Kim GH, Lee JH, Seo SW, et al. Hippocampal volume and shape in pure subcortical vascular dementia. Neurobiol Aging. 2015;36(1):485–91.
Kim HJ, Kim J, Cho H, et al. Individual subject classification of mixed dementia from pure subcortical vascular dementia based on subcortical shape analysis. PLoS One. 2013;8(10):e75602.
Hagmann P, Cammoun L, Gigandet X, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6(7):e159.
Hagmann P, Kurant M, Gigandet X, et al. Mapping human whole-brain structural networks with diffusion MRI. PLoS One. 2007;2(7):e597.
Gong G, He Y, Concha L, et al. Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb Cortex. 2009;19(3):524–36.
Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett. 2001;87(19):198701.
Lawrence AJ, Chung AW, Morris RG, et al. Structural network efficiency is associated with cognitive impairment in small-vessel disease. Neurology. 2014;83(4):304–11.
Tuladhar AM, van Dijk E, Zwiers MP, et al. Structural network connectivity and cognition in cerebral small vessel disease. Hum Brain Mapp. 2016;37(1):300–10.
Tuladhar AM, Reid AT, Shumskaya E, et al. Relationship between white matter hyperintensities, cortical thickness, and cognition. Stroke. 2015;46(2):425–32.
Kim HJ, Im K, Kwon H, et al. Clinical effect of white matter network disruption related to amyloid and small vessel disease. Neurology. 2015;85(1):63–70.
Tuladhar AM, van Uden IW, Rutten-Jacobs LC, et al. Structural network efficiency predicts conversion to dementia. Neurology. 2016;86(12):1112–9.
Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–11.
Papma JM, den Heijer T, de Koning I, et al. The influence of cerebral small vessel disease on default mode network deactivation in mild cognitive impairment. NeuroImage Clin. 2012;2:33–42.
Yi L, Wang J, Jia L, et al. Structural and functional changes in subcortical vascular mild cognitive impairment: a combined voxel-based morphometry and resting-state fMRI study. PLoS One. 2012;7(9):e44758.
Ding W, Cao W, Wang Y, et al. Altered functional connectivity in patients with subcortical vascular cognitive impairment—a resting-state functional magnetic resonance imaging study. PLoS One. 2015;10(9):e0138180.
Liu C, Li C, Yin X, et al. Abnormal intrinsic brain activity patterns in patients with subcortical ischemic vascular dementia. PLoS One. 2014;9(2):e87880.
Zhou X, Hu X, Zhang C, et al. Aberrant functional connectivity and structural atrophy in subcortical vascular cognitive impairment: relationship with cognitive impairments. Front Aging Neurosci. 2016;8:14.
Kim HJ, Cha J, Lee JM, et al. Distinctive resting state network disruptions among Alzheimer’s disease, subcortical vascular dementia, and mixed dementia patients. J Alzheimers Dis. 2016;50(3):709–18.
Kim HJ, Yoon CW, Ye BS, Kim E-J, Seo SW, Jeong JH, Na DL. Vascular dementia. In: Dierckx RAJO, Otte A, de Vries EFJ, van Waarde A, Leenders KL, editors. PET and SPECT in neurology. Berlin, Heidelberg: Springer Berlin Heidelberg; 2014. p. 417–35.
Nagata KEN, Maruya H, Yuya H, et al. Can PET data differentiate Alzheimer’s disease from vascular dementia? Ann N Y Acad Sci. 2000;903(1):252–61.
Brooks DJ. The clinical role of PET in cerebrovascular disease. Neurosurg Rev. 1991;14(2):91–6.
Kuwabara Y, Ichiya Y, Sasaki M, et al. Time dependency of the acetazolamide effect on cerebral hemodynamics in patients with chronic occlusive cerebral arteries. Early steal phenomenon demonstrated by [15O]H2O positron emission tomography. Stroke. 1995;26(10):1825–9.
De Reuck J, Decoo D, Hasenbroekx MC, et al. Acetazolamide vasoreactivity in vascular dementia: a positron emission tomographic study. Eur Neurol. 1999;41(1):31–6.
Yao H, Sadoshima S, Kuwabara Y, et al. Cerebral blood flow and oxygen metabolism in patients with vascular dementia of the Binswanger type. Stroke. 1990;21(12):1694–9.
Tohgi H, Yonezawa H, Takahashi S, et al. Cerebral blood flow and oxygen metabolism in senile dementia of Alzheimer’s type and vascular dementia with deep white matter changes. Neuroradiology. 1998;40(3):131–7.
Tohgi H, Chiba K, Sasaki K, et al. Cerebral perfusion patterns in vascular dementia of Binswanger type compared with senile dementia of Alzheimer type: a SPECT study. J Neurol. 1991;238(7):365–70.
Hanyu H, Shimuzu S, Tanaka Y, et al. Cerebral blood flow patterns in Binswanger’s disease: a SPECT study using three-dimensional stereotactic surface projections. J Neurol Sci. 2004;220(1–2):79–84.
Yang D-W, Kim B-S, Park J-K, et al. Analysis of cerebral blood flow of subcortical vascular dementia with single photon emission computed tomography: Adaptation of statistical parametric mapping. J Neurol Sci. 2002;203–204(Supplement C):199–205.
Shim YS, Yang DW, Kim BS, et al. Comparison of regional cerebral blood flow in two subsets of subcortical ischemic vascular dementia: statistical parametric mapping analysis of SPECT. J Neurol Sci. 2006;250(1–2):85–91.
Wahlund L-O, Bronge L, Savitcheva I, Axelsson R. Neuroimaging in vascular covnitive impairment: Structural and functional imaging in vascular dementia, Oxford textbook of neuroimaging. Oxford University Press, USA, 2015.
Mendez MF, Ottowitz W, Brown CV, et al. Dementia with leukoaraiosis: clinical differentiation by temporoparietal hypometabolism on (18)FDG-PET imaging. Dement Geriatr Cogn Disord. 1999;10(6):518–25.
Pascual B, Prieto E, Arbizu J, et al. Brain glucose metabolism in vascular white matter disease with dementia: differentiation from Alzheimer disease. Stroke. 2010;41(12):2889–93.
Kerrouche N, Herholz K, Mielke R, et al. 18FDG PET in vascular dementia: differentiation from Alzheimer’s disease using voxel-based multivariate analysis. J Cereb Blood Flow Metab. 2006;26(9):1213–21.
Seo SW, Cho SS, Park A, et al. Subcortical vascular versus amnestic mild cognitive impairment: comparison of cerebral glucose metabolism. J Neuroimaging. 2009;19(3):213–9.
Tullberg M, Fletcher E, DeCarli C, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63(2):246–53.
Suri S, Topiwala A, Mackay CE, et al. Using structural and diffusion magnetic resonance imaging to differentiate the dementias. Curr Neurol Neurosci Rep. 2014;14(9):475.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Kim, G.H., Hwang, J., Jeong, J.H. (2020). Neuroimaging Characteristics of Subcortical Vascular Cognitive Impairment. In: Lee, SH., Lim, JS. (eds) Stroke Revisited: Vascular Cognitive Impairment. Stroke Revisited. Springer, Singapore. https://doi.org/10.1007/978-981-10-1433-8_10
Download citation
DOI: https://doi.org/10.1007/978-981-10-1433-8_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-1432-1
Online ISBN: 978-981-10-1433-8
eBook Packages: MedicineMedicine (R0)