Abstract
There are several mechanisms by which cells communicate with each other. Evidence accumulates that the evolutionary oldest mechanisms of cell-cell communication involves extracellular microvesicles (ExMVs). Generally, these circular membrane fragments enriched for mRNA, miRNA, proteins, and bioactive lipids are released by exocytosis from endosomal compartment or are directly formed by budding from cell surface membranes. ExMVs from endosomal compartment called exosomes are smaller in size ~100 nM as compared to larger ones released from cell membranes that are in size up to 1 μM. In this chapter we will present an emerging link between ExMVs and recently identified novel cell-cell communication network involving a new type of cell known as telocyte. Mounting evidence accumulates that telocytes mediate several of their biological effects in several organs by releasing ExMVs enriched in mRNA, miRNA, proteins, and several biological mediators to the target cells.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Extracellular microvesicles (ExMVs)
- Telocytes
- Exosomes
- Ectosomes
- Multivesicular cargo
- Cell-cell communication
- Horizontal transfer of mRNA
- Membrane lipid rafts
3.1 Introduction
Cells communicate with each other and exchange biological information by employing different mechanisms [4, 14, 15, 17, 20–22]. The most important cell-cell communication systems are based on (i) secreted growth factors, cytokines, chemokines, and small molecular mediators (e.g., extracellular nucleotides, bioactive lipids, ROS, and nitric oxide ions), (ii) cell to cell adhesion contacts mediated by sets of specialized adhesion molecule-ligand interactions, (iii) exchanging information by means of tunneling nanotubules, and (iv) what is a subject of this chapter by circular membrane fragments called extracellular microvesicles (ExMVs), a mechanism that for many years has been largely overlooked [4, 14, 20, 21, 23].
ExMVs are small circular membrane fragments secreted from the endosomal compartment known as exosomes or shed from the cell surface by blebbing of cell surface membrane and play an important role in cell-cell communication [10, 20]. This intriguing ExMV-mediated communication system emerged very early during evolution and most likely served as a template for the further development of cell-cell interaction mechanisms involving soluble bioactive mediators and fine-tuned ligand-receptor interactions. ExMVs as mediators of physiological cell-cell communication are different from apoptotic bodies and other cell fragments that emerge in conditions related to irreversible cell damage [4, 20]. Nevertheless, their overall small size and similarity to cellular debris/fragments or apoptotic bodies is one of the reasons that biological significance of ExMVs for many years has been somehow underappreciated.
However, recent augmenting evidence accumulates to show that these tiny membrane fragments orchestrate several biological responses [4, 14, 15, 17, 20–23]. ExMVs contain numerous cell surface proteins and lipids similar to those present in the membranes of the cells from which they originate [12, 19]. As demonstrated, ExMVs may stimulate target cells directly by surface-expressed ligands acting as a kind of “signaling device” [12, 19]. They may also transfer cell surface receptors between various cells [16, 18]. These receptors after transfer may remain functional and change a surface phenotype of the target cells [19]. Furthermore, since they engulf some cytoplasm during membrane blebbing, they may also contain intracellular proteins, mRNA, and miRNA, and as we have demonstrated, they are involved in horizontal transfer of functional mRNA species between cells [5, 16, 18]. ExMVs are also enriched in bioactive lipids (e.g., sphingosine-1-phosphate, ceramide-1-phosphate) and extracellular signaling nucleotides (e.g., ATP, ADP, AMP, adenosine) that all may induce biological responses of the target cells after exposure to ExMVs [20, 21].
More importantly what is highly relevant for this chapter is that ExMVs have been recently implied to be involved in biological effects mediated by novel interstitial (stromal) cell type known as telocytes [5–9]. These intriguing cells are present in different organs including, e.g., the heart, kidney, lung, esophagus, intestine, reproductive system, and skin [7, 10]. Telocytes are CD34+/PDGFα+ cells and are characterized by small cell bodies (9–15 μM) that give rise to extremely long one to five thin tubular processes as compared to cell body, called telopodes [7]. Electron microscopy studies revealed that telopodes are up to 100 μM long yet 80–300 nm in diameter and are not homogenous but consist of short dilatations known as podomes (250–300 nm) and long thin tubes that connect podomes known as podomers (~80 nm) [7, 11]. The overall size of telocyte can reach up to 1,000 μM [7, 11]. Enlarged fragments of telopodes – podomes – are abundant in mitochondria, endoplasmatic reticulum, and planar and invaginated surface membrane lipid rafts [5–9]. As it will be discussed in this chapter, telocyte-derived ExMVs play an important role in several biological effects of telocytes related to physiological adult body homeostasis and tissue/organ regeneration and may even be involved in some pathological processes [10].
Overall, telocytes represent an evolutionary conserved type of interstitial cells and have been described in multiple species including fish, reptiles, birds, and mammals [7]. Telocytes have been also reported to express some stem cell markers including c-kit and Sca-1 (mouse) and even express the intranuclear stem cell pluripotency marker Oct-4 [7, 22]. The telocyte markers, however, may vary between tissues and anatomical location of these cells in a given tissue [7]. This demonstrates somehow some degree of existing heterogeneity among these cells that may be dictated and epigenetically enforced by the microenvironment in which these cells reside.
3.2 The Mechanisms Involving ExMV Release from the Cells
It is well documented that telocytes identified in many tissues secrete ExMVs [2, 5–9]. However, an open question remains if telocyte-derived ExMVs are released into extracellular space as it occurs in all types of cells secreting these circular fragments or in some cases telocytes as postulated may additionally form “bridging nanostructures” to the target cells (e.g., stem cells residing in stem cell niches) as a route for the transfer of exosomes [7, 22, 23]. This latter intriguing possibility has been described, for example, during exosome delivery from telocytes to lung stem cells [7, 22].
Nevertheless, the most common mechanism of ExMV signaling and biological cargo delivery to the cells is mediated by their release from cells by (i) exocytosis from multivesicular bodies or (ii) by shedding from the cell surface membrane [2, 7]. The process of ExMV formation is energy consumption dependent, and it is still not very well known from a mechanistic point of view, although some of the crucial steps have been already identified [1, 3, 12, 13, 23].
The above mentioned endosomal cell membrane compartment-derived smaller exosomes are released from cells during exocytosis often together with proteins secreted from the Golgi apparatus. The first step in the creation of multivesicular bodies enriched for intraluminal vesicles, which are precursors of exosomes, requires involvement of the so-called endosomal sorting complex required for transport (ESCRT) machinery [7]. After intraluminal vesicles are formed, in a next step, multivesicular bodies may fuse with lysosomes, and their content becomes degraded or they may fuse with plasma membrane to release intraluminal vesicles (exosomes) from the cells into extracellular space [12, 13]. This process requires involvement of Rab GTPases (e.g., Rab 27a, Rab 27b, and Rab 11) [13]. Exosomes were reported to express some characteristic surface proteins including Alix, CD63, CD9, CD81, HSP70, and TSG101 (ESCRT machinery) [1]. In contrast cell surface-derived larger ExMVs are shed from the cell surface membrane by blebbing in response to cell stimulation that leads to a cytosolic Ca2+ increase that promotes changes in the structure of cell membrane [10, 19]. Blebbing of cell membrane and formation of ExMVs occur mostly in cholesterol-enriched fragments of cell membrane known as lipid rafts [19].
The cell cytoplasmic membrane consists of a phospholipid bilayer with embedded proteins that is held together via non-covalent interactions between the hydrophobic tails. Moreover, the cytoplasmic membrane has an asymmetric distribution of phospholipids including aminophospholipids, phosphatidylserine, and phosphatidylethanolamine that as demonstrated are specifically sequestered in the inner membrane leaflet [1, 3, 12, 13]. This transmembrane lipid distribution is under the control of three phospholipidic pumps: (i) flippase, (ii) floppase, and (iii) lipid scramblase. The latter phospholipidic pump is responsible for nonspecific redistribution of lipids across the cytoplasmic membrane [10].
It is known that the phospholipid molecules in the cell membrane are in a liquid crystalline state and contain distinguished combinations of glycosphingolipids and protein receptors organized into glycoprotein microdomains that are known in literature as membrane lipid rafts [19]. There are two described types of lipid rafts in cell membranes: (i) planar lipid rafts and (ii) invaginated lipid rafts, called caveolae [19]. Lipid rafts are cholesterol-enriched microdomains in the cell membrane, and cholesterol can be envisioned as a kind of molecular glue that holds the components of lipid rafts together and is important for their integrity [19]. It has been proposed that the loss of phospholipid asymmetry of the cytoplasmic membrane, which leads to phosphatidylserine exposure on cell surface, and a transient phospholipidic imbalance between the externals, at the expense of the inner leaflet caused by lipid scramblase, results in blebbing of the plasma membrane and ExMVs releasing from the areas enriched in lipid rafts [1, 10, 19].
As described for several types of cells during the blebbing process of the cytoplasm membrane, a fragment of cytoplasm that contains mRNA, miRNA, proteins, and even organelles (e.g., mitochondria) is encapsulated into ExMVs [11]. Evidence accumulates that this process of enrichment for mRNA or miRNA species is not random but somehow regulated by proteins involved in mRNA and miRNA storage, transport, and processing [1, 10, 19].
Telocytes as a cell also producing ExMVs are somehow unique. It has been described that telocytes may secrete three types of ExMVs including not only classical (i) endosomal cell membrane compartment-derived exosomes (45 ± 8 nm) and (ii) larger ExMVs (ectosomes) corresponding to small ExMVs shed from the cell membranes (128 ± 28 nm) but also a novel type of ExMVs (iii) described as multivesicular cargo (1 ± 0.4 μM) that are large ExMVs containing tightly packed endomembrane-bound smaller vesicles (145 ± 35 nm) [7]. This interesting new type of ExMVs has been described initially to be secreted by telocytes in myocardium and as postulated involved in paracrine effects of these cells residing in a normal heart where they form tridimensional structure connected with all the types of cells present in this organ including cardiac stem cells and cardiomyocyte progenitors [2, 7].
It is known that ExMVs that originate from the blebbing of cell membrane express several receptors present on the cell surface, and future studies are needed to characterize these receptors on telocyte-derived ExMVs. This will facilitate their detection in the tissues as well as allow to assess if they can contribute to the pool of circulating ExMVs in peripheral blood and lymph [14, 17].
3.3 The Physiological Effects of Telocyte-Derived ExMVs
Mounting evidence accumulates that ExMVs are mediators for several long-distance paracrine functions of telocytes residing in adult organs. Telocytes may affect biology of several cell types including differentiated somatic cells as well as tissue-residing stem cells [7, 16–18]. As reported they are in contact with stem cell niches, blood capillaries, and nerve bundles as well as collagen and elastic fibers [7]. Thus, telocytes most likely regulate blood rheology, muscle tonus, as well as their motoric activity [5–11]. Further work is needed to elucidate how these interactions are regulated via paracrine signals from telocytes including the release of ExMVs.
ExMV-mediated paracrine effects are based, as described for other types of cells [16, 18], on direct stimulation of target cells by ExMV-expressed signaling molecules or horizontal transfer between cells of mRNA and proteins [5, 16]. However, in contrast to telocytes themselves [7], telocyte-derived ExMVs have not been characterized yet for their content of mRNA and miRNA species or proteins. Nevertheless, taking into consideration that telocytes express several miRNAs that possess pro-angiopoietic potential (e.g., miR-126, miR-130, let-7e, miR-100), the horizontal transfer of these miRNAs via ExMVs to the target cells may promote angiogenesis in the damaged tissues. A similar role has been also postulated for ExMVs secreted by other types of cells [3, 16]. Moreover, a fact that telocytes play a role in several organs in ameliorating oxidative stress and aging and stimulate proliferation and inhibit apoptosis lends support for further studies to identify secretome of telocytes along with molecular composition of ExMVs that are involved in all of these processes. Such studies could identify important factors involved in keeping the homeostasis of adult tissues. We could envision that similarly for other ExMV-producing cells [3, 21, 23], telocytes may augment ExMV secretion in response to hypoxia, inflammation, and tissue/organ damage after stimulation by some inflammatory cytokines as well as after exposure to activated components of complement and coagulation cascades. Elucidation of these possibilities may better explain involvement of telocytes in tissue/organ regeneration [7].
Similarly, since telocytes have been reported to play a role in immunosurveillance and interact with cells being involved in innate and acquired immunity, further studies are needed to show how much this interaction involves telocyte-ExMVs and vice versa if telocytes may respond to ExMVs secreted by cells involved in immune responses. We have to remember that ExMV-directed crosstalk between cells is a two-way street [24], and we have to consider that telocytes most likely also respond to ExMVs secreted by surrounding cells.
In frame of the last possibility, it is tempting to hypothesize that telocytes could be involved in the distribution and trafficking of ExMVs secreted by other types of cells and could deliver such “third-party” ExMVs to the target cells via telopodes and the abovementioned “bridging nanostructures” [22]. Moreover, there is no doubt that the development of pharmacological strategies to modulate the secretion of ExMVs from telocytes may turn out to be an important means to enhance at the paracrine level important influences of these cells in maintaining tissue homeostasis.
3.4 The Role of Telocyte-Derived ExMVs in Pathology
Telocytes may ameliorate several pathological processes. For example, since telocyte numbers decrease in infarcted myocardium [7], the therapeutic injection of exogenous telocytes to a damaged heart reduced infarct size and leads to improved heart function [7]. Based on this it is a possibility that telocyte-derived ExMVs could exert a very similar effect and replace treatment by intact telocytes. To support this latter notion, mesenchymal stem cell-derived ExMVs have been demonstrated to have a similar biological therapeutic efficacy as intact cells [3]. On the other hand, one can envision that telocyte-derived induced pluripotent stem cells (iPSCs), as cells endowed with telocyte-characteristic epigenetic memory, could be potentially employed as a source of therapeutic ExMVs [20, 21]. Such ExMVs could also be employed in several other clinical situations where telocytes have been demonstrated to play a positive therapeutic role, e.g., in lung pathology, liver regeneration, ameliorating scleroderma, and improving the function of the digestive and reproductive systems [7].
However, telocyte function is mostly related to their supportive role in organ and tissue homeostasis; it has been recently reported that telocytes may be responsible for the origin of some malignancies. Accordingly, telocytes may promote proliferation of breast cancer cells and inhibit their apoptosis [22]. Obviously, in this particular case the pro-proliferative effect of telocytes has been activated at the wrong time and the wrong place. How much this effect depends on ExMVs and how valid this effect is in other types of malignancies require further studies.
3.5 Conclusions and Future Directions
Evidence has accumulated that paracrine effects of telocytes in adult tissues largely depend on secretion of ExMVs. In this respect telocytes are somehow unique cells since, in addition to classical exosomes and cytoplasmic cell membrane-derived ExMVs, they also secrete large ExMV characteristic for telocytes known as multicellular cargo vesicles [7].
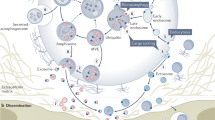
Further studies are necessary to identify employing mRNA and miRNA arrays, proteomics, and lipidomic analysis molecular “cargo” present in telocyte-derived ExMVs as well as to elucidate and understand mechanisms that promote their secretion. Taking into consideration an important role telocytes play in organ and tissue homeostasis development of pharmacological strategies to modulate/enhance secretion of ExMVs, these cells may lead to better treatment strategies in all these situations where telocytes have been demonstrated to be of benefit. Finally, it is tempting to postulate the established telocyte-derived induced pluripotent stem cells (iPSC) that could be employed as ExMV-producing cell lines [20, 21]. Such iPSC immortalized cell lines would be endowed with telocyte-epigenetic memory, and ExMVs harvested from these cells could be employed for therapeutic purposes. All these intriguing possibilities are depicted in Fig. 3.1. We may expect that the next few years will provide us with more information about the paracrine effects of telocytes in regulating body homeostasis and will also lead to the development of therapeutic strategies to employ these cells or telocyte-based ExMVs in the clinic.
Future potential strategies of telocyte-derived ExMVs in modulating bioactive function of telocytes. Panel (a): Telocytes secrete among paracrine factors circular membrane fragments known as ExMVs. Panel (b): Development of pharmacological strategies to augment secretion of ExMVs from telocytes may enhance involvement of these cells in maintaining tissue/organ homeostasis. Panel (c): Telocyte-derived induced pluripotent stem cells (iPSCs) endowed with telocyte-epigenetic memory could be a source of therapeutic ExMVs employed in situations where beneficial effects of telocytes have been demonstrated (e.g., impaired function of myocardium after heart infarct)
References
Adell MA, Vogel GF, Pakdel M, Müller M, Lindner H, Hess MW, Teis D. Coordinated binding of Vps4 to ESCRT-III drives membrane neck constriction during MVB vesicle formation. J Cell Biol. 2014;205(1):33–49.
Albulescu R, Tanase C, Codrici E, Popescu DI, Cretoiu SM, Popescu LM. The secretome of myocardial telocytes modulates the activity of cardiac stem cells. J Cell Mol Med. 2015;19(8):1783–94.
Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7(3):e33115.
Camussi G, Quesenberry PJ. Perspectives on the potential therapeutic uses of vesicles. Exosomes Microvesicles. 2013;1(6). doi:10.5772/57393.
Cismaşiu VB, Popescu LM. Telocytes transfer extracellular vesicles loaded with microRNAs to stem cells. J Cell Mol Med. 2015;19(2):351–8.
Cretoiu D, Gherghiceanu M, Hummel E, Zimmermann H, Simionescu O, Popescu LM. FIB-SEM tomography of human skin telocytes and their extracellular vesicles. J Cell Mol Med. 2015;19(4):714–22.
Cretoiu SM, Popescu LM. Telocytes revisited. Biomol Concepts. 2014;5(5):353–69.
Creţoiu SM, Creţoiu D, Popescu LM. Human myometrium – the ultrastructural 3D network of telocytes. J Cell Mol Med. 2012;16(11):2844–9.
Cretoiu SM, Cretoiu D, Marin A, Radu BM, Popescu LM. Telocytes: ultrastructural, immunohistochemical and electrophysiological characteristics in human myometrium. Reproduction. 2013;145(4):357–70.
Enjeti AK, Lincz LF, Seldon M. Microparticles in health and disease. Semin Thromb Hemost. 2008;34(7):683–91.
Fertig ET, Gherghiceanu M, Popescu LM. Extracellular vesicles release by cardiac telocytes: electron microscopy and electron tomography. J Cell Mol Med. 2014;18(10):1938–43.
Hugel B, Martínez MC, Kunzelmann C, Freyssinet JM. Membrane microparticles: two sides of the coin. Physiology (Bethesda). 2005;20:22–7.
Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30. sup pp 1–13.
Quesenberry PJ, Aliotta JM. The paradoxical dynamism of marrow stem cells: considerations of stem cells, niches, and microvesicles. Stem Cell Rev. 2008;4(3):137–47.
Quesenberry PJ, Aliotta J, Deregibus MC, Camussi G. Role of extracellular RNA-carrying vesicles in cell differentiation and reprogramming. Stem Cell Res Ther. 2015;6:153.
Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20(5):847–56.
Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20(9):1487–95.
Ratajczak J, Kucia M, Mierzejewska K, Marlicz W, Pietrzkowski Z, Wojakowski W, Greco NJ, Tendera M, Ratajczak MZ. Paracrine proangiopoietic effects of human umbilical cord blood-derived purified CD133+ cells – implications for stem cell therapies in regenerative medicine. Stem Cells Dev. 2013;22(3):422–30.
Ratajczak MZ, Adamiak M. Membrane lipid rafts, master regulators of hematopoietic stem cell retention in bone marrow and their trafficking. Leukemia. 2015;29(7):1452–7.
Ratajczak MZ, Kucia M, Jadczyk T, Greco NJ, Wojakowski W, Tendera M, Ratajczak J. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia. 2012;26(6):1166–73.
Ratajczak MZ, Jadczyk T, Pędziwiatr D, Wojakowski W. New advances in stem cell research: practical implications for regenerative medicine. Pol Arch Med Wewn. 2014;124(7–8):417–26.
Smythies J. Intercellular signaling in cancer-the SMT and TOFT hypotheses, exosomes, telocytes and metastases: is the messenger in the message? J Cancer. 2015;6(7):604–9.
Smythies J, Edelstein L. Telocytes, exosomes, gap junctions and the cytoskeleton: the makings of a primitive nervous system? Front Cell Neurosci. 2014;7:278.
Wysoczynski M, Ratajczak MZ. Lung cancer secreted microvesicles: underappreciated modulators of microenvironment in expanding tumors. Int J Cancer. 2009;125(7):1595–603.
Acknowledgments
This work was supported by NIH grants 2R01 DK074720 and R01HL112788, the Stella and Henry Endowment, and Maestro grant 2011/02/A/NZ4/00035 to MZR.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Ratajczak, M.Z., Ratajczak, D., Pedziwiatr, D. (2016). Extracellular Microvesicles (ExMVs) in Cell to Cell Communication: A Role of Telocytes. In: Wang, X., Cretoiu, D. (eds) Telocytes. Advances in Experimental Medicine and Biology, vol 913. Springer, Singapore. https://doi.org/10.1007/978-981-10-1061-3_3
Download citation
DOI: https://doi.org/10.1007/978-981-10-1061-3_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-1060-6
Online ISBN: 978-981-10-1061-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)