Abstract
This chapter summarizes 200 years of the study of cichlid fishes, ranging from the first descriptions of South American and African species in early natural histories to the work of twentieth-century biologists. The rise and influence of evolutionary theory and the development of new knowledge and techniques in the study of genetics are considered central influences in a variety of fields of research involving cichlids, including ecology, ethology, aquaculture, and fisheries. Significant developments and their historical context are considered in relation to the current state of a variety of cichlid research programs, showcasing the extent to which cichlids have become both model species in evolutionary biology and a crucial species in global food production.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Natural history
- Modern science
- Colonial science
- British imperialism
- Darwinian synthesis
- Environmental history
- Tilapia fisheries management
- Biological species
- Allopatric speciation
- International science
1 Cichlids in Natural History
To have more Aristotles, more Alexanders were needed. Positive Natural History requires work and expense that a private person without patronage cannot afford.—Georges Cuvier
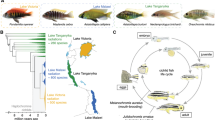
For much of their history as objects of scientific inquiry, cichlid taxonomy has been in question. Today, cichlids are classified as teleosts, an ancient class of ray-finned, “true bone” fishes that account for 95% of extant fish species (Barlow 2000). The family Cichlidae, traditionally belonging to the Perciformes suborder of Labroidei, is most recently classified as a member of the order Cichliformes and includes over 1700 species (Nelson et al. 2016; Fricke et al. 2020). With some estimates as high as 3000 species, cichlids are not only representative of 10% of teleost fishes, they are one of the most diverse families of extant vertebrates (Helfman et al. 2009). Cichlids are believed to have evolved around 160 million years ago (Barlow 2000). During this time, Gondwanaland, the supercontinent composed of present-day Africa and South America, broke up and the southern Atlantic opened, separating founding populations of cichlids that radiated extensively.
The greatest cichlid biodiversity is found in The Great Lakes of East Africa. With estimates on its initial basin formation ranging between 9 and 12 million years before present, Lake Tanganyika is the oldest of the African Great Lakes. It is home to 24 fish families, more than any other lake, with 18 occurring in the tributaries and marshes around the lake. Malawi, between 1 and 2 million years old, is home to over 500 species (possibly as many as 1000 or more). Lake Victoria—the second largest lake on earth and roughly the size of Switzerland—is around 400,000 years old and has dried out completely at least three times over that period. Its basin is believed to have refilled from one such drought a mere 14,700 years ago. Since that time, it has become home to at least 400 species, although it is difficult to say in the wake of recent ecological disasters how many cichlids once existed there or how many now remain (Barlow 2000). Cichlids also inhabit lakes, marshes, floodplains, many river systems in central and northern Africa and Madagascar. Only 20,000 years ago, the Kalahari Desert was an enormous lake called Makgadikgadi. Throughout the Pleistocene, a rapidly evolving radiation of cichlids comparable in morphological diversity to the extant African Great Lakes thrived there (Joyce et al. 2005). Among these were the serranochromine cichlids, and it is now believed that when the lake dried up around 10,000 years ago, the major river systems of Southern Africa were “seeded” with serranochromines (Lowe-McConnell 2006).
In Southeast Asia, and Central and South America, cichlids are predominantly of the riverine variety. In each of these unique locations and environments, species are equipped with a dazzling array of biological variation in coloration, diet, mating, and parental behaviors. This astounding diversity is likely the driver for their popularity among aquarists. It is also the root of a great question surrounding these amazing animals: How have they evolved so quickly? Humans have only recently endeavored to answer this question, but the history between humans and cichlids is far older.
The Nile, with its headwaters at Lake Victoria, is home to tilapiine cichlids, and Egyptians farmed Nile Tilapia as long ago as the third millennium BCE. Ancient Egyptians called the fish Bolti, and revered it as a symbol of rebirth (tilapias recur in Egyptian art with lotus blossoms emerging from their mouths and are thought to signify resurrection). Humans have long treated cichlids as a valuable food source. Ranging from Central Africa to Israel and Jordan, S. galilaeus has historically supplied the fisheries of the Jordan Valley (Barlow 2000). The species is called “St. Peter’s Fish” after a miracle in the Book of Matthew, wherein Peter (following his teacher’s instruction) catches one of the fish, reaches into the tilapia’s buccal cavity (where one might normally find a school of young), and retrieves a coin to pay the temple tax. Tilapias have also supported fisheries carried out by Africans using homemade seines and palm leaf ropes since at least 1865 when David Livingstone documented the practice (Lowe-McConnell 2006). Subsistence fisheries in Africa remain crucial today. For example, Malawi fish provided 75% of the animal protein consumed in Malawi as of 1994 (Barlow, 2000). At the same time, tilapia have become the leading fish for aquaculture in the world in the twenty-first century, with Tel Aviv University in Israel remaining a leading center of tilapia culture research (Beveridge and McAndrew 2000). Cichlid fisheries are both ancient and ongoing practices.
Fishes were often neglected as subjects of natural histories until the middle of the sixteenth century, and descriptions of specific cichlid species do not appear for another two centuries (Cuvier 1828). The first scientific classifications occurred in the late eighteenth and nineteenth centuries when colonial expansion reached its zenith. During this time, the Americas and Africa were explored, mapped, and evaluated as resources. In this new, global arena of research, a naturalist tradition that had been rekindled centuries earlier grew wildly popular among amateurs and professionals alike, bringing the stunning biodiversity of cichlids from both sides of the Atlantic to the attention of the Western scientific world.
1.1 Traditions in Taxonomy: 1750–1800
Until the end of the 1790s, most natural history collections were in personal cabinets containing shells, minerals, ancient coins, and books; they were considered fashionable possessions, every bit as aesthetic as they were scientific (Farber 2000). When the classification of animal specimens became popular, collections were often gathered by medical students who had anatomic knowledge valuable for description. The interest of university scholars in natural specimens, combined with the aristocratic implications of such collections, conveyed social status on owners of such cabinets of curiosities. Royalty, such as the King and Queen of Sweden, had their own cabinets, and in 1751, Carl Linnaeus studied these royal collections (Fernholm and Wheeler 1983). As the first curator of the Swedish Museum of Natural History (known at the time as The Academy Zoological Collection), Linnaeus acquired many descriptions of species and specimens for The Ichthyological Collection Building—founded 1739—through his “apostles” on their journeys abroad. Aside from his more educated volunteers, Linnaeus and other renowned natural historians were aided by enthusiasts who attempted classifications of animals, plants, and minerals using Linnaean binomial taxonomy (Farber 2000). Often, collections were produced by naval officers performing other functions on board their ship (frequently surgeons). Such collections were returned home to the care of wealthy private collectors, and many were given as gifts to royal families or other members of the social elite.
In his 1758 10th edition of Systema Naturae , Linnaeus described the North African Oreochromis niloticus (currently the aquaculture tilapia of choice) and S. galilaeus . He also described the South American Cichlasoma bimaculatum under the genus Labrus , the first species of Cichlasoma ever to be described. The same species was described as if it were a new species altogether—Chromys punctate —by Marcus Elieser Bloch, who from 1782 through 1795 classified various South American, Asian, and African species based on collections sent to Berlin. Misappropriations and errors in systematics were characteristic of taxonomic work involving cichlids in the late eighteenth and the nineteenth centuries. Their remarkably recent speciation, along with frequent cases of convergent evolution, made this family an especially challenging subject in an era in which the concepts of evolution, as well as the disciplines of biology and ichthyology, were just taking shape.
Adolf Frederick and Louise Ulrika of Sweden were not the only royalty to support the growth of Natural History. In 1793, the Royal Garden at Paris was reorganized and renamed the National Museum d’histoire Naturelle under the direction of George-Louis Leclerc Comte de Buffon. It contained collections of fishes formerly of the King’s Cabinet, which was composed of specimens acquired on voyages around the world , including fishes brought back from the Egyptian expedition (1798–1799) of Francois Peron and Charles Lesueur during Nicolas Baudin’s voyage around the world (1800–1804). From 1798 through 1803, Bernard Germain de Lacépède authored Histoire naturelle des poisons (a continuation of Buffon’s volumes of natural history), describing cichlid species of South America, Africa, and Asia. In 1799, Alexander Von Humboldt (considered one of the founders of biogeography, and a profound influence on Charles Darwin and Alfred Russel Wallace) sailed for South America, and his Voyage de Humboldt et Bonpland, Observations de Zoologie (1834) identified several species of Cichla . By establishing a state-sponsored program that offered professorships to trained naturalists and their staff, France had set a new standard (Farber 2000). The Museum of Natural History was a national collection, evidencing the colonial prowess and exploratory accomplishments of an entire nation, and collections grew rapidly in this atmosphere due to the cooperation between museum staff , naval officers, and colonial explorers (Bauchot et al. 1997).
1.2 The Golden Age of Natural History: 1800–1900
By the end of the Napoleonic Wars , the scale of European states’ colonial activities had increased significantly, and in most Western nations, museums were increasingly controlled by municipal governments and associated with the research of renowned universities (Farber 2000). In 1819, the French museum established a program to train traveling naturalists, setting a trend of professionalization of natural history in other countries that coincided with a precipitous rise of naturalists in the field between 1750 and 1850. This can be seen as an important development in the expansion of specific branches of natural history such as zoology (and by the mid-nineteenth century, ichthyology), and it is near the end of this period that the family name Cichlidae first appears, in 1850 (Barlow 2000).
Over the course of the nineteenth century, naturalists from many countries came into contact with cichlid species. Those to describe or classify the most were French, Dutch, German, British, and American. This period marks a general increase in international cooperation between researchers in the effort to establish complete, standardized classifications for species, with trips abroad and contact with other ichthyologists often resulting in loans of interesting specimens, exchanges, and generous gifts to museums (Bauchot et al. 1997). The resulting works were some of the great accomplishments of the naturalist era: Georges Cuvier’s Histoire Naturelle des Poissons (22 volumes published from 1828 to 1849), described over 4000 fish species, fewer than half of which were previously known to science.
One of the first aides at the museum in Paris, and a traveling naturalist, was Cuvier’s student Achillie Valenciennes. In Berlin, Cuvier and Valenciennes studied and described several American cichlid species collected by Bloch and Johann Gottlob Theaenus Schneider, the first to apply the genus Cichla for a group of cichlids native to South America (Bloch and Schneider 1801, Johann Friedrich Hennig edited, expanded, and republished his original work, Systema Ichthyologiae iconibus cx illustratum). The genus name Cichla was based on the Greek “Kichle” for sea fishes like wrasses. In 1829, Valenciennes described Central American cichlids among a collection of Mexican fishes from Ferdinand Depp and Marcus Bloch and in 1831, Cuvier and Valenciennes described additional Asian and South and Central American species. In London, Edward Turner Bennett gave Cuvier access to the collections of the Zoological Society he had described in 1827, as well as the new acquisitions of the British Museum and the Zoological Society. He had identified some South American cichlids himself in 1830. Cuvier also worked alongside the keeper of Zoology at the British Museum of Natural History, John Edward Gray, and Dutch naturalist Laurence Theodore Gronow (also at the British museum) who together collaborated on a catalogue of specimens that included several species of South American cichlids (Gronow 1854).
The Koninklijk Instituut (forerunner of the Royal Netherlands Academy of Sciences) and the Kabinet des Konings were founded in the Netherlands 1806 under Napoleonic rule. After the battle of Waterloo, some natural history collections, confiscated by France during the short-lived government of the French Directory, were returned to Holland and presented to Leiden University by King William in 1815 (Boeseman 1997). Here, Coenraad J. Temminck conceived of an Imperial Dutch Museum that would unite his private collection with the university and royal collection, and in 1820, the Rijksmuseum in Leiden was formed with Temminck as its first director. In 1820, the new museum was named the National or Rijksmuseum van Natuurlijke Historie, and 4 years later, Temminck was on good working terms with Valenciennes, whom he gave access to collections in Leiden. From 1858 on, Pieter Bleeker was responsible for many of these collections (often sent from the East Indies). A Dutch medical doctor for Dutch East Indies Trading Company, Bleeker was a practicing ichthyologist and herpetologist and provided specimens of cichlids from East Asia (1868) and Madagascar (1862–1877). In British East India, cichlids were collected and classified by natural historians and officers of the crown alike, depicted in carefully illustrated panels (e.g., Fig. 1), as in the Orange Chromide depicted below (first classified by Bloch in 1795), found in Sir Francis Day’s 1888 work on the fauna of British East India.
An Orange Chromide. (Reproduced from Day 1888)
Women interested in learning the trade skills of natural illustration, taxidermy, and the classification and cataloguing of natural history specimens found assistant opportunities in museums. Here, they were able to conduct research that universities would have seldom allowed (while some universities offered women undergraduate and graduate degrees in zoology, academic positions were rare). Yet, even in museums where women could contribute to their field, the work was usually considered voluntary; women often received little or no pay (Brown 1994). It was in the Museum of Zoology at the University of Utrecht that Louise Schilthuis, one of the first female ichthyologists, became curatrix and identified species of cichlids in her Collection of fishes from the Congo; with description of some new species.
In 1806, Emperor Franz I opted to enlarge and systematize Germany’s natural history collections, and several large collections of fishes were incorporated. Johann Natterer, assistant at the natural history museum in Vienna, returned from an 1817 expedition to Brazil and recruited the Austrian, Johann Jakob Heckel for museum taxidermy (Herzig-Straschil 1997). Heckel curated the fish collection from 1835, during which time it grew considerably. Heckel remained at Vienna where he catalogued incoming cichlid specimens from the Americas; his extensive paper on Cichlidae in 1840 was based on Natterer’s collections from Brazil.
In 1885, the German government granted Carl Peters, entrepreneur and explorer, an imperial charter to establish a protectorate in the African Great Lakes region (Fig. 2). The colony of East Africa divided Lake Victoria in half and bordered lakes Tanganyika and Malawi (In 1892, the Njassa Sea). The result was the collection and classification of African Lake species of cichlids; In 1888 by F.M. Hilgendorf, in 1893 by Georg Johann Pfeffer, curator of the Natural History Museum at Hamburg (destroyed during World War II), and in 1897 by Max Wilhelm Carl Weber, German professor of anatomy and physiology at University of Amsterdam from 1883. In 1885, Tanzania, Rwanda, and Burundi were placed under the rule of the Imperial German Government, but they were not without competition. Britain had established a colonial administration at Cape Colony in 1807 during the Napoleonic Wars, and Port Elizabeth and Grahamstown received settlers in 1820. In 1858, searching for the source of the Nile, John H. Speke found the headwaters and discovered the southern shore of what local Arabs called “Ukerewe” and the Basuba of Kenya called “Nyanja” (or Nyanza). As the first European to see the lake, he named it in honor of Victoria, Queen of England. In 1875, the American journalist Henry M. Stanley circumnavigated the lake and popularized the name Speke had given it in the Western world (Awange and Ong’ang’a 2006). Soon, Lake Victoria was frequented by missionaries, soldiers, and traders.
Map of Africa. (Reproduced from Johnston and Keane 1878)
Explorations of the other African Great Lakes and central Africa were also underway. In 1859, David Livingstone reached what he called Lake Nyassa (now Malawi), and in 1894, biologist and explorer John Edmund Sharrock Moore led the British Tanganyika Expedition. In the 1890s, Great Britain colonized Kenya and Uganda, followed by Germany’s colonization of the Tanzanian mainland in 1899. The Imperial German Government maintained control over Tanzania, Rwanda, and Burundi until after the First World War, when Tanzania was placed under the British mandate and Rwanda and Burundi came under Belgian rule.
During this time, there were many British and German discoveries of new cichlid species in Africa. From 1834 through 1836, Sir Andrew Smith led a scientific expedition into the interior of South Africa, where many riparian or riverine species of cichlid would be found (Smith 1849). A surgeon and ethnologist, Smith had been ordered to Cape Colony where he met and was inspired by Charles Darwin, who was traveling onboard the H.M.S. Beagle. Darwin himself obtained specimens of several species of South American cichlids during his voyage, which were later classified by Leonard Jenyns in 1842 (an English naturalist who had been offered Darwin’s position on the Beagle first, but apparently turned the opportunity down for health and personal reasons). Considered the father of zoology in South Africa, Andrew Smith’s 1837 work, written upon his return from the colony, was the first to classify cichlids according to the genus Tilapia . Smith was also first superintendent of the South African Natural History Museum at Cape Town, which opened in the 1820s (Gon and Skelton 1997). It is presumed that Smith, who met the Lord Somerset, Governor of the Cape, in 1825 and proposed the museum, had produced his own collection from his time in Grahamstown, which became the museum. By 1840, the museum was essentially derelict, its remaining collections passed to the South African College. Governor Sir George Grey hired E.L. Layard in 1854 to maintain the museum, which was reinstated in the 1850s (Gon and Skelton 1997). Many cichlid specimens were sent to George Albert Boulenger in London, who classified them in 1898. Boulenger had been invited to the British Museum of Natural History by its keeper of zoology, Albert Günther, whose 1864 Descriptions of new species of Batrachians from West Africa was the first description of the Midas cichlid. Both men classified and catalogued a great number of cichlid species from South America, Africa (Fig. 3), and Madagascar in the late nineteenth century.
British and German East African colonial holdings. (Reproduced from Lucas 1897)
South African museums developed out of a need to raise public and official financial support, often creating opportunities for local involvement. The Albany museum for example—which opened 1855—declared that any man in the eastern province could be admitted as a member, the goal being the creation a local scientific research society (Gon and Skelton 1997). However, it was not a man of science, but one Miss Mary Glanville in 1882 who was the first paid curatrix of the Albany Museum. Her hope was that the fisheries increasing on the coast of Port Alfred would drive collections of fish and marine mammals. With the exception of the Albany Museum’s self-trained J.L.B. Smith, no ichthyologists were working in museums as curators or researchers from 1895 to 1950, but the South African Government saw aquatic research as increasingly valuable. Thus, from 1896, the Marine Biological Survey, now called Sea Fisheries Research Institute, was created and run by John D. F. Gilchrist, chair of the Department of Zoology at the South African College, and Honorary Keeper at the South African Museum. He enlisted Boulenger in London to help with species identifications, and many specimens sent by Gilchrist were used for Boulenger’s and Johnston’s catalogues (Johnston 1916) of African freshwater fishes (Gon and Skelton 1997).
On the other side of the Atlantic, cichlids from Central and South America (Fig. 4) remained by far the most represented in collections, and by mid-century, naturalists in Massachusetts, Philadelphia, and Washington all possessed specimens. In 1859, Jean Louis Agassiz, a biologist and geologist who studied with Cuvier and Humboldt in Paris, founded the Museum of Comparative Zoology at Harvard. Austrian zoologist Franz Steidachner visited Agassiz at Harvard to work on South American fishes from the Thayer Expedition in 1869, and collected additional species on a trip to South America with Agassiz in 1871 (Herzig-Straschil 1997).
Map of South America. (Reproduced from Johnson 1862)
Down the coast at the Academy in Philadelphia, Theodore Gill was wildly prolific. In 1862, his Remarks on the relations of the genera and other groups of Cuban fishes identified cichlids in the Caribbean, and in 1877, having moved to the Smithsonian Institution in Washington, DC, Gill and Bransford described central American cichlids in their Synopsis of the fishes of Lake Nicaragua as part of the Panama Canal Surveys then ongoing through the US government. After he left Philadelphia, a personal museum sprang up in Gill’s absence maintained by Edward Drinker Cope. Cope’s work bankrupted him, but he amassed the largest collection of fishes at the Academy in Philadelphia ever; until, that is, Henry Weed Fowler became curator of cold-blooded vertebrates at the Academy of Natural Sciences, Philadelphia and vastly increased the institution’s collection and ichthyological research (Smith-Vaniz and McCracken Peck 1997). Cope’s Contribution to the ichthyology of the Marañon 1869 detailed South American cichlid species. Both men were students of David Starr Jordan.
During his time at Cornell, Cope befriended Jordan, now considered the father of American Ichthyology. Jordan had studied under Agassiz during summer session at his Penikese Island field school in Massachusetts, and worked with Albert Günther before starting his own Ichthyology program at Stanford in 1891 (Brittan 1997). In 1899, along with J.O. Snyder, he identified cichlids from the Americas. Jordan had many prominent students, including Carl Eigenmann, and his wife Rosa Smith Eigennmen, considered the first woman in ichthyology in the United States (Brown 1994). After marrying, the pair left for Harvard to work on Agassiz’s mostly unstudied collections from Brazil. Together, they described many fishes in the Americas, including cichlids, and especially those discovered on a trip to British Guiana funded by the Carnegie Museum (1894). Both Carl and Rosa had studied underneath Jordan at Indiana University; most of the early students of ichthyology in North America were either resident or corresponding members of the academy sharing their knowledge (if not always their specimens) with their fellows (Smith-Vaniz and McCracken Peck 1997).
The natural history museum was an important academic center of this expanding intellectual exchange, both in America and abroad. By 1900, Germany had 150 natural history museums, Great Britain 250, France 300, and the United States 250. Cape Town, São Paulo, and Buenos Aires opened museums that built on local collections and purchased specimens from commercial houses in Europe (Farber 2000). Many of these museums contained cichlid specimens, and each relied on the work of countless naturalists who classified and discovered species all over the globe during the golden age of naturalism. While some names stand out in the history of the taxonomic tradition coming out of centers of learning such as Paris, Berlin, London, or Massachusetts, they are joined by so many contributing amateurs and professionals that to list them all here is impossible. It is difficult to imagine the conditions under which the voyages of 200 years ago were carried out by such individuals. To circumnavigate the world took years and involved travel to locations that had been hitherto entirely unknown. Collections work in natural history had become increasingly scientific, but the motivations for a scientist to go exploring often remained as romantic as they were intellectual.
2 Pioneers of Cichlid Research
Today, when a young scientist can leap into a plane and arrive at a well-equipped laboratory, complete with modern sampling devices and computers, to study some aspect of a specialist subject, it is difficult to realize what was then involved in such a prototype enterprise.—Rosemary Lowe-McConnell
By the turn of the century, natural history was being replaced in academia by biology. This was largely due to the work of Thomas Henry Huxley, who proposed in the late nineteenth century that the science of biology not be restricted to physiology, but should encompass the entire study of life, from the cell to evolution of the organism. This influenced the rise of biology departments offering graduate training in laboratory work with both physiology and morphology, previously the purview of natural history (Farber 2000). Huxley was also popular for his defense of natural selection, which by 1900 was generally accepted as the mechanism of evolutionary change (even if the heritability of this change was not well understood). By this time, ichthyologists interested in evolutionary biology were aware of the biodiversity of the African Great Lakes cichlids, and focus began to shift from African and New World riverine species to those in the Great Lakes (Barlow 2000).
Journeys to and from research locations in Africa could require months of travel time and might expose the traveler to malaria and other tropical diseases; a sleeping sickness epidemic in the Lake George region, for example, prevented cichlid research from 1931 to the late 1940s (Lowe-McConnell 2006). These dangers, along with the occasional need to shoot a crocodile with an elephant gun, made the study of cichlids in the African Great Lakes somewhat prohibitive. Yet, the naturalist penchant for exploration remained, even as natural history as a field was transforming. The ways in which specialized areas of cichlid research were involved in a larger scientific conversation concerning the nature of speciation will be treated in subsequent chapters. Here, we will examine the context in which those pioneering cichlid scientists operated—in this case—often under the aegis of increasing fisheries and aquaculture of tropical species, both in Africa and in Central and South America.
2.1 The Shift from Natural History and the Legacy of The Crown: 1900–1930
Many ichthyologists interested in the study of cichlids retained strong ties to prominent research museums and continued to contribute to classification. Axel Lönnberg, head of the Vertebrate Department of the Swedish Natural History Museum, described cichlid species from Africa in 1903 and would go on an expedition to Africa in 1910. Jacques Pellegrin, who was the Chair of Herpetology and Ichthyology at the Museum d’histoire naturelle in Paris, produced a treatment of cichlids over 3 years beginning in 1903 that described species from Central and South America as well as Madagascar and Africa (Pellegrin 1904). Pellegrin was a prodigious ichthyologist discovering and classifying hundreds of species, many from the family Cichlidae. His work Contribution to the anatomical, biological and taxonomic study of fish of the family Cichlidae constituted the most complete phylogenetic assessment of the family in his time, and was the first major revision to the taxonomy, just ahead of Regan’s in 1905.
Christoph Gustav Ernst Ahl, the Director of the Department of Ichthyology and Herpetology at the Museum fur Naturkunde, Berlin, also classified African and South American species (in his 1927 work), and German zoologist Hermann von Ihering traveled to Brazil in 1880 to take a position at the National Museum of Rio de Janeiro and in 1894 founded the Museu Paulista in São Paulo. His work in 1914, just 6 years before he would leave Brazil forever, identified new species of South American cichlids. Four years later. Alipio de Miranda-Ribeiro, a Brazilian herpetologist and ichthyologist and Director of the Department of Zoology at the National Museum of Brazil, classified several specimens that had been held at von Ihering’s museum in Paulista (Ribeiro 1918). As the focus of research moved from the museum and the crown to the professional in the academy, museums formed ties with higher academic institutions, and some of them—like the Departmento de Zoologia, São Paulo—would eventually begin teaching and supervising graduate students in addition to their research and curatorial activities (Naérico et al. 1997).
During the transitional period between the museum and the lab in the early to mid-1900s, a school of British cichlid study developed. The two earliest figures in this group were Boulenger, and Charles Tate Regan, ichthyologist and Keeper of Zoology at the Natural History Museum in London. Regan and Boulenger did not exactly see eye to eye on the best way to classify these confusing fish. In 1905, Regan’s A revision of the fishes of the American cichlid genus “Cichlasoma” and of the allied genera provided the first comprehensive review of the entire neo-tropical cichlid fauna, assuming relationships based on importance of the anal spine as a primary grouping trait (the first comprehensive catalogue of known African Cichlids was developed around the same time by G.D.F. Gilchrist and W.W. Thompson in South Africa from 1913 to 1917). In his seminal work, Regan isolated Cichla as a sister group to the remaining neo-tropical assemblage. However, in Boulenger’s 1915, Catalogue of the fresh-water fishes of Africa in the British Museum (Natural History) three central genera were instead suggested: Tilapia , Paratilapia , and Pelmatochromis . Regan countered in 1920 and extended his argument to the African Great Lakes in 1922 with The Cichlid Fishes of Lake Victoria. This second work effectively overturned Boulenger’s phylogenetic assignments, replacing them with two lineages (Haplochromis and Tilapia ), a system that endured for the next 50 years.
Another significant figure with direct connections to this early British school of cichlid study was Ethelwynn Trewavas, who began her work with African cichlids as an assistant to Regan in 1928 when he was made Director of the British Museum, joining officially as an Assistant Keeper in 1935. The same year Trewavas became Assistant Keeper, she joined a research expedition with K. Ricardo-Bertram and H.J. Borley who conducted a fishery survey of Lake Malawi (Noakes 1994). Specializing in freshwater fishes of Africa, almost every collection examined caused Trewavas to revise the current understanding of cichlid classification (Trewavas 1935). Her first revision of the cichlid genus was published in 1931 (Trewavas 1931), with her major work on cichlids coming much later.
Prominent groups of ichthyologists with works focusing on cichlids had also been developing in the United States. In 1903, Eigenmann and Clarence Hamilton Kennedy wrote On a collection of fishes from Paraguay, with a synopsis of the American genera of cichlids. Eigenmann continued his contribution to the future study of cichlids with his 1912 work, The freshwater fishes of British Guiana, including a study of the ecological grouping of species. In it, he laid the foundation for ecological work providing an identification key to some 300 species of South American fishes. While the American shift of focus in cichlid research focus to Africa is not dramatic, it began in this period. In 1917, ichthyologists John Treadwell Nichols and Ludlow Griscom were describing African species (Nichols et al. 1917), while only 6 years prior, ichthyologist J.D. Haseman (1911) described his difficulty in distinguishing between two closely related species of cichlid he had collected on an expedition throughout “Bahia” (Brazil).
By 1929, American Museum of Natural History Collectors R. and L. Boulton visited Lake Malawi, helping to bring attention to the diversity of the African Great Lakes cichlids to the United States where most specimens had previously been from Central and South America. Public interest was also on the rise following the 1935 publication of William T. Innes’s Handbook of Exotic Fishes for aquarists, setting the stage for veterans flooding back into universities a decade later when the Second World War ended to grow the cichlid research community substantially, many of them seeking education in biology or fisheries and aquaculture (Brittan 1997).
2.2 Tropical Fisheries and the Growth of Cichlid Research: 1930–1960
In the United States and Great Britain , specialized researchers studying specific aspects of cichlid behavior and biology often emerged from the traditional systematics background of the museum setting described above. Their work was supported by the state largely in connection with the expansion of tropical fisheries (especially in Africa), which grew rapidly in the 1940s (Lowe-McConnell 2006). As scientists directly involved in fisheries struggled to measure and predict the population dynamics of riverine and limnologic systems, this work often brought them head to head with the riddle of cichlid speciation. E. Barton Worthington, for example, concluded based on his biogeography and ecology of the African Lakes that present and past barrier conditions to fish movement led to isolation and speciation, and that when geographical isolation was complete, the degree of differentiation also depended on the organisms concerned. Cichlids, he thought, tended to differentiate based on ecological opportunity wherever they were isolated, whereas lungfish, Protopterus species, did not.
Worthington was a pioneer in studying the ecology and biogeography of the East African lakes and fishes; however, Worthington’s prime directive was not to unravel the evolutionary secrets of cichlids. Rather, he was on assignment from the Crown to survey the Koki Lakes, drainage basins between lakes Victoria and Edward. In this survey, Worthington found no tilapia, which he recommended be introduced. This was done in 1939 to the detriment of the cichlid haplochromine stocks that existed there (Lowe-McConnell 2006).
The history of cichlid research in Africa has been driven in many ways by large-scale fisheries operations. In 1908, the Belgian Congo became a colony, bordering Lake Albert. In 1918, the German protectorate of East Africa, which bordered all three African Great Lakes, was divided between Britain and Belgium and organized as a League of Nations mandate. Each of these Lakes contained valuable stocks of cichlid fauna. In 1929, the Colonial Office Report on the Fish and Fisheries of Lake Victoria by Michael Graham of Lowestoft Laboratory, England, recommended the creation of an East African Fisheries Research Station at the already established Lake Victoria Fisheries Service in order to appropriately manage the resources of Lake Victoria and of the surrounding British territories of Kenya, Uganda and Tanzania. This idea was kept alive by Worthington, who led the first exploratory fishery surveys of many of the East African Lakes and visited in 1944 to discuss proposed plans with representatives from the three territories sharing Victoria’s waters. The Colonial Office of the British government requested that he prepare a memorandum for research and management of freshwater fisheries communities in East Africa in 1944. It was also mandated that for the three territories of Kenya, Tanganyika, and Uganda, a fisheries station would be set up on Lake Victoria at Jinja, Uganda and a Lake Victoria Fisheries Board would be financed and formed by the Colonial Development and Welfare Fund.
British Fisheries efforts overseas were limited in the 1930s due to the development of concerning political conditions on the European continent following the rise of Nazi Germany. Nonetheless, several important surveys and studies were undertaken during this time. In 1936, Kate Ricardo and Janet Owen of Cambridge explored Lake Rukwa and the Bangweulu Swamps, producing a Crown Agent Fisheries Report in 1939, and 2 years later, Ricardo was part of the Lake Nyasa Fishery Survey under Dr. B.S. Platt, which aimed to find methods of improving health and standards of living for the African population. One year after Ricardo’s visit, The Lake Nyasa Survey produced a 178-species outline detailing the cichlid food web, finding along the way that the “one” main commercial tilapia species was in fact three or four closely related tilapias (Bertram and Trant 1991).
Along with Ricardo, Ethylwynn Trewavas was a leading member of the British Colonial Department of World Food Supplies and ichthyologist at the British Museum of Natural History. Her 1935 A Synopsis of the Cichlid Fishes of Lake Nyasa and related works emphasized the variation among the mbuna species flocks of Lake Malawi (then Nyasa), describing both the number of species and their diverse coloration and adaptations. The Second World War interrupted further plans to study the biology of commercial tilapia in Central and East Africa (Lowe-McConnell 2006). From the 1940s through the 1960s, pond culture of tilapia also increased, especially among Belgians living in the Congo and Rwanda who relied on the fish as a food source. At the experimental Fish Culture Research Station in Katanga at Elizabethville (Lubumbashi), Belgians experimented with T. melanopleura .
As the Second World War drew to a close in 1945, Rosemary Lowe-McConnell spent several months at the British Museum’s fish section with Trewavas learning to identify fishes of Lake Nyasa using collections of preserved fishes in the Museum. Through 1947, in a survey commencing just after the Allied victory in Europe, Lowe-McConnell headed to Africa to survey for potential fisheries and aquaculture development (Lowe-McConnell 2006). One year later, she became a member of the newly created United Kingdom Overseas Research Service, overseeing the ichthyological research operating out of the East African Freshwater Fisheries Research Organization (EAFFRO), where entomologists, hydrologists, algologists, and ichthyologists worked together to understand the lakes in a complete sense, including chemical and physical properties that might affect production rates of different species of fishes and fisheries prospects to take pressure off two endemic tilapia species (Lowe-McConnell 2006). During the same period, Trewavas, who 5 years earlier had produced The cichlid fishes of Syria and Palestine concerning tilapias valuable to aquaculture (Trewavas 1942), continued to be an important figure in African aquaculture and fisheries. She was appointed a member of the Fisheries Advisory Committee to the Secretary of State for the Colonies in 1945, and in 1947, she spent 2 months in Nigeria attached to the Fisheries Development unit, working with Mr. Dowsan, the Fisheries Officer (Noakes 1994). The same year, she observed scale eating behavior in Malawi, hypothesizing that Corematodus shiranus with colors mimicking those of Tilapia squamippinis could get close enough to tear the scales off another cichlid and eat them (Barlow 2000).
The end of the war allowed for the construction of fisheries management institutions in Africa that had been delayed for years. The first EAFFRO lab had been built at Lake Victoria in 1946, funded by the British Office of Colonial Development and Welfare. Upon arriving at the intended site, Bobby Beauchamp found that city fathers had placed it in an area designated for “offensive trades.” Construction commenced on the spot previously intended for the houses instead, and was completed in 1949. EAFFRO was formally opened by government of Uganda in 1950, the same year Humphrey Greenwood arrived at the lab (Lowe-McConnell 2006). It was part of a larger scale increase in interest in African fisheries. By 1949, The Uganda Fish Marketing Corporation (TUFMAC) had been built at Lake George, setting up a gillnet fishery to supply dried tilapia by road to mines and other centers. John Barley organized African recorders to provide valuable fisheries statistics; between 1941 and 1943, there was no strict differentiation between commercial and domestic fishermen (Lowe-McConnell 2006). By 1955, a fishmeal plant was established to convert TUFMAC’s fish scrap to cattle food. From 1950 to 1959, annual reports of the Uganda game and fisheries department showed an average catch of over 3 million tilapia a year. Lake George was producing one of the highest yields per unit area recorded for any natural body of water.
In 1954, under the direction of Peter Jackson, Nkata Bay became the headquarters of the Joint Fisheries Research Organization survey of Northern Lake Malawi (where Geoffrey Fryer would conduct his classic study of mbuna cichlids and Derek Iles would study the biology of Utaka cichlids). In the early 1950s, fish farms were set up by the Fishery Departments in all three East African territories to distribute small tilapia and explore the best conditions for culture in pond complexes (Lowe-McConnell 2006). This occurred on both sides of the Atlantic, with the British Guiana Fisheries Division establishing breeding ponds for imported T. mossambica around the same time. Many of these endeavors were fraught with ill consequences. For example, when Kariba (in Zambezi) was stocked with O. macrochir from Lake Mweru in 1959, Chilanga fish farm fingerlings were used. However, these fingerlings were Kafue river stock, later identified as a different species, and their introduction constituted a spectacular failure (the fingerlings were immediately devoured by predators). This underscored the value of taxonomic work in fisheries.
In the 1950s, the importance of Rosemary Lowe-McConnell’s work in the study of tilapia cannot be overstated (Fig. 5). In 1952, Lowe-McConnell found that in Lake Bunyoni, populations of O. niger and O. esculentus had disappeared, replaced by or hybridizing with O. niloticus . She determined that size at maturity varies from lake to lagoon, with lake species at Albert maturing at and growing to a larger size. In 1955, Lowe-McConnell, adding much to our knowledge of tilapiines in African lakes from Turkana in northern Kenya to Jipe in Tanzania, went on a trip that began with a visit to TUFMAC in an attempt to determine if Lakes George and Edwards’ tilapia populations were distinct and where they bred. No evidence of movement between lakes was found (Lowe-McConnell 2006). However, Lowe-McConnell and Peggy Brown found several undescribed species of tilapia related to O. mossambicus at Lake Jipe and the Pangani system at Korogwe, Tanganyika. In the course of their work, it became clear that these species were all part of an interrelated group separate from other East African tilapias. The pair visited tilapia culture experiments in progress at the Kenya Government Experimental Fish Farm in Sagana, but with the onset of the Mau Rebellion, research was halted at RRDC and the associated fish culture unit until 1955 (Lowe-McConnell 2006).
Lowe-McConnell in Guyana in the 1950s. (Reproduced from Stiassny and Kaufman (2015) by permission from Springer Nature)
Traveling to South America, where she lived in British Guiana from 1957 to 1962 with her husband (involved in an ongoing geological survey), Rosemary Lowe-McConnell contributed to the growth of aquaculture and fisheries. While on the continent, she worked for the Fisheries Division of the Department of Agriculture, noting that this work was carried out “at the princely sum of $1 a year…as wives could not be ‘gainfully employed’” (Lowe-McConnell 2006). While in this capacity, Lowe-McConnell nonetheless worked to establish breeding ponds for imported Tilapia mossambica and catalogued countless tropical fish species, pondering as she did so the evolutionary history of cichlids in the Americas in her work Land of Waters. In it, Lowe-McConnell considered the separation of South America and Africa during the breakup of Gondwanaland and subsequent geological events as providing a valuable case of comparative evolutionary biology on both sides of the Atlantic. This work prefigured the growth of cichlid studies in Central and South America in the 1970s, when evolutionary studies would begin to focus on the Great Lakes of Nicaragua alongside the Great Lakes of East Africa as part of valuable comparative studies in an effort to understand the mechanisms of cichlid evolution. Much like in East Africa, this reevaluation of the cichlids as objects of valuable scientific inquiry would sometimes come into conflict with fishing industry interests concerned first and foremost for the economic productivity of the lakes, even as many of the scientists studying cichlids (Lowe-McConnell included) were conducting research in the hopes of informing best practices in fishery management.
In the United States, the study of cichlids was less focused on the aquaculture or fisheries of cichlids than on the taxonomy of American species, but the connection between the two areas of study certainly existed. George S. Myers of Stanford University was both Head of Fishes at United States National Museum and Advisor to the US Fish and Wildlife Service, as well as the fisheries of the Brazilian government. He was the scientific consultant on Innes’s Exotic Aquarium Fishes, which helped to popularize exotic cichlid species in the aquarium trade, species that Myers had in many cases classified himself from South America (Myers 1935). Interest in cichlids stemming from the aquarium trade would receive another boon with the founding of the Tropical Fish Hobbyist magazine by Herbert Axelrod, an aquarium enthusiast who received his doctorate at New York University and for decades strove to produce the most exhaustive aquarium literature available. With regard to cichlids, this endeavor culminated in The Most Complete Lexicon of Cichlids in 1993.
It should be mentioned that the aquarium trade and its corresponding literature, while existing contemporaneously with the growth of cichlid research writ large and contributing to public interest in cichlids, have often proved to be both a valuable resource and a source of great consternation for the scientific community. While prominent importers continued to distribute cichlids to enthusiasts from major centers in America, Germany, and Japan, thousands of people received and circulated often conflicting and confusing information concerning naming, classification, and priority in cichlid identifications. At the same time, liaisons between the industry and academics conducting cichlid studies were not infrequent, with Axelrod and Myers as just two examples. Myers in particular, coming from the strong tradition of ichthyology at Indiana University where he worked as a curator for Carl Eigenmann, was greatly influential at Stanford (where he arrive in 1926), where he contributed to the rise of many prominent researchers (Brown 1994).
Since the early twentieth century, academic positions in US universities and colleges required earning a doctorate. Many women earned doctorates in zoology between 1896 and 1930, but the plight of women in the field of ichthyology had not improved significantly from the late nineteenth to the early twentieth century. Most of the highly credentialed women scientists who chose to remain in higher education could find positions only in women’s colleges or in public schools, none of which were institutions that could afford research (Brown 1994). It is noteworthy that some of the earliest American cichlid research to go beyond the traditional naturalist approach was that of G. K. Noble. In 1939, Noble, at the American Museum of Natural History, investigated sexual identification in the jewelfish with Brian Curtis. By examining the role of visual cues such as the development of the nuchal hump in reproductive behavior, they found that in individual recognition, lifelike movement and facial markings were particularly important. Noble and Curtis also hypothesized that cichlids would fixate on the first young they encountered, an area of study ahead of its time that would later be known as parental imprinting. Their theory was disproved; in fact, many cichlids were found to accept foreign young regardless of previous experience. Nonetheless, Noble and Curtis stand out in this era as pioneers in research concerning cichlid sexual selection.
2.3 Decolonization and the Struggle for Sustainable Fisheries: 1960–1980
From 1945, nationalist movements surrounding Lake Victoria, paired with the political and economic consequences of World War II in Europe, weakened Western domination in Africa. In 1961, Tanzania became independent followed by Uganda, Rwanda, and Burundi in 1962, while Kenya gained independence in 1963. At the same time, there was an increase in manmade lakes (and their ecological study) as dams for hydroelectric power became increasingly common. This occurred behind African dams in Zambezi, and the lower Volta River, Ghana, and Lake Nasser-Nubia. Tilapias flourished in these lakes, thus providing an interest in continued business relations between Western companies and the associations that now controlled regions in which they had established valuable industries (Lowe-McConnell 2006). The legacy of colonial rule over the people of the region had brought with it resource management structures that removed the power from traditional leaders to the central governments of newly sovereign nations such as Kenya, Uganda, and Tanganyika. This meant that the continuing management responsibilities of lake fisheries, often in collaboration with state and private business from Western nations, had been entirely removed from the subsistence fishermen whose families had relied on catches in these lakes for hundreds of years (Awange and Ong’ang’a 2006).
A prime example of this arrangement can be seen at EAFFRO, which continued to operate from Jinja, Uganda through the 1960s. In 1954, to boost tilapia catches, T. zillii (a macrophyte-feeder present in Lake Albert) was introduced to Lake Victoria. With it came O. leucostictus , O. niloticus , and finally, L. niloticus (Lowe-McConnell 2006). Geoffrey Fryer opposed the introduction of the Nile Perch in particular, and in 1960 published an article on the potential disastrous consequences (Fryer 1960a). Nonetheless, Nile perch were officially introduced in 1962 (Goldschmidt 1996). Robin Welcomme studied Lake Victoria ecology in 1963 as a member of the EAFFRO staff, concluding that indigenous species were being extinguished, especially by O. niloticus and L. niloticus . Yet, after a lake-wide survey in the late 1960s showed total fish populations of the lake to be 80% haplochromine, a fishmeal plant became operational in Mwanza in 1976 to take advantage of this available resource. By the end of 1970s, about 10 trawlers were working the Mwanza area, and while the highest yields of haplochromines were recorded in 1977, within a decade, the area showed signs of overfishing.
The International Biological Program was launched for the decade from 1964 to 1974, stimulated by the International Geophysical Year from 1957 to 1958, and intending to study biological productivity and human welfare (the implications of ecological studies for resources use now rapidly gaining acceptance in biological and zoological communities). The Freshwater Productivity section had its headquarters in London with Worthington as Scientific Director. Julian Rzóska, a hydrobiologist who fled Poland during the World War II and arrived at Oxford to continue his research, coordinated the Productivity of Freshwaters section’s 232 ongoing projects from 42 countries by 1969 (Lowe-McConnell 2006). Setting out to obtain internationally comparable observations of basic biological parameters in order to coordinate suitable methods for measuring productivity in natural ecosystems, the Handbook of Freshwater Fish Production was completed by 1968.
In the mid-1960s, Lowe-McConnell and the Uganda International Biological Program team found maturational and full size of catches diminishing due to intensive fishing. Some of these results were published in Welcomme’s (1966), Recent Changes in the Stocks of Tilapia in Lake Victoria. In 1966, the British Royal Society began a 5-year biological study of Lake George, Uganda under Mary Burgis, Lesley McGowna, Tony Viner, Ian Dunn, and George Ganf. The study later included the work of Christine and David Moriarty, Johanna Darlington, Mike Tevlin, and James Gwahaba. Their base of operations was a derelict Uganda Fish Marketing Corporation building at Kasenyi on the lake, damaged by an earthquake, where they proceeded to begin building their lab and accommodations from scratch in collaboration with Makerere University staff. By 1971, a comprehensive ecological picture was in hand, including the discovery (by Dave and Chris Moriarty) that T. nilotica were able to digest cyanobacteria, which had global application for tilapia aquaculture.
Stocking areas in the Americas and Asia (e.g., Fig. 6) resulted in problems as well. The completion of the Panama Canal in 1914 had resulted the creation of the artificial Lake Gatun, quickly colonized by South American fishes (Zaret and Paine 1973). In one notable incident, Peacock cichlids (Cichla ocellaris) introduced into a dammed portion of a Panamanian creek escaped their man-made environment in 1966 when the dam overflowed. The piscivorous C. ocellaris spread into Lake Gatun and surrounding river systems, eventually leading to the extinction of several local species.
Chronicling the spread of Tilapia mossambica. (Reproduced from Riedel (1965) by permission of Springer Nature))
Around the same time tilapia appeared in South East Asia that may have originated from an aquarium shipment to Hong Kong prior to WWII, Peter and Henny Davies relocated cichlids between different parts of the African Great Lakes for their collection business’s convenience (Oliver 2013). As the aquarium trade increased, businesses arrived to collect on the lakes. Stuart Grant was licensed to operate from Malawi in 1973 and the company Aquarist Tropical Fish Ltd. (managed by Eric Fleet) arrived around the same time (Herlong 1999). After several years of a government-sponsored investigation of fish exportation Tony Ribbink recommended that the Davies’ former pilot Norman Edwards, who had transported fishes to Blantyre international airport, be licensed to run the operation at Malawi in 1980 (Herlong 1999). Edwards was licensed to operate the Cape Maclear area following their departure. Until relatively recently, when efforts to reintroduce species and ensure sustainable catch rates gained traction, the aquarium industry joined fisheries and aquaculture in the tropics as a potential threat to biodiversity and ecosystem health.
During the 1970s, expanding African fisheries encountered a variety of problems including transportation demands and increased poaching (Lowe-McConnell 2006). For example, the Ferguson’s Gulf site at Lake Rudolph, Turkana, failed because environmental constraints prevented production from maintaining commercial levels. Fisheries that flourished often did so to the detriment of local subsistence fishermen, who were no longer able to feed themselves or their families from native waters.
In most Great African Lakes where mechanized fishing occurred ecosystems changed significantly throughout the seventies. Of demersal fish in Malawi, 20% of 140 species trawled disappeared from catches within 6 years (Turner 1977). This prompted concern for the lakes’ ecosystems for fisheries and conservation reasons. Anthony Ribbink of Rhodes University, South Africa was sponsored by the JLB Smith Institute of Ichthyology to work toward ensuring national park status for Lake Malawi (Ribbink later became CEO of the Sustainable Seas Trust). In 1972, the Dutch Haplochromis Ecology Survey Team (HEST) from the University of Leiden set up a research station near Mwanza in Tanzania to determine the long-term current status of, and long-term effects of fisheries on lake biodiversity (Goldschmidt 1996). The same year two International Biological Program meetings held in Poland and Reading were almost exclusively limnological (Le Cren and Lowe-McConnell 1980). Their results concerned the effects of physical variables on freshwater production, lake ecology, and stock productivity estimates. Researchers in evolutionary biology and aquaculture and fisheries were becoming increasingly aware of the problems associated with the introduction of new species into fragile ecosystems.
3 A Model Species in Evolutionary Biology
If Darwin had gone not to the Galapagos Islands but to the East African Great Lakes perhaps he would have stumbled on the role played by sexual selection in the origin of species. Perhaps he would have discovered that females, by being fussy in their mate choice, could initiate the origin of species. Perhaps we would have a different theory of evolution today.—Tijs Goldschmidt
Throughout the mid to late twentieth century, cichlid research shifted considerably from the naturalist tradition, growing from a group of ichthyologists interested in taxonomy to a professionally diverse group of scientists with particular interests in social and reproductive behavior, geographic distribution, anatomy, and physiology (Bauchot et al. 1997). At the same time that this expansion and specialization was occurring, random mutation, recombination, and sexual as well as natural selection became foundational to understanding the evolutionary process. Huxley’s vision of a “biology” extending from the smallest components of the cell to the advent of new species became a reality in this half century (Farber 2000). The stage was set for an inquiry into the process of evolution using cichlids as model organisms. Scientists devoted to studying biology, developmental ontology, and lake ecology for the purposes of fisheries science confronted questions of cichlid diversity that had now grown beyond the scope of systematics alone, incorporating evidence from diverse new areas of study to suggest possible evolutionary histories. Sometimes, the work of evolutionary biologists challenged the established taxonomy of the previous generation of naturalists, such as when Wolfgang Wickler challenged Regan’s cichlid phylogeny (Wickler 1963). The new ethological school of cichlid research was critiquing and hoping to refine the systematics of earlier naturalists who did not incorporate behavioral considerations into their determinations of evolutionary histories. In the following sections, the focus will turn to these new types of research and the diverging views they fostered concerning the mechanisms at work in cichlid evolution.
3.1 The Evolutionary Synthesis and Specialization in Cichlid Research: 1930–1970
Lake Malawi, largely by virtue of its cichlid species flocks, contains more species than any other lake on Earth. Indeed, the cichlids of Lake Malawi represent a wider range of niches filled, with less physical variation, than any other vertebrate group (Turner 1994). The high variability of lake levels, and the potential for this to isolate and then recombine groups of cichlids over long periods of time, has been considered one possible explanation for the incredible amount of cichlid diversity. In 1390, Lake Malawi’s water level was about 120–150 meters below current measurements. When the Ngonde King took Mapunda of the neighboring Mwela region as his bride between 1815 and 1835, the water level was so low he was able to walk across the north of the lake for the ceremony (Barlow 2000). By 1860, the water level had risen by approximately 100 meters. Cichlids that inhabited rocky islands in 1869 would have suffocated when those areas were exposed only 30 years prior. This seems to indicate color forms and biological diversification over a period of only a few hundred years (Goldschmidt 1996). Less extreme but of potential importance are more recent changes in water level. For example, between 1914 and 1928, Rodney Wood described breeding locations of both T. squamipinnis and T. saka . In 1945, when Rosemary Lowe McConnell recorded spawning in the lake, it was 10–15 feet deeper.
Although the shift toward evolutionary biology began earlier in the twentieth century, it was not widely seen as compatible with the study of genetics and heredity until the 1930s, when the idea that Darwinian selection and Mendelian inheritance were not mutually exclusive gained acceptance. In 1930, Sir Ronald Aylmer Fisher’s The Genetical Theory of Natural Selection had posed the possibility of “runaway” natural selection; the idea that evolution of sexual preference could establish an effective reproductive isolation between two differentiated parts of a species, even when geographical or other factors were not favorable for such separation. The full importance of this concept would not become commonly agreed upon until the early 1940s, by which point, an understanding of the genetic basis of evolution by natural selection was better understood. Theodosius Dobzhansky (1937) published a milestone work in this synthesis that would come to define modern evolutionary biology: Genetics and the Origin of Species. Alongside him, Julian Huxley strove to bring the fragmented fields of ecology, genetics, paleontology, population genetics, embryology, systematics, comparative physiology, and anatomy under one framework. His 1940 The New Systematics focused on how spatial isolation required for allopatric speciation was achieved, and he published the seminal work Evolution: The Modern Synthesis in 1942. Ernst Mayr is said to have completed the evolutionary synthesis in his Systematics and the Origin of Species of the same year (Mayr 1942). In it, he gave new life to the importance of reproduction as a critical force in evolutionary biology, an approach that embraced interactions between the organism and the environment such as reproductive isolation by sympatric means. This premise served as the main framework for understanding speciation in cichlids via interspecific isolating mechanisms throughout most of the twentieth century, guiding the debate as to the intralacustrine origins of the Great Lakes Cichlids in Malawi, Tanganyika, and Victoria, for which evidence has been consistently sought to confirm either an allopatric (perhaps a micro-allopatric) or a truly sympatric, stasipatric origin (Greenwood 1991).
Interest in the study of cichlid evolutionary biology increased considerably following J. L. Brooks’ 1950 Speciation in Ancient Lakes, which discussed cichlids as models of sympatric speciation. This was the first time researchers began to systematically examine the mechanisms of cichlid speciation in the Great Lakes of Africa, questioning how so many species could coexist in the same lakes in the absence of clear geographic barriers. Cichlids posed what George Barlow (2000) would call a “morphological paradox:” their anatomy from one species to the next was similar, and yet, the differences between species in terms of their environment, diet, reproductive and parental behavior, communication, and coloration were distinct. In 1941, the Dutch biologist Gerard Baerends’ Behavioral Working Group was one of the first teams to address such questions. Working as Niko Tinbergen’s assistant at the University of Leiden Lab from the 1950s, Baerends helped to establish cichlids as a valuable subject of behavioral study, arguing that color and phenotype could be used to assess behavioral or physiological status, and that monogamy was synonymous with substrate guarding.
By this time, ethology and ecology had emerged as important disciplines. Zoogeographic and ecological contributions joined taxonomic and anatomical studies of cichlids to indicate how resource utilization occurred and intralacustrine allopatric speciation took place (Ribbink 1991). There was a growing realization that paleontology, the study most used to determine evolutionary histories, could not provide information on behavior, color, soft tissues, and interrelations between species in their own ecosystems crucial to understanding speciation (Goldschmidt 1996). At Stanford University, George Myers was one of the founders of the Biosystematists: a group of researchers in fields from botany to herpetology and ichthyology who wanted to address common problems in paleontology, biology, and systematics to explain evolutionary problems. Konrad Lorenz’s ethological work throughout the 1960s and 1970s on pair bonding can be seen as a milestone in ushering in the behavioral era of evolutionary biology, laying the groundwork for his 2002 On Aggression. Calling behavior for young was largely classified by Baerands and Baerands van Roon (1950), who detailed “jolting” and “pelvic fin flicking.” They categorized basic cichlid body signals such as lateral and frontal display, aggression inhibition, quivering, and leading. Later, other researchers continued this work, describing fanning, mouthing, churning, leaf lifting, fin digging, and micronipping (Keenleyside 1991). Baerands and Baerands-van Roon also described six systems of chromatophores, describing their color patterns and meaning (especially with regard to reproduction). From 1950 to 1970, investigations of the phenomenon of cichlid speciation expanded to include social behavior (Barlow 1983), bioacoustics revealed a rich vocal repertoire in cichlids including threat behavior in defense of territory (Myrberg et al. 1965; Rodman 1966), and the geographic distribution of species (Poll and Matthes 1962).
Within a decade of Mayr’s crowning work in the evolutionary synthesis, Watson and Crick made their groundbreaking discovery determining the physical structure of DNA, and the study of evolution expanded accordingly to include the genetic factors in phenotypic diversity and the relationship between reproductive behavior and selection of heritable traits (Watson and Crick 1953). As Dobzhansksy (1973) put it, nothing in biology makes sense except in light of evolution. But cichlids presented problems for traditional understandings of natural selection. One problem in explaining the organism (rather than the gene) as the object of selection was the existence of apparently disadvantageous physical traits: extreme characteristics, often coloration, that would attract the attention of predators or in some other way make an organism less capable of surviving in the wild. The question of such “handicaps” became pertinent to the study of cichlid speciation since many Great Lakes cichlids—especially the mbuna of Lake Malawi—possessed a dramatic array of colors and physical forms.
Fischer’s “runaway sexual selection” explained such phenomenon, arguing that traits developed to signal fitness are selected for by the opposite sex, leading to an exaggeration of these traits, leading to the evolution of new forms which might not confer actual fitness on the organism, continually reinforced in the population by sexual selection. The idea that females might influence evolution simply by their mate selection preferences, called the “sexy son” hypothesis, was held to drive such Fisherian runaway (Fisher 1930). A competing model, called the “good genes” hypothesis, stated that in fact handicap traits were biologically affordable for males because of so many positive counterbalances that increased fitness. Thus, no explanation admitting to these males’ lack of real fitness was necessary. Another explanation was sensory bias or sensory exploitation, which—in keeping with the sexy son hypothesis—held that the ability to draw a female’s attention was a greater selective driver than actual fitness.
In 1954 and 1956, Peter H. Greenwood published two works on cichlids, believing the morphology of Lake Victoria cichlids ideal for the study of evolution, and in 1965, he studied coloration and mating behavior as attributes of adaptive radiation in Lake Nabugabo (a satellite lake to Victoria). In 1966, Greenwood’s The Fishes of Uganda was published (an early milestone in his lifelong study of haplochromines), by which point the reproductive behavior of cichlids in Victoria was of interest to scientists around the world in the study of evolution (Lowe-McConnell 2006).
In an effort to understand this process, cichlid reproductive behavior was studied extensively. In many species, such as S. melanotheron and S. occidentalis investigated by Evelyn S. Shaw and Lester R. Aronson (1954), it was not known how long if at all a male would stay with its young in the field, or how long a female required to produce a second clutch of eggs (Aronson 1945, 1949). In 1953, Rosa Kirchshofer—at the Wilhelminberg Biological Station—found that anal fin spots on some species seemed to mimic eggs. In 1965, Wolfgang Wickler found that males presenting these imposter eggs to females would, upon the female’s attempt to gather the extra eggs, orally inseminate the eggs already carried in the female’s buccal cavity. Wickler argued that predation on young might have selected for monogamous behavior among previously haremic males in the open bottom dwelling lamprologines of Lake Tanganyika. However, not all cichlids were found to be monogamous. Apistogramma were found to sometimes “sneak” during reproduction (Loiselle 1985), and both haremic of monogamous behavior depending on environmental conditions (Burchard 1965). In general, mating systems on both sides of the Atlantic have proved to be remarkably plastic. In 1963, George Barlow concluded that the evolution of mating systems in cichlids proceeded from monogamy with bi-parental care to polygamy with maternal care.
One of the earliest explanations for cichlid variation focused on cichlid exploitation of dietary options in the environment, allowing for trophic niches to develop and speciation to occur due to resource competition (Barlow 2000). From 1952 to 1955, N. LeLeup and George Marlier, director of the Belgian research station at Uvira, Lake Tanganyika, observed scale eating behavior; a particularly strange example of this kind of specialization (Marlier and Leleup 1954). Fryer’s 1956 New species of cichlid fishes from Lake Nyasa and 1959 Some aspects of evolution in Lake Nyasa pioneered work in the ecology of cichlids, studying the mbuna of Malawi and relating dentition and jaw structure to feeding behavior. Criticized by Myers (1960), Fryer (1959, 1960b) responded in kind by further demonstrating both habitat stenotopy and specialized feeding mechanisms. Supporting his view, George W. Coulter found in 1967 that the ecological separation of deep-water demersal cichlids in Lake Tanganyika was more likely to be based on diet than habitat preference. One year later, D.J. Randall and Harman showed that South American cichlids ate sponges (highly indigestible to most fish), as well as fruit or seeds that fall into the water. The same year, Albrecht asserted that herbivory evolved as a dietary specialization recently in tropical cichlids, with almost all these species’ young initially eating tiny animals and later switching to other material. Piscivorous adult cichlids were also known. In 1961, Matthes reported hunting behavior for B. microlepis juveniles that followed shoals of clupeids, herding them into shallow bays where adults caught them. Although it was no certainly known to be a driver of speciation, the diversity of cichlid diets was certainly a compelling place to look.
To study cichlid behavior in more detail, new techniques were employed, and the rise of underwater photography became a commonplace research tool to this end. By the 1960s, scuba had revolutionized underwater observations of fish behavior and ecology in clear lakes, pioneered in particular by Hiroya Kawanabe’s studies of freshwater fishes in streams while at Kyoto University, including later studies in Lake Tanganyika (Yuma and Harada 1998; Kawanabe and Nagoshi 1997). The description of new species occurred increasingly as an unintended result, rather than the object, of these scientific studies. Another aid to the discovery of new species was the expanding aquarium trade. From 1950, the aquarium trade for cichlids grew significantly, with Peter and Henry Davies establishing cichlid collection stations (with Cape Maclear as the main station) in the early to mid-1960s, with mbuna from Lake Malawi arriving at fish stores in the United States by 1966. This aided in taxonomic revision by virtue of dedicated aquarists. In 1965, J.E. Burchard pointed out that it was aquarists who discovered that many substrate-guarding Apistogramma species of South America are polygynous, forming harems in which sexual dimorphism is often extreme. Finally, the growth of fisheries also aided in the discovery of new species (e.g., The Belgian Hydrobiological Expedition, ending 1954, which gathered a large collection of Tanganyikan cichlids).
3.2 Sexual Selection and the Curious Adaptive Radiation of Cichlids: 1970–1980
The specialized areas of cichlid research that emerged in the previous 50 years grew significantly in the 1970s. Conceptual shifts in evolutionary biology throughout this decade challenged earlier theories of niche selection in which competition for resources was seen as the primary driver (Goldschmidt 1996). In 1977, Frederick Sanger’s development of DNA sequencing afforded new tools to examine molecular biology and the details of natural selection at the genetic level. Although notable studies with implications for cichlid evolution in the Americas occurred in the late twentieth century—for example, Martin’s (1972) study of the biogeography of the freshwater fishes of Honduras—from 1970 through 1980, the focus in cichlid research moved largely to Africa.
By the end of the decade, most studies in speciation sought to answer two basic questions. The first was whether or not sympatric speciation had occurred in cichlids. If it had, then new species arose in the absence of geographic barriers. Suspicion that cichlids had radiated in a manner unfamiliar to scientists existed since at least 1972, when Trewavas, J. Green, and S.A. Corbet authored Ecological studies on crater lakes in West Cameroon. Fishes of Barombi Mbo commenting on the unusual speciation in cichlids (Trewavas et al. 1972). The same year, Fryer and T.D. Iles’ groundbreaking The Cichlid Fishes of the Great Lakes of Africa: Their Biology and Evolution described “speciation gone wild,” comparing the species of Tanganyika, Malawi, and Victoria.
The second major question in cichlid research followed logically: If sympatric speciation had occurred, then how? This question led to many hypotheses, which fall broadly into two categories. One explanation was that there was a physical driver, something about cichlid anatomy or their ecological niche, together with competition for resources within the seemingly borderless space they inhabited that promoted selection. The second explanation was that the selective force was behavioral, the prime candidate being mate selection. A great deal of study combined aspects of both possibilities, and to date, there appears to be evidence supporting multiple positions on the matter. Not all researchers have maintained one position over the other, and many find cichlids to be such a complicated case that they opt not to take sides.
Studies of anatomy and ecology revealed many unique cichlid characteristics in the 1970s. In 1971, Regnier studied chemical signals between cichlids, termed semiochemicals, and in 1974, Bardach and Villars found taste receptors to be distributed all over cichlid bodies. Two years later, McKaye and Barlow found that parents recognize their young by chemical cues in C. citrinellum , and Meyer, Fernald, and Michael Herzog from Göttingen (an aquarist) sent Barlow evidence that Midas cichlids can change sex, a phenomenon later growing into a significant field of study (Barlow 2000). D. Ohm first documented protogyny in cichlids in the dwarf South American species Crenicara punctulata , 1978. Fernald and Hirata 1979 measured the speed with which male coloration could change the year after. Lanzing and Bower (1974) found that color patterns in a variety of different species used the same 14 units, showing that coloration could be used to determine phylogenetic relationships between species. Voss (1980) compared the color patterns of 20 African species and found that they could be used to discriminate individual species as well as genera and, at the phylogenetic level, discerned different lines of evolution leading to different specializations: display of invariably colored structures in different species, and specializations in quick pattern changes. Supporting the importance of color in behavior and mate selection, Levine and MacNichol demonstrated that cichlids could see color in 1979.
Much of this research was crucial in the quest to unravel the riddle of cichlid speciation. If sufficient physical barriers of some kind had existed, then allopatric speciation must have occurred in a very short window of geological history. If sympatric speciation had occurred, then some physical or behavioral traits unique to cichlids must have played a role in their radiation. Investigations as to what this unique trait might have been frequently focused on diet or feeding. Keenleyside classified three general types of herbivorous fishes: grazers, browsers, and phytoplanktivores (1979). Many cichlids are also piscivorous, some being specialized to consume the young of other cichlids, or even cichlid scales in particular. In 1973, Barlow and Noakes found that young regularly fed from their parents’ bodies, consuming mucus their parents secreted. This was termed “contacting” (Noakes 1979; Noakes and Barlow 1973). In 1970, Knöppel found evidence that central Amazonian cichlids ate ants, plants, bug larvae, algae, and fish. Fryer and Iles thought that the spatulate teeth of E. cyanostictus were used for extracting small insects and crustaceans from algal coverings on rocks. This diversity posed an ecological dilemma. Many cichlid species sharing similar foods coexisted, but in the 1970s, as this was becoming increasingly clear, it had been widely accepted in evolutionary theory that related species with similar diets should not share overlapping environments (Yamaoka 1991).
In their comparison of many African Great Lakes cichlids, Fryer and Iles had detailed cichlid outer jaws (which are particularly dexterous and adaptable) and the pharyngeal jaws (e.g., Fig. 7), which with relatively minor changes can be adapted to many different food types. Cichlids were categorized as feeding by suction, ram feeding, or biting (Liem 1991). In 1978, Greenwood and Karel Liem independently provided evidence based on lab experimentation that the Great Lakes taxa most specialized phylogenetically and morphologically were also “jacks of all trades” when it came to feeding repertoires (Liem 1979). Greenwood studied the size and shape of pharyngeal apophysis in 1978, and one year later, Liem studied feeding in cichlids using X-rays, determining that they could move their outer jaws in a variety of ways to manipulate different food items, allowing the jaws to “take on the properties of a simple hand” (Liem used the term aquatic or hydraulic tongue for this ability). This seemingly paradoxical view contradicted the alternative that the most morphologically specialized taxa were remarkable specialists for particular feeding habits.
Two views of the lower pharyngeal bone of a male Lamprologus ornatipinnis, showing the high degree of specialization of this well-adapted aspect of cichlid morphology. (Reproduced from Gordon and Bills (1999) by permission of Springer Nature)
Cichlid feeding morphology was found to be so variable that it was said to represent a diversification unparalleled in any other vertebrate family (Liem 1991). As a consequence, it was so well studied that it is now better understood in cichlids than in any other teleost family. In 1973, Liem and Greenwood claimed that the cichlid bauplan provided a unique substrate for rapid anatomical change, especially in the evolution of different feeding mechanisms. In 1975, Waddington showed that ecophenotypically induced plasticity existed and argued that its role in speciation was significant, especially if changes were environmentally induced, since potentially reversible morphological changes could then be incorporated into the genome through some form of assimilation. This sentiment was echoed in 1978 by Rosenzweig’s idea of competitive speciation, asserting that the mechanism driving evolution in cichlids was the establishment of niche diets due to trophic resource limitations.
In the previous two decades, a strict focus on genetics in evolutionary biology had been seen as narrow-minded by many ecologists and ethologists. In the 1970s, the synthesis of evolutionary mechanisms at both the micro and macro environmental levels allowed for cichlid research to serve as a bridge between these two worlds of scientific inquiry on the question of speciation. In this era, Irving Kornfield (who, along with Echelle, would support Rosenzweig’s idea of competitive speciation in 1984) laid the foundation for the genetic study of cichlid phylogenies. His The cichlid fishes of Lake Victoria, East Africa: The Biology and evolution of a species in 1975 and his co-authored work with Koehn the same year, Genetic variation and evolution in some New World cichlids, tackled the issue (Kornfield and Koehn 1975). The work continued in 1978 when Kornfield’s Evidence for rapid speciation in African cichlid fishes demonstrated extremely low genetic distance among morphologically divergent cichlid lineages within Lake Malawi, while studies in Tanganyika showed significant differences. Many ecological and ethological studies also directly concerned cichlid taxonomy. Fryer and Iles’ studies led them to challenge Regan’s phylogeny, which had no serious contenders since its establishment in the 1950s. Additional challenges to Regan’s phylogeny came from Heinrich Scheuermann in, A partial revision of the genus “Limnochromis” Regan 1920, 1977 and Greenwood’s 1974 studies of pharyngeal jaws concluded that species from separate Great Lakes (previously believed to be closely related) were, in fact, examples of convergent evolution.
Biogeography and ecology continued to be powerful tools in elucidating cichlid evolutionary history and speciation. Sage and Selander (1975) argued that taxonomic analyses needed to be reconsidered on the grounds that ecophenotypic polymorphism existed in cichlids, and Cichocki’s 1976 study of morphology, ecology, and historical biogeography supported the theory of a monophyletic African radiation. Anthony J. Ribbink and B.J. Hill (1979) found from experimental and field data that cichlid fishes were extant to a depth of 200 meters in Lake Malawi, but that depth distribution was restricted by swimbladder physiology. The same year, Hiroya Kawanabe and a group of Zairean scientists began studies in Lake Tanganyika. Believing as the British ecologist Charles Eton did that communities must be viewed as whole of interactions among organisms, Kawanabe and Nagoshi (1997) sought to uncover the complexity of biotic interactions promoting high species diversity. These underwater studies, lasting into the early twenty-first century, focused on the ecology and ethology of littoral cichlids and revealed much about resource partitioning (Yuma and Harada 1998).
Many studies of cichlid physiology or morphology had important implications for behavior, and from the early 1970s, some studies incorporated aspects of both approaches. Behavioral ontogeny and developmental biology are prime examples. In 1966, Lev Fishelson had found that one significant difference among T. nilotica and macrocephala was the respiratory network of blood vessels in embryos. He suggested that early movements of pectoral fins and whole body “wriggling” produced water currents across these respiratory networks, and found that embryos of guarders had a transitional respiratory network of blood vessels on the anal fin fold, while embryos of bearers have a comparable respiratory network on the surface of the yolk sac. Almost a decade later, in 1975, Hanon found that one species described as intermediate between bearer and guarder had both types of transitional respiratory network. Among a number of alternative suggestions, Balon proposed his terminology for the development of cichlid young: period, phase, and stage (Balon 1975).
Jones (1972) had recently determined that hatching could occur at different stages of development within a species depending on a variety of intrinsic and extrinsic factors, and thus could not be taken as a fixed reference point for either definition or description of normal stages of development. Noakes added to Jones’ assertion that young in guarder species (as opposed to bearer species) hatched at markedly different stages in development. Noakes and Balon would further find in 1982 that embryonic and larval development differed significantly, since—in fact—bearers did not have a larval period in their ontogeny (Noakes and Balon 1982a, b). The physical aspects of such research often entailed the study of biological development in the two groups (“bearers” and “guarders”), which Noakes and Barlow termed precocial (young that begin exogenous feeding when large and with a well-developed sensory and motor control), and altricial (young that begin feeding with a less developed alimentary canal, typically consisting of a simple, straight tube). Bearer young were found to be in a fully formed juvenile state when released, whereas guarder young begin swimming as incompletely differentiated larvae. These differences in size, mobility, and digestive capacity were the purview of physiology and anatomy, but carried important implications for behavior. Noakes and Barlow (1976) investigated an array of issues concerning parent–young behavioral interactions previously unanswered, including the recognition of parents and young by each other. Here, they concluded that in C. citrinellum , parents learned to recognize their young during each parental cycle, but that this memory was somewhat short term, and that parents would accept conspecific young younger than or the same age as their own dry, but not older.
While many behavioral studies simply sought a deeper understanding of the species in question, more often than not, they were concerned with the relationship between speciation and reproduction. This inquiry drew attention to the process of breeding in cichlids, which was discovered to be remarkably diverse across species. In 1972, Keenleyside found that reproductively mature males sometimes possessed female or juvenile coloration, enabling them to steal fertilizations during the reproduction of others. John Maynard Smith applied game theory to such biological phenomena in 1979, arguing the tendency to “cheat” as well as fighting has an important reproductive role as both imply greater fitness in animals. At the same time, Amotz Zahavi argued that natural selection should favor signals that could not be cheated since the existence of “cheatable” signals allowed inferior contestants to succeed, whereas they should find the biological costs too great to bear and fail since they are not, in fact, the fittest (i.e., handicaps should only work if the male is also higher quality).
Through the 1970s, attention was increasingly drawn to the cichlid species of Central and South America as valuable objects of evolutionary biological study. In particular, the cichlids of the Great Lakes of Nicaragua, where McKaye and Barlow (1976a, b) found that C. citrinellum at Lake Jiloá were size dimorphic according to color morphs “gold” and “gray,” with golds pairing and breeding at smaller sizes than grays, and more successfully at greater depths, while grays mated more successfully at lower depths. In a similar investigation, published the same year, Barlow and John Munsey found introgressive hybridization between C. labiatum and C. citrinellum in Lakes Masaya and Jiloá. Studies with the potential to examine incipient speciation events and apparent cross-species hybridization brought these Nicaraguan lakes to the fore, alongside those in Africa, as valuable study sites (Barlow and Munsey 1976). In 1976, Jeffrey Baylis called for a renewed endeavor to examine New World as well as Old World species for their interest to ethologists in his own study of the complex social behavior of H. multispinosa (Baylis 1976a, b). Gerald Meral found female cichlids in Nicaragua competed for nest sites and courted passing males, a very unusual situation in the animal kingdom, and certainly among fishes, and George Myers outlined the ways in which South American and African species were similar, rooting his analysis in the geological history of continental drift and of the area from the Isthmus of Tehuantepec to eastern Panama dating back to the Tertiary period (Myers 1966). Future work examining the species of Nicaraguan lakes would continue in this tradition (see Torres-Dowdall and Meyer 2021).
As with the work being conducted in Nicaragua, work in East Africa continued to focus on sexual selection in cichlids, finding that, on both continents, cichlids display a diverse range of mating types. In polygyny, one male fertilizes eggs of more than one female. In polygynandry, each male fertilizes eggs of more than one female and each female has eggs fertilized by more than one male. In polyandry, one female reproduces with multiple males, while each male reproduces with only that female. Almost all cichlids have biparental care of young, unusual, since in fish usually childcare is male dominated (Barlow 2000). In 1972, Trivers found that biparental care effectively removed both parents from further reproduction during care, and argued that each parent should therefore be tempted to desert in order to invest in additional matings (Trivers 2002). Yet, parents observed in field were persistently monogamous despite communal brooding, and despite the fact that the female invests more in the egg than the male does in the sperm (Ward and Wyman 1977). There were, of course, exceptions. In the presumably primitive Etroplinae , such as the Orange Chromide, males were found to sometimes invest equal time in care of eggs as compared to females (Ward and Samarakoon 1981).
The parental systems of cichlids have been particularly well studied in search of an explanation of cichlid diversity via sexual selection, and fall broadly into two categories: those of mouth brooders and of substrate brooders. It was once supposed that mouth brooding was causally related to buccal morphology (Fryer and Iles 1972). However, mouthbrooders of Malawi and Tanganyika were found to have radically different diets and feeding morphology, complicating this view (Barlow 1991). Anthony J. Ribbink found that for maternal mouthbrooders (almost all species in Lake Malawi), maturing young were released at a safe site and recalled into the mouth during danger (Ribbink et al. 1980). After several days, the young no longer responded to recall signals. It was found that in general, the female concentrated on activities directly with brood while males guarded or patrolled territory (Barlow and Munsey 1976; Keenleyside 1983), although in S. melanotheron , it is the male that provides parental mouthbrooding (Pauly 1976).
Although cichlid parents usually guard their young ferociously, some species pass parental duties onto other cichlids. Ward (1975) found creching, the pooling of young in large schools in which they are cared for communally. Sjölander thought that the adopting of foreign young (sometimes of different species) might be a response to young-eating predation pressure (Sjölander and Fernö 1973). McKaye found in Lake Xiloá, Nicaragua, that altruistic males guarding foreign (interspecific) brood (sometimes more than one) would drive away approaching predators (McKaye and Barlow 1976a, b). This allowed mothers to spend less time defending and resulted in higher reproduction success rates. This phenomenon, known as the dilution effect, explained voluntary incorporation of foreign young into a parent’s brood as increasing the chances of survival for the parent’s offspring by extension. McKaye also observed that in creching among Central American cichlids, foreign young were not always just accepted into another brood, they were sometimes kidnapped (McKaye and McKaye 1977). Experimental manipulations in the lab and field produced the generalization that adults caring for young would accept conspecific young from other broods into their own if the sizes matched. These findings were supported by observations from other researchers (Noakes and Barlow 1973; Myrberg 1975).
In Darwinian selection, individuals compete for food and refuge. In sexual selection, no other species are involved in the competition: males compete for access to female eggs. When lekking was discovered in cichlids, it became an area of focus for this problem. In lekking, males display in an arena (or lek) and females view the performance at a distance and select their preferred mate. This practice granted researchers an opportunity for direct observation of the runaway sexual selection that was held to lead to such rapid speciation. The good genes hypothesis stated that female choice was indicative of real fitness. In the lek, then, males on display were selected for their overall high genetic quality. But this was a problematic premise. If it were true, why would unsuccessful males remain? For the lek to evolve, the number of females attracted per male had to be higher than those lured to a solitary male. Direct observations show no such advantage. Assuming good genes hypothesis was correct, lekking seemed great for females, but bad for most males (Barlow 2000). So why should it persist?
The curious adaptive radiation of cichlids is a stark contrast to Darwin’s finches, which constituted 14 species all on separate islands. In the Great Lakes of Africa, hundreds of cichlid species existed (many of which still thrive), mating and acquiring a variety of resources in overlapping territories lacking obvious geographic barriers. If sexual selection was driving this mysterious diversity, then the question—simply put—was how do females choose and why? There was still no consensus on an explanation in the late seventies. This era of inquiry into cichlid evolution—marked by ecology, ethology , and physiology—now saw something new on the horizon, a field of research that has changed and continues to change the life sciences in profound ways; genetics.
4 The Future of Cichlid Research
I am by nature a cheerful and optimistic person. Growing up in the age of atomic weapons, I learned to practice denial, which was essential for mental health.—George Barlow
Keenleyside argued that genetic research involving cichlids could be broken into three general, but often overlapping, categories: Descriptive studies characterizing attributes of the family or groups of taxa, studies examining genetics as they bear on systematic problems (e.g., phylogeny or taxonomy), and studies exploiting genetics to examine the processes of evolution (Keenleyside 1991). Since accurate evolutionary histories required knowledge from each area of study, and since speciation could not be understood without phylogenetic history, the central roles of the men and women researching genetics in these areas were interconnected (Kornfield 1991). From the late twentieth into the early twenty-first century, the diversity of opinion on the issue of cichlid evolutionary history—and the ways in which it was studied—radiated so significantly that any simple classification becomes misleading. As one example, studies in physiology became crucial to understanding behavior, since studies found chemical cues that prime sexual behavior also aid in species or sex recognition in mate selection (Barlow 2000).
Cichlid research had never existed in particularly well-defined categories, but in this period, interdisciplinary studies became the rule rather than a common exception. In spite of this diversity of study, the importance of genetic studies became a common element in almost all these allied fields, seeming to unify previously disparate groups under the same programmatic end result: verification from genetic evidence.
4.1 The Gene and Cichlid Research: 1980–1990
From the 1980s onwards, cichlid research was increasingly the work of an active and diverse network of men and women whose subjects of study became inextricably interrelated even as they continued to provide different and specialized solutions to the same basic problems. One such problem was the continuing debate between allopatric and sympatric explanations of cichlid radiation. Since the 1950s, it had been suggested that cichlids represented some type of speciation different than the classical sort, possibly due to runaway sexual selection. Some supported the argument that geographical limits to species distribution alone drove speciation (Witte 1984). Others argued for the importance of sexual selection in speciation, a framework that would largely endure until the early 1990s (Dominey 1984).
Lande supported the latter premise, arguing that runaway sexual selection would heavily modify male traits such as size, color, and courtship behavior (Lande 1976). Lande’s polygenic model considered genetic change of a continuous character in a population in which variation could be produced both by several genes and by the environment (Lande 1980, 1981); such a character could be the color or form of a cichlid nest (known as a “bower”). However, many researchers preferred to posit both sorts of speciation as mechanisms in cichlid evolution. McKaye and Gray (1984) suggested that despite Malawi’s historical lack of geographic barriers, geologically brief pond formation around the lake edge could drive classical, allopatric speciation. Seismic surveys of sediments showed that Lake Tanganyika was in fact once three distinct lakes, seeming to support a wider application of this explanation (Scholz et al. 2003). However, McKaye and co-authors also agreed with the basic premise that runaway sexual selection could explain the diversity of Malawi (McKaye et al. 1984).
If sexual selection was in fact the driving force behind speciation, then the most obvious place to look for evidence of this would be in the behavior of spawning and breeding cichlids. From 1980 to 1983, McKaye described cichlid leks in Lake Malawi, which are the largest among vertebrates in the world, with 5000 to 50,000 males in a 4-km long stretch of shore constituting the mating “arena” (McKaye et al. 1990). A bower is built by the male and is the indicator of fitness on display. McKaye and colleagues studied the reproductive ecology of 50 Haplochromines in Malawi, finding that bowers could be divided into ten types, supporting the conclusion that bowers arose from a common ancestor dividing into rapidly evolving species in which local ecological conditions were not the driving evolutionary force in bower form. McKaye (1984) also observed that among mbuna males of P. williamsi , defense of transient territories was common, but of 70 species that spawned over sand, two did not establish territories; the transient spawning territories of these Malawi cichlids were considered unique. Knowledge of the stunning variety of spawning sites among cichlids by species continued to grow. It was found that, in the case of some dimorphic species too large to enter the nest—or in the case of females that spawn in small snail shells—the male could only approach the threshold and hope to fertilize the eggs from beyond (a fact observed by Meek as early as 1907). For South American convict cichlids (Cichlasoma nigrofasciatum ), however, eggs were attached to the ceiling or sides of a cave (Lavery 1991), with some species in Tanganyika doing the same in the lake bottom (as is also the case in Lake Gatun, Panama, where algae is known to be used as a substrate). Such apparent cases of similar traits and behaviors prompted continuing investigation to elucidate evolutionary relationships between species.
Parental behavior among cichlids is also extremely varied and served as another significant line of inquiry on the issue of speciation. Wittenberger (1981), for example, studied different mating systems and found that they often derive from differences in feeding habits. Townshend and Wootton (1984) added to this position, comparing fecundity and size of C. nigrofasciatum in captivity and in Nicaraguan lake, fish were smaller and less fecund, discovering that food resource availability constrained reproductive behavior. Aquarists found that South American earth eaters (Geophagus brasiliensis ) showed a wealth of mating types in captivity extending from monogamous through haremic to open polygamous and from substrate guarding through primitive and advanced mouthbrooding (Barlow 2000). Schwanck (1987) found monogamy to be uncommon in African mouthbrooders, probably because maternal mouthbrooding provides the opportunity for males to abandon their mates. According to Keenleyside, monogamous male cichlids tended to flee and females to remain with the young when danger threatened (Keenleyside et al. 1990). In general, males spent more time away from the brood than females. Keenleyside (1983) found that in experimental ponds with a female-biased sex ratio, the tendency of H. multispinosa males to desert their first mate to get a second increased along with the number of surplus females, and that most of these males spawned within six days of deserting. Evidence accumulated during this time for a tendency to desert among males of many presumably monogamous species, with studies on desertion and polygamy carried out separately by Carlisle, and by Townshend and Wootton (Carlisle 1985; Townshend and Wootton 1985a, b).
Some links between parental behavior and evolutionary history were pointed to by Loiselle (1985), who found that transitional South American mouth brooding species showed a progression from monogamy to polygyny with the deciding factor appearing to be how long the eggs stuck to their substrate. Further elucidating this phenomenon, Trewavas (1983) reported on comparative studies indicating a progression from a delay in taking the eggs into the mouth to picking them up instantly across species. Kuwamura et al. (1989) also described parallel behavior among the haremic lamprologines of Lake Tanganyika, ranking 17 species on a gradient from biparental to maternal care, showing that more maternal species’ young tended to become benthic rather than nektonic. In another comparative study by Kuwamura (1986), 28 of 35 species examined were found to be maternal mouthbrooders, while seven others had delayed or some other variation on mouth brooding. Yanagisawa and Nshombo (1983) found that in mouthbrooders (which predominate in the lakes of Africa), scale eaters immediately shelter non-adhesive eggs in their mouth after spawning. In most polygynous mouth brooders, the male would drive the female away, but in this case, the parents become a monogamous pair, jointly sheltering the young when they are spit out one week later.
In addition to the ability of the eggs to adhere to different substrate, Loiselle (1985) found that predation on young has been a primary selective force for monogamous behavior among previously haremic males in the open bottom dwelling lamprologines of Lake Tanganyika. According to the evolutionary theory of parental investment, offspring become increasingly valuable as they reach maturation (Dawkins 1976). However, it was argued by Loiselle, Lavery and others that in some species—such as convict cichlids—it was not age, but vulnerability to predation, that was the best indicator of parental investment (Barlow 2000). Around this time, it was also shown that when a clutch is too large and there are too many young, the fitness of individual young is diminished (Lavery and Keenleyside 1990a, b). However, optimal clutch size for different cichlids and how this might be determined remain unknown. Ward and Samarakoon (1981) reported species differences in the defense of young with some parents alternating care to take time to feed and, in other cases, found that both parents remain with the young constantly. Tamsie Carlisle (1985) in Panama showed that a greater number of young led to greater defensive behavior in parents. Yanagisawa (1986) found that in some species, the female broods the eggs and the embryos, and the pair of parents jointly defends the young. Yanagisawa’s observations led him to believe that two parents were in fact not required to protect the young, but that male care was favored because it permitted the female to feed at a normal rate and shorten the interval between broods. Shine (1978) had outlined a principle based on a similar phenomenon, referred to as the “safe harbor.” In many species, newly mobile cichlids were found to stay near the spawning site requiring protection, usually from both parents, rather than disperse and search for food (as do the young of most species with male-only care).
Regardless of its relationship to monogamy, polygamy, or polygyny, mouthbrooding has been of enduring interest to evolutionary biologists. Despite the increasing evidence that categorizing species as mouthbrooders or substrate brooders was an over-simplistic dichotomy, emphasis remained on these reproductive methods as crucial to understanding evolution in cichlids (Kuwamura 1986). A behavior of special interest was intra- and interspecific mouth brooding, in which a species might carry young in its mouth from another parent, or even from another parent of a different species entirely, a phenomenon called creching, which was described by Ribbink et al. (1980) in females of several species, including African tilapiine cichlids. Tetsu Sato (1986) in Tanganyika showed that mouth brooders would even carry young catfish. McKaye described reliable interspecific creching in mouth brooding African cichlids that mix their young with those of catfish around the same time (McKaye 1985). It was suggested that kidnapping another animal’s brood might be an effective anti-predator adaptation, increasing young size to increase a single offspring’s odds of survival. However, Pitcher (1986) argued that this dilution effect could only work if predation did not increase with brood size. The exact evolutionary origins and function of creching remained unclear.
With increasing emphasis placed on cichlid broods themselves, two areas of study concerning the reproduction of cichlids that gained traction in the mid to late 1980s were behavioral ontogeny and developmental biology, which focused on growth from the larval state. The issue of conspecific recognition emerged as a critical question in the study of speciation. Shaw and Innes (1980) worked with A. pulcher and implied that parental recognition of foster young depended on the behavior of the young, as well as chemical and visual cues, and that young might be capable of learning simple responses. The question of sexual imprinting had bearing on how new species of cichlids originated, since learned species recognition would be an important factor in adult sexual selection. This recognition was found to occur in cases where species in overlapping physical areas had no overt differences in shape or coloration. Russock and Schein had investigated the effects of early social experience on filial and subsequent adult social preferences in S. mossambicus as early as 1977, and through 1986 found that although young had an initial predisposition to respond to the general characteristics of their mother, they would respond similarly to a range of objects.
The question of whether early imprinting affected later social behavior was concluded to concern only the mechanism at work (Noakes 1991), and researchers focused on this since these effects could reveal how cichlids came to show their usual social reference as a consequence of early experience. Such factors were also considered of potential significance in cases where young were reared by foster parents of their own or other species. Recognition or association between cichlids was also implicated in the phenomenon of “helping” by Taborsky (1984), who found that members of successive broods might be found together in a territory since helpers from an older brood often remained behind to assist with the new. The behavioral consequences of early social experience attracted much interest, but the physiological conditions and changes in ontogeny related to cichlid development were found to be equally important avenues of study. Crapon de Caprona (1982) found that at least some of the early chemical and visual signals of H. burtoni influenced social responses, and social interactions among juveniles were shown to affect growth rate. Such studies also had implications for mating style, diet, and evolutionary history, as in the case of Noakes and Balon (1982a, b) who report that bearer species do not have a larval period in their development. The issue of sex change during development was also of special interest. This phenomenon could be employed strategically in aquaculture production since small male fish are excluded by large males during reproduction, but small females are just as viable (Barlow and Munsey 1976).
When a large female could hold more eggs, however, the size situation was found to be reversed. For years, the plasticity of hermaphroditism was thought to be confined mostly to marine fishes, demonstrated in only a few freshwater species (Cole 2010). However, Richard Francis (1992) showed that many fishes are protogynous, starting life as a female and then staying female or becoming male. Water pH and temperature were found to be important factors; for dwarf cichlids in Apistogramma , for example, higher temperatures produced more males (Heiliginberg 1965). Francis and Barlow found that in some cichlids, growth rate determines sex change, with slower rates leading to female development. Size was also found to be related to dominance and territorial holdings for the purpose of reproduction, with dominance behavior, neuronal changes, and hormones contributing to the development of testes in dominant males (Francis and Barlow 1993). Brem (1988) found that, as with tilapias, sex reversal in haplochromines could be induced by hormonal treatment early in development.
While many researchers maintained that rapid speciation must have occurred due to runaway sexual selection (Dominey 1984), the most significant competing explanation was still viewed by many to be the argument from feeding morphology (since expanded upon) of Liem and Greenwood (1981). They argued that the cichlid “bauplan” provided a uniquely adaptable system capable of rapid change in diet and behavior that, under competitive resource pressure, allowed for niche selection. Throughout the 1980s, diet, feeding behavior, and morphology all remained significant areas of study. Focusing on cichlid diet, McKaye, McKenzie, and Kocher in 1982 and 1983 discovered at least three species of paedophages in Malawi, and McKaye and Marsh (1983) showed that zooplankton would be favored as a food source when abundant. Heinz Bremer and Ulrich Walter (1986) discovered secretocytes in some species, specialized cells in the mucus eaten by young, potentially to acquire valuable gut flora. The fact that young feed on different food types for the first 8.5 months prompted Meyer (1989) to propose the heterochronic model, accounting for the fact that young also differ from the adult form morphologically, especially in head measurements reminiscent of different feeding behavior phenotypes. Meyer (1987) manipulated the diet of C. managuense from Nicaragua and quantified changes in head morphology that resulted during development. Diet was also found to impact coloration, demonstrated by Barlow (1983).
While some researchers studied cichlid diet, others focused on the adaptability of feeding behavior. Barel (1983) proposed that feeding behavior in cichlids has two morphotypes, biters and suckers (though others showed these to be nonrigid categories). Van Oijen (1982) found piscivores to be the most speciose group in Lake Victoria (accounting for up to 40% of all species), and Hori (1983) found that hunting either nocturnally or diurnally in piscivores involved turning over stones and pursuit or ambush tactics. But while much research was still devoted to specialized diets, others began to suspect that feeding behavior alone could not explain the extent of cichlid speciation. Between 1980 and 1983, Barel, Galis and Hoogerhound argued that, in contrast to prior opinions that adaptive radiation mainly concerned the feeding apparatus, more recent research suggested that all anatomical systems (gills, eyes, ovaries, etc.) were involved.
Cichlid functional design had long been held to allow specialization into trophic and ecological niches, but there were problems with this explanation. More accurate phylogenetic assignments were required to clarify persistent issues in questions of cichlid evolutionary history pertinent to speciation, and the taxonomy remained confounding even as genetic research advanced by leaps and bounds. Kocher and Meyer established the first estimates of divergence between New World and Old World fauna, but McKaye (1980) followed by Liem and Kaufman (1984) found that South American cichlids presented problems for hypotheses based on studies of African cichlids. Kullander (1988) pointed out that Crenicichla cichlids had specializations similar to those in African rheophilic cichlids, but that this did not necessarily indicate historical evolutionary relationships accurately. Stiassny (1982) found South American Cichla to be a morphologically aberrant taxon, with their closest relatives in fact being the neo-tropical pike-cichlids of the genus Renicichla . Morphological markers of taxonomic relationships were continually debated, with Kullander (1982) rejecting Regan’s “three spines vs more than three spine” dichotomy, positing instead that Cichla resembled distant percoid relatives because of an ancient unchanged lineage. Stiassny (1987) found that the presence of six rather than seven lateralis canal foramina in the preoperculum was a grouping characteristic that conflicted with Kullander’s 1983–1988 descriptions of characters shared by Crenicichla , and not found in Cichla .
Meanwhile, the taxonomy in Africa was also hotly debated. From 1981 to 1984, Stiassny, Zihler, and Gaemers (among others in their field) worked to establish that the family Cichlidae was monophyletic (Gaemers 1983). Oliver supported the monophyly of African radiation with a morphological feature study (1984), and recognized the Zairean genus Heterochromis as a taxon removed from remaining African types, a major advance in understanding high-level relationships. Greenwood joined a growing group of researchers challenging Regan’s preeminent phylogeny. To get around errors and ambiguities associated with the less accurate process of protein electrophoresis, Dorit (1986) applied restriction enzyme analysis of mtDNA to questions in systematics concerning fauna in Lake Victoria, demonstrating heterogeneity of mtDNA phenotype frequencies in several species that supported Greenwood’s taxonomic revisions. Greenwood’s major revision of the haplochromines taxonomic system based on the shape of the skull, teeth, and mouth and several other characteristics, reflected his belief that the species flocks in the Great African lakes in fact formed a superflock (Goldschmidt 1996).
Tijs Goldschmidt, too, began to consider the possibility that the cichlids of Victoria might represent one huge species with “hundreds of masks” behind which one genome was “hiding,” an assertion akin to the superflock that Greenwood imagined (Goldschmidt 1996). Researchers were beginning to question the basic premise that some form of physical adaptability had allowed niche selection in cichlids; now they wondered whether or not resource partitioning or competition was even involved at all. Goldschmidt (1989) argued that the number of resources a species might exploit was limited by the compatibility of the anatomical requirement for coping with these resources. It was a common ecological assumption that competition must have occurred in the distant past. However, in the course of his investigation, Goldschmidt found no evidence of such competition in Furu species at Lake Victoria (Goldschmidt 1996). Similarly, Hori (1987) had found that mutualism in Tanganyika could be studied from the viewpoint that it raised species richness in each feeding habit group without resource partitioning. If behavior in cichlids could diverge by virtue of the connections among three kinds of interspecific relationships in feeding, commensalism, mutualism and competition in ecological communities within tropical ecosystems, then this might circumvent the need for resource partitioning as a driver of speciation, but do so without discounting that speciation had, in fact, occurred. However, if specimens classified as belonging to different taxonomic species did not interbreed in nature, and if there were no barriers to interbreeding between these organisms, then the taxonomic and population-genetic species would coincide, suggesting the possibility of a superflock.
For Liem and Greenwood (1981), this was the case. He held that similarities in the patterns of diversity of cichlids—like the Hawaiian honeycreepers and Darwin’s finches—were more apparent than real. Therefore, complex ecosystems of cichlids could develop in the absence of geographical borders without initially violating the basic premises of classical evolution. The unique combinations of diet, feeding behavior, and habitat found by Van Oijen (1982) in Lake Victoria piscivorous haplochromines, or the commensal and mutualistic relationships in Tanganyikan rocky shore groups between predatory and epilithic algal-feeding cichlids found by Takamura and Hori (1983), might then be entirely superficial indicators of speciation. Ribbink reawakened the controversy over the very concept of “species,” with a review in 1986 critiquing several controversial points concerning speciation. Kornfield et al. (1982) found that different trophic morphs in Mexico were actually members of the same species, and Sultan (1987) found, contrary to what had been previously accepted about cichlid adaptability driving speciation, that plasticity might be a form of “inertia” against speciation. This position was later taken up by Meyer, who began to question the role of trophic polymorphism in speciation from at least 1989. Despite recent origins and close affinities, it was now clear that similarities between many Victoria and Malawi species were due to convergence and perhaps largely superficial. When it came to cichlid evolutionary history, anything seemed possible.
At the same time that evidence from genetics and morphology was suggesting that cichlid species were more apparent than real, evidence was mounting from biogeography to show that these species were not only real but had emerged by allopatric mechanisms. In Tanganyika and Malawi, cichlids restricted to shallower coastal areas due to water depths greater than 200 meters—which lacked enough dissolved oxygen—were contrasted with species in the shallower waters of Victoria where more diversity had developed over a comparatively shorter geological timespan. From 1981 to 1987, Kawabata, Mihigo, and Yamaoka found communities distributed according to depth and lake-bottom physiography showing similar general trends in resource partitioning (Kawabata and Mihigo 1982). Lewis and Van Oijen (1980, 1981) showed a sharp contrast between Lakes Malawi and Tanganyika in terms of habitat distribution, with Victoria habitats usually lake-wide. Goudswaard and Witte (1984) showed that demersal cichlid species’ composition was strongly influenced by water depth and the nature of nearby substrates (many researchers supported this premise with similar findings). Similarly, Bell-Cross and Minshull (1988) found that the occurrence and abundance of Serranochromis species was determined by preference for shallow reedy lakes or swamps.
Sharp and Marsh had already found that mbuna existed where water was well oxygenated, and that their territorial requirements were species-specific. In a 1983 study that involved 121 stations in 14 regions, 196 mbuna species were identified with only two lithophilic species occurring in every study region, showing simply that the smaller a rocky “island” or pinnacle is, the fewer species it supports (Marsh et al. 1983). Species were found to occur in a depth range of 3–10 m where well-oxygenated water and high algal productivity were present. The distribution of other species was more difficult to explain, since species inhabiting similar northern and southern habitats were, for unclear reasons, not present in similar habitats in central regions. Variation within populations at particular sites was small, but across a series of habitats, changes in colors and size were obvious.
The argument that resource partitioning or physical separation drove cichlid speciation, although formed in previous decades as an antipode to the suggestion that sexual selection might drive sympatric speciation, seemed now inextricably linked with behavior and reproduction. Similarly, sexual selection and ontogenetic development had been found to connect intimately with environment, diet, and other physical factors. Ringler (1983) found that feeding preferences and specializations could develop either as a result of experience or through maturational changes, and Ribbink (1984) found that Docimodus evelynae developed into a scale eater from a juvenile “cleaner.” Similarly, Gottfried (1986) showed that Midas cichlids changed from suckers as juveniles to biters as adults. Noakes and Balon (1982a, b) suggested mouth brooders and substrate brooders as alternative styles of life history and ontogeny, guarders being altricial and bearers being precocial. The complexities of these studies were nicely encapsulated by the works of Nagoshi (1983), in which spawning and parental care were found to contribute an additional dimension to the utilization of space and other resources behavioral differences associated with alternative methods of pair formation (Brichard 1978).
The general conclusion of the wide array of work done in the 1980s was that the process of natural selection in cichlids must be complicated and defies explanation according to any single factor. Selection operates with respect to different colorations and mating systems, environmental change, diet, morphology and developmental biology, territoriality, and other behavior, and these variables do not affect selection independently. In particular, a recent speciation or inability to discriminate other species, kidnapping and ability to recognize individuals and sexual dimorphism (or lack of) are crucial and interrelated factors in sexual selection (Barlow 2000). These aspects of the organism are deeply interconnected, and they have been found to demand interdisciplinary approaches if any understanding of the complexities of their roles in speciation is to be gained.
4.2 The Tragedy and Promise of Fisheries and Aquaculture: 1980–2000
While the use of new technology and research in genetics clearly aided a variety of research programs related to evolutionary biology, these advances were also directly applicable to fisheries and aquaculture for commercial production. At the end of the 1970s, Ramshaw et al. had found that standard starch gel electrophoresis underestimated within-taxon variation by 30% or more (because co-migrating bands may not be identical or buffer systems may not resolve slight differences in net charge), and Takezaki and Nei (1996) argued that estimations concerning genetic divergence were highly dependent on the number and identity of loci examined. Similarly, Hillis (1987) found that small sample sizes might significantly bias estimates of variation and reduce the ability to detect alleles. These technical problems and inaccuracies would have to be surmounted for considerable progress to be made in areas related to genetic study. A significant step forward was the development of the Polymerase Chain Reaction (PCR) by Kary B. Mullis (1994), which allowed for advancement beyond earlier technological limits to genetic study.
Advances made in genetics have had important benefits for the aquaculture of tilapias since 1983. Gjedrem (1985) showed the value of genetics for selection in aquaculture, and Seyoum (1989) conducted mtDNA characterizations of tilapias from East Africa and distinguished seven subspecies clearly, with direct applicability for defining wild and cultured stocks. Much research aimed at increasing the efficiency of aquaculture production through better control of breeding and the maximization of yield, whether genetic in nature or not. Lowe- McConnell, as well as Noakes and Balon, considered mixed-sex, freely-breeding tilapia populations kept in confined spaces and found that under these circumstances, tilapia mature early and breed prolifically, overpopulating the pond with undersized fish and thus undercutting the maximum economic return (Pullin and Lowe-McConnell 1982).
Another problem in aquaculture was the ratio of male-to-female production. Maximizing economic yield requires more males, since male tilapia grow to a larger size, with more metabolic energy channeled directly to growth. Females direct energy into ovarian growth, which is considerably greater than testicular growth. Avtalion and Don (1990) found that females tended to occur in monosex systems because autosomal genes contribute to sex determination. While alleles at supposed sex determining loci could be manipulated by hybridization, simple crosses might not control for these additional sources of allelic variation, confounding attempts to maximize yield (Avtalion 1982). Further complicating this issue, Scott et al. (1989) found that spontaneous sex reversal in tilapias, while infrequent, was common to all commercial stocks. It was also found that YY “supermales” could occur if a phenotypic female (a genetic XY male) mated with a male (in fact, this phenomenon was discovered accidentally).
Genetic studies were not merely a means of understanding how to best maximize the output of farmed tilapias, it was a means of actively manipulating the genetic code to achieve this goal. Only 2 years after the International Center for Living Aquatic Resource Management (ICLARM) 1980 conference on The Biology and Culture of Tilapias, Philippart and Ruwet (1982) collated an immense amount of information available on ecology and distribution of tilapias, Lovshin provided a review of tilapia breeding and hybridization in Brazil and elsewhere, and the textbook The Biology and Culture of Tilapias appeared (Pullin and Lowe-McConnell 1982). Two years later, Trewavas summarized the distinguishing features of tilapiini (Trewavas 1983, 1982). In 1989, the ICLARM officially recommended tilapias and carps as species of focus for the Consultative Group on International Agricultural Research. It was in accordance with this goal that the maximization of commercial tilapia stocks was sought by means of genetic manipulation. Wolters (1982) found that in polyploids, gonadal development might be suppressed so that additional energy could be channeled into increased physical growth. Chourrout and Itskovich (1983) produced triploid tilapias through cold and heat shocks. Myers (1986) induced tetraploidy in tilapias using thermal and pressure shocks, and Brem (1988) demonstrated the expression of human growth hormone in tilapias microinjected with DNA early in development. Kocher et al. (1989) produced universal mtDNA primers for cichlid PCR studies, just one year after Wachtel argued that the identification of sex specific regulatory loci in cichlids could, and should, progress rapidly if findings in other vertebrate systems were exploited. The ICLARM was, by this time, a world leader in fostering research to support genetic objectives in tilapia (Kornfield 1991).
As the species of cichlid first to appear in written human history and in early depictions of controlled breeding—Nile tilapia (Oreochromis niloticus) —quickly became the most popular fish for modern aquaculture methods in the world, with its exponential production commencing by the end of the 1980s (Teletchea 2018, and see Fig. 8). In cichlid rich lakes, wild fisheries castatrophes were becoming more commonplace, seemingly providing all the more reason to raise commercial stocks of tilapias in isolation from fragile ecosystems. In the late 1980s, in response to dangerous changes in African Lake ecology, Lowe-McConnell and Fritz Roest of the Fish Section of the International Agricultural Centre at Wageningen Netherlands organized a symposium on Resource Use and Conservation of the African Great Lakes at University of Burundi in Bujumbura, 1989 (Lowe-McConnell et al. 1992). Recommendations from the symposium were used to apply for international funds for fisheries and diversity projects on African lakes. Yet, even as concern over lake ecologies mounted, officials in Bujumbura consulted with a Swiss firm to determine a building site for a pipeline and town authorities in Burundi consulted with an Austrian university concerning the building of a sewage outfall pipe, both of which would pollute valuable freshwater fisheries. Similarly, it was amid concerns of oil drilling in Lake Tanganyika that the International Limnological Association formed the African Great Lakes Group (also convened by Lowe-McConnell, this time in New Zealand, 1987). Lake Victoria and Tanganyika were not the only lakes threatened, and by the early 1990s, Lake Malawi’s history was recounted by Tweddle (1991), showing that the use of beach seines since the 1970s—leading to reduced catches in the 1980s—finally expanded to such an enterprise that in the early 1990s, fish stocks in the southeastern arm of the lake and those of Lake Malombe collapsed. By 1996, Malawi Fishery Department records showed a decline in endemic tilapia catches to negligible amounts, and today, beach seines are rare in the South east Arm of Lake Malawi not because local authorities have enforced strict regulations, but simply because so few fish remain to be caught (Lowe-McConnell 2006).
Global aquaculture production (in million metric tons) from 1950 to 2016 of the Nile tilapia Oreochromis niloticus; data from the FAO database. (Reproduced from Teletchea 2018, licensed under CC by 3.0)
Of the tales of fishery collapse on the African Lakes, none is as poignant as Lake Victoria. After 1980, annual fish catches in Lake Victoria consisted of 80% Nile perch (Barlow 2000). This noticeably affected catches of native cichlid species by 1987, species that had been the primary food source of Nile perch in the Mwanza area (Barel et al. 1991). From 1977 to 1983, the University of Leiden HEST team caught occasional Nile perch, but the populations seemed low enough that they were not alarmed. By 1985, the situation had changed considerably, with Tijs Goldschmidt finding that normally common species were entirely missing from catches (Goldschmidt 1996). Haplochromine stocks at Victoria were almost entirely obliterated by 1987, and the same year, benthic prawn and cyprinid fishes replaced these cichlids as the main food source for L. niloticus , completely changing the ecology of the lake (Barel et al. 1991). Although cichlid stocks at Lake Victoria served as a crucial resource to local populations for hundreds of years, by the twenty-first century, it was the exotic Nile perch that drove the economies of the surrounding nations (Awange and Ong’ang’a 2006). Since the 1970s, total catches increased between four- and five-folds, making Victoria the world’s largest freshwater fishery, and the number of fish harvested at Lake Victoria peaked between 1991 and 1992 (Awange and Ong’ang’a 2006).
Over 3 million people depend on the fishery at Lake Victoria for their livelihood in Tanzania alone, and in Uganda, fishing is an important source of high-quality food, employment revenue, and has led to development of infrastructure, and is currently the second most important export commodity next to coffee (Awange and Ong’ang’a 2006). Yet, Lowe-McConnell and others noted that as of 1995, many locals were no longer able to provide fish for their families; cichlid catches were no longer substantial, or legally attained (Lowe-McConnell 2006). Surrounded by five countries forming one of the densest and poorest rural populations in the world, the Lake Victoria basin had a population of approximately 26 million in 1999 with a growth rate of about 3% annually (Awange and Ong’ang’a 2006). Increasing pollution pressures on Lake Victoria and pollution impact by municipal and industrial discharges became visible in some of the rivers feeding the Lake and along the shoreline; small-scale gold mining increased in part of the Tanzanian catchment area, leading to mercury contamination of the waterway (Awange and Ong’ang’a 2006). Continued commercial overfishing in the south drove many cichlid species to extinction and left many rare or endangered.
Phosphorous and nitrogen concentrations rose, and algal growth increased five-fold from the 1960s to the 1990s (Awange and Ong’ang’a 2006). This algal growth was exacerbated by the extinction of local species that once consumed it. Artisanal fishing by lakeshore villagers also became a significant threat in some areas, very long and fine-meshed nets that catch even juvenile cichlids in large numbers now being widely used. Intensive fishing of furu cichlids was no longer possible, as the Nile perch introduced to Victoria had decimated their populations, also consuming the catfish that had once been the furu’s main predator, and thus removing over one hundred species from the ecosystem. Following the disappearance of the furu cichlids that consumed them, prawn populations increased and Nile Perch began to feed intensively on prawn (Goldschmidt 1996). However, the prawn had replaced detritus eating furu cichlids, and small sardines had taken the place of zooplankton eating furu cichlids. Species capable of keeping the algae levels of the lake in check effectively vanished from the food chain. In turn, massive increases in blue-green algae were left to be decomposed by oxygen consuming bacteria. By the late nineties, 50–70% of the lake was deoxygenated year-round, making it unlivable for any fish species, including haplochromine cichlids and the Nile perch that had driven many of them to extinction (Goldschmidt 1996).
The fisheries of Lake Victoria have not yet collapsed, but the threat is imminent: ecosystems that have been greatly simplified by humans are often only productive for a short period (Goldschmidt 1996). The decline is already noticeable—the biomass and abundance of Nile perch decreased from 790,000 tons in 1999 to 530,000 tons in 2001 (Awange and Ong’ang’a 2006). It has been argued that enforcing a regional fishing ban would cost industry stakeholders, fish processors, traders, exporters, and transporters billions of dollars. It is also estimated that export losses would be significant. Any ban, however ecologically reasonable, would cause considerable economic turmoil—an outcome that may be inevitable, given the fishery’s impending decline. In response to this dilemma, the Lake Victoria Fish Processors Association of Tanzania volunteered to cease fishing for 4 months of breeding to allow the Nile Perch populations to recover. The firm is currently demanding that similar moves be undertaken by Kenya and Uganda. However, such a proposal would bring 32,000 small fishermen into conflict with Lake Patrols preventing subsistence fisheries (Awange and Ong’ang’a 2006).
Recently, there has been an effort in each of the three East African Countries to pursue sustainable fisheries. A Convention for the Establishment of the Lake Victoria Fisheries Organization (LVFO), currently supported by the EU through the Lake Victoria Fisheries Research Project, drafted with FAO assistance was signed by Kenya, Uganda, and Tanzania in 1994. It intends to promote better fisheries management and conservation. More recently, three of the East African countries collaborated through the support of the Food and Agriculture Organization (FAO) Committee for Inland Fisheries in Africa (CIFA), Sub-Committee for Lake Victoria.
Witte et al. found in 1991 that 300 species had apparently gone extinct in Victoria. This reality was rendered even more tragic by Johnson and Scholz’s geological work in 1996, which showed that the biodiversity of Lake Victoria was likely only 12,400 years old. The fish in this lake represented the most diverse, most rapidly radiating group of living animals known to man, and in a few short decades, the opportunity to study this phenomenon was almost entirely destroyed. In the late twentieth and early twenty-first centuries, evidence of these changes has continued to garner some helpful responses. A growing number of biologists, limnologists, and anthropologists became actively involved in trying to conserve Lake Victoria and its biological diversity. The furu were included in the book of endangered species of the International Union for the Conservation of Nature, and a Captive Breeding Program was launched. Some 40 species were sent to Europe and the United States, each fish packed in a plastic bag where they were housed in US and European zoos and bred.
A great deal of research, both based at Lake Victoria and abroad, has aimed to better understand and resolve issues of ecological overexploitation. The Lake Victoria Research and Conservation Team, which included the HEST group from Leiden, contributed significantly to this project. The Lake Tanganyika Research Project of Osse Lindqvist’s team at University Kuopio, Finland from 1992 to 1998 on production and optimal management of pelagic fisheries, as well as the UNDP/GEF Lake Tanganyika Biodiversity Project (through 2000), aimed to deal with pollution control and protect biodiversity. Another long-term proponent of holistic ecological health in the biological sciences has been Hiroya Kawanabe. In 1991, Kawanabe founded the Center for Ecological Research at Kyoto University, and in 1992, Kawanabe organized the “SymBiosphere: ecological complexity for promoting biodiversity international” workshop there. Kawanabe also worked with the Organization for Tropical Sciences at Laselva, Costa Rica, and pushed for the Center for Ecological Research (CER), Kyoto, to start the “International summer seminar on the global environment and ecology.”
As amateur enthusiasts and aquarists interested in cichlids became concerned, so too did the aquarium trade begin to organize events designed to bring about positive change. Michael Tlusty (2002), for example, promoted the aquaculture of cichlids for efficient aquarium trade production and for species conservation; the Species Survival Program of the American Zoological Association now manages 28 such captive populations of haplochromine species. It is hoped that this might relieve pressure placed on local populations by hobbyists or aquarists, especially for desirable rare or newly discovered species. However, even ornamental fish breeders rely on fresh wild stock periodically, and as a result, the benefit to wild populations may be minimal except in cases of extreme population loss (as has occurred at Lake Victoria). An example of successful reintroduction of species based on this model is the work conducted by Larry Johnson (a cichlid enthusiast who discovered an interesting feeding behavior in Sciaenaochromis fryeri and photographed a new species of Lethrinops for the first time), Ad Konings, and associates through the Stuart M. Grant Conservation Fund. Together, they returned P. saulosi to Taiwanee Reef, Lake Malawi. These fish were produced by Stuart M. Grant Limited that operates the largest cichlid export facility on Lake Malawi. This procedure was repeated in 2014 and was planned again for 2015 (Konings 2013).
As fisheries around the world have encountered numerous ecological issues, the aquaculture of tilapia has continued to expand. By the 1990s, a consensus emerged that research into tilapia genetics should move away from all male hybridization and toward a systematic application of quantitative genetics in breeding. The tools available to genetic study were advancing significantly—exemplified by the technology and techniques necessary to sequence the human genome between 1990 and 2003—a task that has grown more efficient and accessible to researchers with each passing year. Such advances have continued to aid in the aquaculture of tilapia in a variety of ways, as can be seen in the 1994 work of Franck, Kornfield, and Wright concerning tilapia phylogeny, as well as that of Andreas R. Dunz and U.K. Schliewen (2013), whose molecular study re-classified and created a split in Smith’s 1840 Tilapia genus.
4.3 The Continuing Role of Genetics in Cichlid Phylogeny: 1990–2000
In E.O. Wilson’s (1998) Biodiversity, a species is regarded conceptually as a population or series of populations within which free gene flow occurs under natural conditions. This means that most individuals at a given time are capable of breeding with members of the same species, or at least that they are capable of being linked genetically to them through chains of other breeding individuals. By definition, they do not breed freely with members of other species. However, this creates a dilemma when observing a species: Which is the more relevant grouping feature, morphology of reproductive isolation? Demonstrating the genetic relationship between organisms is without a doubt the most powerful modern tool available in the effort to unlock the complex and ever-changing riddle of how new species form. However, owing to Aristotelian notions of the function of organisms and the relationship of function to form, early natural philosophers believed form to follow logically from function. Thus, in the mind of most learned men prior to the late nineteenth century, morphology could be thought of as the ideal design to meet the intended purpose of physiology. As a result, the tradition of classification within natural history has long been rooted in observable morphology and anatomy rather than the reproductive relationship between large groups of individual organisms.
Throughout the late twentieth century, genetic evidence mounted to overturn taxonomic assumptions based primarily on outward appearances. Using the HENNIG86 program, Stiassny (1991); Stiassny and De Pinna 1994 used the outgroup method of phylogenetic analysis to produce a cladogram of the Neotropical lineages with three trees, and later proposed Retroculus to be the most basal cichlid in South America. Kullander (1998) supported this idea with genetic analysis. Pairing this with morphology, Stiassny found evidence that the ethmovomerine region of the neurocranium shared by the Neotropical assemblage might serve as a grouping feature, finally offering a stable substitution for Regan’s phylogeny, which had been repeatedly challenged since the 1950s.
In many cases, the search for “stable” phylogenies meant confronting and accounting for apparent incidents of interspecific breeding. Meyer et al. (1990) determined a monophyletic origin of species in Lake Victoria, concluding that the species of Lake Victoria are a genuine species flock and overturning Greenwood’s polyphyletic model. Martin et al. (2006) found “introgression,” or gene flow between species in the lakes, or in the past between different lake (and even river) populations. Oppen et al. (1997) showed that in four species of mbuna, there was small but measurable gene flow between intraspecific populations, which were genetically distinct, in a sympatric situation. This seemed to argue against the conclusion that cichlids were in fact many species, or at least against the fact that sympatric speciation occurred.
On the other hand, Fabrice Duponchelle and Tony Ribbink, now the Director of SADC/GEF Lake Malawi/Nyasa Biodiversity Conservation Project, worked to determine cichlid evolutionary relationships using mbuna DNA samples and found that the results indicated a paraphyletic lineage. The presence of a well-differentiated mtDNA polymorphism confounded cladistic analysis (two divergent and statistically distinct mtDNA lineages being present). This pattern suggested lineage sorting, the random fixation of ancestral mtDNA clones within isolated populations characteristic of classical, allopatric speciation. Additionally, some endemic non-mbuna haplochromines were found to possess distinctive mtDNA profiles identical to members of the mbuna. This indicated that as currently defined, mbuna were a paraphyletic group. However, McKaye et al. 1993) found that genetic analysis could not discriminate unambiguously among species of mbuna (although they cautioned against assuming too much from this based on the fact that biological species may have identical cleavage profiles).
Evidence of cichlid introgression and the existence of geographic isolation seemed to undermine the conclusion that sympatric speciation (especially via the popular mechanism of sexual selection or reproductive isolation) played a role in cichlid radiation; yet, there was also evidence to support the conclusion that sympatric mechanisms were at work in addition to traditional allopatric means. In Turelli et al.’s (2001) Theory and Speciation, a convincing general model of sympatric speciation delimiting testable or observable conditions is called for to specify how the process can and cannot occur, especially in the case of reproductive divergence despite continual (but limited) gene flow. Much work in cichlid research in the 1990s and beyond attempted to answer this call, particularly by examining sexual selection and reproductive isolation mechanisms.
Many studies focused on the issue of recognition in sexual selection, since any system of recognition of a proto-species might contribute to speciation and reduce interspecific breeding. Jennifer Holder (1991) argued that the high possibility of hybridization due to overlapping physical regions in cichlids should encourage behavior that distinguishes between species. She showed that in monogamous cichlids, visual and chemical cues are needed to recognize species in the absence of behavioral cues, supporting Baylis’ “multiplicity of cues” hypothesis. Similarly, Kenji Karino (1997) showed that the size of the castle/bower does not matter in mate selection, implying that different bower types function as cues in species recognition for reproduction. Supporting the idea that mate selection is cued by a variety of factors, Phillip Lobel (1998) found that Malawi lekking cichlids use vocalizations to approach females but their exact role in communication or mate assessment is still unknown. Balshine-Earn and Lotem (1998) found that N. pulcher from Tanganyika recognized a video image of her mate (1998). Paterson (1993) proposed the specific mate recognition system (SMRS) for conspecific reproduction as a solution to this problem.
The strongest evidence for sympatric speciation comes from examples of incipient speciation involving sexual selection. Adaptation of sensory and signaling systems to local environmental signal transmission conditions can cause speciation when the sensory or signaling systems affect mate choice, a phenomenon known as “Sensory drive speciation.” Seehausen et al. (1998) demonstrated that sympatric populations living at different water depths evolved different male breeding coloration, undergoing divergent evolution in visual pigments and adapting to local light. Mairi E. Knight and George F. Turner (2004) found that lab mating trials indicate incipient speciation by sexual selection among P. zebra , in which females show preference for different male courtship colors that are indicative of their own geographic region. Elisabeth Martin and Michael Taborsky (1997) found that P. pulcher red males are monogamous or haremic, while yellow males are only monogamous, a contrast in mating style accompanied by behavioral differences in territoriality. Red males were found to produce three times the offspring, while yellow “satellite” males might occasionally equal “Red” fitness by sneaking (the first time for any animal a reproductive equivalency of fitness has been shown for sneaking compared to conventional means). Despite these examples of incipient speciation, color may not always be a key factor. By contrast, Barlow (1998) showed that coloration played little role in speciation among substrate brooding species in Central America.
Morphology, physiology, behavior, and genetics are all important factors in identifying a species and determining its evolutionary relationship to other organisms. Once the theory of evolution gained sufficient acceptance, all these traits could be seen as randomly varying within a population, with selective pressures determining how alterations in form over time might allow organisms to function in new and adaptive ways, thus driving reproductive isolation and speciation. The late twentieth century generally continued to see the study of relationships between cichlid species shift to molecular genetics, but our modern endeavors to classify organisms have never entirely caught up to this shift. Researchers continue to grapple with the possibility that, if we are observing evolution in action rather than a static set of species as would have been assumed in the early years of the naturalist tradition, we may be confounding pattern with process. At the same time that genetic studies were ascendant in cichlid systematics (i.e., from the late twentieth century on), an emerging body of work conducted in Iceland tackled the problem from outside the world of cichlids by examining polymorphisms (phenotypic diversity such as different morphs of forms) within a single species: the Arctic charr, Salvelinus alpinus (Jonsson et al. 1988). This approach reduces the analysis to the ultimate contrast with African Great Lake cichlids by selecting Icelandic lakes with only a single model species. The hypothesis is that if we can understand the origin of sympatric polymorphism in Arctic charr, then we can extend that mechanism to the origin of cichlid species (Kapralova et al. 2011).
As many as four morphs of S. alpinus occupy different habitats in one land-locked lake, separated by trophic specializations and reproductively isolated from each other (Skúlason et al. 1996). These morphs differ substantially in appearance (especially with regard to the anatomy and physiology of feeding) but are conspecific by some definitions (Skulason et al. 1989). Life history differences among morphs appear to be partly genetically based (Skúlason et al. 1996), some differences possibly being controlled by a single locus, with phenotypic plasticity playing a proximate role (Smith and Skúlason 1996). Further, it has been shown that the four morphs exist at differing levels of phenotypic and genotypic segregation, with one morph completely reproductively isolated despite all morphs in question sharing an intralacustrine origin (Gislason et al. 1999).
It has been suggested that the evolution of resource-based polymorphisms is driven by flexible behavior (especially related to trophic adaptations) in the early phases with morphological divergence occurring as speciation advances. Studies have shown that in some fish species the evolution of sympatric morphs occurs locally, following postglacial invasions of common ancestors (Snorrason and Skúlason 2004; Kristjánsson et al. 2011, 2012). Evidence has mounted in favor of sympatric trophic polymorphism as a viable explanation for this diversity (Noakes 2008).
Contemporary and recent patterns of restricted gene flow have apparently been conducive to the evolution and maintenance of adaptive genetic variation of S. alpinus (Kristjánsson et al. 2011). These and other similar studies have added to the argument that, in cases where phenotypic diversity seems related specifically to resource acquisition in the absence of clear physical barriers (as is the case with many African cichlids), different trophic conditions might be driving speciation within a population, visible first as the increasing diversification of resource polymorphisms (i.e., stable phenotypes sensitive to environmental selective forces) within a species. The broader application and interpretation of these hypotheses of intraspecific polymorphism and speciation appear to be a likely area for our understanding of cichlid evolution.
4.4 Looking Back and Moving Forward
The field of cichlid research has changed dramatically in the more than 250 years since the family’s first appearance in Linnaean taxonomies. Whereas the 1880s saw fisheries operations in colonial holdings such as Africa take root, the 1980s saw those enterprises at the height of a drastic expansion that changed both the lakes and surrounding areas. In 1880, national natural history museums devoted kingly sums to collecting and categorizing species that were entirely new to the Western science, and by 1982, Kullander determined in his revision of South American cichlasomines that, in fact, some of these groups had existed since at least the Tertiary Period. At the same time that they were discovered to be ancient in South America, cichlids were discovered to be startlingly young in Africa; in 1982, Cromie suggested that Lake Malawi could be only 100,000 years old.
We continue to live in a time of great discovery in cichlid research. About 400 species have been described from Malawi, and between 450 and 500 additional suspected species have been collected, photographed underwater, or both, but remain undescribed. Visits to any remote, rocky shore still produce new discoveries, as do deep-water trawls in unsampled areas. In 1996, Ole Seehausen discovered that, despite its ecological devastation, Lake Victoria still retains a previously unknown group of rock reef cichlids that evaded the extensive predation of Nile perch. Seehausen produced a book on the ecology of this recently discovered flock of nearly 100 colorful rock-dwelling “Mpipi” cichlid species in Victoria, a diversity rivaling the mbuna of Malawi. Many river systems are still unexplored and information on little known cichlids is scattered in aquarium literature, reports, and collections made over a wide area.
Just as surely as many species remain to be discovered even in the twenty-first century, there are many avenues of research just opening up for exploration. Cues like olfaction and sound were left largely unexamined even in the 1990s, with Barlow’s study of the effects of acoustic signals between cichlids being the first to catalogue the relatively rich sonic repertoire of some cichlids from a young age. Another line of inquiry concerns interspecific dominance hierarchies , examined by Andries and Nelissen (1990), a new area of research emerging in the late 1980s. The study of energetics in relation to mating systems is yet another research program that is still in its infancy and might be expanded.
Cichlids have been increasingly hailed as model species for the study of evolutionary biology (Kornfield and Smith 2000). However, despite the current importance of developmental biology, there are few programs using cichlids, though their availability (depending on species), great diversity, and ease of raising and observing make them ideal candidates. Significant advances in the understanding of cichlid taxonomy and systematics could be combined with ontogenetic study and life history. Comparative behavioral studies in development could have implications for the role of behavioral ontogeny in evolution writ large. Even though much work has been done on the subject of cichlid speciation, each species has a unique combination of ecological attributes that might serve a variety of functions in speciation, and the precise mechanisms by which these have allowed for niche segregation in the Great Lakes of East Africa remain unknown. On the other hand, the problem of distinguishing between known species continues to require more attention. In contrast to significant efforts on cichlid supraspecific classification, for example, very little has been published on the technical problems of haplochromine species distinction. Possibly in response to this inadequacy, there has been a return to the collections tradition in modern cichlid fieldwork. Researchers like Ad Konings (2011) have exhumed the original museum collections specimens from centuries past to reclassify known species and reconsider existing phylogenies according to new knowledge of these animals, some of which are now extinct. Neither is it the case that the “old ways” ever entirely left us. Indeed, the connection between cichlid research and ichthyology in the more traditional sense (systematics and specimen collection) has been retained in many ways, perhaps due to the ongoing central importance of cichlid classification in the study of this family of fishes. Melanie Stiassny, as just one example, continues to act as Curatrix of Ichthyology at the American Museum of Natural History.
Although much has changed in 200 years of cichlid study, those who pioneered it and those who study cichlids today have a great deal in common. Separated by vast amounts of time they are nonetheless bound together by a common interest to determine the place of things—a sense for the “order” of organisms, so critical to natural history—a question that remains despite the myriad scientific discoveries and advances of our present day. It is a question that is unanswered not because the study of cichlids has failed to progress, but because with each question answered, ever more curiosities arose. Inquiries into the deceptively simple question of cichlid history are branching and radiating, like the specializing fields of cichlid research that endeavor to arrive at a solution. Like the rapidly evolving cichlids themselves, they are multiplying—spreading out, changing, adapting and becoming unique—yet they remain inextricably related. It is because the subjects of cichlid research are so diverse, complex, and fascinating that they have taken us centuries to scratch the surface. Our understanding of their behavior and biology now fills volumes, yet the origin of so many species in a span of time so short remains a mystery.
One is struck by the sensibility of the dilemma. If the answers were easy, we would have them already! The complexities of biological evolution—genetic, behavioral, and environmental—demand a more holistic, yet simultaneously a more precise, understanding. This is a combination we still find elusive. In time, as we continue to watch cichlids evolve, perhaps they will enter the history books as another scientific triumph (a chapter easily closed). Or perhaps not. Perhaps new species will still be found. Perhaps aquarists at home will call scientists at universities and reveal tidbits of cichlid behavior that are still surprising. Perhaps it will require hundred more years of work with these animals only to realize what we are just beginning to learn: That we are still standing on the frontier, and the pioneers of cichlid research are not consigned to dusty tomes. They are remembered, they are in the field and in the lab, and they are preparing a new generation to embark on voyages of discovery that have not yet been written.
References
Ahl E (1927) Einge neue Fische der Famile Cichlidae aus dem Nyassa-see. Schrift Berlin Gesellschaft Natur Freunde Berl 1926:51–62
Andries S, Nelissen MHJ (1990) A study of the dominance hierarchy in four mbuna-species: Melanochromis Johanni, M. auratus, Pseudtropheus ornatus, and P. lombardoi (Teleostei: Cichlidae). Belg J Zool 120(2):165–193
Aronson LR (1945) Influence of the stimuli provided by the male cichlid fish, Tilapia macrocephala, on the spawning frequency of the female. Physiol Zool 18(4):403–415
Aronson LR (1949) An analysis of reproductive behavior in the mouthbreeding cichlid fish, Tilapia macrocephala (Bleeker). Zool Sci Contrib N Y Zool Soc 34(3):133–158
Avtalion RR (1982) Genetic markers in Sarotherodon and their use for sex and species identification. In: International Conference on the Biology and Culture of Tilapias, Bellagio Italy, 2–5 Sep 1980
Avtalion RR, Don J (1990) Sex-determining genes in tilapia: a model of fenetic recombination emerging from sex ratio results of three generations of diploid gynogenetic Oreochromis aureus. J Fish Biol 37(1):167–173
Awange JL, Ong’ang’a O (2006) Lake Victoria: ecology, resources, environment. Springer, Dordrecht
Axelrod HR (1993) The most complete colored lexicon of cichlids: every known cichlid illustrated in color. Neptune City, NJ, T.F.H. Publications
Baerends GP, Baerends-van Roon JM (1950) An introduction to the study of the ethology of the cichlid fishes. Behaviour 1:111–243
Balon EK (1975) Terminology of intervals in fish development. J Fish Board Canada 32(9):1663–1670
Balshine-Earn S, Lotem A (1998) Individual recognition in a cooperatively breeding cichlid: evidence from video playback experiments. Behaviour 135(3):369–386
Barel CDN, Ligtvoet W, Goldschmidt T, Witte F, Goudswaard PC (1991) The haplochromine cichlids in Lake Victoria: an assessment of biological and fisheries interests. In: Keenleyside Miles HA (ed) Cichlid fishes: behavior, ecology and evolution. Chapman & Hall, London, pp 258–279
Barlow GW (1983) The benefits of being gold: behavioral consequences of polychromatism in the Midas cichlid, Cichlasoma citrinellum. Environ Biol Fishes 8(3–4):235–247
Barlow GW (1991) Mating systems among cichlid fishes. In: Keenleyside Miles HA (ed) Cichlid fishes: behavior, ecology and evolution. Chapman & Hall, London, pp 173–190
Barlow GW (1998) Sexual-selection models for exaggerated traits are useful but constraining. Am Zool 38(1):59–69
Barlow GW (2000) The cichlid fishes: nature’s grand experiment in evolution. Perseus, Cambridge, MA
Barlow GW, Munsey JW (1976) The Red Devil-Midas-Arrow cichlid species complex in Nicaragua. In: Investigations of the Ichthyofauna of Nicaraguan Lakes. Available via Digital Commons. http://digitalcommons.unl.edu/ichthynicar/24
Bauchot ML, Daget J, Bauchot R (1997) Ichthyology in France at the beginning of the 19th century: the Histoire Naturelle des Poissons of Cuvier (1769-1832) and Valenciennes (1794-1865). In: Pietsch TW, Anderson WD (eds) Collection building in ichthyology and herpetology. American Society of Ichthyologists and Herpetologists, Lawrence, pp 27–80
Baylis JR (1976a) A quantitative study of long-term courtship: II. A comparative study of the dynamics of courtship in two New World cichlid fishes. Behavior 54:117–161
Baylis JR (1976b) The behavior and ecology of Herotilapia multispinosa (Teleostei Cichlidae). In: Investigations of the Ichthyofauna of Nicaraguan Lakes. Available via Digital Commons. http://digitalcommons.unl.edu/ichthynicar/35/
Bell-Cross G, Minshull JL (1988) The fishes of Zimbabwe. National Museums and Monuments of Zimbabwe, Harare, Zimbabwe
Bennett ET (1830) Observations on a collection of fishes from the Mauritius, presented by Mr. Telfair, with characters of new genera and species. Proc Comm Sci Corres Zool Soc Lond 31:165–169
Bertram CKR, Trant JO (1991) Letters from the Swamps East Africa, 1936-1937. Newtown, Montgomeryshire
Beveridge MC, McAndrew B (2000) Tilapias: biology and exploitation. Fish & Fisheries Series, vol 25. Springer, New York
Bleeker P (1868) Description de trois espèces inédites de Chromidoïdes de Madagascar. Versl Akad Amsterdam 2(2):307–314
Bloch ME, Schneider JG (1801) Systema ichthyologiae: iconibus CX illustratum 1. Available via Biodiversity Heritage Library. https://www.biodiversitylibrary.org/page/5475805
Boeseman M (1997) Collectors and fish collections of the Rijksmuseum van Natuurlijke Histoire in Leiden, Netherlands (1820-1980). In: Pietsch TW, Anderson WD (eds) Collection building in ichthyology and herpetology, vol 3. American Society of Ichthyologists and Herpetologists, Lawrence, pp 27–80
Boulenger GA, Moore JES (1898) Report on the collection of fishes made by Mr. JES Moore in Lake Tanganyika during his expedition, 1895-96. Zool Soc Lond 64(1):217–224
Brem G, Brenig B, Hörstgen-Schwark G, Winnacker EL (1988) Gene transfer in tilapia (Oreochromis niloticus). Aquaculture 68(3):209–219
Brichard P (1978) Fishes of Lake Tanganyika. T.F.H. Publications, Neptune City
Brittan MR (1997) The Stanford school of ichthyology: eighty years (1891–1970) from Jordan (1851–1931) to Myers (1905–1985). In: Pietsch TW, Anderson WD (eds) Collection building in ichthyology and herpetology, vol 3. American Society of Ichthyologists and Herpetologists, Lawrence, pp 233–268
Brooks JL (1950) Speciation in ancient lakes (concluded). Q Rev Biol 25(2):131–176
Brown PS (1994) Early women ichthyologists. In: Balon EK, Bruton M, Noakes DLG (eds) Women in ichthyology: an anthology in honour of ET, Ro and Genie. Kluwer Academic Publishers, Dordrecht, pp 9–33
Burchard JE (1965) Family structure in the dwarf cichlid Apistogramma trifasciatum (Eigenmann and Kennedy). Zeitschrift für Tierpsychologie 22(2):150–162
Chourrout D, Itskovich J (1983) Three manipulations permitted by artificial insemination in tilapia: induced diploid gynogenesis, production of all-triploid populations and intergeneric hybridization. In: Fishelson L, Yaron Z (eds) Int symp on tilapia in aquaculture. Nazareth, Israel, pp 246–255
Caprona MDC (1982) The Influence of Early Experience on Preferences for Optical and Chemical Cues Produced by both Sexes in the Cichlid Fish Haplochromis burtoni (Astatotilapia burtoni, Greenwood 1979). Zeitschrift für Tierpsychologie 58(4):329–361
Carlisle TR (1985) Parental response to brood size in a cichlid fish. Anim Behav 33(1):234–238
Cole KS (2010) Reproduction and sexuality in marine fishes. University of California Press, Berkeley
Cope ED (1869) Contribution to the ichthyology of the Marañon. Proc Am Philos Soc 11(81):559–570
Cuvier G (1828) Natural history fish, vol 1. Chez F. G. Levrault, Paris
Dawkins R (1976) The selfish gene. Oxford University Press, Oxford
Day F (1888) Supplement to the fishes of India: being a natural history of the fishes known to inhabit the seas and fresh water of India, Burma and Ceylon. Williams and Norgate, London. Available via Internet Archive. https://archive.org/details/fishesofindiabei03dayf. Accessed 14 June 2019
Dobzhansksy T (1973) Nothing in biology makes sense except in the light of evolution. Am Biol Teacher 35(3):125–129
Dobzhansky T (1937) Genetics and the origin of species. Columbia University Press, New York
Dominey WJ (1984) Effects of sexual selection and life history on speciation: species flocks in African cichlids and Hawaiian Drosophila. In: Echelle AA, Kornfield I (eds) Evolution of fish species flocks. University of Maine Press, Orono, pp 231–249
Dorit RL (1986) Molecular and morphological variation in Lake Victoria haplochromine cichlids (Perciformes: Cichlidae). PhD thesis, Harvard University, Cambridge
Dunz AR, Schliewen UK (2013) Molecular phylogeny and revised classification of the haplotilapiine cichlid fishes formerly referred to as “Tilapia”. Mol Phylogenet Evol 68(1):64–80
Eigenmann CH (1912) The freshwater fishes of British Guiana, including a study of the ecological grouping of species and the relation of the fauna of the plateau to that of the lowlands, vol 5. Carnegie Institute, Troy, MI
Eigenmann CH, Kennedy CH (1903) On a collection of fishes from Paraguay, with a synopsis of the American genera of cichlids. Proc Acad Nat Sci Phil 55:497–537
Farber PL (2000) Finding order in nature: the naturalist tradition from Linnaeus to EO Wilson. JHU Press, Baltimore, MA
Fernald RD, Hirata NR (1979) The ontogeny of social behavior and body coloration in the African cichlid fish Haplochromis burtoni. Zeitschrift für Tierpsychologie 50(2):180–187
Fernholm B, Wheeler A (1983) Linnaean fish specimens in the Swedish Museum of Natural History, Stockholm. Zool J Linn Soc 78(3):199–286
Fisher RA (1930) The genetical theory of natural selection. Oxford University Press, Oxford
Francis RC (1992) Sexual lability in teleosts: developmental factors. Q Rev Biol 67(1):1–18
Francis RC, Barlow GW (1993) Social control of primary sex differentiation in the Midas cichlid. Proc Nat Acad Sci 90(22):10673–10675
Franck JP, Kornfield I, Wright JM (1994) The utility of SATA satellite DNA sequences for inferring phylogenetic relationships among the three major genera of tilapiine cichlid fishes. Mol Phylogenet Evol 3(1):10–16
Fricke R, Eschmeyer WN, Fong JD (2020) Eschmeyer’s catalog of fishes: genera/species by family/subfamily name. http://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp
Fryer G (1959) Some aspects of evolution in Lake Nyasa. Evolution 13(4):440–451
Fryer G (1960a) Concerning the proposed introduction of Nile Perch Into Lake Victoria. East Afr Agric J 25(4):267–270
Fryer G (1960b) Evolution of fishes in Lake Nyasa. Evolution 14(3):396–400
Fryer G, Iles TD (1972) The Cichlid fishes of the great lakes of Africa: their biology and evolution. T.F.H. Publications, Neptune City, NJ
Gaemers PA (1983) Taxonomic position of the Cichlidae (Pisces, Perciformes) as demonstrated by the morphology of their otoliths. Netherlands J Zool 34(4):566–595
Gill T (1862) Remarks on the relations of the genera and other groups of Cuban fishes. Proc Acad Nat Sci Phil 14:235–242
Gill T, Bransford JF (1877) Synopsis of the fishes of Lake Nicaragua. Proc Acad Nat Sci Phil 29:175–191
Gíslason D, Ferguson MM, Skúlason S, Snorrason S (1999) Rapid and coupled phenotypic and genetic divergence in Icelandic Arctic char (Salvelinus alpinus). Can J Fish Aquat Sci 56(12):2229–2234
Gjedrem T (1985) Improvement of productivity through breeding schemes. GeoJournal 10(3):233–241
Goldschmidt PT (1989) An ecological and morphological field study on the haplochromine cichlid fishes Pisces, Cichlidae of Lake Victoria. University of Washington Press, Seattle, WA
Goldschmidt T (1996) Darwin’s dreampond: drama in Lake Victoria. MIT Press, Cambridge
Gon O, Skelton PH (1997) A history of the fish collections of South Africa. In: Pietsch TW, Anderson WD (eds) Collection building in ichthyology and herpetology. American Society of Ichthyologists and Herpetologists, Allen Press, Lawrence, pp 133–168
Gordon AK, Bills IR (1999) Aspects of the feeding and reproductive biology of the Lake Tanganyikan cichlid, Lamprologus ornatipinnis (Pisces, Cichlidae). Env Biol of Fish 55:431–441
Gottfried MD (1986) Developmental transition in feeding morphology of the Midas cichlid. Copeia 1986(4):1028–1030
Goudswaard PC, Witte F (1984) Observations on Nile perch Lates niloticus (L.), 1758 in the Tanzanian waters of lake Victoria
Greenwood PH (1954) On two species of Cichlid fishes from the Malagarazi River (Tanganyika), with notes on the pharyngeal apophysis in species of the Haplochromis group. J Nat Hist 7(78):401–414
Greenwood PH (1956) The monotypic genera of cichlid fishes in Lake Victoria. Bull Brit Mus (Nat Hist) 3(7):295–333
Greenwood PH (1965) The cichlid fishes of Lake Nabugabo, Uganda. Bull Brit Mus (Nat Hist) 12(9):313–357
Greenwood PH (1978) A review of the pharyngeal apophysis and its significance in the classification of African cichlid fishes. Bull Brit Mus (Nat Hist) 33:297–323
Greenwood PH (1991) Speciation. In: Keenleyside MHA, Miles HA (eds) Cichlid fishes: behavior, ecology and evolution. Chapman & Hall, London, pp 86-102
Gronow LT (1854) A Catalogue of Fish Collected and Described by Laurence Theodore Gronow now in the British Museum. Order of the Trustees. Trustees of the British Museum, London
Günther A (1864) Descriptions of new species of batrachians from West Africa. Proc Zool Soc Lond 32:479–482
Haseman JD (1911) An annotated catalog of the cichlid fishes collected by the expedition of the Carnegie Museum to central South America, 1907-10. Ann Carnegie Mus 7(3–4):329–373
Heckel JJ (1840) Johann Natterer’s neue Flussfische Brasilien’s nach den Beobachtungen und Mittheilungen des Entdeckers beschrieben. Erste Abtheilung: Die Labroiden. Ann Wien Mus Naturges 2:326–470
Heiligenberg W (1965) Colour polymorphism in the males of an African cichlid fish. Proc Zool Soc Lond 146(1):95–97
Helfman G, Collette BB, Facey DE, Bowen BW (2009) The diversity of fishes: biology, evolution, and ecology. Wiley, Hoboken, NJ
Herlong D (1999) Interview with: Stuart Grant, Jul-85. In: Cichlid room companion. Available via Cichlid Room Companion. http://www.cichlidae.com/article.php?id=275
Herzig-Straschil B (1997) Franz Steidachner (1834-1919) and other prime contributors to the ichthyological collection of the Naturhistorisches Museum Wien. In: Pietsch TW, Anderson WD (eds) Collection building in ichthyology and herpetology. American Society of Ichthyologists and Herpetologists, Lawrence, pp 391–405
Hilgendorf FM (1888) Fische aus dem Victoria-Nyanza (Ukerewe-See), gesammelt von dem verstorbenen In: Fischer GA (ed) Sitzungsberichte der Gesellschaft Naturforschender Freunde zu Berlin, pp 75–79
Hillis DM (1987) Molecular versus morphological approaches to systematics. Ann Rev Ecol Syst 18:23–42
Holder JL (1991) The mechanisms of mate choice in the Mida Cichlid, Cichlasoma citrinellum. University of California, Berkeley
Hori M (1987) Mutualism and commensalism in a fish community in Lake Tanganyika. In: Kawano S, Connell JH, Hidaka T (eds) Evolution and coadaptation in biotic communities. University of Tokyo Press, Tokyo, pp 219–239
Hori M, Yamaoka K, Takamura K (1983) Abundance and micro-distribution of cichlid fishes on a rocky shore of Lake Tanganyika. Afr Study Monogr 1983(3):25–38
Huxley JS (1940) The new systematics. The Clarendon Press, Oxford
Huxley JS (1942) Evolution. The modern synthesis. Harper & Brothers Pub, New York
Jenyns L (1842) Part 4: Fish. In: Darwin C (ed) The zoology of the voyage of HMS Beagle during the years 1832 to 1836. Smith Elder & Co., London
Johnson AJ (1862) Johnson’s new illustrated (steel plate) family atlas with descriptions, geographical, statistical, and historical. 1862 Johnson map of South America. Image also available in File:1862 Johnson Map of South America – Geographicus – SouthAmer-johnson-1862.jpg via Wikimedia Commons. https://commons.wikimedia.org/wiki/File:1862_Johnson_Map_of_South_America_-_Geographicus_-_SouthAmer-johnson-1862.jpg. Accessed 14 June 2019
Johnston AK, Keane AH (1878) Africa. Edward Stanford, London. Available via Internet Archive. https://archive.org/details/africa00johnrich. Physical map of Africa, opposite p 11. Image also available in File:Africa (1878) (14796102343).jpg via Wikimedia Commons. https://commons.wikimedia.org/wiki/File:Africa_(1878)_(14796102343).jpg. Accessed 14 June 2019
Johnson TC, Scholz CA, Talbot MR, Kelts K (1996) Late Pleistocene desiccation of Lake Victoria and rapid evolution of cichlid fishes. Science 273(5278):1091
Johnston HH (1916) Catalogue of the fresh-water fishes of Africa in the British Museum (Natural History). Nature 97(2428):218
Jones A (1972) Studies on egg development and larval rearing of turbot, Scophthalmus maximus L., and brill, Scophthalmus rhombus L., in the laboratory. J Mar Biol Assoc UK 52(04):965–986
Jonsson B, Skúlason S, Snorrason S, Sandlund OT, Malmquist HJ, Jónasson PM, Cydemo R, Lindem T (1988) Life history variation of polymorphic Arctic Charr (Salvelinus alpinus) in Thingvallavatn, Iceland. Can J Fish Aquat Sci 45(9):1537–1547
Joyce DA, Lunt DH, Bills R, Turner GF, Katongo C, Duftner N, Sturmbauer C, Seehausen O (2005) An extant cichlid fish radiation emerged in an extinct Pleistocene lake. Nature 435(7038):90–95
Kapralova KH, Morrissey MB, Kristjánsson BK, Ólafsdóttir GÁ, Snorrason SS, Ferguson M (2011) Evolution of adaptive diversity and genetic connectivity in Arctic charr (Salvelinus alpinus) in Iceland. Heredity 106(3):472
Karino K (1997) Female mate preference for males having long and symmetric fins in the Bower-holding Cichlid Cyathopharynx furcifer. Ethology 103(11):883–892
Kawabata MH, Mihigo NY (1982) Littoral fish fauna near Uvira, northwestern end of Lake Tanganyika. Afr Study Monogr 2:133–143
Kawanabe MH, Nagoshi M (1997) Fish Communities in Lake Tanganyika. Kyoto University Press, Kyoto
Keenleyside MH (1983) Mate desertion in relation to adult sex ratio in the biparental cichlid fish Herotilapia multispinosa. Anim Behav 31(3):683–688
Keenleyside MH (1991) (ed) Cichlid Fishes: behavior, ecology and evolution. Chapman & Hall, London
Keenleyside MH, Bailey RC, Young VH (1990) Variation in the mating system and associated parental behaviour of captive and free-living Cichlasoma nigrofasciatum (Pisces, Cichlidae). Behaviour 112(3):202–220
Kirchshofer R (1953) Aktionssystem des Maulbrütters Haplochromis desfontainesii. Zeitschrift für Tierpsychologie 10(2):297–318
Knight ME, Turner GF (2004) Laboratory mating trials indicate incipient speciation by sexual selection among populations of the cichlid fish Pseudotropheus zebra from Lake Malawi. Proc R Soc Lond Ser B Biol Sci 271(1540):675–680
Kocher TD, Thomas WK, Meyer A, Edwards SV, Pääbo S, Villablanca FX, Wilson AC (1989) Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc Natl Acad Sci U S A 86:6196–6200
Konings A (2011) Some critical remarks on Cynotilapia and Microchromis. In: The Cichlid Room Companion. Available via The Cichlidae Information Center. https://www.cichlidae.com/article.php?id=369
Konings A (2013) Stuart M. Grant Cichlid Conservation Fund. https://www.cichlidpress.com/smgfund/
Kornfield IL (1978) Evidence for rapid speciation in African cichlid fishes. Experientia 34(3):335–336
Kornfield I (1984) Descriptive genetics of cichlid fishes. In: Turner BJ (ed) Evolutionary genetics of fishes. Springer, New York, pp 591–616
Kornfield I (1991) Genetics. In: Keenleyside MHA (ed) Cichlid fishes: behavior, ecology and evolution. Chapman & Hall, London, pp 103–128
Kornfield IL, Koehn RK (1975) Genetic variation and speciation in New World cichlids. Evolution 29(3):427–437
Kornfield I, Smith PF (2000) African cichlid fishes: model systems for evolutionary biology. Annu Rev Ecol Syst 31:163–196
Kornfield I, Smith DC, Gagnon PS, Taylor JN (1982) The cichlid fish of Cuatro Cienegas, Mexico: direct evidence of conspecificity among distinct trophic morphs. Evolution 36(4):658–664
Kristjánsson BK, Malmquist HJ, Antonsson FI, Snorrason SS, Skúlason S (2011) Relationships between lake ecology and morphological characters in Iceland charr, Salvelinus alpinus. Biol J Linn Soc 103(4):761–771
Kristjánsson BK, Snorrason SS, Skúlason S (2012) Fine-scale parallel patterns in diversity of small benthic Arctic charr (Salvelinus alpinus) in relation to the ecology of lava/groundwater habitats. Ecol Evol 2(6):1099–1112
Kullander SO (1982) Cichlid fishes from the La Plata basin. Part IV. Review of the Apistogramma species, with description of a new species (Teleostei, Cichlidae). Zool Scr 11(4):307–313
Kullander SO (1988) Teleocichla, a new genus of South American rheophilic cichlid fishes with six new species (Teleostei: Cichlidae). Copeia 1988(1):196–230
Kullander SO (1998) A phylogeny and classification of the South American Cichlidae (Teleostei: Perciformes). In: Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS (eds) Phylogeny and classification of neotropical fishes. Edupucrs, Porto Alegre, pp 461–498
Kuwamura T (1986) Parental care and mating systems of cichlid fishes in Lake Tanganyika: a preliminary field survey. J Ethol 4:129–146
Kuwamura T, Nagoshi M, Sato T (1989) Female-to-male shift of mouthbrooding in a cichlid fish, Tanganicodus irsacae, with notes on breeding habits of two related species in Lake Tanganyika. Environ Biol Fish 24:187. https://doi.org/10.1007/BF00001223
Lacépède BG (1798–1803) Histoire Naturelle des Poisons. Plassan, Paris
Lande R (1976) Natural selection and random genetic drift in phenotypic evolution. Evolution 30(2):314–334
Lande R (1980) Genetic variation and phenotypic evolution during allopatric speciation. Am Nat 116(4):463–479
Lande R (1981) Models of speciation by sexual selection on polygenic traits. Proc Natl Acad Sci 78(6):3721–3725
Lanzing WJR, Bower CC (1974) Development of colour patterns in relation to behaviour in Tilapia mossambica (Peters). J Fish Biol 6(1):29–41
Lavery RJ (1991) Physical factors determining spawning site selection in a Central American hole nester, Cichlasoma nigrofasciatum. Environ Biol Fish 31(2):203–206
Lavery RJ, Keenleyside MH (1990a) Parental investment of a biparental cichlid fish, Cichlasoma nigrofasciatum, in relation to brood size and past investment. Anim Behav 40(6):1128–1137
Lavery RJ, Keenleyside MH (1990b) Filial cannibalism in the biparental fish Cichlasoma nigrofasciatum (Pisces: Cichlidae) in response to early brood reductions. Ethology 86(4):326–338
Le Cren ED, Lowe-McConnell RH (1980) The functioning of freshwater ecosystems. Cambridge University Press, Cambridge, UK
Levine JS, MacNichol EF, Kraft T, Collins BA (1979) Intraretinal distribution of cone pigments in certain teleost fishes. Science 204(4392):523–526
Lewis D (1980) Preliminary comparisons between the ecology of the haplochromine cichlid fishes of Lake Victoria and Lake Malawi. Netherlands J Zool 31(4):746–761
Liem KF (1979) Modulatory multiplicity in the feeding mechanism in cichlid fishes, as exemplified by the invertebrate pickers of Lake Tanganyika. J Zool 189(1):93–125
Liem KF (1991) Functional morphology. In: MHA K (ed) Cichlid fishes: behavior, ecology and evolution. Chapman & Hall, London, pp 129–149
Liem KF, Greenwood PH (1981) A functional approach to the phylogeny of the pharyngognath teleosts. Am Zool 21(1):83–101
Liem KF, Kaufman LS (1984) Intraspecific macroevolution: functional biology of the polymorphic cichlid species Cichlasoma minckleyi. In: Echelle AA, Kornfield I (eds) Evolution of fish species flocks. Univ. Maine Press, Orono, pp 203–215
Linnaeus C (1758) Systema naturae, sive regna tria naturae systematice proposita per classes, ordines, genera & species. Lugduni Batavorum (Haak)
Lobel PS (1998) Possible species specific courtship sounds by two sympatric cichlid fishes in Lake Malawi, Africa. Environ Biol Fish 52(4):443–452
Lönnberg E (1903) On a collection of fishes from the Cameroon containing new species. J Nat Hist 12(67):37–46
Lorenz K (2002) On aggression. Psychology Press, London
Lowe-McConnell R (2006) The tilapia trail: the life story of a fish biologist. MPM Publishing, Ascot
Lowe-McConnell R, Crul R, Roest F (1992) Symposium on resource use and conservation of the African-Great Lakes, Bujumbura, 1989. Mitt Int Verein Limnol 23:1–128
Lucas CP (1897) South and East Africa. A historical geography of the British colonies, vol 4. Clarendon Press, Oxford. Available via Internet Archive. https://archive.org/details/southeastafrica04lucauoft. British East Africa, opposite p 106. Image also available in File:South and East Africa (1897) (14595850330).jpg via Wikimedia Commons. https://commons.wikimedia.org/wiki/File:South_and_East_Africa_(1897)_(14595850330).jpg. Accessed 14 June 2019
Marlier G, Leleup N (1954) A curious ecological niche among the fishes of Lake Tanganyika. Nature 174:935–936
Marsh BA, Marsh AC, Ribbink AC, Sharp BJ (1983) A preliminary survey of the cichlid fishes of rocky habitats in Lake Malawi. South Afr J Zool 18(3):149–310
Martin M (1972) A biogeographic analysis of the freshwater fishes of Honduras. Unpublished Ph.D. University of Southern California, Los Angeles
Martin E, Taborsky M (1997) Alternative male mating tactics in a cichlid, Pelvicachromis pulcher: a comparison of reproductive effort and success. Behav Ecol Sociobiol 41(5):311–319
Martin NH, Bouck AC, Arnold ML (2006) Detecting adaptive trait introgression between Iris fulva and I. brevicaulis in highly selective field conditions. Genetics 172(4):2481–2489
Mayr E (1942) Systematics and the origin of species, from the viewpoint of a zoologist. Harvard University Press, Cambridge
McKaye KR (1980) Seasonality in habitat selection by the gold color morph of Cichlasoma citrinellum and its relevance to sympatric speciation in the family Cichlidae. Environ Biol Fishes 5(1):75–78
McKaye KR (1985) Cichlid-catfish mutualistic defense of young in Lake Malawi, Africa. Oecologia 66(3):358–363
McKaye KR, Barlow GW (1976a) Competition between color morphs of the Midas Cichlid, Cichlasoma citrinellum, in Lake Jiloá, Nicaragua. In: Thorson TB (ed) Investigations of the Ichthyofauna of Nicaraguan Lakes. University of Nebraska School of Life Sciences, Lincoln, pp. 467-475. https://digitalcommons.unl.edu/ichthynicar/1/34
McKaye KR, Barlow GW (1976b) Chemical recognition of young by the Midas cichlid, Cichlasoma citrinellum, in Lake Jiloá, Nicaragua. Copeia 1976: 277–282
McKaye KR, Marsh A (1983) Food switching by two specialized algae-scraping cichlid fishes in Lake Malawi, Africa. Oecologia 56(2–3):245–248
McKaye KR, McKaye NM (1977) Communal care and kidnapping of young by parental cichlids. Evolution 31:674–681
McKaye KR, Gray WN (1984) Extrinsic barriers to gene flow in rock-dwelling cichlids of Lake Malawi: macrohabitat heterogeneity and reef colonization. In: Echelle A, Kornfield I (eds) Evolution of fish species flocks. University of Maine, Orono Press, Orono, pp 169–184
McKaye KR, Kocher T, Reinthal P, Harrison R, Kornfield I (1984) Genetic evidence for allopatric and sympatric differentiation among color morphs of a Lake Malawi cichlid fish. Evolution 38(1):215–219
McKaye KR, Louda SM, Stauffer JR Jr (1990) Bower size and male reproductive success in a cichlid fish lek. Am Nat 135(5):597–613
McKaye KR, Howard JH, Stauffer R Jr, Morgan RP, Shonhiwa F (1993) Sexual selection and genetic relationships of a sibling species complex of bowe building cichlids in Lake Malawi, Africa. Japn J Ichthyol 40:15–21
Meek SE (1907) Synopsis of the fishes of the fishes of the Great Lakes of Nicaragua. Chicago Field Mus Nat Hist 7(4):97–132
Meyer A (1987) Phenotypic plasticity and heterochrony in Cichlasoma managuense (Pisces, Cichlidae) and their implications for speciation in Cichlid fishes. Evolution 41(6):1357–1369
Meyer A (1989) Cost of morphological specialization: feeding performance of the two morphs in the trophically polymorphic cichlid fish, Cichlasoma citrinellum. Oecologia 80(3):431–436
Meyer A, Kocher TD, Basasibwaki P, Wilson AC (1990) Monophyletic origin of Lake Victoria cichlid fishes suggested by mitochondrial DNA sequences. Nature 347(6293):550
Mullis KB (1994) PCR and scientific invention: the trial of DuPont vs Cetus. The Polymerase chain reaction. Springer, New York, pp 427–441
Myers GS (1935) Four new fresh-water fishes from Brazil, Venezuela and Paraguay. Proc Biol Soc Washington 48:7–14
Myers GS (1960) Fish evolution in Lake Nyasa. Evolution 14(3):394–396
Myers GS (1966) Derivation of the freshwater fish fauna of Central America. Copeia (4):766–773
Myrberg AA (1975) The role of chemical and visual stimuli in the preferential discrimination of young by the cichlid fish Cichlasoma nigrofasciatum (Gunther). Z Tierpsychol:274–297
Myers JM, Hershberger WK, Iwamoto RN (1986) The induction of tetraploidy in salmonids. J World Aquacult Soc 17(1–4):1–7
Myrberg AA, Kramer E, Heinecke P (1965) Sound production by cichlid fishes. Science 149(3683):555–558
Naérico A, Menezes JL, Figueiredo D, Britski HA (1997) Ichthyological collection building at the Museu de Zoologia da Universidade de São Paulo, Brazil. In: Pietsch TW, Anderson WD (eds) Collection building in ichthyology and herpetology. American Society of Ichthyologists and Herpetologists, Lawrence
Nagoshi M (1983) Distribution, abundance and parental care of the genus Lamprologus (Cichlidae) in Lake Tanganyika. Afr Study Monogr 3:39–47
Nelson JS, Grande TC, Wilson MVH (2016) Fishes of the world, 5th edn. John Wiley and Sons, Hoboken
Nichols JT, Griscom L, Lang H, Chapin JP (1917) Fresh-water fishes of the Congo basin obtained by the American Museum Congo expedition, 1909-1915. In: Bulletin of the American Museum of Natural History. Available via Research Library Digital Repository. http://digitallibrary.amnh.org/handle/2246/1069. Accessed 25 June 2019
Noakes DLG (1979) Parent-touching behavior by young fishes: incidence, function and causation. Environ Biol Fish 4(4):389–400
Noakes DLG (1991) Ontogeny of behavior in cichlids. In: Keenleyside MHA (ed) Cichlid fishes: behavior, ecology and evolution. Chapman & Hall, London, pp 209–224
Noakes DLG (1994) The life and work of Ethelwynn Trewavas: beyond the focus on tilapiine cichlids. Environ Biol Fish 41(1):33–50
Noakes DLG (2008) Charr truth: sympatric differentiation in Salvelinus species. Environ Biol Fish 83(1):7–15
Noakes DLG, Balon E (1982a) Life histories of tilapias: an evolutionary perspective. In: Paper presented at the International Conference on the Biology and Culture of Tilapias, Bellagio, Italy, 2–5 Sep 1980
Noakes DLG, Balon EK (1982b) Life histories of tilapias: an evolutionary perspective. In: RSV P, Lowe-McConnell R (eds) The biology and culture of tilapias. International Center for Living Aquatic Resources Management, Manila, pp 61–82
Noakes DLG, Barlow GW (1973) Ontogeny of parent-contacting in young Cichlasoma citrinellum (Pisces, Cichlidae). Behaviour 46(3):221–255
Noakes DLG, Barlow GW (1976) Cross-fostering and parent-offspring responses in Cichlasoma citrinellum (Pisces, Cichlidae). Zeitschrift für Tierpsychologie 33(2):147–152
Noble GK, Curtis B (1939) The social behavior of the jewel fish, Hemichromis bimaculatus Gill. American Museum of Natural History, New York
Oijen MV (1982) Ecological differentiation among the haplochromine piscivorous species of Lake Victoria. Netherlands J Zool 32:336–363
Oliver MK (1984) Systematics of African cichlid fishes: determination of the most primitive taxon, and studies on the haplochromines of Lake Malawi (Teleostei: Cichlidae). PhD Dissertation, Yale University
Oliver MK (2013) A Passion for Cichlids. In: Blogs. Available via Seriously Fish. http://www.seriouslyfish.com/a-passion-for-cichlids/
Paterson HEH (1993) Variation and the specific-mate recognition system. Perspect Ethol 10:209–227
Pauly D (1976) The biology, fishery and potential for aquaculture of Tilapia melanotheron in a small West African lagoon. Aquaculture 7(1):33–49
Pellegrin J (1904) Contribution à l’étude anatomique, biologique et taxinomique des poissons de la famille des Cichlidés. Bigot frères, Lille
Pfeffer G (1893) Ostafrikanische Reptilien und Amphibien, gesammelt von Herrn Dr. F. Stuhlmann im Jahre 1888 und 1889. Jahrbuch der Hamburgischen Wissenschaftlichen Anstalten 10:69–105
Philippart JC, Ruwet JC (1982) Ecology and distribution of tilapias. In: The biology and culture of tilapias, vol 7. International Center for Living Aquatic Resources Management, Manila, pp 15–60
Pitcher TJ (1986) Functions of shoaling behaviour in teleosts. In: Pitcher TJ (ed) The behaviour of teleost fishes. Springer, New York, pp 294–337
Poll M, Matthes H (1962) Trois poissons remarquables du lac Tanganikya. Musée Royal de l’Afrique Centrale 111:1–26
Pullin RSV, Lowe-McConnell R (1982) The biology and culture of tilapias. ICLARM, Manila
Regan CT (1905) A revision of the fishes of the American Cichlid Genus Cichlasoma and of the allied genera. J Nat Hist 16(91):60–77
Regan CT (1922) The Cichlid Fishes of Like Victoria. Proc Zool Soc Lond 92(1):157–191
Ribbink AJ (1984) Is the species flock concept tenable? Evolution of fish species flocks. In: Echelle A, Kornfield I (eds) Evolution of fish species flocks. University of Maine at Orono Press, Orono, pp 21–25
Ribbink AJ (1991) Distribution and ecology of the cichlids of the African Great Lakes. In: Keenleyside MHA (ed) Cichlid fishes: behavior, ecology and evolution. Chapman & Hall, London, pp 36–59
Ribbink AJ, Hill BJ (1979) Depth equilibration by two predatory cichlid fish from Lake Malawi. J Fish Biol 14(6):507–510
Ribbink AJ, Marsh A, Marsh B, Sharp B (1980) Parental behavior and mixed broods among cichlid fish of Lake Malawi. Afr Zool 15(1):1–6
Ribeiro ADM (1918) Dos gêneros e três espécies novas de peixes Brasileiros determinados nas coleçoes do Museu Paulista. Revta Mus Paulista, Sao Paulo 10:787–791
Riedel D (1965) Some remarks on the fecundity of Tilapia (T. mossambica) and its introduction into Middle Central America (Nicaragua) together with a first contribution toward the limnology of Nicaragua. Hydrobiologia 25(3–4):357–388
Ringler NH (1983) Variation in foraging tactics of fishes. In: DLG N, Lindquist DG, Helfman GS, Ward JA (eds) Predators and prey in fishes: Proceedings of the 3rd biennial conference on ethology and behavioral ecology of fishes, held at Normal, Illinois, U.S.A., May 19–22, 1981. Springer, Dordrecht, pp 159–171
Rodman DT (1966) Sound production by the African cichlid Tilapia mossambica. Ichthyol Aquar J 38:279–280
Rosenzweig ML (1978) Competitive speciation. Biol J Linn Soc 10(3):275–289
Russock HI, Schein MW (1977) Effect of age and experience on the filial behaviour of Tilapia mossambica fry (Pisces: Cichlidae). Behaviour 61(3):276–302
Sage RD, Selander RK (1975) Trophic radiation through polymorphism in cichlid fishes. Proc Natl Acad Sci 72(11):4669–4673
Sato T (1986) A brood parasitic catfish of mouthbrooding cichlid fishes in Lake Tanganyika. Nature 323(6083):58–59
Scheuermann H (1977) A partial revision of the genus Limnochromis Regan 1920. Cichlidae 3:69–73
Scholz CA, King JW, Ellis GS, Swart PK, Stager JC, Colman SM (2003) Paleolimnology Lake Tanganyika, East Africa, over the past 100kyr. J Paleolimnol 30(2):139–150
Schwanck E (1987) Reproductive behaviour of a monogamous cichlid fish Tilapia mariae. PhD thesis. University of Stockholm
Scott AG, Penman DJ, Beardmore JA, Skibinski DOF (1989) The ‘YY’supermale in Oreochromis niloticus (L.) and its potential in aquaculture. Aquaculture 78(3-4):237–251
Seehausen O, Lippitsch E, Bouton N, Heleen Z (1998) Mbipi, the rock-dwelling cichlids of Lake Victoria: description of three new genera and fifteen new species (Teleostei). Ichthyol Explor Freshw 9:129–228
Seyoum S (1989) Stock identification and the evolutionary relationship of the genera Oreochromis, Sarotherodon and Tilapia (Pisces: Cichlidae) using allozyme analysis and restriction endonuclease analysis of mitochondrial DNA. University of Waterloo, Waterloo, ON
Shaw ES, Aronson LR (1954) Oral incubation in Tilapia macrocephala. Bull. Am. Mus Nat Hist, 103:379–415
Shaw E, Innes K (1980) The “infantilization” of a cichlid fish. Dev Psychobiol 13(2):131–139
Shine R (1978) Propagule size and parental care: the “safe harbor” hypothesis. J Theor Biol 75(4):417–424
Sjölander S, Fernö A (1973) Sexual imprinting on another species in a cichlid fish, Haplochromis burtoni. Rev Comp Animal 7:77–81
Skúlason S, Noakes DLG, Snorrason SS (1989) Ontogeny of trophic morphology in four sympatric morphs of arctic charr Salvelinus alphinus in Thingvallavatn, Iceland. Biol J Linn Soc 38(3):281–301
Skúlason S, Snorrason SS, Noakes DLG, Ferguson MM (1996) Genetic basis of life history variations among sympatric morphs of Arctic charr, Salvelinus alpinus. Can J Fish Aquat Sci 53(8):1807–1813
Smith A (1849) Illustrations of the zoology of South Africa: consisting chiefly of figures and descriptions of the objects of natural history collected during an expedition into the interior of South Africa, in the years 1834, 1835, and 1836; fitted out by “the Cape of Good Hope Association for Exploring Central Africa”: together with a summary of African zoology, and an inquiry into the geographical ranges of species in that quarter of the globe. Smith, Elder and Co., London
Smith B, Skúlason S (1996) Evolutionary significance of resource polymorphisms in fishes, amphibians and birds. Ann Rev Ecol Syst 27:111–133
Smith-Vaniz WF, Peck RM (1997) Contributions of Henry Weed Fowler (1878-1965), with a Brief Early History of Ichthyology at The Academy of Natural Sciences of Philadelphia. In: Pietsch TW, Anderson WD (eds) Collection building in ichthyology and herpetology. American Society of Ichthyologists and Herpetologists, Lawrence, pp 173–191
Snorrason SS, Skúlason S (2004) Adaptive speciation in Northern freshwater fishes. In: Dieckmann U, Doebeli M, Metz JAJ, Tautz D (eds) Adaptive speciation. Cambridge University Press, Cambridge, pp 210–228
Stiassny MLJ (1982) Relationships of the Neotropical genus Cichla (Perciformes, Cichlidae): a phyletic analysis including some functional considerations. J Zool 197(3):427–453
Stiassny ML (1987) Cichlid familial intrarelationships and the placement of the neotropical genus Cichla (Perciformes, Labroidei). J Nat Hist 21(5):1311–1331
Stiassny ML (1991) Phylogenetic intrarelationships of the family Cichlidae: an overview. In: Keenleyside MHA (ed) Cichlid fishes: behaviour, ecology and evolution. Chapman & Hall, London, pp 1–35
Stiassny ML, De Pinna MC (1994) Basal taxa and the role of cladistic patterns in the evaluation of conservation priorities: a view from freshwater. Syst Assoc Special 50:235–235
Stiassny ML, Kaufman LS (2015) Rosemary Lowe-McConnell, obituary. Env Biol Fish 98:1719–1722
Sultan SE (1987) Evolutionary implications of phenotypic plasticity in plants. Evol Biol 21:127–178
Taborsky M (1984) Broodcare helpers in the cichlid fish Lamprologus brichardi: their costs and benefits. Anim Behav 32(4):1236–1252
Takezaki N, Nei M (1996) Genetic distances and reconstruction of phylogenetic trees from microsatellite DNA. Genetics 144(1):389–399
Teletchea F (2018) Fish domestication: an overview. In: Teletchea F (ed) Animal domestication. IntechOpen. https://doi.org/10.5772/intechopen.79628
Tlusty M (2002) The benefits and risks of aquacultural production for the aquarium trade. Aquaculture 205(3–4):203–219
Torres-Dowdall J, Meyer A (2021) Sympatric and allopatric diversification in the adaptive radiation of Midas cichlids in Nicaraguan lakes. In: Abate ME, Noakes DLG (eds) The behavior, ecology and evolution of cichlid fishes. Springer Nature, Dordrecht, pp 175–216. https://doi.org/10.1007/978-94-024-2080-7_6
Townshend TJ, Wootton RJ (1985a) Adjusting parental investment to changing environmental conditions: the effect of food ration on parental behavior of the convict cichlid, Cichlasoma nigrofasciatum. Anim Behav 33(2):494–501
Townshend TJ, Wootton RJ (1984) Effects of food supply on the reproduction of the convict cichlid, Cichlasoma nigrofasciatum. J Fish Biol 24(1):91–104
Townshend TJ, Wootton RJ (1985b) Variation in the mating system of a biparental cichlid fish, Cichlasoma panamense. Behaviour 95(3):181–197
Trewavas E (1931) A revision of the Cichlid fishes of the genus Lethrinops, Regan. J Nat Hist 7(37):133–152
Trewavas E (1935) A synopsis of the Cichlid fishes of Lake Nyasa. J Nat Hist 16(91):65–118
Trewavas E (1942) The cichlid fishes of Syria and Palestine. J Nat Hist 9(55):526–536
Trewavas E (1982) Genetic groupings of Tilapiini used in aquaculture. Aquaculture 27(1):79–81
Trewavas E (1983) Tilapiine fishes of the genera Sarotherodon, Oreochromis and Danakilia. British Museum of Natural History, London
Trewavas E, Green J, Corbet SA (1972) Ecological studies on crater lakes in West Cameroon fishes of Barombi Mbo. J Zool 167(1):41–95
Trivers R (1972) Parental investment and sexual selection. Aldine Publishing Company, Chicago
Trivers R (2002) Natural selection and social theory: selected papers of Robert Trivers. Evolution and cognition. Oxford University Press, Oxford
Turelli M, Barton NH, Coyne JA (2001) Theory and speciation. Trends Ecol Evol 16(7):330–343
Turner JL (1977) Some effects of demersal trawling in Lake Malawi (Lake Nyasa) from 1968 to 1974. J Fish Biol 10(3):261–271
Turner GF (1994) Speciation mechanisms in Lake Malawi cichlids: a critical review. Ergebnisse der Limnologie 44:139–139
Tweddle D (1991) Twenty years of fisheries research in Malawi: a brief review of Malawi Government Fisheries Department Research Programmes conducted since 1970. Montfort Press, Limbe
Van Oijen MJP (1981) Ecological differentiation among the piscivorous haplochromine cichlids of Lake Victoria (East Africa). Netherlands J Zool 32(3):336–363
Van Oppen MH, Turner GF, Rico C, Deutsch JC, Ibrahim KM, Robinson RL, Hewitt GM (1997) Unusually fine–scale genetic structuring found in rapidly speciating Malawi cichlid fishes. Proc R Soc Lond B Biol Sci 264(1389):1803–1812
Von Humboldt A (1834) Voyage de Humboldt et Bonpland, Observations de Zoologie
Von Ihering H (1914) Os bugios do gênero Alouatta. Revista do Museu Paulista 9:231–256
Voss J (1980) Color patterns of African cichlids. TFH Publications
Ward JA (1975) The cichlids of the Resplendent Isle. Oceans Mag 8:42–47
Ward JA, Samarakoon JI (1981) Reproductive tactics of the Asian cichlids of the genus Etroplus in Sri Lanka. Environ Biol Fish 6(1):95–103
Ward JA, Wyman RL (1977) Ethology and ecology of cichlid fishes of the genus Etroplus in Sri Lanka: preliminary findings. Environ Biol Fish 2(2):137–145
Watson JD, Crick FH (1953) Molecular structure of nucleic acids. Nature 171(4356):737–738
Welcomme RL (1966) Recent changes in the stocks of tilapia in Lake Victoria. Nature 212:52–54
Wickler W (1963) In order to classify the Cichlidae, using the example of the genera Tropheus, Petrochromis, Haplochromis and Hemihaplochromis n. Gen. (Pisces, Perciformes). Senckenbergiana Biol 44:83–96
Wickler W (1965) Mimicry and the evolution of animal communication. Nature 208:519–521
Wilson EO (1998) The current state of biological diversity. In: Wilson EO (ed) Biodiversity. National Academy Press, Washington, DC, pp 3–18
Witte F (1984) Consistency and functional significance of morphological differences between wild-caught and domestic Haplochromis squamipinnis (Pisces, Cichlidae). Netherlands Journal of Zoology 36:596–612
Witte F, Goldschmidt T, Goudswaard PC, Ligtvoet W, Van Oijen MJP, Wanink J (1991) Species extinction and concomitant ecological changes in Lake Victoria. Netherlands J Zool 42(2):214–232
Wolters WR, Libey GS, Chrisman CL (1982) Effect of triploidy on growth and gonad development of channel catfish. Trans Am Fish Soc 111(1):102–105
Yamaoka K (1991) Feeding relationships. In: MHA K (ed) Cichlid fishes: behavior, ecology and evolution. Chapman & Hall, London, pp 151–172
Yanagisawa Y (1986) Parental care in a monogamous mouthbrooding cichlid Xenotilapia flavipinnis in Lake Tanganyika. Jpn J Ichthyol 33(3):249–261
Yanagisawa Y, Nshombo M (1983) Reproduction and parental care of the scale-eating cichlid fish Perissodus microlepis in Lake Tanganyika. Physiol Ecol Jap 20(1):23–31
Yuma M, Harada E (1998) The life and work of Hiroya Kawanabe: the priest ecologist. Environ Biol Fish 52(1–3):11–35
Zaret TM, Paine RT (1973) Species introduction in a tropical lake. Science 182(2):449–455
Acknowledgments
Regrettably, much valuable information is left out of a brief history spanning over two centuries. To adequately cover the work of the professional men and women and supporters outside academia who have been instrumental to the advancement of cichlid studies would be a much larger project. It is my hope that this introduction might serve as a resource for future endeavors. Without the guidance and help of Michael Osborne, Anita Guerrini, David Noakes, Maria Abate, and Tori Spence, this would not have been possible.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature B.V.
About this chapter
Cite this chapter
McConnell, M. (2021). Frontiers in Cichlid Research: A History of Scientific Advancement. In: Abate, M.E., Noakes, D.L. (eds) The Behavior, Ecology and Evolution of Cichlid Fishes. Fish & Fisheries Series, vol 40. Springer, Dordrecht. https://doi.org/10.1007/978-94-024-2080-7_2
Download citation
DOI: https://doi.org/10.1007/978-94-024-2080-7_2
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-024-2078-4
Online ISBN: 978-94-024-2080-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)