Abstract
Pediatric pelvic tumors are challenging because a surgeon must balance the challenges of treating a child, who is likely to have a better response to preoperative chemotherapy, with the challenges of resecting and reconstructing a tumor in the immature skeleton. Because young children typically have a far better preoperative chemotherapy response than most adults and young adults, they are often candidates for a smaller, more conservative tumor resection than the typical adult. The extent of the resection should be based on a careful preoperative assessment of the response to preoperative chemotherapy, as demonstrated by a comparison of preoperative MRI and PET scan imaging. Pediatric allografts can be an excellent reconstructive option for pelvic resections, which have a greater likelihood of healing in children. Patients under age 10 years may be candidates for nonoperative resection of their primary tumor because of a good response to chemotherapy. Surgical complications vary by multiple factors including tumor size, the type of resection and reconstruction, the age of patient, the length of the surgery and extent of surgical blood loss, and the surgeon’s experience. Tumor recurrence is a greater risk after pelvic resections than after extremity resections, and careful diligence in the documentation of operative margins is critical. Careful preoperative planning and preoperative discussions are an essential requirement for a good postoperative result.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The care of pediatric pelvic tumors adds the challenges of caring for children to the challenges of caring for pelvic tumors. The pelvis is a less common and more complex anatomic location for tumor resections than extremity tumors, and pelvic sarcomas require more planning for resection than extremity tumors. Pelvic tumors are typically larger than extremity tumors and present more challenging resections because pelvic sarcomas may encroach upon the adjacent iliac vessels, sacral nerve roots, acetabulum, sacrum, or bladder and bowel. Surgical pelvic resection has historically been defined by the Enneking classification that recognizes tumor resections that are located in the posterior (sacroiliac, Type I), lateral (acetabular, Type II), or anterior (obturator, Type III) pelvis (Fig. 17.1) [1]. The most common osseous pelvic sarcoma diagnoses include Ewing’s sarcoma (20–25% pelvic primary tumors) and osteosarcoma (10% pelvic primary tumors) [2, 3]. Chondrosarcoma and metastatic pelvic tumors are uncommon in children, while benign bone tumors in the pelvis are relatively common.
2 Pediatric Versus Adult Pelvic Resections
The resection of tumors located in the immature pediatric pelvis is sometimes less challenging to resect than adult pelvic sarcomas because of the greater sensitivity of many pediatric sarcomas to preoperative chemotherapy compared to adult sarcoma patients. That greater sensitivity to chemotherapy allows preoperative treatment that results in a tumor that is physically less invasive and more easily removed. This sensitivity also allows closer surgical margins and more effective surgery with better local control of their tumor for children younger than age 16–18 years. Assessing pediatric sarcomas or their preoperative chemotherapy response is an essential part of making surgical decisions regarding their surgical treatment.
Surgical resection in the skeletally immature patient also becomes less challenging if the acetabular and femoral epiphyses are spared by surgical resection. That option is easier to achieve in a younger patient who is more likely to have a good response to chemotherapy, indicated by greater tumor necrosis, and is more likely to have a resection with acceptable osseous margins. Making that decision is the essence of preoperative planning for pediatric pelvic sarcoma surgery. The resection of bone sarcomas in patients under the age of 14–16 years also requires accommodation for skeletal growth, planning for a life expectancy that may exceed oncologic implant survival, and an expectation for levels of postoperative physical activity that exceeds the usual adult expectations.
While preoperative chemotherapy is an important adjuvant therapy for both pelvic osteosarcoma and Ewing’s sarcoma, preoperative radiation therapy has historically been utilized mostly with Ewing’s sarcoma patients. Ewing’s sarcoma is a relatively radiosensitive tumor, and radiotherapy can be employed pre- or postoperatively or in palliative situations as an effective treatment for local control. Osteosarcoma has historically not been considered to be a radiosensitive tumor. Surgical pelvic resections are not contraindicated in skeletally immature, unless the patient is younger than 10 years old and has acetabular involvement. Patients under the age of 10 years old with a pelvic Ewing’s sarcoma involving the acetabulum should be considered for radiation therapy without resection for local control rather than surgical resection, if the patients have demonstrated a good response to preoperative chemotherapy on preoperative imaging. Patients of any age without a good response to neoadjuvant chemotherapy (based on preoperative imaging) are at a greater risk for local recurrence after radiation only and are at greater risk for requiring amputation. Nonsurgical treatment for local control (radiation without resection) of pelvic sarcomas does carry a greater risk of local recurrence than the use of both resection and adjuvant radiation therapy for local control [4, 5].
3 Predicting and Achieving Surgical Margins with Pediatric Pelvic Tumors
Adequate tumor resection margins are the most important goal for a successful tumor procedure in any patient. Resections should be based on a careful preoperative assessment of the response to preoperative (neoadjuvant) chemotherapy that is accomplished with careful review of preoperative MRI, CT, and positron emission tomography (PET) imaging that should assess the tumor size, soft tissue and osseous involvement, tumor inflammation, and proposed surgical margins [6]. The best assessment of the patient’s response to preoperative chemotherapy involves a comparison of both MRI and PET scan imaging before and after the first 10–12 weeks of preoperative chemotherapy [7, 8]. That comparison of imaging before and after preoperative chemotherapy is a critical sign of whether the patient’s tumor is responding to preoperative chemotherapy. The assessment of the neoadjuvant chemotherapy “response” with a comparison of MRI and PET imaging is an essential part of planning surgical margins in all patients treated with preoperative chemotherapy, because a good response to chemotherapy will allow closer surgical margins at resection and the possible preservation of important anatomic structures (Fig. 17.2). Close or smaller tumor margins (1.0–2.0 cm), however, are at higher risk for tumor contamination and subsequent tumor recurrence, whereas wider, greater margins (2.0 cm or greater), with the presence of a normal cuff of tissue, are theoretically at less risk of tumor recurrence [3, 9]. Evidence-based guidelines for determining the adequacy and quality of a tumor margin and the pathologic description of surgical margins are challenging and become more complex in the pelvis [10, 11].
The process of evaluating preoperative imaging should be determined with imaging guidelines. On T2 or STIR MR imaging sequences, the increased signal surrounding the tumor indicates inflammation and possible microscopic tumor at the tumor interface with surrounding normal tissues. With effective neoadjuvant preoperative therapy, this zone may disappear or shrink and allow a closer margin of resection [12] (Fig. 17.2). PET scan imaging is a quantitative assessment of the glucose uptake by the tumor as reflected in the standard uptake value (SUV). A 50% reduction in the PET SUV will typically reflect a good response when comparing pre- and post-systemic therapy PET scans.
A “good response” to preoperative chemotherapy may shrink the peritumor inflammatory zone and/or may result in a small decrease in tumor diameter as visualized on preoperative MRI [12,13,14]. A good response to chemotherapy may also result in apparent necrosis within the tumor mass and a subsequent reduction in pre- and post-chemotherapy PET scans. Multiple published adult sarcoma studies regarding the assessment of treatment response to neoadjuvant chemotherapy and radiation therapy using fluorodeoxyglucose PET show that a significant (50% or greater) decrease in tumor fluorodeoxyglucose uptake is predictive of patient survival [6, 8]. Criteria for acceptable or successful bony margins are difficult to interpret from the literature, but a comparison to extremity sarcomas is helpful [3, 9,10,11, 15, 16]. A review of pediatric sarcomas involving the distal femur by Zimel et al. demonstrated that resections with larger surgical margins compared to intercalary joint-sparing resections with smaller margins did not show a significant difference in local recurrence [17]. The role of local recurrence following resection is associated with the adequacy of soft tissue and osseous surgical margins and should be carefully evaluated for all pelvic resections [18, 19]. The surgical goal for extremity surgical margins of 1.0 cm should be increased to 2.0 cm, if possible, for pelvic margins because of the higher risk of recurrence. In addition, recent margin assessments in osteosarcoma and Ewing’s sarcoma suggest that soft tissue tumor margins are more challenging than bony margins and are more likely to contain microscopic, margin contamination, leading to local recurrence [3, 10].
Computer navigation assistance with pelvic resection is an attractive planning tool for pelvic resection because of its ability to improve and document the accuracy of resection in the pelvis and to improve the documentation of the resected specimen and the associated margins. The surgical experience with navigation requires accurate system “registration,” reporting of surgical margins, and a comparison of local recurrence with or without the technique in order to demonstrate surgical efficacy [20]. Navigation requires documentation of the preoperative and intraoperative surgical margins that can be compared to the intraoperative and final postoperative plan and margins. Those comparisons represent important metrics for confirming the effectiveness of navigation for sarcoma resections. Demonstrating navigation accuracy with minimal radiation exposure (intraoperative CT “registration”) is another issue to be resolved by future navigation protocols for pediatric patients [1, 21, 22]. Intraoperative navigation requires the “registration” or downloading of preoperative MRI and/or CT imaging and the intraoperative “registration” of the patient to that imaging [17,18,19] (Fig. 17.3e). The use of intraoperative navigation is able to provide a “GPS” function that will improve the accuracy and planning of both surgical tumor margins and reconstructions and is particularly useful in the pelvis.
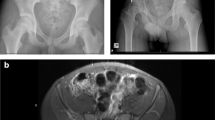
Type II pelvic Resection and reconstruction—preoperative imaging (a–c), PET scan (d), navigation margin (e), postoperative composite allograft/THR (f). (a) Preoperative pelvic X-ray. (b) Preoperative pelvic MRI (coronal T2). (c) Preoperative pelvic MRI (axial T2). (d) PET scan image of tumor. (e) 3D image of navigated pelvic procedure. (f) Postoperative Type II alloprosthetic reconstruction
4 Ewing’s Sarcoma and Osteosarcoma of the Pelvis
Surgical resection of Ewing’s sarcoma in the pelvis should consider multiple factors including the presence of metastatic disease, size of primary tumor, chemotherapy response, the anatomic location of the tumor, and patient age. Metastatic disease at presentation may serve as a contraindication for resection, although 20–30% of patients with lung metastases survive their disease (compared to 16% with bony disease) [4]. Patients with Ewing’s sarcoma should be very carefully assessed at presentation for both pulmonary and osseous metastases. Patients with osseous metastases or multiple lung metastases should be considered for pelvic surgery with careful discussion of the risk of progressive disease.
Osteosarcoma of the pelvis is less common than Ewing’s sarcoma and may be a more challenging tumor to resect with adequate margins. It has the same challenges as Ewing’s of the pelvis with regard to surgical margins and is more common in older patients. Radiation therapy has historically not been effective for osteosarcoma, and preoperative chemotherapy is a critical pre-resection systemic therapy.
5 Pelvic “Composite” Alloprosthetic and Implant Reconstructions
Cadaveric pelvic allografts are an excellent reconstructive option for both Type I and II pelvic resections. Type III anterior, obturator resections without acetabular involvement are best managed with a Gor-Tex, allograft dermis, or other synthetic patch to reconstruct the inguinal canal and associated pelvic defect. When acetabular resection (Type II) is involved, a composite pelvic allograft and total hip reconstruction or custom implant should be considered to reconstruct the graft’s acetabulum and patient’s femoral head [23] (Fig. 17.3a–e).
Type II pelvic reconstruction can also be achieved with either custom or modular pelvic implants that incorporate a total hip reconstruction (Fig. 17.3f). Custom implants may be replaced by modular pelvic implants that incorporate sciatic notch fixation and modular acetabular components. “Saddle” prosthesis reconstruction has lost their popularity for pelvic reconstruction because of postoperative pain and instability at the “yoke”-patient osseous iliac interface [24]. While extremity sarcoma patients have been managed with “growing” implants, those implants remain associated with a high complication risk including implant aseptic loosening, sepsis, and failure of lengthening. There are significant limits regarding how large a segment these devices can lengthen (4–6 cm), and their use in the pelvis and hip joint is extremely limited. Flail reconstructions are a reasonable option for patients with large tumors and have demonstrated surprisingly good function postoperatively, and pelvic amputations should always be considered as a possible surgical option, especially in patients with large or recurrent tumors [5].
Type I and Type II pelvic allografts should be fixed to the remaining adjacent pelvis with plate and screw fixation at both the anterior pubic and posterior iliac osteosynthesis sites (Fig. 17.3f). That fixation should include at least six cortices of fixation at both host junction sites. Additional sacroiliac fixation should include two or three carefully placed large diameter sacroiliac screws if the graft involves the sacroiliac joint. If radiographic evidence of bony healing is not apparent on radiographs/CT imaging at the osseous junction site at 10–12 months postoperatively, fixation revision and autogenous bone grafting should be considered as an additional procedure [25,26,27]. Initial selection of pelvic allografts for reconstruction should be selected with consideration of patient and donor gender, age, and acetabular diameter. Pelvic allograft sizing can be achieved by the assessment of acetabular diameter for both the donor graft and, if possible, patient gender and age of grafts versus patients should be considered with graft selection [28]. Pelvic allografts carry a risk of postoperative infection but can achieve bony union at the host bone-graft interface and may offer better soft tissue attachment.
6 Surgical Exposure After Pelvic Resection
Pelvic resection, surgical exposure, and skin incisions are a critical consideration for a successful pelvic sarcoma resection and reconstruction. Choices for the surgical skin incision include the standard ilioinguinal incision from the pubis to the posterior lateral iliac crest. Patients may require more surgical exposure posteriorly or anteriorly depending on the location of their tumor, and their incision, exposure, and positioning should be adjusted accordingly.
Skin incision “extensions” of the standard ilioinguinal incision, transversing the groin and iliac crest, should include an anterior/longitudinal skin incision for obturator ring resection (Type III) with the associated exposure of the femoral vessels. A Type II resection with acetabular resection usually requires an anterolateral extension for total hip exposure and reconstructions. Posterior Type I resections may require a longitudinal sacral extension of the skin incision.
7 Postoperative Complications and Rehabilitation
Surgical complications vary by tumor size and location, the type of resection and reconstruction, age of patient, length of the surgery, extent of surgical blood loss, operative time, and surgeon experience and level of skill. Choices of pelvic reconstruction range from no reconstruction (flail limb) to nonanatomic endoprosthetic implants (i.e., saddle prosthesis) or anatomic composite pelvic allograft/total hip reconstruction. The most recent surgical trends for pelvic reconstruction favor “composite” alloprosthetic reconstructions, modular pelvic implant, or no reconstruction, frequently referred to as a “flail” pelvis. Pelvic amputation or hemipelvectomy is always an option for surgical resection.
One of the most significant factors in predicting surgical challenges and a higher incidence of postoperative complications is the inclusion of the acetabulum in the pelvic resection (Type II resections). Acetabular reconstructions require a decision to reconstruct the peri-acetabular ilium with a pelvic implant or alloprosthetic implant or a decision to leave the pelvis flail and unreconstructed. Acetabular reconstructions carry the risk of sciatic nerve injury, hip dislocation, and the challenges of fixation and graft healing. Pelvic amputation (hemipelvectomy) is usually reserved for patients with large tumors, neurovascular involvement, a poor response to chemotherapy, or a tumor recurrence after initial resection. When considering amputation as the primary resection, the surgeon should be aware of the recurrence risk for larger tumors because of the challenges of achieving adequate margins at the sacrum, spine, and/or other critical midline structures. Pelvic tumors with sacral or neurovascular involvement should be assessed preoperatively with caution for the adequacy of those surgical margins.
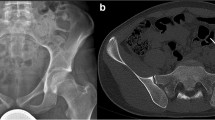
The risks of postoperative infections (20–30%), local recurrence, neurovascular injury, and massive intraoperative blood loss are the most common surgical risks with osseous pelvic resections [18, 19]. Critical surgical issues during tumor resection include the control of the external and common internal iliac and femoral vessels during operative exposure and resection (Fig. 17.4). The lack of adequate iliac vascular control is associated with a higher risk of massive intraoperative hemorrhage and perioperative death. It is also important to identify other critical structures including the sacral, sciatic, obturator, and femoral nerves and ureter. Damage to the peripheral nerve roots is most common for the femoral nerve, obturator nerve, and sciatic nerve. Those risks all increase with larger pelvic tumors, older patients, recurrent tumors, and revision surgeries.
Deep infections and delayed wound healing are the most common complications following pelvic resection. Those complications can be minimized with copious irrigation during surgery, shorter procedure times, lower blood loss, healthy wound closure, and attention to appropriate postoperative intravenous antibiotics. Approximately 50–60% of patients who undergo surgical resection and radiation therapy require a second operation, and approximately 15–20% of patients will have a major complication requiring multiple additional procedures. The risk of tumor local recurrence in pelvic sarcomas is 25–30% and related to tumor size and sensitivity to adjuvant chemotherapy. The incidence of revision surgery for pelvic allograft complications ranges from 25% to 50% with revision surgery most commonly occurring for graft infections, wound necrosis, nonunion of the pelvic allograft, instability of the hip arthroplasty, or tumor recurrence [15, 29]. The most common postoperative complication is postoperative infection, which occurs in 20–30% of patients within the first 4–6 weeks postoperatively and requires surgical washout with or without graft removal and extended antibiotics. Amputations are indicated for most tumor local recurrences and on rare occasions for postoperative complications. Wound infections can also be avoided with careful consideration of appropriate surgical incisions and the judicious use of myocutaneous flaps for persistent drainage postoperatively. Published reviews for pelvic sarcoma resections demonstrate an overall risk of revision to amputation of approximately 10–15% [30, 31].
Postoperative rehabilitation following pelvic resections should be approached with a standard protocol specific to your practice and focus on preoperative and postoperative goals (Table 17.1). That protocol should be focused on the immediate postoperative management for the first 3 days, the first 3 weeks, and the first 3 months. The first 3 days should focus on a patient’s blood loss during and after surgery, their vital signs, and nerve function. Patient pain control and neurovascular monitoring of the extremity should also be a high priority in the first 24 h and first 3 days. Routine Doppler vascular ultrasound should be carried out to confirm good arterial and venous flow in the iliac and femoral vessels. Venous Doppler to detect thrombosis should be repeated weekly for the first 4–6 weeks in all patients. Any problem with excessive postoperative bleeding at the surgical site in the first 48 h should be managed with intravenous embolization and appropriate transfusions.
Postoperative infection prophylaxis requires IV antibiotics to cover both gram negative and gram positive bacteria for the first 7–14 days postoperatively. All patients should be followed for possible infections with weekly CBCs/WBC for 3 weeks postoperatively to detect possible leukocytosis secondary to postoperative infections. Postoperative imaging should routinely include pelvic X-ray and a postoperative pelvic CT scan and venous Doppler. Pediatric patients are usually placed in a postoperative pelvic/hip brace for approximately 6–12 weeks with toe-touch ambulation with a walker or crutches for 6 months or until bony pelvic graft and implant healing is documented on postoperative radiographs. Transfer to a rehabilitation facility is typically considered at 2–3 weeks postoperatively.
Pelvic resection rehabilitation usually involves a postoperative hospitalization of 1–2 weeks. Patients are partial weight bearing for 3–12 months depending on the size of their resection and age. Patients receiving systemic therapies such as chemotherapy need to be able to resume their postoperative chemotherapy schedule in 2–3 weeks as a delay greater than 3 weeks can affect patient survival. Recovery for allograft reconstructions requires 6–12 months of partial weight bearing, while prosthetic reconstructions require 3–6 months. “Flail” pelvic resection patients and patients treated with hemipelvectomy also are able to recover their weight bearing status at 3–6 months.
8 Growth in the Pediatric Patient
The onset of puberty in children (age 10–14 years) is a critical milepost signaling the beginning of the last phase of skeletal growth. Younger preadolescent children have 10–20% of their overall skeletal growth and 15–25% of their femoral growth remaining at age 10 years. Girls 8–10 years of age have 6–10 cm of remaining femoral and proximal tibial growth, and boys have 9–12 cm remaining. This remaining growth makes surgical decisions for younger patients with osteosarcoma or Ewing’s sarcoma more challenging [32]. Pelvic resection in the skeletally immature should not involve significant leg length discrepancies unless their resection is in a patient under the age of 12 years or involves interruption of the acetabular and or proximal femoral epiphysis (Fig. 17.5). Loss of height or length with iliac or pelvic resections is difficult to predict and measure and should be carefully measured radiographically along with length assessment of the lower extremities at regular intervals.
9 Physeal Biology and Predicting Pelvic Growth Issues
An epiphysis occurs in a weight bearing long bone, while an apophysis represents a growth center associated with an insertion point for a major muscle group (i.e., gluteus).
“Pressure” epiphyses, or normal growth plate “physes,” such as the proximal femur or acetabular physes and iliac and trochanteric apophyses account for both hip development and longitudinal growth in the pelvis, acetabulum, and proximal femur. For example, the femoral neck can be significantly narrowed as a result of the placement of an intramedullary rod in the proximal femur in a child under the age of 12 years. Traction epiphyses or apophyses, such as the greater trochanter, or iliac apophysis does not involve the joint but does serve as a major muscle insertion point for the gluteal and abdominal muscles [33, 34]. The acetabular physis is a “triradiate” growth plate that produces acetabular/pelvic growth in three planes until it reaches maturity at approximately age 12 years (Fig. 17.5) [35,36,37]. Other apophyseal and epiphyseal maturation points serve as an important sign of growth in immature patients.
The growth plate is composed of chondrocytes in various stages of differentiation termination with endochondral ossification [36, 38]. The growth plate is composed of three zones: the resting, proliferative, and hypertrophic zones. In the resting or reserve zone, chondrocytes rarely divide, and nutrients are stored. In the proliferative zone, chondrocytes divide rapidly and arrange themselves vertically in columns. In the hypertrophic zone, chondrocytes enlarge and proliferate. As a result of the cellular division, the proliferative zone is at greater risk for being affected by neoadjuvant chemotherapy and radiation therapy physeal growth. Regulation occurs via a parathyroid hormone-related protein (PTHrP) feedback loop [39, 40]. This feedback loop affects the expression of Indian hedgehog (Ihh) which is responsible for chondrocyte proliferation and maturation and the rate at which this process takes place [39]. If disruption of the growth plate circulation or bone bridge formation occurs via trauma or surgical resection, angular growth deformities and/or limb-length discrepancies can result [40].
10 Managing Leg Length Discrepancies in Children
The most important factor in assessing patients with a leg length discrepancy after surgery is the consistent tracking and documentation of the patients’ bilateral extremity growth at regular 6-month intervals. It is critical to begin that “tracking” in the first 2 postoperative years such that postoperative epiphyseodeses are not delayed for 1–2 years. Assessing and predicting pelvic and acetabular growth is challenging because of the acetabular triradiate and more complex bony anatomy. Significant apophyseal growth occurs at the iliac crest (e.g., Risser sign) and traditionally has been used as an easy and common skeletal maturity assessment for pediatric orthopedic surgeons [34, 41]. Growth milestones for the acetabulum have not been well studied or documented in children, but the acetabular physis is thought to naturally reach maturity and “close” at 12–14 years of age [41,42,43,44,45,46,47]. Routine scanogram assessment of leg length should include assessment of the ipsilateral iliac and acetabular sciatic notch.
The management of leg length discrepancies can be simplified for small discrepancies that are less than 2 cm through the use of a shoe lift and nonoperative management. These shoe lifts can be made in variable sizes and built into a patient’s shoes. They do not require any invasive procedures but have cosmetic limitations on how large a discrepancy can be corrected without patient complaints. Surgical methods can be utilized to partially correct anticipated leg length discrepancy concerns. At the time of pelvic resection, a reconstruction can be carried out in a way that effectively lengthens the limb by placing a pelvic implant or allograft that is slightly (1–2 cm) larger than preoperative length for the correction of future leg length discrepancies. This method is limited to corrections of 10–15 mm and requires precise pelvic reconstructive metrics that include the proximal femur and acetabulum and ilium.
Arresting the physes of the contralateral “normal” leg is a popular and effective option for addressing leg length discrepancy after resection and could be considered for a leg length discrepancy after pelvic resection with arrest of the contralateral distal femoral physis. This method should be carried out within the first year postoperatively in order to minimize the magnitude of the discrepancy. Depending on the number of physes arrested and the timing of epiphysiodeses, a discrepancy of 2–3 cm could be addressed with an epiphysiodesis of the contralateral distal femur but at the expense of the patient’s ultimate overall height at skeletal maturity. This procedure usually occurs 1 year after limb salvage in a skeletally immature patient. An alternative leg length discrepancy can be corrected by skeletal shortening via femoral osteotomy and fixation. The longer contralateral “normal” extremity can be shortened surgically by 2–3 cm if necessary in a procedure that typically occurs 2–3 years after limb salvage and is best achieved as femoral shortening fixed with a femoral rod or plate [48,49,50]. Femoral osteotomy for the correction of leg length discrepancy usually heals at 6 weeks and has a low complication risk.
In cases for whom the discrepancy is projected to be greater than 2 cm and shortening of the contralateral limb is refused, distraction osteogenesis could be considered as an alternative to lengthen the short extremity. Bone lengthening is accomplished with an osteotomy and gradual lengthening through the use of an Ilizarov-style external fixator, which is difficult to implement after chemotherapy and requires 6–12 months of postoperative rehab. This procedure may be limited by the amount of remaining viable bone that is suitable for distraction osteogenesis and has limited applications in the pelvis and in sarcoma patients. The entire course of lengthening requires multiple operative procedures with significant risk of pin sepsis and in not an attractive alternative in patients who have just completed chemotherapy and/or radiation treatment for a sarcoma [51].
11 Future Directions
Future directions for the treatment of pediatric sarcoma patients include three promising areas for advancement: (1) improved assessment and documentation of surgical margins by intraoperative technology; (2) the development of more sophisticated modular, 3D-printed oncologic pelvic biologic implants; and (3) improved methods to preserve or reproduce skeletal growth after sarcoma treatment.
In the near future, improvements in the intraoperative assessment of surgical tumor margins will involve the ability to assess the adequacy of surgical margins with intraoperative transducers and biologic tumor imaging. Immunofluorescence imaging techniques or other intraoperative real-time assessment tools of critical tumor margins will facilitate that process. The development of quantitative and “co-registered” (overlapping) MRI and PET should also allow for the improved preoperative assessment of tumor viability and the improved intraoperative assessment of tumor margins. Current studies are investigating the role of intraoperative navigation systems in the planning and documentation of adequate surgical margins [22].
New oncologic implant 3D printing manufacturing processes should allow for the improved attachment of the gluteal and hip muscles and other tendon attachments at the greater trochanter, lesser trochanter, and hip capsule. As manufacturing technology continues to advance, the options for improved pediatric pelvic implants should allow for improved fixation, osseous healing, and physical function. Ideally, these new implants will allow for modularity, possible noninvasive lengthening, and improved function. An improved viable biologic alternative for pelvic iliac allografts and physeal acetabular cartilage may provide dramatic improvements for pediatric patients with viable pelvic grafts that will achieve bony healing in 3 months instead of the current 6–12 months. Improved pelvic grafts might include a collagen matrix incorporating osteoblastic stem cells to be used in conjunction with demineralized 3D-printed allografts to allow for earlier bony union of hemipelvic reconstructions. The current 12–24-month delay for bony healing of pelvic allografts is a mandate for better options and progress with both biologic and molecular mechanisms for cartilage and bone transplants in the pelvis and extremities.
Limb salvage for sarcoma patients gained popularity in the 1970s and 1980s with the success of chemotherapy regimens. Adult surgical resections have shown significant improvements in the last two decades in the function and survival of extremity oncologic implants. The focus is now on developing better reconstruction options for children with a better capacity for growth and improved methods for antibacterial therapies. Other challenges in pediatric oncologic limb salvage currently involve significant recent improvements in perioperative imaging and expectation for improved pediatric pelvic implants and more successful methods for skeletal growth. With advances in technology and biologic research, these improvements will likely be accomplished in the next 5–10 years and have significant consequences for the skeletally immature patients who have the greatest risks and rewards after tumor resection.
References
Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–6.
Eary JF, O’Sullivan F, Powitan Y, Chandhury KR, Vernon C, Bruckner JD, Conrad EU. Sarcoma tumor FDG uptake measured by PET and patient outcome: a retrospective analysis. Eur J Nucl Med. 2002;29(9):1149–54.
Fernebro J, Wiklund M, Jonsson K, Bendahl PO, Rydholm A, Nilbert M, Engellau J. Focus on the tumour periphery in MRI evaluation of soft tissue sarcoma: infiltrative growth signifies poor prognosis. Sarcoma. 2006;2006:21251.
Eary JF, Conrad EU, O’Sullivan J, Hawkins DS, Schuetze SM, O’Sullivan F. Sarcoma mid-therapy [F-18]fluorodeoxyglucose positron emission tomography (FDG PET) and patient outcome. J Bone Joint Surg Am. 2014;96(2):152–8. https://doi.org/10.2106/JBJS.M.00062.
Ozaki T, Hillmann A, Hoffmann C, Rube C, Blasius S, Dunst J, Jurgens H, Winkelmann W. Significance of surgical margin on the prognosis of patients with Ewing’s sarcoma. A repost from the cooperative Ewing’s sarcoma study. Cancer. 1996;78(4):892–900.
Sampo M, Tarkkanen M, Huuhtanen R, Tukiainen E, Böhling T, Blomqvist C. Impact of the smallest surgical margin on local control in soft tissue sarcoma. Br J Surg. 2008;95(2):237–43.
Trovik CS, Skjeldal S, Bauer H, Rydholm A, Jebsen N. Reliability of margin assessment after surgery for extremity soft tissue sarcoma: the SSG experience. Sarcoma. 2012;2012:290698. https://doi.org/10.1155/2012/290698.
Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–20.
Takahashi M, Sato K, Miura T. MR imaging of musculoskeletal sarcomas: the clinical significance of peritumoral low signal intensity lines in planning surgical margins. J Jpn Orthop Assoc. 1993;67(10):881–96.
Holscher HC, Bloem JL, Vanel D, Hermans J, Nooy MA, Taminiau HT, Henry-Amar M. Osteosarcoma: chemotherapy induced changes at MR imaging. Radiology. 1992;182(3):839–44.
Gherlinzoni F, Picci P, Bacci G, Campanacci D. Limb sparing versus amputation in osteosarcoma. Correlation between local control, surgical margins and tumor necrosis: Istituto Rizzoli experience. Ann Oncol. 1992;3(Suppl 2):S23–7.
Mankin HJ, Gebhardt MC, Jennings C, Springfield DS, Tomford WW. Long-term results of allograft replacement in the management of bone tumors. Clin Orthop Relat Res. 1996;324:86–97.
Zimel MN, Cizik AM, Rapp TB, Weisstein JS, Conrad EU. Megaprosthesis versus Condyle-sparing intercalary allograft: distal femoral sarcoma. Clin Orthop Relat Res. 2009;467(11):2813–24.
Ng V, Jones R, Bompadre V, Punt S, Conrad EU. The effect of surgery with radiation on pelvic ewing sarcoma. J Surg Oncol. 2015;112(8):861–5.
Ayvaz M, Bekmez S, Mermerkaya MU, Caglar O, Acaroglu E, Tokgozoglu AM. Long-term results of reconstruction with pelvic allografts after wide resection of pelvic sarcomas. Scientific World Journal. 2014;2014:605019. https://doi.org/10.1155/2014/605019.
Zang J, Guo W, Yang Y, Xie L. Reconstruction of the hemipelvis with a modular prosthesis after resection of a primary malignant peri-acetabular tumour involving the sacroiliac joint. J Bone Joint J Br. 2014;96-B(3):399–405. https://doi.org/10.1302/0301-620X.96B3.32387.
Stoll KE, Miles JD, White JK, Punt SE, Conrad EU, Ching RP. Assessment of registration accuracy during computer-aided oncologic limb salvage surgery. Int J Comput Assist Radiol Surg. 2015;10(9):1469–75. https://doi.org/10.1007/s11548-01401146-1.
Ieguchi M, Hoshi M, Takada J, Hidaka N, Nakamura H. Navigation-assisted surgery for bone and soft tissue tumors with bony extension. Clin Orthop Relat Res. 2012;470:275–83.
Cheong D, Letson GD. Computer-assisted navigation and musculoskeletal sarcoma surgery. Cancer Control. 2011;18(3):171–6.
Gerbers JG, Stevens M, Ploegmakers JJ, Bulstra SK, Jutte PC. Computer-assisted surgery in orthopedic oncology: technique, indications, and a descriptive study of 130 cases. Acta Orthop. 2014;85(6):663–9. https://doi.org/10.3109/17453674.2014.950800.
Donati D, Yin J, Di Bella C, Colangeli M, Bacci G, Ferrari S, et al. Local and distant control in non-metastatic pelvic Ewing’s sarcoma patients. J Surg Oncol. 2007;96:19–25.
Krasin MJ, Constine LS, Friedman DL, Marks LB. Radiation-related treatment effects across the age spectrum: differences and similarities or what the old and young can learn from each other. Semin Radiat Oncol. 2010;20(1):21–9.
Takami M, Ieguchi M, Takamatsu K, Kitano T, Aono M, Ishida T, Yamano Y. Functional evaluation of flail hip joint after periacetabular resection of the pelvis. Osaka City Med J. 1997;43:173–83.
Donati D, Bella C, Frisoni T, Cevolani L, Groot H. Alloprosthetic composite is a suitable reconstruction after periacetabular tumor resection. Clin Orthop Relat Res. 2011;469:1450–8. https://doi.org/10.1007/s11999-011-1799-9.
Frisoni T, Cevolani L, Giorgini A, Dozza B, Donati DM. Factors affecting outcome of massive intercalary bone allografts in the treatment of tumours of the femur. J Bone Joint Surg Br. 2012;94(6):838–41.
Ogilvie CM, Crawford EA, Hosalkar HS, King JJ, Lackman RD. Long-term reconstruction results for limb salvage with osteoarticular allograft reconstruction. Clin Orthop Relat Res. 2009;467:2685–90.
Aljassir F, Beadel GP, Turcotte RE, Griffin AM, Bell RS, Wunder JS, Isler MH. Outcome after pelvic sarcoma resection reconstructed with saddle prosthesis. Clin Orthop Relat Res. 2005;438:36–41. https://doi.org/10.1097/00003086-200509000-00009.
Campanacci D, Chacon S, Mondanelli N, et al. Pelvic massive allograft reconstruction after bone tumour resection. Int Orthop. 2012;36(12):2529–36. https://doi.org/10.1007/s00264-012-1677-4.
Carter SR, Eastwood DM, Grimer RJ, Sneath RS. Hindquarter amputation for tumours of the musculoskeletal system. J Bone Joint Surg Br. 1990;72(3):490–3.
Hillmann A, Hoffmann C, Gosheger G, Roedl R, Winkelmann W, Ozaki T. Tumors of the pelvis: complications after reconstruction. Arch Orthop Trauma Surg. 2003;123:340–4. https://doi.org/10.1007/s00402-003-0543-7.
Morris CD, Teot LA, Bernstein ML, Marina N, Krailo MD, Villaluna D, Janeway KA, DuBois SG, Gorlick RG, Randall RL. Assessment of extent of surgical resection of primary high-grade osteosarcoma by treating institutions: a report from the Children’s Oncology Group. J Surg Oncol. 2016;113:351. https://doi.org/10.1002/jso.24145.
Anderson M, Messner MB, Green WT. Distribution of lengths of the normal femur and tibia in children from one to eighteen years. J Bone Joint Surg Am. 1964;46:1197–202.
Heimkes B. [The great apophyses: functional strain and relevance]. Orthopade. 2016;45:206.
Wang WW, Xia CW, Zhu F, Zhu ZZ, Wang B, Wang SF, Yeung BH, Lee SK, Cheng JC, Qiu Y. Correlation of Risser sign, radiographs of hand and wrist with the histological grade of iliac crest apophysis in girls with adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2009;34(17):1849–54. https://doi.org/10.1097/BRS.0b013e3181ab358c.
Ponseti IV. Growth and development of the acetabulum in the normal child. Anatomical, histological, and roentgenographic studies. J Bone Joint Surg Am. 1978;60(5):575–85.
Pichler K, Schmidt B, Fischerauer EE, Rinner B, Dohr G, Leithner A, Weinberg AM. Behaviour of human physeal chondro-progenitor cells in early growth plate injury response in vitro. Int Orthop. 2012;36:1961.
Hansman CF. Appearance and fusion of ossification centers in the human skeleton. Am J Roentgenol Radium Ther Nucl Med. 1962;88:476–82.
Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol. 2000;16:191–220.
van Leeuwen BL, Kamps WA, Jansen HW, Hoekstra HJ. The effect of chemotherapy on the growing skeleton. Cancer Treat Rev. 2000;26:363–76.
Wattenbarger JM, Gruber HE, Phieffer LS. Physeal fractures, part I: histologic features of bone, cartilage, and bar formation in a small animal model. J Pediatr Orthop. 2002;22(6):703–9.
Menelaus MB. Correction of leg length discrepancy by epiphysial arrest. J Bone Joint Surg Br. 1966;48(2):336–9.
Green WT, Anderson M. Experiences with epiphyseal arrest in correcting discrepancies in length of the lower extremities in infantile paralysis: a method of predicting the effect. J Bone Joint Surg Am. 1947;29:659–75.
Paley D, Bhave A, Herzenberg JE, Bowen JR. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000;82-A:1432–46.
Moseley CF. A straight-line graph for leg-length discrepancies. J Bone Joint Surg Am. 1977;59:174–9.
Risser JC. The classic: the iliac apophysis: an invaluable sign in the management of scoliosis. 1958. Clin Orthop Relat Res. 2010;468(3):643–53. https://doi.org/10.1007/s11999-009-1096-z.
Yang JH, Bhandarkar AW, Suh SW, Hong JY, Hwang JH, Ham CH. Evaluation of accuracy of plain radiography in determining the Risser stage and identification of common sources of errors. J Orthop Surg Res. 2014;9:101. https://doi.org/10.1186/s13018-014-0101-8.
Hallel T, Salvati E. Premature closure of the triradiate cartilage. Clin Orthop. 1977;124:278–81.
Dimeglio A. Growth in pediatric orthopaedics. J Pediatr Orthop. 2001;21(4):549–55.
Bucholz R, Ezaki M, Ogden J. Injury of the acetabular triradiate physeal cartilage. J Bone Joint Surg Am. 1982;64-A:600–9.
Hasler CC, Krieg AH. Current concepts of leg lengthening. J Child Orthop. 2012;6(2):89–104. https://doi.org/10.1007/s11832-012-0391-5.
Stanitski DF. Limb-length inequality: assessment and treatment options. J Am Acad Orthop Surg. 1999;7:143–53.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature B.V.
About this chapter
Cite this chapter
Zamora, R., Punt, S., Conrad, E.U. (2020). Pelvic Tumor Surgery in Children. In: Guo, W., Hornicek, F., Sim, F. (eds) Surgery of the Pelvic and Sacral Tumor. Springer, Dordrecht. https://doi.org/10.1007/978-94-024-1945-0_17
Download citation
DOI: https://doi.org/10.1007/978-94-024-1945-0_17
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-024-1943-6
Online ISBN: 978-94-024-1945-0
eBook Packages: MedicineMedicine (R0)