Abstract
There is an increasing interest for analytical methods aimed to detect biological sulfur-containing amines, because of their involvement in human diseases and metabolic disorders. This work describes an improved HPLC method for the determination of sulfur containing amino acids and amines from different biological matrices. We optimized a pre-column derivatization procedure using dabsyl chloride, in which dabsylated products can be monitored spectrophotometrically at 460 nm. This method allows the simultaneous analysis of biogenic amines, amino acids and sulfo-amino compounds including carnosine, dopamine, epinephrine, glutathione, cysteine, taurine, lanthionine, and cystathionine in brain specimens, urines, plasma, and cell lysates. Moreover, the method is suitable for the study of physiological and non-physiological derivatives of taurine and glutathione such as hypotaurine, homotaurine, homocysteic acid and S-acetylglutathione. The present method displays good efficiency of derivatization, having the advantage to give rise to stable products compared to other derivatizing agents such as o-phthalaldehyde and dansyl chloride.
With this method, we provide a tool to study sulfur cycle from a metabolic point of view in relation to the pattern of biological amino-compounds, allowing researchers to get a complete scenario of organic sulfur and amino metabolism in tissues and cells.
The original version of this chapter was revised. An erratum to this chapter can be found at 10.1007/978-94-024-1079-2_97
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Analysis of biogenic and sulfur-containing amines is becoming increasingly important in clinical and biochemical research (Curran et al. 2016). Taurine is a sulfur-containing β-amino acid present in different human body areas and is one of the most important and studied sulfurous organic bioactive molecules involved in human health (Jacobsen and Smith 1968). It is one of the end-products of cysteine metabolism and is excreted in urines. Taurine concentration in humans ranges from high millimolar in plasma, heart and brain, to micromolar amounts in tissue and body fluids such as urines (Schuller-Levis and Park 2003). Apart from its important physiological role as a neuroactive molecule (Wade et al. 1988), it displays a wide range of pharmacological effects including membrane stabilization, cytoprotective effects, antioxidant and anti-inflammatory action (Chaturvedi et al. 2015; Abdel-Moneim et al. 2015). In addition taurine is able to restore muscle function and performance in different pathological conditions (Chan-Palay et al. 1982a, b, c; De Luca et al. 2015). Recently, a number of studies have shown that taurine may have a beneficial effect against metabolic syndrome, preventing obesity regulating glucose metabolism and lowering cholesterol plasma concentration (Bai et al. 2016; Zhang et al. 2016a, b; Chen et al. 2016).
Moreover, many sulfurous amino compounds and taurine metabolic derivatives such as cysteic acid, homocysteic acid, hypotaurine and homotaurine (Fig. 1) have important biological roles (Jacobsen and Smith 1968) and researchers are in need of analytical methods for their accurate determination. Accumulation of sulfite, taurine, S-sulfocysteine and thiosulfate contributes to the severe neurological impairment in molybdenum cofactor deficiency, a severe autosomal recessive inborn error of metabolism (Atwal and Scaglia 2016).
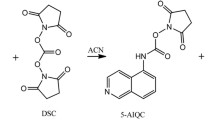
For this purpose we developed a High Performance Liquid Chromatography (HPLC) analytical method for the determination of taurine, sulfur-containing molecules and other biogenic amines from different biological matrices. We improved a dabsyl chloride (DABS) pre-column derivatization method (Krause et al. 1995). DABS (4-N,N-dimethylaminoazobenzene-4′ sulfonyl chloride), is an amine derivatizing agent (Fig. 2) able to give rise to stable products that can be easily monitored spectrophotometrically at 460 nm (Krause et al. 1995; Lin and Wang 1980).
2 Materials and Methods
2.1 Chemicals
Amino acid standards were obtained from Sigma-Aldrich (St. Louis, MO, USA). Gradient grade solvents used for chromatographic analyses were purchased from Carlo Erba Reagents (Milan, Italy). DABS was purchased from Supelco (595 North Harrison Road, Bellefonte, PA). All other reagents were analytical grade products from Sigma-Aldrich. S-Acetylglutathione (SAG) was purchased from GNOSIS S.p.A. (Desio, MB, Italy).
2.2 Derivatization Procedure
The amino acid standards were dissolved in 0.1 M HCl containing 0.2% thiodiglycolic acid (TDGA) to prevent—SH oxidation. One microliter of amino acid standards at different concentrations were added to 14 μL of reaction buffer (1 M NaHCO3, pH 8.6) and then to 25 μL of 15 mM DABS in acetone. After vigorous vortexing, the standards were incubated at 40 °C for 30 min, vortexing at the first minute, twelfth minute and twenty-eighth minute. The resultant Dabsyl derivatives were put in an ice bath for 5 min and then centrifuged at 14000 g for 20 min. Supernatants were diluted 1:10 in mobile phase, filtered onto 0.2 μm filters, and then 50 μL were injected onto the column.
2.2.1 Treatment of Plasma and Urine Samples
Hundred microliter of fresh human plasma or urines were deproteinized by treatment with 100 μL of 10% TCA for 30 min at 4 °C and centrifuged at 14000 g for 30 min at room temperature. The supernatant was then lyophilized. Dry material was resuspended in 15 μL of reaction buffer and 25 μL of 15 mM DABS and derivatized as described above.
2.2.2 Treatment of Brain Tissues and Cultured Cell Samples
Selected mouse brain samples from either cortical or striatal regions (100 mg wet weight) and neuroblastoma cells (SH-SY5Y) pellet derived from 25 cm2 flask were treated with 500 μL of 0.1 M HCl containing 0.2% TDGA, sonicated for 10 min (only for brain tissue), and then centrifuged at 14000 g for 30 min. The supernatant was freeze-dried. 50 μL of reaction buffer and 100 μL of 15 mM DABS were added to the tube and derivatized as described above.
2.3 High-Performance Liquid Chromatography (HPLC)
The apparatus consisted of a Waters HPLC 600 pump equipped with a controller, a Waters autosampler mod. Seven hundred and seventeen and a UV-Vis photodiode array detector mod. 2996. The chromatographic column was a reverse phase X-Bridge C18 column, 4.6 mm × 150 mm, 5 μm particle size, with a 10 mm guard column of the same material. Data analysis was performed using a dedicated application (Millennium32). Elution was performed with a binary gradient system with sodium acetate (30 mM, pH 6.5) and acetonitrile in a ratio of 80:20 (v/v) (solvent A) and propan-2-ol with acetonitrile 50:50 (v/v) (solvent B). The gradient was: 0–4 min, 5% B; 4–8 min, 20% B; 8–15 min, 25% B; 15–27 min, 60% B; 27–28 min, 100% B; 28–32 min, 100% B; 32–33 min, 5% B; 33–60 min, 5% B. The column was equilibrated for 20 min with 5% B at 1 mL/min and was maintained at 40 °C. Dabsylated products were monitored spectrophotometrically at 460 nm.
3 Results and Discussion
A variety of pre-column amine derivatizing methods are reported in the literature, among them o-phthalaldehyde (OPA) and dansyl chloride (DANS) are the main derivatizing agents (Fig. 3) (Kang et al. 2006; Bertollini et al. 2012; Mou 1997).
OPA reacts in the presence of thiols specifically with primary amines above their isoelectric point. One of the limits of this procedure is that not all of the isoindolic fluorescent OPA derivatives are stable, moreover the analyses with this method could be hampered by interferences of unknown thiol moieties potentially present in biological samples. Thiols can displace the organic thiolic additive for the reaction generating different isoindolic adducts for the same investigated molecule (Kand’ar et al. 2007; Mopper and Delmas 1984).
DANS derivatization is a good versatile tool in terms of chromatographic separation and determination but the instability of the products and the need of a fluorimetric detector, as for OPA derivatization, makes this method inaccessible to a large number of research, commercial and clinical laboratories (Loukou and Zotou 2003). By contrast dabsyl chloride, 4-N,N-dimethylaminoazobenzene-4′ sulfonyl chloride (DABS), is an amine derivatizating agent that provides a simple derivatization, good stability, good reproducibility and good analytical detection limit (Lin and Wang 1980; Krause et al. 1995). HPLC analyses with DABS show a good separation of a large number of amines and amino acids, which are detected in the visible region at 460 nm (Krause et al. 1995). DABS, like the other amine derivatization agents, can give rise to mono-dabsyl and bis-dabsyl derivatives in the presence of multiple amino groups. Furthermore with respect to OPA derivatization, DABS can react with primary and also with secondary amines.
In our experimental conditions the chromatographic method allows the separation and the analysis of a wide range of dabsylated amino-derivatives from different complex biological matrices such as plasma, brain tissue, urines and cell lysate samples. As shown in Fig. 4 the following molecules were analyzed in this elution order: Aspartate (Asp), Glutamate (Glut), reduced Glutahione (GSH), Serine (Ser), Threonine (Threo), Glycine (Gly), Hypotaurine (HTau), Taurine (Tau), ɣ-aminobutirric acid (GABA), Proline (Pro), Oxidized glutathione (GSSG), Methionine (Met), Phenylalanine (Phe), Lanthionine (LAN), Cystathionine (CYT), Cysteine (Cys), Carnosine (CAR), Dopamine (DPN) and Norepinefrine (NOR).
Dabsylated amino acids and amphoteric molecules are eluted and separated according to their isoelectric point, from lower to higher value. DABS derivatization displays a good sensitivity (pmole level), linearity and efficiency of derivatization and has the important advantage of giving rise to stable derivatized products (24 h at 4 °C and at least 14 days at −20 °C) (Jansen et al. 1991; Kang et al. 2006; Krause et al. 1995).
With respect to other similar methods using DABS as a derivatizing agent, we introduced some important modifications of the procedure (Krause et al. 1995; Vendrell and Aviles 1986; Drnevich and Vary 1993).
Firstly, the derivatizing procedure was modified and the volumes of reaction were reduced to allow a better recovery of all the analytes from biological samples. The mobile phase was modified and no organic additives such as triethylamine or tetrahydrofuran were added to the hydrophilic phase (Solvent A, 30 mM sodium acetate pH 6.5 containing 20% CH3CN). Many published methods in literature report the use of organic modifiers in the hydrophilic phase to avoid the interaction of the analytes with siloxan and silanol residues in non completely endcapped C18 stationary phases (Krause et al. 1995; Romero et al. 2000). In our setting the use of modifiers was not necessary because of the chemistry of the column. X-bridge columns have replaced a certain number of siloxan groups with ethylene bridges reducing the hydrophilic interaction of the molecules with the stationary phase and improving the stability of the column. The ethylene bridge also reduces the number of free silanols, minimizing adverse interactions with the injected sample and improving both chromatographic resolution and peaks shape.
The second point is the separation of some strictly related compounds and acidic molecules that is strongly dependent on the pH of the mobile phase. In our setting it is important to maintain mobile phase at pH 6.5. Higher pH values lead to loss of chromatographic resolution for taurine and GABA, with concomitant improvement in aspartate and glutamate peak separation. Conversely, with lower pH values a more suitable separation of taurine and GABA is obtained, at the expenses of aspartate and glutamate peaks resolution. This could be explained by the increase of the total protonated forms of acidic compounds at lower pH values.
The third important factor that we want to underline is the condition of derivatization reaction with DABS. We set up our method on the basis of some considerations with respect to the basicity of the reaction environment and the temperature. We chose values of pH 8.6 and 40 °C for 30 min after a series of tests. Many papers report a reaction temperature of 70 °C with different reaction times. We tested the efficiency of different derivatizing reaction conditions by modifying reagents concentration, pH, temperature and reaction times, based on the chemical stability of the molecules of interests, and we found that some conditions are more appropriate than others for the detection of specific compounds. For example, our results indicated that for plasma samples it is necessary to increase the ionic strength of reaction buffer to guarantee the same pH value for all the samples. For the analysis of such biological samples (Figs. 5 and 6) after deproteinization with acid precipitants such as TCA, the use of a concentrated reaction buffer (0.5–1 M) is strongly recommended to ensure the buffering of the acidity.
As regards the pH of derivatization, we tested different reaction buffers (pH 8.0, 8.6 or 9.0). In our conditions the best derivatization efficiency of brain tissue samples is obtained with a reaction buffer at pH 9.0, with a reaction time of 15 min at 70 °C (Fig. 7).
Same results can be obtained by lowering the pH of the buffer and increasing the time of derivatization at lower temperatures. It should be underlined that for some molecules such as catecholamines, pH 8.0 is more convenient because of the lability of these molecules and their tendency to be oxidized to form quinones with subsequent degradation or loss of derivatization. At variance, some authors reported the derivatization of this molecules on the –OH moiety at pH 11.5 by forming phenolate which acts as nucleophile (Cai et al. 2010). In our conditions, the derivatization efficiency of dopamine is strictly dependent on the pH value of the reaction buffer; indeed, at pH values higher than 9.0 the derivatization is not efficient probably because of the oxidation of dopamine to the o-quinonic form. For this reason in our final protocol we adopted a pH 8.6 for the reaction buffer.
The last parameters that we adjusted in the procedure of derivatization were temperature and time. We tested the efficiency and the effect of different reaction conditions on the deacetylation of an S-acetylated sulfo-amino derivative, the S-acetylglutathione (SAG) (Fig. 8). SAG is the thioester of GSH and its detection in biological specimens could be very important as regards the study of thioesters (Fig. 9).
We tested different reaction conditions by analyzing the deacetylation of the molecule at different temperatures, pH and time of reaction. Dabsylation reaction carried out at pH 9 for 15 min at 70 °C gives a deacetylation of the thioester moiety of about 45%. Conversely, reaction conditions of 40 °C, 30 min and pH 8.6 reduces the extent of the deacetylation to about 8%. It is important to emphasize that reaction at pH values lower than 8.6 gives heterogeneous efficiencies of derivatization due to the fact that some amines have pKa values around 8.0. Hence, pH 8.6 is the best compromise that can ensure the homogeneous derivatization of all the studied amines and can minimize the degradation and the oxidation of labile molecules.
We also evaluated the derivatization of GSSG with DABS. As shown in Fig. 10, being GSSG a GSH dimer, its derivatization gives rise to the formation of two derivatives, the mono- and bis-dabsylated forms.
Our data indicate that the dabsylation occurs in the same manner on the two amino groups and the rate of mono- and bis-dabsylated derivatives formation is constant in our reaction conditions.
As regards GSH and thiols in general, we used TDGA to protect thiol moieties from oxidation. The use of TDGA, together with the low temperature and the mild reaction conditions, allows the derivatization of GSH peptide to form a stable dabsyl derivative that can be monitored concomitantly with its bis-dabsylated oxidized form (GSSG) within the same chromatographic analysis. The simultaneous analysis of reduced/oxidized GSH forms, whose ratio is a useful index of oxidative stress (Zitka et al. 2012; Lakritz et al. 1997) enable the study of the redox state of cells or tissues.
GSH analysis shows the same linearity of derivatization and quantification as all the other studied amines, but it is important to underline that, when other thiols and sulfur-containing molecules are present in the derivatization process, some unknown byproducts are formed with a retention time that is strictly similar to GSH and in some cases not completely resolved from DABS peak.
We tested our chromatographic method by analyzing some important sulfur containing organic bioactive compounds. We analyzed simultaneously cysteic acid, homocysteic acid, hypotaurine, taurine and homotaurine (Fig. 11). As for GABA, taurine and homotaurine, resolution is improved at lower mobile phase pH, i.e. pH 6.45. This is due to the strictly related chemical structures and properties that homotaurine and GABA have.
As regards detectable sulfur-containing amines in the biological specimens analyzed, our data indicate that taurine relative content is higher in brain tissue samples with respect to all other biological samples analyzed. As described in the literature, the brain is one of the major body district in which taurine is present. Also in neuroblastoma cells the pattern of amino compounds (Fig. 12) reveals a relative content of taurine significantly higher with respect to other amines, whereas plasma and urine samples present lower relative amounts of taurine (Figs. 5 and 6).
It is well known that taurine is one of the end-products of cysteine metabolism. It is noteworthy, in brain tissues and neuroblastoma cells (Figs. 7 and 12) samples, we observed a lower amount of cysteine relative content along with higher amounts of taurine and hypotaurine, in agreement with cysteine metabolic cycle.
As regards the bioactive peptides carnosine and GSH, it can be noticed that urinary content of these two molecules is higher with respect to the other samples. GSH content in brain tissue samples is also relatively higher than in cells and in plasma, emphasizing the important role that this thiol tripeptide has at the cerebral level as a bioactive molecule.
4 Conclusion
The revised version of a relatively old analytical procedure, has enabled the improvement of a low-cost chromatographic method with high resolution and high diagnostic potential in order to investigate qualitatively and quantitatively many substances which can be implicated in the pathogenesis of several diseases.
This method represents a good tool to study sulfur biochemical cycle from a metabolic point of view in relation to the pattern of biologic amines and provides a complete scenario of organic sulfur and amino metabolism. It allows also the study of amine level variations in mice and human samples derived from different treatments or environmental conditions (pollutants, drugs, etc.).
The use of spectrophotometric detection makes this method accessible to a large number of research, commercial and clinical laboratories that do not have access to fluorimetric or mass spectrometric detectors. Apart from the obvious advantages of lower costs and larger number of samples that can be analyzed within a working day, more importantly, an undoubted advantage from a qualitative and methodological point of view is the possibility to simultaneously analyze in each single sample a large number of amines, aminoacids and sulfur-amino compounds including GSH, GSSG, cysteic acid, cysteine, taurine, hypotaurine, lanthionine and cystathionine.
In conclusion, the use of this chromatographic method on different complex biological matrices, could pave the way for a new diagnostic tool.
Abbreviations
- DABS:
-
4-N,N-Dimethylaminoazobenzene-4′-sulfonyl chloride
- DANS:
-
5-Dimethylaminonaphthalene-1-sulfonyl chloride
- HPLC:
-
High performance liquid chromatography
- OPA:
-
o-phthalaldehyde
- TCA:
-
Trichloroacetic acid
- TDGA:
-
Thiodiglycolic acid
References
Abdel-Moneim AM, Al-Kahtani MA, El-Kersh MA, Al-Omair MA (2015) Free radical-scavenging, anti-inflammatory/anti-fibrotic and hepatoprotective actions of taurine and silymarin against CCl4 induced rat liver damage. PLoS One 10(12):e0144509. doi:10.1371/journal.pone.0144509
Atwal PS, Scaglia F (2016) Molybdenum cofactor deficiency. Mol Genet Metab 117(1):1–4. doi:10.1016/j.ymgme.2015.11.010
Bai J, Yao XF, Jiang LP, Zhang QT, Guan H, Liu S, Wu W, Qiu TM, Gao N, Yang L, Yang G, Sun XC (2016) Taurine protects against As2O3-induced autophagy in livers of rat offsprings through PPAR gamma pathway. Sci Rep 6:27733. doi:10.1038/Srep27733
Bertollini C, Murana E, Mosca L, D’Erme M, Scala F, Francioso A, Catalano M, Limatola C, Bregestovski P, Di Angelantonio S, Ragozzino D (2012) Transient increase in neuronal chloride concentration by neuroactive aminoacids released from glioma cells. Front Mol Neurosci 5:100. doi:10.3389/fnmol.2012.00100
Cai HL, Zhu RH, Li HD (2010) Determination of dansylated monoamine and amino acid neurotransmitters and their metabolites in human plasma by liquid chromatography-electrospray ionization tandem mass spectrometry. Anal Biochem 396(1):103–111. doi:10.1016/j.ab.2009.09.015
Chan-Palay V, Engel AG, Palay SL, Wu JY (1982a) Synthesizing enzymes for four neuroactive substances in motor neurons and neuromuscular junctions: light and electron microscopic immunocytochemistry. Proc Natl Acad Sci U S A 79(21):6717–6721
Chan-Palay V, Lin CT, Palay S, Yamamoto M, Wu JY (1982b) Taurine in the mammalian cerebellum: demonstration by autoradiography with [3H]taurine and immunocytochemistry with antibodies against the taurine-synthesizing enzyme, cysteine-sulfinic acid decarboxylase. Proc Natl Acad Sci U S A 79(8):2695–2699
Chan-Palay V, Palay SL, Wu JY (1982c) Sagittal cerebellar microbands of taurine neurons: immunocytochemical demonstration by using antibodies against the taurine-synthesizing enzyme cysteine sulfinic acid decarboxylase. Proc Natl Acad Sci U S A 79(13):4221–4225
Chaturvedi SK, Alam P, Khan JM, Siddiqui MK, Kalaiarasan P, Subbarao N, Ahmad Z, Khan RH (2015) Biophysical insight into the anti-amyloidogenic behavior of taurine. Int J Biol Macromol 80:375–384. doi:10.1016/j.ijbiomac.2015.06.035
Chen W, Guo JX, Zhang YZ, Zhang J (2016) The beneficial effects of taurine in preventing metabolic syndrome. Food Funct 7(4):1849–1863. doi:10.1039/c5fo01295c
Curran C, Weimer J, Ludwig C, Brown J (2016) High-taurine consumption by adolescent C57BL/6J mice alters biogenic amines in a sex-dependent manner. Neurotoxicol Teratol 55:72–72
De Luca A, Pierno S, Camerino DC (2015) Taurine: the appeal of a safe amino acid for skeletal muscle disorders. J Transl Med 13:243. doi:10.1186/S12967-015-0610-1
Drnevich D, Vary TC (1993) Analysis of physiological amino-acids using dabsyl derivatization and reversed-phase liquid-chromatography. J Chromatogr 613(1):137–144. doi:10.1016/0378-4347(93)80207-K
Jacobsen JG, Smith LH (1968) Biochemistry and physiology of taurine and taurine derivatives. Physiol Rev 48(2):424–511
Jansen EHJM, Vandenberg RH, Bothmiedema R, Doorn L (1991) Advantages and limitations of precolumn derivatization of amino-acids with dabsyl chloride. J Chromatogr 553(1–2):123–133. doi:10.1016/S0021-9673(01)88480-6
Kand’ar R, Zakova P, Lotkova H, Kucera O, Cervinkova Z (2007) Determination of reduced and oxidized glutathione in biological samples using liquid chromatography with fluorimetric detection. J Pharm Biomed Anal 43(4):1382–1387. doi:10.1016/j.jpba.2006.11.028
Kang X, Xiao J, Huang X, Gu Z (2006) Optimization of dansyl derivatization and chromatographic conditions in the determination of neuroactive amino acids of biological samples. Clin Chim Acta 366(1–2):352–356. doi:10.1016/j.cca.2005.11.011
Krause I, Bockhardt A, Neckermann H, Henle T, Klostermeyer H (1995) Simultaneous determination of amino-acids and biogenic-amines by reversed-phase high-performance liquid-chromatography of the dabsyl derivatives. J Chromatogr A 715(1):67–79. doi:10.1016/0021-9673(95)00578-B
Lakritz J, Plopper CG, Buckpitt AR (1997) Validated high-performance liquid chromatography-electrochemical method for determination of glutathione and glutathione disulfide in small tissue samples. Anal Biochem 247(1):63–68. doi:10.1006/abio.1997.2032
Lin JK, Wang CH (1980) Determination of urinary amino acids by liquid chromatography with “dabsyl chloride”. Clin Chem 26(5):579–583
Loukou Z, Zotou A (2003) Determination of biogenic amines as dansyl derivatives in alcoholic beverages by high-performance liquid chromatography with fluorimetric detection and characterization of the dansylated amines by liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. J Chromatogr A 996(1–2):103–113
Mopper K, Delmas D (1984) Trace determination of biological thiols by liquid chromatography and precolumn fluorometric labeling with o-phthaladehyde. Anal Chem 56(13):2557–2560
Mou D (1997) Determination of amino acids by precolumn derivatization with o-phthaldialdehyde (OPA) and reversed-phase high performance liquid chromatography. Se Pu 15(4):319–321
Romero R, Bagur MG, Sanchez-Vinas M, Gazquez D (2000) Optimization of experimental variables in the dabsyl chloride derivatization of biogenic amines for their determination by RP-HPLC. Chromatographia 51(7–8):404–410. doi:10.1007/Bf02490476
Schuller-Levis GB, Park E (2003) Taurine: new implications for an old amino acid. FEMS Microbiol Lett 226(2):195–202
Vendrell J, Aviles FX (1986) Complete amino-acid-analysis of proteins by dabsyl derivatization and reversed-phase liquid-chromatography. J Chromatogr 358(2):401–413. doi:10.1016/S0021-9673(01)90354-1
Wade JV, Olson JP, Samson FE, Nelson SR, Pazdernik TL (1988) A possible role for taurine in osmoregulation within the brain. J Neurochem 51(3):740–745. doi:10.1111/j.1471-4159.1988.tb01807.x
Zhang L, Yuan YS, Tong Q, Jiang SM, Xu QR, Ding J, Zhang L, Zhang R, Zhang KZ (2016a) Reduced plasma taurine level in Parkinson’s disease: association with motor severity and levodopa treatment. Int J Neurosci 126(7):630–636. doi:10.3109/00207454.2015.1051046
Zhang YF, Wang G, Jin YF, Deng Y, Zhao YY (2016b) Effects of high hydrostatic pressure processing on purine, taurine, cholesterol, antioxidant micronutrients and antioxidant activity of squid (Todarodes pacificus) muscles. Food Control 60:189–195. doi:10.1016/j.foodcont.2015.07.044
Zitka O, Skalickova S, Gumulec J, Masarik M, Adam V, Hubalek J, Trnkova L, Kruseova J, Eckschlager T, Kizek R (2012) Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol Lett 4(6):1247–1253. doi:10.3892/ol.2012.931
Acknowledgments
This work has been supported by FILAS project (Prot.Filas_RU.2014.1020) and by GNOSIS S.p.A. Authors wish to thanks Dr. Martino Luigi Di Salvo for its critical reading of the manuscript. English language has been revised by Ms. Jane Reynolds.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media B.V.
About this paper
Cite this paper
Francioso, A. et al. (2017). HPLC Determination of Bioactive Sulfur Compounds, Amino Acids and Biogenic Amines in Biological Specimens. In: Lee, DH., Schaffer, S.W., Park, E., Kim, H.W. (eds) Taurine 10. Advances in Experimental Medicine and Biology, vol 975. Springer, Dordrecht. https://doi.org/10.1007/978-94-024-1079-2_42
Download citation
DOI: https://doi.org/10.1007/978-94-024-1079-2_42
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-024-1077-8
Online ISBN: 978-94-024-1079-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)