Abstract
Hepatitis E (HE) virus infection is not limited to spread from human to human but also occurs between animals and more importantly as zoonotic spread from animals to humans. Genotyping of strains from hepatitis E virus-infected patients has revealed that these infections are not all caused by genotypes 1 or 2 but often by genotypes 3 or 4. Therefore, it is important to understand the striking difference between the spread of genotypes 1 and 2 in countries with poor sanitary standards and the spread of genotypes 3 and 4 in countries with good sanitary standards. The number of animal species known to be infected with HEV is expanding rapidly. The finding of HEV in new host species always raises the question regarding the zoonotic potential of these newfound strains. However, as new strains are found, the complexity increases.
Certain genotypes are known to have the ability of zoonotic spread from certain animal species and these animals may even constitute an infection reservoir. Some animal species may contribute to zoonotic infections albeit on a smaller scale, while others are believed to be of minor or no importance at all. This chapter reviews possible sources of zoonotic hepatitis E virus infection.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction to Zoonotic HEV Infections
Large disease outbreaks of hepatitis E (HE) occur in countries with poor sanitary conditions as described elsewhere in this book. The disease is usually endemic in these countries and spread indirectly from person to person through contaminated water, food and toilets especially in refugee camps and other poor dwellings. It causes epidemics from time to time. However, it has been well documented that hepatitis E (HE) also occurs sporadically in persons living in countries with good sanitary standard. The previously widely accepted assumption, that all human infections in countries with good sanitary standards were acquired while travelling or living in countries with an endemic HEV situation, has been challenged for a long time and is not in line with current scientific evidence. It is known that a part of these infections are caused by genotype 1 (or 2) and occur in patients who recently travelled to endemic regions. However, another large part of these infections occurred in patients who did not travel to endemic regions during the calculated incubation time, or not at all travelled abroad, and that was not caused by genotypes 1 or 2 but rather by genotypes 3 or 4. The increased awareness of this situation has in turn led to more samples from patients being sequenced and genotyped. Thus it has been demonstrated that several strains causing disease in humans demonstrate a high degree of similarity to strains detected in animals and food of animal origin. The application of serological test for detection of HEV antibodies in the human population in industrialized countries has demonstrated a surprisingly high (1–53 %) antibody prevalence in several countries [17]. Furthermore, HEV RNA, mainly of genotype 3, has been detected in blood products from donations given by healthy humans. For example, in Germany, 1 out of 4500 and, in Sweden, 1 out of 8000 healthy blood donors had HEV RNA in the blood at the time of donation. Since these HEV-positive samples came from healthy blood donors and the viraemic stage is rather short, the number of humans that are infected during their lifetime can be expected to be much higher. All facts taken together have led to the conclusion that there is an autochthonous source of HEV present in industrialized countries that cause infections and disease in humans and cannot be disregarded [6, 20]. While HEV has been detected in many animal species, only HEV strains belonging to genotypes 1–4 are regarded as possibly zoonotic.
4.2 Introduction to HEV Infection in Animals
The list of animal species susceptible to infection with HEV has been expanding rapidly during the two last decades [29]. The list is now extensive and still continues to grow. The list is based on results from PCR amplification of nucleic acid (RNA) but can be made significantly longer if serological results are also taken into account. Such serological results are generally generated by ELISA. There are now a number of commercially available ELISAs that can be used for testing of animal sera for antibodies against HEV. However, several reports suggesting infection of animal species are based on a small number of serologically positive samples. If there are no previous experiences of analysing sera with the assay, the results should be interpreted with caution. Single serological results are difficult to evaluate because a confirmatory assay, a gold standard, is lacking. The commercially available ELISA assays for antibodies to HEV that have been developed all suffer from a lack of specificity, and different assays will not give identical results for a given selection of sera. This is not a unique feature of HEV ELISA assays as almost all assays will show some disagreement when compared to each other. However, when applied to one or a few samples only and without prior experience of the assays compatibility with sera from the species, the disagreement becomes a critical factor. Therefore, reports of isolated, serologically positive individuals from animal species not previously reported as HEV hosts cannot be relied on and thus need to be confirmed by PCR amplification with sequencing, seroconversion or by other confirmatory assay.
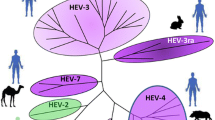
While the genotypes 1 and 2 infect only humans and primates and are known to cause large disease outbreaks or endemic disease in humans in countries with poor sanitary conditions, the genotypes 3 and 4 are found in swine and wild boar and are able to infect humans. It has been demonstrated that that porcine HEV strains of genotype 3 can infect primates and that human genotype 3 strains can infect swine. Contrary to genotypes 1 and 2, these genotypes cause only sporadic cases of overt disease. Reports of disease, caused by genotypes 3 and 4, have been restricted to humans living in countries with good sanitary conditions. However, genotypes 3 and 4 may also circulate in countries where gt1 or 2 is present but is not observed due to the dominance of gt1 and 2. Furthermore, HEV of genotype 3 has also been found in various deer species, and infection of humans through consumption of contaminated deer meat has been demonstrated [42].
4.3 Taxonomical Considerations
The rapidly expanding number of proven HEV-positive animal species and the zoonotic potential has made a revision of the previously accepted taxonomy almost unavoidable, because too many species remain unassigned at the genus level in the former HEV taxonomy. The previously accepted taxonomy contained only one genus, Hepevirus, with one species, hepatitis E virus and four genotypes, and one unassigned genus containing one species, avian hepatitis E virus with several genotypes. The currently accepted taxonomy divides the Hepeviridae family in two genus, Orthohepevirus with four species and Orthohepevirus A–D and Piscihepevirus with only one species, Piscihepevirus A. The Orthohepevirus has been proposed to contain six or seven genotypes ([14] release).
4.4 Swine HEV
While it has been known since 1980 that a non-A or B hepatitis virus, later to be named hepatitis E, caused outbreaks of disease in humans [45], it was not until 1995 that HEV in swine was detected first in Nepal and subsequently also in the USA, and further studies revealed more than 90 % similarity between swine and human HEV strains [4, 24]. This was followed by similar reports from several non-endemic countries. The disease in humans was described in detail and proven by experimental infection in 1983. The finding and characterization of HEV in swine followed studies in swine and primates showing that they could produce HEV antibodies naturally and that HEV RNA could be detected in swine faeces and sera and thus should have been infected with HEV or similar virus previously. This finding of HEV RNA and antibodies in swine raised concerns that HEV could be a zoonotic virus. Indeed, partial sequencing and comparison of the swine and human HEV genome demonstrated a high degree of similarity. However, more thorough studies demonstrated that the genomic sequence of swine HEV differed substantially from genotypes 1 and 2 in humans. Subsequently, two different genotypes, gt 3 and gt 4, have been identified in swine. A couple of years ago, two additional genotypes were suggested for wild boar due to their high divergence with known HEV sequences [40, 41]. Reports of seemingly food-related infections from Japan and elsewhere supported the assumption that HEV could be a zoonosis. However, the long incubation time, 3–8 weeks (average 40 days), creates difficulties to prove a causal link as in many cases the food items have since long been discarded when clinical symptoms first appear in the consumer. Several studies from Europe and Japan have now proven the link [2, 8, 11, 32, 42]. A high prevalence of gt 3 in swine and wild boar has been demonstrated in many parts of the world, and HEV is now regarded as a worldwide infection of swine. The gt 3 is completely dominating in European swine and can be found almost all over the world. Moreover, it is the only genotype detected, until now, in European wild boar and causes almost all autochthonous human HEV infections in Europe. Indeed, for several years only gt 3 was found in Europe and it was not until 2011 that gt 4 was detected in European swine [12] for the first time. Genotype 4 has been found in swine from Italy, Belgium and Denmark. The gt 4 is more frequent than gt 3 in China and is the predominant cause of hepatitis E in humans in China. In Chinese swine both gt3 and 4 can be found, but the gt 3 in China seem to cause only a few cases of human hepatitis E [39]. The gt 4 can also be found in swine in other Asian countries like Japan, India, Indonesia, Korea and Taiwan. At present it seems that in China the gt 4 causes almost all the autochthonous human infections detected so far, while in Japan it is either gt 3 or gt 4 that is the cause of autochthonous human infections. On the other hand, in Europe only a few autochthonous human infections caused by gt 4 have been identified. These cases were identified in France, Germany, Denmark, the United Kingdom, Italy and possibly also in Russia. It has been speculated that genotype 3 originated in Europe in the early nineteenth century reached Asia 100 years later and was spread from there to North America and the rest of the world. Genotype 4 is thought to have originated in Japan in the early nineteenth century.
In swine the infection route is, as in humans, faecal-oral, and the disease normally follows a subclinical course inducing mild to moderate lesions in the liver and regional lymph nodes. The litter size of the sow is not affected. The combined prevalence of markers for current (RNA) or past (antibodies) HEV infection is usually very high in swine as demonstrated by publications from several countries around the world. The peak of viraemia and virus excretion occurs at 2–4 months of age when the maternal antibodies have waned and piglets of different origin are mixed. This is followed by seroconversion. With increasing age, the prevalence of virus-positive (RNA) swine goes down, while the prevalence of antibody positive goes up since the infection is self-limiting and antibodies protect against reinfection, at least for some time. However, some studies indicate that the immunity against reinfection is rather short and that swine may be infected several times during their life span [3]. The antibody prevalence goes up with age due to continuous exposure to infected swine excreting virus that remain stable in the environment for a long time. Infected swine start excreting virus approximately 1 week after infection, remain viraemic for 1–2 weeks and excrete virus for approximately 3 weeks. The virus can be detected in the liver for 4 weeks; however, other studies indicate that the virus could remain in the liver for 3 months. The virus has also been detected in muscle samples. Excretion in faeces is an important route of infection for swine but other routes, like urine, are also important. Furthermore, excretion of HEV genotype 3 for a period of more than 5 months has been demonstrated in a wild boar [38].
Given the normal path of HEV infection in swine, the risk for human exposure through pork products should be rather low at the time of slaughter. However, several studies have demonstrated that swine of slaughter age can still be virus positive. Indeed, in a study in the Netherlands, four out of 62 swine livers were positive for HEV at slaughter. In the Netherlands this could be extrapolated to roughly 1800 contaminated livers being consumed annually. This may be due to primary infection late in life, repeated infections due to poor immunity and prolonged virus persistence in organs as stated above.
What is stated above for HEV infection of swine generally also applies to wild boar since it is the same species. However, the epidemiology of HEV in wild boar may differ considerably from swine because of the differences between the natural habitat of wild boars and the rearing in swine farms [36, 37].
4.5 Rabbit HEV
Zhao et al. [49] reported the finding of HEV in farmed rabbits (Oryctolagus cuniculus) in China. This was followed by several reports of HEV detection from farmed rabbits in China [9], Mongolia, France [15] and the USA [5]. In France HEV was also detected in wild rabbits, and an HEV virus from a human with clinical symptoms, in France, demonstrated a high degree of similarity with rabbit HEV. The sequence identity with gt 1–4 varies between 73 and 79 %. Inoculation of pigs and cynomolgus monkeys with rabbit HEV demonstrated replication; increase of liver enzymes, indicating liver damage; and excretion of virus. It has now been accepted to place rabbit HEV in gt 3 as it forms a distant gt 3 subgroup ([14] release). These results demonstrate that rabbits may constitute a risk for zoonotic infection of humans. However, the prevalence of HEV infection in rabbits is not known.
4.6 Avian HEV
The disease known by two names, hepatitis-splenomegaly (HS) in North America and big liver and spleen disease (BLS) in Australia, is caused by avian hepatitis E virus (AHEV). It is present in, at least, three genotypes and was first described in Canada [34]. In 1999 [30] a virus that could be connected to the disease was detected and partially sequenced in the USA and Australia. Further studies revealed that the Australian and North American virus both are distantly related variants of AHEV and caused the same disease. Since AHEV share part of the sequence with swine HEV and human HEV, it was important to determine if AHEV could infect humans. Therefore, experiments with non-primate monkeys were performed. These experiments concluded that AHEV did not cause viraemia or seroconversion in rhesus macaques [13]. Further sequencing of AHEV showed that only 50 % of the sequence is shared with human and swine HEV and phylogenetical comparisons of the HEV family members indicate only a distant relationship. Thus, the three genotypes of AHEV are related distantly. However, AHEV is even more distantly related to HEV in swine and humans. Over 20 years of experience with AHEV without detection of avian HEV in humans gives a strong indication that AHEV does not infect and cause disease in humans. Even if not fully proven, these are good reasons to believe that AHEV is not a zoonotic virus.
4.7 Other Animal Species Infected by HEV
At present only strains belonging to genotypes 1–4 are regarded as zoonotic. However, the increasing number of HEV strains detected in several animal species makes the separation between zoonotic and non-zoonotic strains more difficult. The list of animal species known to be susceptible to HEV infection is long and continues to expand as new species are investigated. Currently, apart from the species mentioned above, the list contains primates (experimentally and naturally infected; cynomolgus monkeys, Japanese macaque (Macaca fuscata), etc. [22, 33, 47]), wild boar (Sus scrofa, [36, 44]), five bat species from three families ((Hipposideridae, Vespertilionidae and Phyllostomidae, [7]), Norwegian rat (Rattus norvegicus, [16]), black rat (Rattus rattus [25]), cotton rat (Sigmodon hispidus), greater bandicoot rat (Bandicota indica), other rat species (Rattus spp., [25])), Asian musk shrew (Suncus murinus, [10]), tree shrew [48], roe deer (Capreolus capreolus), red deer (Cervus elaphus, [26, 43]), mongoose (Herpestes javanicus, [27]), moose (Alces alces, [21]), sika deer (Cervus nippon [42], Rex rabbits (Oryctolagus cuniculus, [49]), ferret (Mustela putorius), farmed mink (Neovison vison, [18]), camel (Camelus dromedarius, [46]) and cutthroat trout (Oncorhynchus clarkii, [1]). Even more animal species have been implicated as potential host animals. These are, for example, Asian black bear (Selenarctos thibetanus), clouded leopard (Neofelis nebulosa), dog and cats (Canis lupus, [23]), fox (Vulpes vulpes), horse (Equus caballus, [35]), cattle (Bos taurus), yak (Bos grunniens), goat (Capra aegagrus) and sheep (Ovis aries). However, these isolated findings remain to be confirmed by other studies using different methods or repeating the finding in other individuals. This is also the case with the reported findings of antibodies to HEV in goat, sheep, cat and dog as well as for the finding of HEV in fox. The HEV detected in fox was found in faeces and could therefore have originated from an animal that was eaten by the fox. Bioaccumulation in mussels has also been demonstrated (Mytilus galloprovincialis).
The wild boar belongs to the same species as pigs, so it is not at all surprising to find that it can also be infected by HEV genotype 3.
The HEV infecting mongoose also belongs to genotype 3 and can therefore be regarded as a zoonotic virus. However, no human cases caused by infection from mongoose have been described. However, humans and mongoose rarely come close to each other, and the risk for oral ingestion of infected material from a mongoose by a human seems quite remote. If mongoose also can be infected by genotype 4 remains to be shown.
The HEV found in bats forms a separate phylogenetic branch distinct from the known zoonotic hepeviruses. There are no known cases of humans becoming infected with HEV from bats. Therefore, bat hepevirus is currently not believed to be zoonotic. However, the same reasoning as for mongoose could be applied to bats. The prevalence of HEV in bats seems to be low, humans and bats rarely have contact, and oral ingestion, by humans, of infected material from bats is unlikely. However, fruit bats eating fruits from trees, for example, mango, may contaminate fruit pieces, with bat saliva or urine, subsequently falling down to the ground. Bats may also contaminate date palm sap, later to be ingested by humans as is suspected for Nipah transmission in Bangladesh [31]. For HEV transmission from bats to humans, this is just a hypothetical reasoning since the HEV virus in bats is so different from genotypes 1 to 4 and the risk for exposure probably is low.
Several species of rats can be infected with HEV but mainly by a variant that is distant to genotypes 1–4 and forms a separate branch in phylogenetic trees. This virus is not thought to be zoonotic. However, rats in the USA have also been infected by genotype 3 of HEV and could therefore constitute a zoonotic risk [19]. If rats also can be infected with genotype 4 is not known. Ferrets can be infected by HEV that cluster with HEV in rats. However, the phylogenetic distance between rat HEV and ferret HEV is larger than between genotypes 1 and 2 of humans. HEV found in mink is also related but distinct to ferret HEV as well as to rat HEV. Similar to HEV in rats (except genotype 3 variants found in the USA) and ferret, the distant relation to genotypes 1–4 and the clustering with rat and ferret HEV support the assumption that HEV from mink is not zoonotic.
Moose are frequently infected by HEV as has been demonstrated [21]. However, the HEV infecting moose is distantly related to HEV genotypes 1–4 and there are no indications that this virus should be zoonotic. It should be noted that moose is traditionally hunted and the meat is eaten but the liver is not. This may contribute to reducing the risk for human infections both in the short, infected material is not eaten, and the long run since HEV in moose and genotype 4 with time are becoming even more distantly related.
Importantly, roe deer, red deer, sika deer and other deer species can be infected by HEV genotypes 3 or 4. The seroprevalence varies between 2 and 30 %, while the RNA prevalence is approximately 30 %. The zoonotic potential of HEV-infected deer has been documented. In Japan several members of a family became infected with HEV and fell sick after eating sika deer meat [42]. The HEV sequence recovered from the patients and from frozen meat was almost identical.
Hepatitis E virus has also been found in mussels. Mussels become contaminated with HEV through bioaccumulation while filtering large volumes of seawater. The risk is higher if the mussel cultivations are placed close to river mouths. Rivers may be contaminated by runoff water from pig farms or from sewage that is treated in water-cleaning plants that cannot stop HEV from passing through.
4.8 Conclusions
Research on zoonotic hepatitis E has taken big strides forward. The research field is still very dynamic with new hosts being found frequently. In little more than two decades, the number of known variants has increased from two infecting two animal species (poultry and humans, not counting experimental infections of monkeys) to over 20 infecting over 20 animal species. The hepeviruses are also very variable in genome sequence. While a large number of strains, encompassing genotypes 1–4, show a high degree of sequence similarity and a similar host pattern, other strains are significantly more different, compared to genotypes 1–4, like HEV in rats and HEV in moose. Avian HEV is only 50 % similar to genotypes 1–4 and cuttroat trout HEV is only very distantly related to other HEVs. On the other hand, rabbit HEV was before considered not to belong to genotypes 1–4, but it is now accepted that this virus belongs to genotype 3. This demonstrated how dynamic and complex is the HEV family. Furthermore, it demonstrates the difficulties in drawing a sharp line between zoonotic and non-zoonotic HEV strains.
References
Batts W, Yun S, Hedrick R, Winton J (2011) A novel member of the family Hepeviridae from cutthroat trout (Oncorhynchus clarkii). Virus Res 158(1–2):116–123
Bouwknegt M, Frankena K, Rutjes SA, Wellenberg GJ, de Roda Husman AM, van der Poel WH, de Jong MC (2008) Estimation of hepatitis E virus transmission among pigs due to contact-exposure. Vet Res 39(5): 40
Chandra V, Taneja S, Kalia M, Jameel S (2008) Molecular biology and pathogenesis of hepatitis E virus. J Biosci 33(4):451–464
Clayson ET, Innis BL, Myint KS, Narupiti S, Vaughn DW, Giri S, Ranabhat P, Shrestha MP (1995) Detection of hepatitis E virus infections among domestic swine in the Kathmandu Valley of Nepal. Am J Trop Med Hyg 53:228–232
Cossaboom CM, Córdoba L, Dryman BA, Meng XJ (2011) Hepatitis E virus in rabbits, Virginia USA. Emerg Infect Dis 17(11):2047–2049
Dalton HR, Kamar N, Izopet J (2014) Hepatitis E in developed countries: current status and future perspectives. Future Microbiol 9(12):1361–1372
Drexler JF, Seelen A, Corman VM, Fumie Tateno A, Cottontail V, Melim Zerbinati R, Gloza-Rausch F, Klose SM, Adu-Sarkodie Y, Oppong SK, Kalko EK, Osterman A, Rasche A, Adam A, Müller MA, Ulrich RG, Leroy EM, Lukashev AN, Drosten C (2012) Bats worldwide carry hepatitis E virus-related viruses that form a putative novel genus within the family Hepeviridae. J Virol 86(17):9134–9147
Garbuglia AR, Alessandrini AI, Pavio N, Tessé S, Grignolo S, Viscoli C, Lapa D, Capobianchi MR (2015) Male patient with acute hepatitis E in Genoa, Italy: figatelli (pork liver sausage) as probable source of the infection. Clin Microbiol Infect 21(1):4–6
Geng J, Fu H, Wang L, Bu Q, Liu P, Wang M, Sui Y, Wang X, Zhu Y, Zhuang H (2011) Phylogenetic analysis of the full genome of rabbit hepatitis E virus (rbHEV) and molecular biologic study on the possibility of cross species transmission of rbHEV. Infect Genet Evol 11(8):2020–2025
Guan D, Li W, Su J, Fang L, Takeda N, Wakita T, Li TC, Ke C (2013) Asian musk shrew as a reservoir of rat hepatitis E virus, China. Emerg Infect Dis 19(8):1341–1343
Guillois Y, Abravanel F, Miura T, Pavio N, Vaillant V, Lhomme S, Le Guyader FS, Rose N, Le Saux JC, King LA, Izopet J, Couturier E (2016) High proportion of asymptomatic infections in an outbreak of Hepatitis E associated with a Spit-Roasted Piglet, France, 2013. Clin Infect Dis 62(3):351–357
Hakze-van der Honing RWE, van Coillie AF, Antonis AF, van der Poel WH (2011) First isolation of hepatitis E virus genotype 4 in Europe through swine surveillance in the Netherlands and Belgium. PLoS One 6(8):e22673. doi:10.1371/journal.pone.0022673
Huang FF, Sun ZF, Emerson SU, Purcell RH, Shivaprasad HL, Pierson FW, Toth TE, Meng XJ (2004) Determination and analysis of the complete genomic sequence of avian hepatitis E virus (avian HEV) and attempts to infect rhesus monkeys with avian HEV. J Gen Virol 85:1609–1618
ICTV, virus taxonomy 2015 release. http://www.ictvonline.org/virustaxonomy.aspEC 47, London, UK, July 2015. Email ratification 2016 (MSL #30)
Izopet J, Dubois M, Bertagnoli S, Lhomme S, Marchandeau S, Boucher S et al (2012) Hepatitis E virus strains in rabbits and evidence of a closely related strain in humans, France. Emerg Infect Dis 18:1274–1281
Johne R, Dremsek P, Reetz J, Heckel G, Hess M, Ulrich RG (2014) Hepeviridae: an expanding family of vertebrate viruses. Infect Genet Evol 27:212–229
Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, Dalton HR (2012) Hepatitis E. Lancet 379(9835):2477–2488
Krog JS, Breum SO, Jensen TH, Larsen LE (2013) Hepatitis E virus variant in farmed mink, Denmark. Emerg Infect Dis 19:2028–2030
Lack JB, Volk K, Van Den Bussche RA (2012) Hepatitis E virus genotype 3 in wild rats, United States. Emerg Infect Dis 18(8):1268–1273
Lapa D, Capobianchi MR, Garbuglia AR (2015) Epidemiology of hepatitis E Virus in European countries. Int J Mol Sci 16(10):25711–25743
Lin J, Karlsson M, Olofson AS, Belák S, Malmsten J, Dalin AM, Widén F, Norder H (2015) High prevalence of hepatitis e virus in Swedish moose–a phylogenetic characterization and comparison of the virus from different regions. PLoS One 10(4) http://dx.doi.org/10.1371/journal.pone.0122102
McCaustland KA, Krawczynski K, Ebert JW, Balayan MS, Andjaparidze AG, Spelbring JE, Cook EH, Humphrey C, Yarbough PO, Favorov MO, Carson D, Bradley DW, Robertson BH (2000) Hepatitis E virus infection in chimpanzees: a retrospective analysis. Arch Virol 145(9):1909–1918
McElroy A, Hiraide R, Bexfield N, Jalal H, Brownlie J, Goodfellow I, Caddy SL (2015) Detection of hepatitis E virus antibodies in dogs in the United Kingdom. PLoS One 10(6):e0128703
Meng XJ, Purcell RH, Halbur PG, Lehman JR, Webb DM, Tsareva TS, Haynes JS, Thacker BJ, Emerson SU (1997) A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci U S A 94(18):9860–9865
Mulyanto DSN, Sriasih M, Takahashi M, Nagashima S, Jirintai S, Nishizawa T, Okamoto H (2013) Frequent detection and characterization of hepatitis E virus variants in wild rats (Rattus rattus) in Indonesia. Arch Virol 158(1):87–96
Neumann S, Hackl SS, Piepenschneider M, Vina-Rodriguez A, Dremsek P, Ulrich RG, Groschup MH, Eiden M (2016) Serologic and molecular survey of hepatitis E virus in German Deer populations. J Wildl Dis 52(1):106–113
Nidaira M, Takahashi K, Ogura G, Taira K, Okano S, Kudaka J, Itokazu K, Mishiro S, Nakamura M (2012) Detection and phylogenetic analysis of hepatitis E viruses from mongooses in Okinawa, Japan. J Vet Med Sci 74(12):1665–1668
Oliveira D, Rivadulla E, Abreu-Silva J, Varela MF, Romalde JL, Maria SJ (2016) Nascimento Hepatitis E virus genotype 3 in mussels (Mytilus galloprovincialis), Spain. Food Microbiol 58:13–15
Pavio N, Meng XJ, Doceul V (2015) Zoonotic origin of hepatitis E. Curr Opin Virol 10:34–41
Payne CJ (2003) Big liver and spleen disease. In: Saif YM, Barnes HJ, Glisson JR, Fadly AM, McDougald LR, Swayne DE (eds) Diseases of poultry, 11th edn. Iowa State Press, Ames, pp 1184–1186
Rahman MA, Hossain MJ, Sultana S, Homaira N, Khan SU, Rahman M, Gurley ES, Rollin PE, Lo MK, Comer JA, Lowe L, Rota PA, Ksiazek TG, Kenah E, Sharker Y, Luby SP (2012) Date palm sap linked to Nipah virus outbreak in Bangladesh, 2008. Vector Borne Zoonotic Dis 12(1):65–72
Renou C, Roque-Afonso AM, Pavio N (2014) Foodborne transmission of hepatitis E virus from raw pork liver sausage, France. Emerg Infect Dis 20(11):1945–1947
Ribeirão P (1998) The use of non-human primates as animal models for the study of hepatitis viruses. Braz J Med Biol Res 31(8):1035–1048
Ritchie SJ, Riddell C (1991) Hepatitis–splenomegaly syndrome in commercial egg-laying hens. Can Vet J 32:500–501
Saad MD, Hussein HA, Bashandy MM, Kamel HH, Earhart KC, Fryauff DJ, Younan M, Mohamed AH (2007) Hepatitis E virus infection in Work Horses in Egypt. Infect Genet Evol 7(3):368–373
Schielke A, Sachs K, Lierz M, Appel B, Jansen A, Johne R (2009) Detection of hepatitis E virus in wild boars of rural and urban regions in Germany and whole genome characterization of an endemic strain. Virol J 6:58
Schlosser J, Eiden M, Vina-Rodriguez A, Fast C, Dremsek P, Lange E, Ulrich RG, Groschup MH (2014) Natural and experimental hepatitis E virus genotype 3-infection in European wild boar is transmissible to domestic pigs. Vet Res 45:121
Schlosser J, Vina-Rodriguez A, Fast C, Groschup MH, Eiden M (2015) Chronically infected wild boar can transmit genotype 3 hepatitis E virus to domestic pigs. Vet Microbiol 180(1–2):15–21
Si F, Yang Q, Zhu Y, Dong S, Yu R, Shen S, Li Z (2012) Adaptation of genotype 3 hepatitis E virus in Eastern China and inverse correlation with genotype 4 hepatitis E virus. Intervirology 55(5):356–364
Takahashi K, Terada S, Kokuryu H, Arai M, Mishiro S (2010) A Wild boar-dely representing so far unidentified “genotype 5”. Kanzo 51:536–538
Takahashi M, Nishizawa T, Sato H, Sato Y, Jirintai S, Nagashima S, Okamoto H (2011) Analysis of the full-length genome of a hepatitis E virus isolate obtained from a wild boar in Japan that is classifiable into a novel genotype. J Gen Virol 92:902–908
Tei S, Kitajima N, Takahashi K, Mishiro S (2003) Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 362(9381):371–373
Thiry D, Mauroy A, Saegerman C, Licoppe A, Fett T, Thomas I, Brochier B, Thiry E, Linden A (2015) Belgian Wildlife as Potential Zoonotic Reservoir of Hepatitis E Virus. Transbound Emerg Dis 31
Vina-Rodriguez A, Schlosser J, Becher D, Kaden V, Groschup MH, Eiden M (2015) Hepatitis E virus genotype 3 diversity: phylogenetic analysis and presence of subtype 3b in wild boar in Europe. Viruses 7(5):2704–2726
Wong DC, Purcell RH, Sreenivasan MA, Prasad SR, Pavri KM (1980) Epidemic and endemic hepatitis in India: evidence for a non-A, non-B hepatitis virus aetiology. Lancet 2(8200):876–879
Woo PC, Lau SK, Teng JL, Tsang AK, Joseph M, Wong EY, Tang Y, Sivakumar S, Xie J, Bai R, Wernery R, Wernery U, Yuen KY (2014) New hepatitis E virus genotype in camels, the Middle East. Emerg Infect Dis 20(6):1044–1048
Yamamoto H, Suzuki J, Matsuda A, Ishida T, Ami Y, Suzaki Y, Adachi I, Wakita T, Takeda N, Tian-Cheng L (2012) Hepatitis E virus outbreak in monkey facility, Japan. Emerg Infect Dis 18(12):2032–2034
Yu W, Yang C, Bi Y, Long F, Li Y, Wang J, Huang F (2016) Characterization of hepatitis E virus infection in tree shrew (Tupaia belangeri chinensis). BMC Infect Dis 16(1):80. doi:10.1186/s12879-016-1418-1
Zhao C, Ma Z, Harrison TJ, Feng R, Zhang C, Qiao Z, Fan J, Ma H, Li M, Song A, Wang Y (2009) A novel genotype of hepatitis E virus prevalent among farmed rabbits in China. J Med Virol 81(8):1371–1379
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Widén, F. (2016). Hepatitis E as a Zoonosis. In: Wang, Y. (eds) Hepatitis E Virus. Advances in Experimental Medicine and Biology, vol 948. Springer, Dordrecht. https://doi.org/10.1007/978-94-024-0942-0_4
Download citation
DOI: https://doi.org/10.1007/978-94-024-0942-0_4
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-024-0940-6
Online ISBN: 978-94-024-0942-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)