Abstract
The present study is aimed to the preparation of synthetic zeolites of a defined Na-X type (Faujasite) from the fly ash (FA) generated by the incineration of Bulgarian lignite coals. FA zeolitization was carried out by three different methods: classical alkaline conversion, two-stage fusion-hydrothermal synthesis, and atmospheric aging, all with sodium hydroxide (NaOH) as an alkaline activator. The nature, morphology and chemistry of the zeolites were studied by X-ray diffraction, scanning electron microscopy (SEM) and energy-dispersive X-ray (EDX) analyses. The results from the classical alkaline conversion show the formation of an aluminosilicate hydrogel on the surface of the FA particles but a crystallization of any zeolitic phase does not take place. Zeolites with a dominant Na-X phase were obtained under by a two-stage synthesis under the following conditions: fusion at 550 °C, hydrothermal treatment at 90 °C for 2 h of duration, and NaOH/FA ratios of 2.0–2.4. Zeolite Na-X is also obtained by atmospheric aging for 1 year at a NaOH/FA ratio of 1.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Mineral waste is one of the most spread solid by-products produced in enormous amounts all over the world, the so-called fly ash (FA), from coal combustion plants. It is normally reused in cement and concrete production, but recently, different advanced technologies are under development for its reduction by the component recovery. Due to the specific chemical composition and the amorphous-crystalline structure, FA is a suitable starting material for the synthesis of zeolites [1]. Zeolites are natural or synthetic crystalline aluminosilicates with a structural framework built-up of SiO4/2 and [AlO4/2]− tetrahedra linked each to other at the corners, sharing an oxygen bridge. Zeolites are characterized by a highly mesoporous structure and a large surface area which are favorable for surface phenomena and determine their applications as adsorbents, catalytic carriers, molecular sieves, etc. [2]. This investigation is a part of a broad experimental program on the conversion of lignite coal fly ash into synthetic zeolites and their applications in the flue gas cleaning systems.
2 Materials and Methods
2.1 Starting Material
FA generated from the combustion of local lignite coals in the largest thermal power plant in Bulgaria named TPP “Maritza-East 2”, was subjected to zeolitization. The FA was preliminary studied with respect to its chemical and phase composition, and its thermal properties [3]. The FA used as a raw material for this study was found to contain 52.7 wt% SiO2 and 23.4 wt% Al2O3; the amorphous phase was determined to 43 % vs. the crystalline one. The grain size distribution of the FA particles, determined by sieve separation, was found to be between 125 and 250 μm. The density of the FA is ~3.07 g/cm3 .
2.2 Classic Alkaline Conversion
This method is based on the combination of different ratios of activatоr and FA, with the temperature and the reaction time as parameters to obtain different zeolites types. For the purpose of this study a mixture of 5 g FA and 6 g NaOH was subjected to magnetic stirring in 100 ml distilled water for 12 h. The solution obtained was filled into an autoclave, and the synthesis was performed at 90 °C for different times (2, 3 and 4 h). After the hydrothermal activation, the solid part was separated by filtration, washed with distilled water and dried.
2.3 Two Stage Fusion-Hydrothermal Synthesis
The fusion stage prior to classical hydrothermal treatment is directed to obtain soluble sodium silicate and aluminate, which after dissolution under continuous stirring are converted to a hydrogel. Further, crystallization takes place in the gelous medium during the hydrothermal synthesis. Mixtures of NaOH and FA were prepared in different ratios: 1.6, 2.0, and 2.4. They were treated in two steps: fusion prior to hydrothermal synthesis. The first stage was performed at a temperature of 550 °C for 1 h. Thereafter, the sintered alloys were crushed, mixed with distilled water and subjected to magnetic stirring for 16 h. The water suspensions obtained were filled into an autoclave; the hydrothermal synthesis stage was performed at 90 °C with a duration of 2 h. Then, the solid part was separated by filtration, thoroughly washed with distilled water and dried at 105 °C. In general, the process of zeolite synthesis, applying a fusion stage before the hydrothermal treatment, passes through the following reaction scheme:
The atmospheric synthesis aimed to simulate a mechanism of formation of natural zeolites which is associated with alkaline activation of volcanic rocks. A mixture of 10 g FA, 10 g NaOH and 100 ml distilled water was prepared and filled into a tube. Part of the suspension was separated after 8, 10 and 12 months and dried at atmosphere conditions.
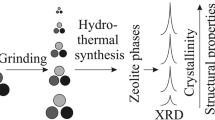
In Fig. 21.1 the principle schemes of the three different synthesis mechanisms performed in this research to obtain the zeolite Na-X are summarized.
3 Results and Discussions
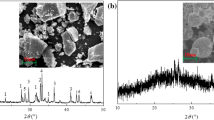
XRD patterns and SEM images of the samples obtained by different approaches are presented in Figs. 21.2 and 21.3, respectively. The most of the reflections typical for zeolite Na-X are well expressed for the self-crystallized product. Some intensive peaks of different mineral components from the parent FA composition, mostly those of mullite (2Al2O3SiO2) and magnetite (γ-Fe3O4) are preserved (Fig. 21.2a). Those two components need to be treated at higher temperature to achieve a transformation of their structure.
The influence of the duration of the atmospheric aging on the type of the synthesized materials was investigated by storing samples in a closed tube for 8, 10 and 12 months, respectively. It can be seen that with increasing self-crystallization time the characteristic peaks of the zeolite Na-X become more intensive. The intensity of the peaks belonging to the unreacted FA remains constant.
After the hydrothermal activation, the treated sample still contains parts of the raw material, as the reflections of mullite still exist in the XRD pattern (Fig. 21.2b, type II) and the FA particles remain unchanged (Fig. 21.3a). In addition, aluminosilicate hydrogel formation and its initial crystallisation over the FA particles can be observed in the SEM micrograph, confirmed by the appearance of a diffraction peak at 2θ = 7.4, typical for zeolite A.
Samples obtained by the two-stage syntheses at different NaOH/FA ratios of 1.6, 2.0, and 2.4 do not contain traces of the raw aluminosilicates (Fig. 21.2b, type III). The product obtained at the lowest NaOH/FA ratio of 1.6 possess cubic crystals of zeolite A (Fig. 21.3b). It can be seen also that the surface of the cubic crystals is covered by nanoscale particles probably belonging to some other zeolitic phase. Further, hexaoctahedral crystallites of Na-X appear at a higher NaOH/FA ratio of 2.0 (Fig. 21.3c), while some zeolite A phase is still preserved. Crystallites and reflections belonging only to Na-X zeolite are observed for a NaOH/FA ratio of 2.4 (Figs. 21.3d and 21.2, type III). The crystallite size changes with varying NaOH/FA ratios from the nanoscale at 2.0 to microcrystallites at 2.4. A morphology analysis shows dimensions of 0.3–0.9 μm for the sample with the lower NaOH/FA ratio, and of 2.1–5.5 μm for the sample with the higher NaOH/FA.
4 Conclusions
The zeolitizaton of aluminosilicates by hydrothermal synthesis and atmospheric aging does not ensure complete alkaline conversion of the mullite from the raw FA. The hydrothermal process can be improved by increasing the duration and the concentration of the alkaline activator but this will result in a mixture of different zeolitic phases. The self-crystallization and the two-stage fusion-hydrothermal synthesis both result in a Na-X zeolitic material. The self-crystallization mechanism is the most energy efficient but results in a mixture of zeolitic phases and unreacted raw aluminosilicates. The two-stage synthesis is controllable to obtain different types of zeolites with defined morphology. With an increase of the NaOH/FA ratio, the crystallite size changes from nano- to microscales.
References
Ahmaruzzaman M (2010) Prog Energy Combust Sci 36:327
Querol X, Moreno N, Umana JC, Alastuey A, Hernandez E, Lopez-Soler A, Plana F (2002) Int J Coal Geol 50:413
Boycheva S, Zgureva D, Vassilev V (2013) Fuel 108:639
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this paper
Cite this paper
Zgureva, D., Boycheva, S. (2015). Synthesis of Highly Porous Micro- and Nanocrystalline Zeolites from Aluminosilicate By-Products. In: Petkov, P., Tsiulyanu, D., Kulisch, W., Popov, C. (eds) Nanoscience Advances in CBRN Agents Detection, Information and Energy Security. NATO Science for Peace and Security Series A: Chemistry and Biology. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9697-2_21
Download citation
DOI: https://doi.org/10.1007/978-94-017-9697-2_21
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9696-5
Online ISBN: 978-94-017-9697-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)