Abstract
Exercise-induced proteinuria has been observed and studied for more than a century. It was found that different sport disciplines alter the urinary proteome in different ways. Moderate-intensity exercise results in increased glomerular filtration, meaning that medium-sized proteins are excreted in higher amounts, while high-intensity exercise of short duration also increases the excretion of low molecular weight proteins as a result of tubular dysfunction. Exhaustive exercise may lead to the excretion of hemoglobin or myoglobin, which changes the urinary proteome considerably. Studies comparing protein maps of different sport types compared to a control group showed that quality and quantity of urinary proteins are interindividually different. In addition, urine samples collected before and after exercise exhibit substantially different protein patterns even from the same person. Therefore, further studies investigating the urinary proteome are desirable. As the variation of protein content and composition in urine are generally much higher than in other matrices, respective studies need to be well controlled and homogenous groups of volunteers should be chosen. In addition to the sport-related physiological and biochemical interest, exercise-induced protein changes also need to be considered for biomarker measurements from urine samples for kidney or other diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Analysis of Proteins in Urine After Exercise
First reports about exercise-induced albuminuria are from Williams and Arnold 1899 in the context of the Boston marathon, where the amounts of urinary albumin were found to be higher after the marathon than prior to the competition [35]. Dunhill and Patterson [5] as well as Collier and Lond [4] later demonstrated that exercise-induced albuminuria is reversible and probably not a sign of an impaired kidney function or Brights’ disease, but a commonly occurring phenomenon following exercise. It was also recognized already at that time that the amount of protein in urine relates to the amount of work that was done. Before these studies and the conclusion that proteinuria is a frequently observed exercise-induced and reversible reaction, Collier and Lond and several other physicians recommended athletes to cease sports and live quiet lives if protein was found in urine. The assumption at that time was that the vascular system was not strong enough, or the organs were too weak for high-intensity exercise. In the early days, protein determination measurements were taken using the nitric acid or acetic acid test and the Esbach’s albuminometer [9] measuring the height of the protein precipitate.
The protein/creatinine ratio is used today as normalized value for the excreted protein amounts and allows the evaluation of spot urine samples. Children have a higher total protein concentration and protein/creatinine ratio than adults [13]. In the 1960s [24], the differentiation between glomerular and tubular proteinuria was observed [8] and Poortmans was the first to analyze different urinary proteins instead of total protein (namely tryptophan-rich prealbumin, albumin, α1-acid glycoprotein, α1-antitrypsin, ceruloplasmin, haptoglobin, Gc-globulin, transferrin, hemopexin, β2-glycoprotein I, γA-globulin, and γG-globulin) before exercise and after a marathon run [28]. Urinary protein changes were monitored for different sport types, and a few studies performed proteomics for the untargeted investigation of protein alterations.
2 Mechanism of Exercise-Induced Proteinuria
In contrast to the wide field of kidney diseases, the impact of exercise on renal function(s) is not investigated as intensely. Nevertheless, several studies were dedicated to the elucidation of the mechanisms of post-exercise proteinuria, and an early observation in that regard was that proteinuria depends on exercise intensity rather than exercise duration [30].
The mechanisms of exercise-induced proteinuria are not entirely understood, but it is established that glomerular proteinuria appears at lower exercise intensity than tubular proteinuria. Glomerular proteinuria is characterized by the excretion of medium-sized proteins such as albumin, while tubular failure leads to increased excretion of smaller proteins such as α1-microglobulin. At high-intensity exercise, a mixed-type proteinuria is detected with elevated amounts of small- and medium-sized proteins. Glomerular filtration is determined by the blood flow in the Bowman’s capsule as well as the permeability of the glomerular basement membrane, which is defined mainly by negative charges from heparan sulfate proteoglycans [8]. Acidity changes in the circulation and therefore charge alterations of the glomerular membrane as well as proteins were discussed as one reason for increased excretion of specific proteins. A direct and exclusive connection of lactate concentration to the quality and quantity of proteinuria was excluded after lactate injection at rest did not induce proteinuria [26]. Nevertheless, there is a strong correlation between blood lactate and proteinuria. Although the mechanism is unclear, it is assumed that a decrease in charge of the glomerular basement membrane results in higher permeability and filtration rate [29]; hence, if the charge of the proteins plays a major role, the relative amounts of proteins would change with altered blood acidity. An influence of the adrenergic system was also proposed and shown by the application of an α2-adrenergic agonist, resulting in a reduced catecholamine response during exercise [31]. The renal blood flow drops as a result of renal vasoconstriction, which increases the glomerular filtration rate. It was found that prostaglandin inhibition attenuated increased exercise-induced protein excretion, but that inhibition of angiotensin-converting enzyme did not alter the protein excretion after exercise, indicating that the renin–angiotensin system regulating blood pressure as well as water and electrolyte equilibrium is not responsible for proteinuria [20]. In contrast, angiotensin II inhibition in rats attenuated proteinuria [6]. Tubular reabsorption is mainly receptor-mediated and may be saturated when glomerular filtration increases. In addition, inhibition mechanisms leading to a further increase in the excretion of low molecular weight proteins are discussed [3].
3 Hematuria and Myoglobinuria
Although the mechanisms of hematuria and myoglobinuria will not be part of this report, these two alterations have to be mentioned as they may significantly change the urinary proteome and lead to the detection of hemoglobin or myoglobin. Hematuria originates from mechanical stress, e.g., in long-distance runners in the foot capillaries as well as molecular mechanisms including filtration of erythrocytes in the glomerulus, decreased renal blood flow, damage to the nephrons, or dehydration increasing the molarity of the blood [3, 14, 23]. Myoglobinuria is a result of the rupture of muscle membranes from extreme exercise. In that case, myoglobin as well as hemoglobin may be found in urine [3].
4 Different Exercise Types and Intensities and Their Effects on Urinary Proteins
A number of different studies have been performed that analyze the effect of different types of exercise on proteinuria or specific urinary proteins. Proteinuria was found in 61 % of male and 66 % of female elite badminton players after competition. In addition, the presence of leukocytes (men = 43.5 % and women = 50.0 %) and erythrocytes in urine was investigated (men = 50.0 % and women = 21.7 %) [1]. A two-hour karate training session (elite athletes, female) did not result in elevated protein/creatinine ratios [33]. Moreover, the impact of different swimming distances (100, 600, 2,000 m) on proteinuria was assessed, demonstrating that the endurance distance yielded only elevated albumin levels, while the shorter distances caused an increase in the tubular marker ß2-microglobulin that gradually increased with increasing swim speed. Further, Poortmans found out that a 100-km run resulted in glomerular but not tubular proteinuria [27], while intermittent exercise was reported to have a higher impact on protein excretion than continuous cycling on a bicycle ergometer [21].
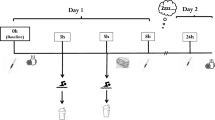
Running exercise at 70 % of the calculated maximal heart rate at normoxia condition and at hypoxia simulating 2,750, 3,250, and 3,750 m altitude showed that excreted protein amounts did not differ significantly. In contrast, specific proteins, namely albumin and β2-microglobulin, were significantly increased when training in hypoxia conditions [16] (Fig. 12.1).
5 Exercise and Acute or Chronic Kidney Injury
As exercise results in proteinuria, the question arises if exercise may lead to acute or chronic kidney injury. Investigation of urine samples from marathon runners showed that 40 % had elevated markers for acute kidney injury after the race. Acute kidney injury was determined by serum creatinine and urinary markers such as cystatin C, neutrophil gelatinase-associated lipocalin, and kidney injury molecule 1 supported the diagnosis. Cardiovascular magnetic resonance imaging results led to the suggestion that athletes were not volume-depleted, which could have triggered false conclusions. Eventually, all markers returned to normal baseline values after 24 h; however, repetitive long-distance running exercise has to be investigated concerning potential long-term alterations of kidney functions [19]. Urinary markers for renal damage were analyzed from patients with chronic kidney disease after a 20-min treadmill walk at 40–60 % exercise intensity. L-type fatty acid-binding protein (a new marker of tubular function in chronic kidney disease and acute kidney injury) as well as the common markers urinary albumin, N-acetyl-beta-D-glucosaminidase, and α1-microglobulin was not significantly influenced after exercise [11]. Junglee et al. [15] recently showed that muscle-damaging exercise prior to exercise in the heat can be seen as a risk factor for acute kidney disease.
Exercise of 15-min duration to maximal heart rate or exhaustion was shown to result in albuminuria. Within 24 h, measures returned to baseline in all subjects. It was therefore concluded that urinary parameters connected to disease or protein excretion should not be measured within 24 h after exercise [10].
6 Urinary Proteomics
In contrast to other body fluids, fewer studies were performed on the urinary proteome. Besides the fact that plasma or serum is the most common matrix in clinical studies, urine has a high salt concentration and is relatively dilute regarding proteins (~150 mg of protein/day) in general but still retains the issue of highly abundant albumin. On the other hand, urine can be sampled noninvasively and is available in large volumes of 1–2 l/day. Gel-based and gel-free proteomics was used to map and categorize urinary proteins [2, 25, 34]. Adachi et al. [2] reported on an unexpectedly high percentage of membrane proteins. Compared to the entity of Gene Ontology entries, extracellular lysosomal and plasma membrane proteins were enriched and the latter were proposed to originate from renally eliminated exosomes. Such exosomes were subsequently investigated as potential source of new biomarkers [12].
7 Effect of Sports on the Urinary Proteome
Studies of untargeted analyses of urinary proteins or a certain fraction are rare. As summarized in the previous sections, exercise may change the urinary proteome differently depending on exercise intensity but also on temperature, hydration status as well as physiological condition. Besides parameters that result in variation of the proteome, Gür et al. described that the amount of protein excreted does not depend on age, duration of running or training, or athletic background of athletes as shown by examinations of participants of a half marathon [7]. The same exercise intensity may lead to different changes such as muscle damage or hematuria in some but not all athletes leading, e.g., to the excretion of myoglobin or hemoglobin and their fragments, which are usually not detected in urine. Therefore, even when measuring protein amounts and analyzing same amounts of proteins, substantial inter- and intra individual qualitative differences can occur. In addition to differences in renal filtration and reabsorption, proteins may not only originate from the kidney filtrate but could be post-renal, e.g., from parts of ureter, bladder, or urethra including proteins from the inner membranes as well as bacterial contamination through infection or other external contamination.
A pilot study using two-dimensional gel electrophoresis for comparison of the urinary proteome of elite athletes performing different types of exercise (endurance exercise, strength sport, and team sport) showed considerable differences within as well as between the groups [17]. This study was performed in a doping control context addressing the question how much the urinary protein varies and if that may influence the detection of, especially peptide and protein based, prohibited substances such as erythropoietin, insulin, or chorionic gonadotrophin. The idea was that in addition to the existing blood passport of athletes, a urinary passport or protein map may allow indication of, e.g., gene doping. In addition, from a sport physiological point of view, the urinary proteome could provide indications as to the nutritional and training status of an athlete. That way, muscle damage may be identified by myoglobin and its fragments in urine or erythrocytosis by the detection of hemoglobin or other erythrocyte-derived proteins. These parameters may be used for training control and modulation.
Protein maps of strength sport athletes, endurance sport athletes, and team sport athletes (10 each) showed that respective 2D patterns were too different within as well as between groups to allow a software-based comparison. Therefore, visual inspection was performed for evaluation of relevant differences. Proteinuria (>15 mg protein/mmol creatinine) was found in 2/10 strength sport athletes, 5/10 team sport samples and 10/10 endurance sport samples and 0/10 samples from the control group. Endurance and team sport samples showed comparable protein patterns with protein spots considered as ‘elevated’ that contained transferrin, albumin, prostaglandin-H2 D-isomerase, immunoglobulin kappa chain and alpha-2-glycoprotein 1, gelsolin isoform b fragment, CD 201 antigen, kininogen 1, and clusterin isoform 1. In comparison, strength sport samples showed a higher amount of low molecular weight proteins (elevated spots contained transthyretin, CD 59 antigen, GM 2 ganglioside activator, and apolipoprotein A) including also fragments from high molecular weight proteins (albumin, transferrin, hemopexin, or IgG fragments) [17]. In this case, lactate cannot be the reason for increased protein excretion as the exercise time is too short for a sufficient production of lactate. Adrenergic activity is also discussed to be connected to proteinuria and results in higher blood pressure. As a matter of fact, blood pressure is extremely increased during weight lifting to values up to 370/360 mm Hg [22] and may be the reason for proteinuria in this case.
Within the same study, stability of urine samples was tested and it was found that the protein pattern did not change within four weeks of storage at 4 °C.
In a consecutive project, marathon runners who participated in the same marathon competition were investigated. As control groups, competitive athletes at rest with a mean endurance sport measure of 13–20 h/week (triathlon, biking, and running) were used. In addition, a control group of healthy volunteers performing occasional exercise (5 h/week) was acquired. No differences were found between the two control groups. Nine out of ten marathon runners had protein/creatinine ratios of >15 mg/mmol (15–73 mg/mmol). A relative decrease in acidic proteins was observed after exercise, which may be interesting for variations in EPO levels in doping control. Manual evaluation of gels showed six spots to be clearly elevated in marathon runners compared to healthy volunteers. Orosomucoid may be elevated because of increased glomerular filtration. If it can be shown that there is an increase in plasma as well, it could indicate an unspecific immune response due to muscle lesions. Another spot observed in marathon runners but not in controls contained hemopexin and may indicate hemolysis. The same phenomenon applies to a spot containing carbonic anhydrase I, which is responsible for the rehydration of carbon dioxide to bicarbonate within erythrocytes. Zinc α-2-glycoprotein 1, which is assumed to have a stimulatory effect on lipolysis, may be increased in plasma as well because of an increased energy consumption and demand. The increase of transferrin, an iron transport protein, may be due to glomerular filtration changes or to increased transferrin synthesis as athletes often have reduced iron levels [18]. For confirmation of the reason for higher concentration of these proteins in urine, plasma samples should be collected in addition in future studies.
In addition, it was investigated how the protein patterns change from rest to post-exercise and back in one volunteer in a yet unpublished study. The protein patterns changed after exercise and, within a few hours, returned back to the pattern observed prior to the intervention. Besides, it was found that high altitude (3,500 m, isobaric, 1 h) does not have an influence on the protein pattern or protein amount. That is in contrast to residence in high altitude, where increased protein excretion is reported [32]. Exercise in altitude resulted in qualitatively similar protein patterns. Nevertheless, oxygen saturation was lower at high altitude, and power on a bicycle ergometer was lower.
Examples of urinary profiles of athletes from different sports categories are shown in Fig. 12.2 to illustrate the variability. Figure 12.3 shows different kinds of possible contaminations. The sample containing a high number of albumin fragments (encircled) is atypical, and the reason for the excretion of that many fragments in this individual case is unclear. The same is true for the gel which lacks albumin in usual amounts. For the sample containing myoglobin isoforms and fragments, the explanation may be that exercise has been muscle damaging. Nevertheless, there are several forms at different pI, which has not been described before.
Selected reasons for the variation of the urinary proteome and effect on the protein pattern. Samples with albumin fragments and myoglobin were from marathon runners after the race, and the samples with bacterial contamination as well as with the lack of albumin were from recreational endurance sport athletes after a time trial on a bicycle ergometer
In addition, other factors in athletes, such as special diets or training status, may lead to changes in metabolism and therefore also changes in the urinary protein pattern, which necessitates further investigations in future studies.
In summary, it can be concluded that it is critical to further investigate exercise-induced changes in protein patterns for different kinds of exercise conditions. Although the variations observed are very high, it is possible to find systematic changes for certain groups and it is important to expand the knowledge on exercise-induced changes for doping control, sport physiology as well as biomarker discovery in disease as levels of specific proteins may be changed after exercise.
References
Abián-Vicén J, Del Coso J, González-Millán C et al (2012) Analysis of dehydration and strength in elite badminton players. PLoS ONE 7:e37821
Adachi J, Kumar C, Zhang Y et al (2006) The human urinary proteome contains more than 1500 proteins, including a large proportion of membrane proteins. Genome Biol 7:R80
Bellinghieri G, Savica V, Santoro D (2008) Renal alterations during exercise. J Ren Nutr 18:158–164
Collier W, Lond F (1907) Functional albuminuria in athletes. Br Med J 1:4–6
Dunhill TP, Patterson SW (1902) Albuminuria following severe exercise in healthy persons. Intercol Med J Australia 2:334
Gündüz F, Kuru O, Şentürk ÜK (2005) Angiotensin II inhibition attenuates postexercise proteinuria in rats. Int J Sports Med 26:710–713
Gür H, Küçükoğlu S (1994) Effects of age, training background and duration of running on abnormal urinary findings after a half-marathon race. Br J Sports Med 28:61–62
Haraldsson B, Nyström J, Deen W (2008) Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev 88:451–487
Hatcher W, Webb A (1979) Georges Hubert Esbach and the tube albuminometer. Med Lab Sci 36:185–190
Heathcote K, Wilson MP, Quest DQ et al (2009) Prevalence and duration of exercise induced albuminuria in healthy people. Clin Invest Med 32:E261–E265
Hiraki K, Kamijo-Ikemori A, Yasuda T et al (2013) Moderate-intensity single exercise session does not induce renal damage. J Clin Lab Anal 27:177–180
Hoorn EJ, Pisitkun T, Zietse R et al (2005) Prospects for urinary proteomics: exosomes as a source of urinary biomarkers. Nephrology 10:283–290
Houser M (1984) Assessment of proteinuria using random urine samples. J Pediatr 104:5–8
Jones G, Newhouse I (1997) Sport-related hematuria: a review. Clin J Sports Med 7:119–125
Junglee NA, Di Felice U, Dolci A et al (2013) Exercising in a hot environment with muscle damage: effects on acute kidney injury biomarkers and kidney function. Am J Physiol Enal Physiol 305:F813–F820
Kohanpour MA, Sanavi S, Peeri M et al (2012) Kidney diseases effect of submaximal aerobic exercise in hypoxic conditions on proteinuria and hematuria in physically trained young men. Iran J Kidney Dis 6:192–197
Kohler M, Franz S, Regeniter A et al (2009) Comparison of the urinary protein patterns of athletes by 2D-gel electrophoresis and mass spectrometry—a pilot study. Drug Test Anal 1:382–386
Kohler M, Walpurgis K, Thomas A et al (2010) Effects of endurance exercise on the urinary proteome analyzed by 2-D PAGE and Orbitrap MS. Proteomics Clin Appl 4:568–576
McCullough PA, Chinnianyan KM, Gllagher MJ et al (2011) Changes in renal markers and acute kidney injury after marathon running. Nephrology 16:194–199
Mittleman K, Zambraski E (1992) Exercise-induced proteinuria is attenuated by indomethacin. Med Sci Sports Exerc 24:1069–1074
Montelpare W, Klentrou P (2002) Continuous versus intermittent exercise effects on urinary excretion of albumin and total protein. J Sci Med Sport 5:219–223
Narloch JA, Brandstater ME (1995) Influence of btreathing technique on arterial blood pressure during heavy weight lifting. Arch Phys Med Rehabil 76:457–462
Patel DR, Torres AD, Greydanus DE (2005) Kidneys and sports. Adolesc Med Clin 16:111–119
Peterson PA, Evriun PE, Berggard I (1969) Differentiation of glomerular, tubular, and normal proteinuria: determinations of urinary excretion of beta-2-microglobulin, albumin, and total protein. J Clin Invest 48:1189–1198
Pieper R, Gatlin CL, McGratz AM et al (2004) Characterization of the human urinary proteome: a method for high-resolution display of urinary proteins on two-dimensional electrophoresis gels with a yield of nearly 1400 distinct protein spots. Proteomics 4:1159–1174
Poortmans JR (1984) Exercise and renal function. Sports Med 1:125–153
Poortmans JR, Haralambie G (1979) Biochemical changes in a 100 km run: proteins in serum and urine. Eur J Appl Physiol Occup Physiol 40:245–254
Poortmans JR, Jeanloz RW (1968) Quantitative immunological determination of 12 plasma proteins excreted in human urine collected before and after exercise. J Clin Invest 47:386–393
Poortmans JR, Vanderstraeten J (1994) Kidney function during exercise in healthy and diseased humans. Sports Med 18:419–437
Poortmans JR, Labilloy D (1988) The influence of work intensity on postexercise proteinuria. Eur J Appl Physiol Occup Physiol 57:260–263
Poortmans JR, Haggenmacher C, Vanderstraeten J (2001) Postexercise proteinuria in humans and its adrenergic component. J Sports Med Phys Fitness 41(1):95–100
Rennie D, Marticorena E, Monge C et al (1971) Urinary protein excretion in high-altitude residents. J Appl Phyisiol 3:259–275
Shavandi N, Samiei A, Afshar R et al (2012) The effect of exercise on urinary gamma-glutamyltransferase and protein levels in elite female karate athletes. Asian J Sports Med 3:41–46
Thongboonkerd V, McLeish KR, Arthur JM et al (2002) Proteomic analysis of normal human urinary proteins isolated by acetone precipitation or ultracentrifugation. Kidney Int 62:1461–1469
Williams H, Arnold H (1899) The effects of violent and prolonged muscular exercise upon the heart. Trans Am Climatol Assoc 15:267–285
Acknowledgments
The study was conducted with support of the Manfred-Donike-Institute, Cologne, Germany, and Antidoping Switzerland, Berne, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Kohler, M., Schänzer, W., Thevis, M. (2015). Effects of Exercise on the Urinary Proteome. In: Gao, Y. (eds) Urine Proteomics in Kidney Disease Biomarker Discovery. Advances in Experimental Medicine and Biology, vol 845. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9523-4_12
Download citation
DOI: https://doi.org/10.1007/978-94-017-9523-4_12
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9522-7
Online ISBN: 978-94-017-9523-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)