Abstract
Ethylene and auxin have overlapping effects on growth and development of young seedlings, with either synergistic or antagonistic actions depending on the developmental process. This chapter introduces the growth and developmental processes that are regulated by these two hormones and explores recent studies that provide insight into the mechanistic basis for the regulation of these processes. Ethylene and auxin both inhibit root elongation and stimulate root hair elongation, while acting in opposition on lateral root development and hypocotyl elongation. The interplay between the hormones is even more complex in differential growth processes, such as gravitropism, nutation, and apical hook opening. The presence of well characterized mutants with altered ethylene and auxin signaling and synthesis, as well as auxin transport, has been essential to demonstrate the mechanistic basis of crosstalk between these hormones. As both of these hormones lead to profound changes in gene expression, genome-wide transcript abundance data sets are identifying additional genes that are induced or repressed through this hormonal crosstalk. Experimental tests of predicted regulatory networks involving these genes will likely yield the next set of new insights into the mechanisms by which these hormones control plant growth and development.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Ethylene and Auxin Regulate Root Growth and Development
10.1.1 Primary Root Elongation
The complex interactions between ethylene and auxin on growth and development have been studied in greatest detail in roots. The ability of auxin to regulate primary root elongation and gravitropism, as well as the initiation, emergence, and elongation of lateral and adventitious roots has been studied for decades (Woodward and Bartel 2005; Overvoorde et al. 2010; Bellini et al. 2014). The inhibition of root elongation by auxin has been used as the screen to isolate auxin insensitive mutants, including aux1 and axr1-axr6, revealing important features of the auxin signaling pathways that control a diversity of growth and developmental processes (Mockaitis and Estelle 2008). Like auxin, ethylene, and its precursor, 1-aminocyclopropane-1-carboxylic acid (ACC) inhibit root elongation, as shown in Fig. 10.1. The root elongation inhibition by both hormones is rapid, within 5 min of treatment (Le et al. 2001; Rahman et al. 2007) and occurs predominantly in the central root elongation zone (Swarup et al. 2007; Ruzicka et al. 2007; Strader et al. 2010;Rahman et al. 2007; Alarcon et al. 2013), consistent with these two hormones acting to regulate growth through converging signaling pathways. Genetic approaches have demonstrated that ethylene regulates elongation through the canonical signaling pathways that mediate the triple response in dark grown seedlings and fruit ripening (Muday et al. 2012). Mutants with reduced sensitivity to ethylene, including etr1, ein2, ein3, and eil1 in Arabidopsis and never ripe (nr) and green ripe (gr) in tomato have elevated primary root growth (Barry and Giovannoni 2006; Ruzicka et al. 2007; Stepanova et al. 2007; Swarup et al. 2007; Negi et al. 2010). In contrast, roots of seedlings with enhanced ethylene signaling or synthesis, ctr1 and eto1, respectively, exhibit reductions in the rate of root elongation (Kieber et al. 1993).
Auxin and ethylene alter root growth and development. Seedlings were transferred to medium containing indole 3-acetic acid or the ethylene precursor ACC and the tip of roots at time of transfer was marked by a black dot. When roots were imaged two days later, both auxin and ethylene decreased Arabidopsis root elongation relative to an untreated control as judged by the length of root that forms below the black dots. In contrast, auxin treatment enhances lateral root formation and elongation while ethylene treatment inhibits both of these processes
10.1.2 Lateral Root Development
In contrast to primary root elongation, lateral root development is oppositely regulated by auxin and ethylene. The role of auxin in most aspects of lateral root initiation has been well studied in Arabidopsis, including priming pericycle cells in the basal meristem, initiating cell cycle progression and asymmetric division and driving the emergence and elongation of lateral roots (Overvoorde et al. 2010; Lavenus et al. 2013; van Norman et al. 2013). Recent genetic studies in Arabidopsis and tomato have shown that ethylene negatively regulates lateral root formation (Ivanchenko et al. 2008; Negi et al. 2008, 2010). Elevated levels of ethylene through treatments with ethylene or ACC inhibits lateral root formation at early stages of root initiation (Ivanchenko et al. 2008). Arabidopsis and tomato ethylene insensitive mutants exhibit elevated numbers of lateral roots (Negi et al. 2008, 2010; Strader et al. 2010). Although most of these studies examined root growth on agar medium, the increase in lateral root formation seen in Nr is even more striking in seedlings grown in soil (Negi et al. 2010), suggesting that ethylene may have even more profound effects on roots during standard cultivation. These opposite effects of auxin and ethylene on lateral roots are evident in Fig. 10.1. This image also illustrates that the stimulation of auxin on lateral root development is greatest in mature regions of the primary root near the root shoot junction, while ethylene inhibits lateral root formation near the root tip in regions of the primary root formed after exposure to ethylene.
10.1.3 Adventitious Root Development
The reported effects of ethylene on adventitious root formation are more complex, as ethylene increases formation in some species (reviewed in De-Klerk et al. 1999; Bellini et al. 2014), while decreasing adventitious root formation in others (Coleman et al. 1980; Nordstrom and Eliasson 1984). The effect of ethylene on adventitious root formation has only been examined in genetic models in a small number of cases (Clark et al. 1999; Kim et al. 2008; Negi et al. 2010; Sukumar 2010). In tomato, elevated endogenous or exogenous ethylene levels increase adventitious root formation, while ethylene insensitive nr produces fewer adventitious roots (Clark et al. 1999; Kim et al. 2008; Negi et al. 2010). In contrast in Arabidopsis, ACC treatment, as well as the eto1 and ctr1 mutations, results in reduced adventitious root formation (Sukumar 2010). A recent study looking for suppressors of the sur2 (superroot2) mutation, which has elevated auxin synthesis and proliferation of adventitious roots, identified genes linked to both auxin and ethylene synthesis, consistent with roles of both of these hormones in this process (Pacurar et al. 2014).
10.1.4 Root Gravitropism and Waving
Auxin’s role in regulating differential growth processes, such as root gravitropism and waving has been well studied (Muday and Rahman 2008; Baldwin et al. 2013). These processes are readily observed when plants are grown on agar surfaces. Gravitropism is observed after root reorientation relative to the gravity vector, and waving is observed when seedlings are grown on impenetrable agar surfaces. When roots are reoriented relative to gravity, auxin is redistributed across the root tip, accumulating on the lower side, where it inhibits root elongation (Baldwin et al. 2013). The resulting auxin gradients across the root have been observed with numerous approaches (Geisler et al. 2014), with the asymmetric expression of the auxin responsive DR5 promoter driving GFP as illustrated in Fig. 10.2a. Inhibition of auxin transport with inhibitors or by mutations in genes encoding auxin transport proteins blocks the gravity response (Muday and Rahman 2008; Geisler et al. 2014). The differential growth required for root gravitropism and root waving is also altered by ethylene. Ethylene treatment inhibits the gravitropic response in maize and Arabidopsis and increases the amplitude and frequency of the root waving response (Lee et al. 1990; Chang et al. 2004; Buer et al. 2003, 2006; Lewis et al. 2011b). The inhibition of gravitropism by ethylene is lost in ein2 and etr1 mutants (Buer et al. 2006), suggesting an intact ethylene signaling pathway is necessary for the effect of ethylene on differential growth.
Ethylene negatively regulates gravity response. Treatment of roots with either ethylene or its biosynthetic precursor, ACC, reduces root gravitropic curvature. a The images of roots include both root tip angles at low magnification and confocal images of roots at 8 h after reorientation 90° relative to the gravity vector. The angle of gravity is indicated by an arrow. The confocal image shows individual cells after propidium iodide staining (red) and the asymmetric expression of the auxin responsive DR5-GFP reporter across the untreated root (green). In contrast, ACC treatment leads to DR5-GFP fluorescence on the upper side, which then minimizes the auxin gradient across the root reducing gravitropic curvature. Scale bars = 100 μm. b The model indicates the presence of elevated auxin transport protein synthesis and enhanced auxin accumulation in the root tip. The increases in auxin transport are accompanied by increases in flavonol accumulation in the elongation zone of ethylene treated seedlings, which combine to prevent auxin export from the root tip, reducing formation of the gradient of auxin across a gravity stimulated root tip that is needed for gravitropic curvature. Reprinted with permission from Trends in Plant Science (Muday et al. 2012)
10.1.5 Root Hair Initiation and Elongation
In contrast to the negative effects of ethylene on root elongation and lateral root formation, root hair initiation and elongation are synergistically increased by ethylene and auxin (Rahman et al. 2002; Pitts et al. 1998; Kieber et al. 1993; Schiefelbein 2000). Auxin-insensitive signaling mutants (axr2/iaa7, axr3/iaa17, slr1/iaa14, and iaa28) develop fewer and shorter root hairs (Wilson et al. 1990; Leyser et al. 1996; Rogg et al. 2001; Fukaki et al. 2002), as do ethylene insensitive mutants, such as ein2 (Rahman et al. 2002). Root hair elongation is enhanced by auxin treatment (Rahman et al. 2002) and in mutants that have elevated auxin synthesis, including sur1 and yucca (Boerjan et al. 1995; Zhao et al. 2001). The constitutive activation of ethylene signaling or synthesis by eto1 or ctr1, treatment with exogenous ethylene, or ACC also promotes root hair elongation (Pitts et al. 1998; Dolan 2001; Strader et al. 2010). In contrast, root hairs are significantly shorter in ethylene insensitive mutants (Masucci and Schiefelbein 1994; Pitts et al. 1998; Rahman et al. 2002) and seedlings treated with inhibitors of ethylene synthesis or signaling (Dolan 2001; Zhang et al. 2003). Auxin is able to rescue root hair elongation defects in ethylene insensitive mutants, and inhibition of auxin influx exacerbates the ein2 root hair phenotype (Rahman et al. 2002). Application of ACC or IAA to the root hair deficient mutant, rhd6, restores root hair initiation (Masucci and Schiefelbein 1994). Collectively, these results indicate that ethylene and auxin responses are both required for maximal root hair initiation and elongation and that these two hormones act in concert on this process.
10.2 Auxin and Ethylene Regulate Hypocotyl Growth and Development
10.2.1 Hypocotyl Elongation
In hypocotyls, the ability of ethylene to control elongation has received considerable study, while the role of auxin in controlling the maintenance of the apical hook and control of hypocotyl elongation has more recently been linked to modulation of ethylene responses. Ethylene inhibits hypocotyl growth as part of the triple response, while auxin is best known for its ability to enhance hypocotyl growth. The effects of these hormones on shoot growth also differ by environmental conditions and species. In the dark, ethylene causes growth inhibition of Arabidopsis hypocotyls (Bleecker et al. 1988; Guzman and Ecker 1990), while in the light it stimulates growth (Smalle et al. 1997; Collett et al. 2000). Auxin is more effective in stimulating growth and auxin transport levels in light-grown, than in dark-grown seedlings (Sargent et al. 1974; Yang and Hoffman 1984; Hall et al. 1985; Jensen et al. 1998; Rashotte et al. 2003; Poupart et al. 2005; Liu et al. 2011).
10.2.2 Apical Hook Formation
Most research examining auxin-ethylene crosstalk in the hypocotyl has focused on apical hook development. There are three phases to hook development (formation, maintenance, and opening) with hook formation and maintenance involving asymmetrical growth caused by both altered cell division and elongation (Raz and Ecker 1999; Gupta et al. 2012). Evidence that auxin is involved in hook development comes from the observation that seedlings with auxin transport blocked by auxin transport inhibitors or in mutants with altered auxin transport have a reduced apical hook (Garbers et al. 1996; Lehman et al. 1996; Friml and Palme 2002; de Grauwe et al. 2005; Muday et al. 2006; Abbas et al. 2013). However, in at least one case, the defect in auxin transport may be tied to elevated ethylene synthesis (Skottke et al. 2011). Similarly, wild type seedlings in the presence of high levels of exogenous auxin or mutants that over-accumulate auxin lack an apical hook (Boerjan et al. 1995; King et al. 1995; Lehman et al. 1996; Zhao et al. 2001), and auxin resistant mutants have reduced or no hook (Lehman et al. 1996; Tian and Reed 1999; Dharmasiri et al. 2005). Application of ethylene to wild-type plants or mutations in eto1 and ctr1 lead to exaggerated apical hooks (Guzman and Ecker 1990; Kieber et al. 1993). In contrast, ethylene insensitive mutants fail to form an exaggerated apical hook in the presence of applied ethylene (Roman et al. 1995). Time-lapse imaging of apical hooks shows that ethylene prolongs the formation phase in apical hook development leading to an increased hook angle (Vandenbussche et al. 2010; Zadnikova et al. 2010) during a limited developmental window (Raz and Ecker 1999).
10.2.3 Hypocotyl Nutation and Gravitropism
Auxin-ethylene crosstalk has also been studied in the context of differential growth leading to hypocotyl curvature. Nutational bending and gravitropism of shoots has been linked to alterations in auxin transport and auxin accumulation in the zone of bending (Britz and Galston 1982; Hashiguchi et al. 2013). Ethylene also stimulates nutations in hypocotyls of etiolated Arabidopsis seedlings (Binder et al. 2006). These nutations are eliminated by application of NPA suggesting that ethylene-stimulated nutations require normal auxin transport. Interestingly, ETR1 ethylene receptor is both necessary and sufficient for nutations, but is not necessary for apical hook formation (Binder et al. 2006; Kim et al. 2011). Thus ETR1, but not the other receptor isoforms, may be subtly influencing auxin transport or accumulation. Ethylene has been reported to negatively impact shoot gravitropism (Gupta et al. 2012), consistent with complex interactions between tropistic growth and ethylene in this tissue.
10.3 Auxin and Ethylene Signaling Pathways
10.3.1 Auxin Signaling Pathways
To explore the mechanisms by which auxin and ethylene act together or in opposition to control growth and development, it is necessary to summarize the signaling pathways that control these responses. The signaling cascade that drives auxin-regulated gene expression has been well characterized (as reviewed by Chapman and Estelle 2009). Auxin causes profound and rapid increases in expression of genes with changes in the AUX/IAA, GH3, and SAUR families being the best described (Chapman and Estelle 2009). With the advent of genome-wide transcriptional profiling, additional rapidly regulated auxin-dependent transcripts have been identified (Vanneste et al. 2005; Paponov et al. 2008; Lewis et al. 2013). Genetic approaches have provided insight into the function of several transcriptional targets and other proteins that mediate auxin signaling and transcriptional responses. Auxin binds to the TRANSPORT INHIBITOR RESPONSE 1 (TIR1) receptor or another auxin F-box (AFB) protein, which are E3-ubiquitin ligases that add ubiquitin tags to AUX/IAA transcriptional repressor proteins (Chapman and Estelle 2009). This ubiquitination targets AUX/IAA proteins for proteolytic destruction (Dharmasiri et al. 2005; Kepinski and Leyser 2005). Destruction of AUX/IAA repressors release auxin response factors (ARFs), which are transcription factors that mediate auxin-dependent transcription (Abel et al. 1995a; Ulmasov et al. 1999). Mutations in genes encoding ARFs and TIR1/AFBs alter auxin responses, as do mutations that stabilize AUX/IAA proteins, preventing their proteolytic destruction (Dharmasiri et al. 2005; Lee et al. 2009). The core auxin transcriptional response machinery drives the synthesis of proteins that modulate auxin dependent growth processes. The right half of Fig. 10.3 depicts the auxin signaling pathway, culminating in the transcription of auxin-responsive genes.
Model of auxin/ethylene crosstalk. In Arabidopsis, ethylene, and auxin responses are initiated by binding the ETR1 and TIR1 receptors, respectively. Ethylene binds to and inhibits ETR1 activity, which in turn leads to inhibition of the CTR kinase, a negative regulator of EIN2 activity. EIN2 activates the EIN3 and EIN3-like (EIL) family of transcription factors, which in turn promote transcription of genes containing an ethylene responsive element (ERE) in their promoter region. Auxin signaling is mediated by proteasome-dependent degradation of AUX/IAA transcriptional repressors, which release ARF transcription factors to activate transcription of genes with auxin responsive elements (AUXRE) in their regulatory region. Primary crosstalk occurs by activation of genes that contain both AUXRE and ERE in their promoter region allowing both signaling pathways to directly regulate transcription. Secondary crosstalk occurs through expression of genes that are either auxin or ethylene responsive, but whose activities control expression of genes that regulate the other hormones synthesis, signaling, or response. Reprinted with permission from Trends in Plant Science (Muday et al. 2012)
10.3.2 Auxin Transport
Another mechanism that controls the available auxin for signaling is its redistribution throughout the plant from sites of synthesis. Auxin is transported from cell to cell in a polar fashion. In shoots, indole 3-acetic acid (IAA), the predominant naturally occurring auxin, moves unidirectionally from the apex to the base (Zazimalova et al. 2010). In roots, auxin transport is more complex, with two distinct polarities. IAA moves acropetally or in the rootward direction through the central cylinder and basipetally (shootward) through the outer layers of root cells. In Arabidopsis roots, both polarities of IAA movement control distinct processes and are mediated by unique suites of auxin transporters and their accessory proteins. Rootward (acropetal) movement of IAA from the shoot toward the root apex has been implicated in the control of lateral root formation and elongation (Reed et al. 1998; Casimiro et al. 2001; Bhalerao et al. 2002; Lewis et al. 2011a). AUX1 (Marchant et al. 2002; Negi et al. 2008; Lewis et al. 2011a), ABCB19 (Wu et al. 2007), and PIN 1, 3, and 7 (Benkova et al. 2003; Laskowski et al. 2008; Lewis et al. 2011a) participate in rootward auxin transport and lateral root initiation. Shootward (basipetal) movement of IAA in roots from the root apex is required for gravity response (Rashotte et al. 2000). AUX1 (Bennett et al. 1996; Marchant et al. 1999), PIN2 (Chen et al. 1998; Müller et al. 1998; Rashotte et al. 2000), and ABCB4 (Lewis et al. 2007) mediate this polarity of IAA transport and mutants of these genes exhibit altered gravitropic responses. By using plants with mutations in these genes, it is possible to separate ethylene’s effect on auxin transport from its potential effect on auxin signaling and elucidate the mechanisms governing the developmental processes that are influenced by these two hormones.
10.3.3 Ethylene Signaling Pathways
The ethylene signal transduction pathway has been identified through genetic analyses and the details of this pathway have been reviewed elsewhere (Giovannoni 2007; Kendrick and Chang 2008; Stepanova and Alonso 2009; elsewhere in this book). Important proteins in the ethylene signaling and synthesis pathways are briefly summarized here, as plants with mutations in these genes have been used to examine crosstalk with auxin. Physiological and genetic characterization of ethylene mutants has revealed a linear signaling pathway that begins with ethylene binding to and turning off its receptor proteins, including ETR1 and its tomato ortholog, NEVER-RIPE (NR) (Chang et al. 1993; Wilkinson et al. 1995). CTR1, a protein kinase with sequence similarity to the catalytic domain of RAF protein kinase (a mitogen-activated protein kinase kinase kinase) is downstream from ETR1 and functions as a negative regulator of signaling (Kieber et al. 1993; Huang et al. 2003). EIN2 is an essential, positive modulator of ethylene signaling (Alonso et al. 1999) that regulates the activity of transcription factors, including EIN3 and EIN3-like (EIL) proteins, whose targets include ETHYLENE RESPONSE FACTOR1 (ERF1) and EDF1 though 4 (ETHYLENE RESPONSE DNA BINDING FACTOR 1 through 4) (Solano et al. 1998; Alonso et al. 2003). As a result of this hierarchical transcriptional cascade, ethylene either positively or negatively regulates diverse genes encoding proteins that mediate the growth response to ethylene (as reviewed by Stepanova et al. 2007; Kendrick and Chang 2008). Figure 10.3 contains a simplified schematic of the Arabidopsis ethylene signaling pathway with these proteins indicated. This detailed understanding of ethylene signaling, and the associated genetic resources, provide an excellent framework in which the role of ethylene in root growth and development can be examined.
10.4 Examination of Crosstalk Between Auxin and Ethylene Signaling Pathways
10.4.1 Signaling Crosstalk Regulating Root Elongation
The ability of auxin and ethylene to control root elongation is linked to ethylene enhancing auxin signaling. Auxin-dependent gene expression is strongly enhanced by elevated levels of ethylene. The ability of ethylene to induce auxin signaling is readily observed after ethylene or ACC treatment or in the eto1 background by increased expression of auxin-inducible reporters in the root elongation zone, including DR5-GUS (Ruzicka et al. 2007; Stepanova et al. 2007; Negi et al. 2008; Strader et al. 2010), DR5-GFP (Ruzicka et al. 2007; Lewis et al. 2011a), DR5-vYFP (Lewis et al. 2011b) and IAA2-GUS (Swarup et al. 2007). An example of DR5-vYFP induction after ACC treatment is shown in Fig. 10.4. Ethylene’s ability to regulate root growth depends on the presence of a functional auxin signaling network (Ruzicka et al. 2007; Stepanova et al. 2007; Swarup et al. 2007). The auxin transcriptional machinery that controls this response is not yet clear. Two studies have examined the role of ARF7/ARF19 in ethylene-mediated primary root growth inhibition with contradictory findings (Li et al. 2006; Ruzicka et al. 2007). A recent study indicated that ARF7 or ARF19 function is required for ethylene response in etiolated hypocotyls (Robles et al. 2012).
Auxin and ethylene synergistically inhibit root elongation and antagonistically regulate lateral root development. In untreated roots, lateral root formation occurs in the lateral root forming zone (LRFZ) as a result of localized auxin maxima. This accumulation may occur as a result of depletion of PIN3 and PIN7 proteins that prevents auxin movement away from these points, enhancing auxin accumulation. Ethylene increases rootward auxin transport by increasing transcription and translation of PIN3 and PIN7 in the central cylinder, which may then deplete the lateral root-forming region of auxin, while increasing auxin accumulation in the root apex or meristematic zone (MZ). This effect may be responsible for ethylene’s negative regulation of lateral root formation. At the root tip, increases in transcription and translation of AUX1 and PIN2 enhance shootward transport of the auxin into the elongation zone (EZ). Accumulation of quercetin and kaempferol, two flavonoids, increases upon ACC treatment (shown in yellow), which limits shootward auxin transport leading to enhanced accumulation at the root tip. Insets show confocal micrographs of root tips expressing nuclear localized DR5:vYFP in the presence and absence of ACC treatment. Reprinted and modified with permission from Trends in Plant Science (Muday et al. 2012)
The evidence is much less clear on whether a fully functional ethylene signaling pathway is required for maximal auxin dependent root growth inhibition. Two groups report different growth effects of exogenous auxin on the ethylene insensitive mutants, ein2 and etr1, with one report of insensitivity to NAA (Ruzicka et al. 2007), and another report of normal response to IAA (Stepanova et al. 2007). Finally, etr1 and ein2 mutants exhibit a wild-type response to IAA when transcript abundance of genes encoding auxin transport proteins and flavonol biosynthetic enzymes are examined, even though these mutations block the transcriptional responses to ACC (Lewis et al. 2011a, b). These results suggest that ethylene signaling is not necessary in some cases either for the effect of IAA on transcription of target genes or on growth inhibition. In a genome-wide transcript data set in which IAA and ethylene treated dark grown roots were examined, 28 % of the IAA responsive transcripts showed altered response in the ein2 mutant (Stepanova et al. 2007), suggesting that the transcriptional response of some IAA responsive genes does require ethylene signaling.
10.4.2 Signaling Crosstalk Regulating Lateral Root Development
The interactions between auxin and ethylene signaling pathways have also been examined in control of lateral root formation, a process in which auxin and ethylene have antagonistic effects. Although the expression of the DR5rev:GFP reporter increases in the root tip in response to ACC treatment, consistent with the synergistic inhibition of growth in this region, in the mature regions of the root where lateral roots form, DR5rev:GFP fluorescence was reduced after ACC treatment with opposite responses in both regions to the ethylene synthesis inhibitor, aminoethoxyvinylglycine (AVG) (Lewis et al. 2011a). The ACC-dependent auxin transport protein transcript changes are lost in ethylene signaling mutants, ein2 and etr1, while IAA-induced changes are not (Lewis et al. 2011a). Similarly, in tir1, auxin-induced gene expression changes are lost, but ACC-induced transcription and repression of root branching are maintained (Lewis et al. 2011a). These results are consistent with independent auxin and ethylene signaling pathways controlling transcription of the same target genes.
10.4.3 Signaling Crosstalk Occurs at the Level of Transcriptional Responses
A model depicting the potential layers of crosstalk that may control transcriptional responses to auxin and ethylene is shown in Fig. 10.3. Although auxin and ethylene may both enhance expression of certain genes, these transcriptional effects may operate through independent signaling pathways and lead to primary transcriptional crosstalk, in which both auxin and ethylene dependent transcription factors directly bind to and regulate expression of target genes. Consistent with this possibility, examination of the upstream regulatory region of genes encoding flavonol biosynthetic enzymes, whose transcription is induced by both auxin and ethylene, contained regions with sequence similarity to both AuxRE (auxin-responsive element) and ERE (ethylene-responsive element). AuxRE and ERE are known sites of ARF or EIN3/EIL binding, respectively. In contrast, the upstream regulatory region of one flavonol biosynthesis gene (TT7), which was induced by auxin, but not ethylene, had a potential AuxRE, but no identifiable ERE-like sequence (Lewis et al. 2011b).
To dissect the interactions between auxin and ethylene at the level of transcription more globally, a microarray analysis of dark grown Arabidopsis roots was performed using wild-type, ein2, and the auxin insensitive aux1 mutant with and without auxin and ethylene treatment (Stepanova et al. 2007). This experiment uncovered numerous genes that were only regulated by auxin or ethylene, but also found that 33 % of ethylene regulated genes and 23 % of auxin regulated genes were in both data sets (Stepanova et al. 2007). Of the transcripts whose expression changed after auxin treatment, 38 % required EIN2 function for this induction, while 28 % of ethylene altered transcripts required AUX1 (Stepanova et al. 2007). Interpretation of the later result is complex, since AUX1 is an auxin transport protein and mutations in AUX1 cause auxin insensitivity as a secondary phenotype. When the 5′-UTR of the genes in this large data set were examined, ARF binding sites were found to be enriched in the genes whose transcripts increased after auxin treatment, especially those that were not induced by ethylene. In contrast, EIN3/EIL and AP2/EREBP binding sites were not enriched in any of the populations identified in this study. The authors concluded that other types of transcription factors may be necessary for the ethylene response. They also concluded that ethylene and auxin may mostly act independently to control transcription (Stepanova et al. 2007). This possibility is also indicated in Fig. 10.3 as a secondary crosstalk, in which targets of ethylene and auxin crosstalk are further downstream from primary auxin- or ethylene-responsive genes.
To fully understand the crosstalk between auxin and ethylene in regulation of gene expression additional experimentation is required. The experiment described above (Stepanova et al. 2007) and many others focused on ethylene effects on gene expression used dark grown plants in which root development is minimal. In contrast, experiments focused on auxin induced transcript changes used light grown seedlings and have demonstrated strong interconnected networks of light and auxin signaling (Cluis et al. 2004; Sibout et al. 2006). Furthermore, many of the microarray studies of auxin response were performed with RNA extracted from whole seedlings, rather than root tissue, further complicating interpretation of transcriptional events linked to root growth and development, as is clear from a recent meta-analysis of whole seedling versus root specific auxin transcriptomes (Lewis et al. 2013). In two root specific auxin-transcriptome data sets from light grown seedlings, transcripts linked to ethylene signaling and synthesis have been identified (Vanneste et al. 2005; Lewis et al. 2013). A recent study of the transcriptome of light grown Arabidopsis roots identified 240 transcripts that are differentially expressed after ACC treatment, with only 7 of these transcripts linked to auxin signaling, synthesis, or transport (Markakis et al. 2012). It is clear that understanding this crosstalk will require additional genome-wide transcript studies to understand these transcriptional interactions. Together, these studies show that auxin signaling networks are required for ethylene effects on some growth and gene expression responses, but additional experiments provided evidence that crosstalk between auxin and ethylene also occurred at other levels including regulation of the synthesis and transport of auxin.
10.5 Ethylene Modulates Auxin Transport
Ethylene regulates elongation growth, gravitropism, and lateral root development in part by modulating auxin transport. Early investigations into the mechanisms of ethylene action focused predominately on plant shoots, with several implicating auxin transport regulation as part of ethylene’s mode of action in a range of plant species (Burg and Burg 1967; Beyer and Morgan 1971; Suttle 1988). More recent studies have focused on roots of genetically tractable species, including Arabidopsis and tomato. These studies have shown that elevated ethylene levels caused by treatment with the ethylene precursor, ACC, or by elevated synthesis in the eto1 and epi mutants positively regulate both shootward and rootward auxin transport (Negi et al. 2008, 2010). The elevated IAA transport after ACC treatment is lost in the Arabidopsis and tomato ethylene signaling mutants etr1 and ein2, and nr, respectively (Negi et al. 2008, 2010). Plants with mutations in genes encoding auxin transport proteins were used to identify the specific proteins that mediate ethylene regulated auxin transport (Negi et al. 2008; Lewis et al. 2011a).
10.5.1 Ethylene Regulated Auxin Transport Controls Root Elongation
The rapid growth inhibition caused by ethylene or ACC treatment is dependent in part upon regulation of auxin transport within the root tip. Forward genetic screens have identified ethylene insensitive mutant alleles of PIN2 and AUX1, suggesting normal auxin transport capacity is necessary for the full inhibition of growth by ethylene (Pickett et al. 1990; Luschnig et al. 1998). This necessity is specific to this set of transport proteins, as pin1, pin4, and pin7 are normally sensitive to ethylene’s effect on root elongation (Ruzicka et al. 2007). Ethylene increases the accumulation of AUX1 and PIN2 transcripts and promoter or protein fusion reporters (Ruzicka et al. 2007; Lewis et al. 2011b), which is linked to enhanced auxin accumulation at the root tip. Treatment of aux1 with low levels of NAA, which bypasses AUX/LAX proteins (Yamamoto and Yamamoto 1998), partially reverses ethylene insensitivity (Rahman et al. 2001), while IAA, which requires influx proteins for entry into cells, does not. This result suggests that auxin influx proteins are essential for ethylene inhibition of elongation. Reduced cell expansion may be caused by the enhanced accumulation of auxin in the elongation zone mediated by elevated expression of PIN2 and AUX1 in epidermal cells.
10.5.2 Ethylene Regulates Auxin Transport to Modulate Root Gravitropism
The inhibition of root gravitropism by ethylene may also act through modulation of auxin transport. Root gravitropism is driven by formation of an auxin gradient across a root tip, which requires AUX1 and PIN2 mediated shootward IAA transport. Both decreases and increases in auxin transport can impair formation of this gradient (Muday and Rahman 2008). For instance, the reduction in shootward auxin transport in eir1/agr1 and pid-9 or perturbation of transport by auxin transport inhibitors reduces or abolishes root gravitropic curvature (Chen et al. 1998; Rashotte et al. 2000; Sukumar et al. 2009). Similarly, an enhancement in shootward transport of auxin is accompanied by a reduced rate of gravity response in rcn1 and tt4 mutant roots (Rashotte et al. 2001; Buer and Muday 2004). Ethylene increases shootward IAA transport (Negi et al. 2008), upon which gravitropism relies for differential growth (Rashotte et al. 2000), suggesting that ethylene may modulate gravitropism by altering auxin transport. Consistent with ethylene targeting auxin transport, mutants with defects in genes encoding auxin transport proteins have defects in gravitropism and ethylene responses. These mutants include the auxin influx mutant aux1 (Maher and Martindale 1980) and the auxin efflux mutants agr1/eir1/pin2/wav6 (Chen et al. 1998; Luschnig et al. 1998; Müller et al. 1998; Utsuno et al. 1998). Similarly, mutations that alter auxin signaling are also associated with reduced ethylene sensitivity and altered gravitropic curvature, including axr1 (Estelle and Somerville 1987), tir1-1 (Ruzicka et al. 2007; Shibasaki et al. 2009), axr2 (Wilson et al. 1990 ), axr3 (Leyser et al. 1996; Ruzicka et al. 2007), and dgt of tomato (Zobel 1973; Muday et al. 1995). A recent genetic screen identified the are1-1 mutant as a second site modifier of the ctr1-1 mutant, which conveys root specific ethylene insensitive root growth and gravitropism (Shin et al. 2013). This mutant has altered auxin distribution and transport (Shin et al. 2013). Taken together, these results provide evidence that auxin and ethylene may act in concert to regulate the root gravity response.
However, mutants that are ethylene resistant, but auxin sensitive, show more variable gravitropic phenotype. Both ein2 and etr1 show wild-type root gravity response in the absence of ethylene (Rahman et al. 2001; Buer et al. 2006). In contrast, in the presence of elevated ethylene, which reduces gravitropic response in wild-type, etr1, ein2, and the ACC insensitive alh1 (ACC related long hypocotyls), exhibit enhanced root gravity response compared with wild-type (Vandenbussche et al. 2003; Buer et al. 2006). Many ethylene insensitive plants have wild-type root gravitropism when ethylene is limiting such as when plants are grown along the surface of agar plates. However, when plants are grown in soil, ethylene levels are likely to be much higher due to more limited diffusion, yielding greater differences in response between wild-type and ethylene insensitive mutants. In support of this possibility, the root phenotype of the ethylene insensitive Nr mutant is enhanced when roots are growing in soil relative to roots grown on agar medium (Negi et al. 2010). Additionally, when Arabidopsis plants are grown along the surface of agar media in plates that are wrapped to prevent diffusion of ethylene gas, root waving is impaired relative to plants grown where diffusion is not limited (Buer et al. 2006).
The demonstration that exogenous application of ACC enhances shootward auxin transport (Ruzicka et al. 2007; Negi et al. 2008) could explain the observed ACC inhibition of root gravitropism. Ethylene-elevated shootward transport of auxin would prevent the formation of an auxin gradient required for the early phase of gravitropic response, as shown in the model in Fig. 10.2. In particular, in the presence of ethylene, auxin may be elevated on both sides of the root, rather than simply on the lower side, as shown using DR5-GFP reporter constructs in Fig. 10.2.
10.5.3 Ethylene Regulates Auxin Transport During Mechanical Stimulation
The above model also explains the roles of ethylene and auxin in the altered growth of mechanically impeded roots. During mechanical impedance, root morphology is modulated by enhanced ethylene signaling (Okamoto et al. 2008). Not surprisingly, the change in ethylene response is coupled with a change in root auxin response. Mechanical impedance also induces the expression of Anthranilate Synthase (AS) AS-α and AS-β genes, whose gene products catalyze the first committed step of biosynthesis of tryptophan, an auxin precursor (Radwanski et al. 1996; Okamoto et al. 2008). Further analyses of the auxin responsive reporters DR5-GUS and IAA2-GUS revealed the formation of an auxin gradient with greater accumulation in the lower side of the mechanically impeded roots (Okamoto et al. 2008). Taken together, these results suggest that localized enhancements in ethylene signaling in mechanically impeded roots stimulates auxin production in the root tip, and promotes its asymmetric redistribution, which is an absolute requirement for root gravity response (Muday and Rahman 2008). Consistent with this hypothesis, the aux1 mutant is insensitive to the mechanical impedance response (Okamoto et al. 2008). Further support to this idea comes from a recent report demonstrating that tomato root penetration in soil was completely blocked by ethylene signaling inhibitors, which also altered auxin dependent gene expression at the root apex (Santisree et al. 2011).
10.5.4 Ethylene Regulates Synthesis of Flavonols, Which Act as Endogenous Inhibitors of Auxin Transport
Ethylene reduces root elongation and gravitropism at least partially through modulation of auxin transport by inducing the synthesis of flavonols, which are negative regulators of auxin transport (Buer et al. 2006; Lewis et al. 2011b). Flavonols have been shown to regulate auxin transport in vivo, by demonstration of elevated auxin transport in the tt4 mutant, which synthesizes no flavonols (Brown et al. 2001; Buer and Muday 2004; Peer et al. 2004) and in vitro by inhibition of auxin efflux either in tissue segments (Jacobs and Rubery 1988) or through ABCB proteins expressed in a heterologous system (Geisler et al. 2005; Bailly et al. 2008). The full inhibition of gravitropism by ethylene requires the presence of flavonols, with genetic evidence indicating that quercetin is the active molecule (Buer et al. 2006; Lewis et al. 2011b). Treatment of Arabidopsis seedlings with ethylene induces flavonol accumulation (Buer et al. 2006; Lewis et al. 2011b), as shown in Fig. 10.4, in an ETR1 and EIN2 dependent fashion (Lewis et al. 2011b). Taken together, these data support the model shown in Fig. 10.4 in which the coordinated increases in auxin transport from the root tip to the elongation zone (via PIN2 and AUX1) and inhibition of auxin transport within the elongation zone (by flavonols) together elevate the auxin concentration in expanding root cells to growth inhibiting ranges.
10.5.5 Ethylene Regulates Lateral Root Development by Altering Auxin Transport
A mechanism by which ethylene regulates auxin transport and inhibits root branching have recently been identified. Ethylene increases rootward auxin transport and decreases lateral root number in Arabidopsis and tomato (Negi et al. 2008, 2010; Lewis et al. 2011a, b), a surprising result given the generally positive correlation between shoot to root transport of auxin and root branching. Mutants in auxin transport proteins that are less sensitive to the effect of ethylene on lateral root formation and rootward auxin transport include pin3, pin7, aux1, and lax3. In contrast, neither abcb19 nor pin2 show any difference in ethylene responsiveness in either process. Additionally abundance of transcripts encoding PIN3, PIN7, and AUX1 and fluorescent protein reporters fused to these proteins decreased in the presence of the ethylene synthesis inhibitor, AVG, and increased after ACC treatment, as illustrated in the model in Fig. 10.4. Specifically, local depletions of PIN3 and PIN7 protein below (or on the rootward side) of developing lateral root primordia has been suggested to lead to local auxin maxima that drive lateral root formation (Laskowski et al. 2008). The local reduction in PIN3 and PIN7-GFP fluorescence is abolished after treatment with ACC, replaced by global elevation of PIN3 and PIN7 abundance through the whole root that correlates with enhanced long distance auxin movement (Lewis et al. 2011a). While this enhanced rootward transport depletes auxin in the regions from which lateral roots initiate, it likely contributes to the elevated auxin levels in the root tip, which inhibit root elongation. This effect is also shown schematically in Fig. 10.4.
10.5.6 Ethylene and Auxin Transport Regulate Root Hair Elongation
Recent evidence suggests that an intracellular threshold of auxin, controlled by auxin influx and efflux processes, is required for optimal elongation of root hairs. The auxin influx mutant aux1 develops shorter root hairs (Okada and Shimura 1994; Pitts et al. 1998; Rahman et al. 2002). The restoration of the root hair length of aux1 to wild-type level with a minute amount of NAA, which does not affect the intracellular ethylene response, further confirms that besides ethylene, auxin also plays a major role in regulating the root hair elongation process (Rahman et al. 2002). The auxin efflux mutant eir1/pin2 also shows a short root hair phenotype, which has been attributed to the reduced auxin supply from the root tip to the hair differentiation zone (Cho et al. 2007). The requirement of auxin transport in regulating root hair elongation has been confirmed in several recent studies (Schlicht et al. 2008; Jones et al. 2009; Ganguly et al. 2010), including the report that the long root hair phenotype of ethylene over production mutant eto1 can be reversed by specifically blocking auxin influx in an aux1 eto1 double mutant (Strader et al. 2010).
10.6 Ethylene Regulate Auxin Transport, Signaling, and Synthesis in Hypocotyls
10.6.1 Ethylene Modulates Auxin Transport in Hypocotyls
Ethylene-auxin crosstalk in hypocotyls also acts through modulation of auxin transport. Ethylene inhibits auxin transport in excised pea stems (Burg and Burg 1966; Suttle 1988) and high concentrations of IAA increase ethylene production that, in turn, diminishes growth. Application of NPA inhibits hypocotyl growth of light-grown Arabidopsis seedlings (Jensen et al. 1998), but has minimal effect on hypocotyl growth of dark-grown seedlings (Garbers et al. 1996; Lehman et al. 1996; Jensen et al. 1998), suggesting that auxin transport is important for hypocotyl elongation in the light, but not in the dark. Consistent with this, auxin transport in hypocotyls of Arabidopsis (Rashotte et al. 2003) and tomato (Liu et al. 2011) is lower in dark-grown seedlings compared to light-grown seedlings. However, ethylene-stimulated hypocotyl nutational bending of dark-grown Arabidopsis seedlings is blocked by NPA, suggesting that altered auxin transport may have subtle growth effects on dark-grown hypocotyls (Binder et al. 2006). Auxin transport is important for the effects of ethylene since NPA blocks hook formation in eto1 and ctr1 mutants (Lehman et al. 1996), while application of auxin restores hook formation in ethylene insensitive mutants (Vandenbussche et al. 2010).
10.6.2 Ethylene Regulates Auxin Signaling and Synthesis in Hypocotyls
Further evidence for ethylene’s effect on auxin accumulation or signaling comes from reporter-gene and physiological experiments using auxin-deficient mutants. DR5-GUS accumulates on the concave side of the apical hook and ethylene treatment results in higher levels of accumulation (Li et al. 2004; Zadnikova et al. 2010). When the apical hook opens, this expression of DR5-GUS becomes more diffuse (Zadnikova et al. 2010). Additionally, application of ethylene enhances TAR2-GUS levels on the concave side of the hook and the apical hook is eliminated in wei8 tar2 double mutants (Stepanova et al. 2008; Vandenbussche et al. 2010). In contrast, ethylene reporter gene analysis shows that ethylene signaling is homogenous across the hook in air (Vandenbussche et al. 2010), although, application of exogenous ethylene leads to an asymmetrical distribution of ACO2 in the apical hook (Raz and Ecker 1999). Thus, ethylene may accentuate the apical hook by increasing auxin levels on the concave side through both increased synthesis, signaling, and transport.
10.6.3 Ethylene Alters Auxin Transport During Apical Hook Formation
Several auxin transporters were found to play a role in normal hook development. Subtle effects on the apical hook are found in pin3, pin4, pin7, aux1, and lax3 mutants (Vandenbussche et al. 2010; Zadnikova et al. 2010). Many of these transporters have overlapping roles in the apical hook since pin4 pin7 and aux1 lax3 double mutants have more severe apical hook phenotypes than the single mutants (Vandenbussche et al. 2010; Zadnikova et al. 2010). ACC increases DR5-GUS, PIN3-GFP, and AUX1-GUS expression on the concave side of the hook during the period of maximal curvature (Vandenbussche et al. 2010; Zadnikova et al. 2010) suggesting that ethylene exerts at least some of its effects on hook curvature through both auxin influx and efflux carriers.
10.6.4 Ethylene Regulates Auxin Signaling in the Apical Hook
In addition to altering auxin levels across the apical hook, it is also likely that ethylene alters molecular components involved in auxin signaling. The expression of IAA3-GUS, IAA12-GUS, and IAA13-GUS are elevated on the concave side of the hook (Zadnikova et al. 2010). The asymmetric distribution of these reporters’ signal is enhanced upon application of ACC, while NPA diminishes the asymmetric distribution of IAA13-GUS (Zadnikova et al. 2010). It has also been reported that nph4 mutant has diminished auxin response and asymmetric growth including apical hook formation (Stowe-Evans et al. 1998; Harper et al. 2000). However, these mutants show normal triple responses (including an exaggerated apical hook when ethylene is added) (Stowe-Evans et al. 1998; Harper et al. 2000). Thus, there appears to be complex interactions between ethylene and auxin that affect hook curvature.
The HLS1 gene was identified in a screen in which it failed to form an apical hook in the presence of exogenous ethylene (Guzman and Ecker 1990) and was used extensively to examine ethylene-auxin crosstalk in the apical hook. Overexpression of HLS1 causes an exaggerated apical hook (Lehman et al. 1996). Epistasis analysis placed HLS1 downstream of CTR1 (Roman et al. 1995; Lehman et al. 1996). The mRNA levels for HLS1 increase when seedlings are treated with ethylene and decrease in ein2 mutants (Lehman et al. 1996). In the hls1 mutant both SAUR-Ac1 transcripts and DR5-GUS signal are reduced in the region where an apical hook should form (Li et al. 2004), suggesting that disrupted auxin distribution leads to a failure to form an apical hook (Lehman et al. 1996). A hls1 suppressor screen identified ARF2 as a down-stream component in this regulation where hls1 arf2 double mutants showed partially restored hook curvature, responsiveness to ethylene, and DR5-GUS expression (Li et al. 2004). Application of ethylene causes a decrease of ARF2 protein in wild-type plants, while hls1 mutants over-accumulate ARF2 (Li et al. 2004). Thus, HLS1 appears to negatively regulate the levels of ARF2 and may thereby lead to changes in auxin induced gene expression.
10.7 Reciprocal Regulation of Auxin and Ethylene Synthesis
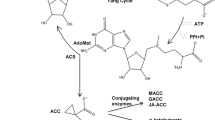
An interesting reciprocal regulation of auxin and ethylene synthesis has been reported. Elevated levels of auxin lead to increased ethylene synthesis (Morgan and Hall 1962), due to increased synthesis of specific members of the ACC synthase (ACS) family, which catalyze the rate limiting step in ethylene synthesis (Abel et al. 1995b; Stepanova et al. 2007). Additionally, ethylene may also positively regulate auxin synthesis (Basu et al. 2011). Free IAA levels increase in the root tip after treatment with 100 µM ACC and this response is lost in etr1 or after treatment with the ethylene synthesis inhibitor AVG (Ruzicka et al. 2007; Swarup et al. 2007). The rate of IAA synthesis was also measured after ACC treatment and in one case, 10 and 100 µM ACC increased IAA synthesis (Swarup et al. 2007), but in another case, 100 µM ACC did not have a significant effect on auxin synthesis (Ruzicka et al. 2007). Yet, reductions in free IAA were detected by both groups in the presence of AVG (Ruzicka et al. 2007; Swarup et al. 2007) leading to the hypothesis that ethylene increases auxin biosynthesis. The demonstration that constitutive ethylene signaling resulted in a fivefold increase in auxin concentration over wild-type in the ctr1 root apex (Ikeda et al. 2009) further supports this idea.
10.7.1 Ethylene Increases Auxin Synthesis
Strong evidence that ethylene can induce auxin synthesis can be found in a screen for weak ethylene insensitive (wei) mutants that identified several genes encoding proteins that function in ethylene-induced auxin synthesis (Stepanova et al. 2005, 2008). These include the alpha and beta subunits of anthranilate synthase, ASA1/WEI2/TIR7 and ASB1/WEI7, respectively, which catalyze the first step of tryptophan biosynthesis and thereby contribute to IAA precursor availability (Stepanova et al. 2005). The WEI8 gene encodes a tryptophan aminotransferase (TAA) that functions in the indole-3-pyruvic acid branch of IAA synthesis and its cloning led to identification of the TAR (TAA-related) gene family (Stepanova et al. 2008). A wei8 tar2 mutant has significant reductions in free IAA, DR5-GUS expression at the root tip in the presence and absence of ACC, and root growth inhibition (Stepanova et al. 2008). The identification of a small molecule, L-kynurenin, which inhibits TAA/TAR proteins, further supports the presence of a feedback loop between auxin biosynthesis and ethylene signaling (He et al. 2011). A recent study identified an additional aminotransferase, VAS1, which is involved in both auxin and ethylene biosynthesis (Zheng et al. 2013). Loss of function of VAS1 causes auxin and ACC levels to increase (Zheng et al. 2013) suggesting this gene regulates the homeostasis of both hormones. The interplay between auxin and ethylene synthesis may vary between organisms, as Brachypodium shows different interactions between ethylene and auxin synthesis (Pacheco-Villalobos et al. 2013). Together, these results suggest that ethylene and auxin enhance each other’s synthesis as one of the mechanisms by which these two hormones synergistically inhibit root elongation.
10.8 Concluding Remarks
Ethylene alters many features of auxin-dependent seedling growth. In all cases examined to date, these ethylene responses alter some feature of auxin function; either signaling, synthesis, or transport, and in most cases all three. Ethylene mediates these effects on seedling growth by acting through the canonical ethylene signaling pathway. What is most fascinating about these interactions is that although ethylene and auxin synergistically affect many processes, such as root elongation and root hair formation, they act antagonistically in other processes, such as lateral root formation. The interactions are even more complex for processes in which auxin is asymmetrically accumulated to drive differential growth, such as gravitropism or hook opening, where ethylene prevents this asymmetry by either lowering or raising auxin accumulation on both sides of these organs.
The level of understanding of ethylene’s modulation of auxin transport, synthesis, and signaling is quite divergent. The evidence for ethylene altering auxin transport by altering the activity or synthesis of auxin transport proteins is now clearly demonstrated, facilitated by the extensive understanding of the proteins that mediate auxin transport. With recent insight into the pathways for auxin synthesis, the mechanisms by which these two hormones regulate each other’s synthesis are now being clarified. The areas in which additional insights are likely to emerge in the near future are the identification of the precise transcriptional factor networks that regulate these synergistic and antagonistic activities of ethylene and auxin in controlling growth and developmental responses.
References
Abbas M, Alabadi D, Blazquez MA. Differential growth at the apical hook: all roads lead to auxin. Front Plant Sci. 2013;4:441.
Abel S, Nguyen MD, Theologis A. The PS-IAA4/5-like family of early auxin-inducible mRNAs in Arabidopsis thaliana. J Mol Biol. 1995a;251:533–49.
Abel S, Nguyen MD, Chow W, Theologis A. ACS4, a primary indoleacetic acid-responsive gene encoding 1-aminocyclopropane-1-carboxylate synthase in Arabidopsis thaliana. Structural characterization, expression in Escherichia coli, and expression characteristics in response to auxin. J Biol Chem. 1995b;270:19093–9.
Alarcon MV, Lloret PG, Salguero J. Auxin-ethylene interaction in transversal and longitudinal growth in maize primary root. Botany. 2013;91:680–5.
Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science. 1999;284:2148–52.
Alonso JM, Stepanova AN, Solano R, Wisman E, Ferrari S, Ausubel FM, Ecker JR. Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis. Proc Natl Acad Sci USA. 2003;100:2992–7.
Bailly A, Sovero V, Vincenzetti V, Santelia D, Bartnik D, Koenig BW, Mancuso S, Martinoia E, Geisler M. Modulation of P-glycoproteins by auxin transport inhibitors is mediated by interaction with immunophilins. J Biol Chem. 2008;283:21817–26.
Baldwin KL, Strohm AK, Masson PH. Gravity sensing and signal transduction in vascular plant primary roots. Am J Bot. 2013;100:126–42.
Barry CS, Giovannoni JJ. Ripening in the tomato Green-ripe mutant is inhibited by ectopic expression of a protein that disrupts ethylene signaling. Proc Natl Acad Sci USA. 2006;103:7923–8.
Basu P, Brown KM, Pal A. Detailed quantitative analysis of architectural traits of basal roots of young seedlings of bean in response to auxin and ethylene. Plant Physiol. 2011;155:2056–65.
Bellini C, Pacurar DI, Perrone I. Adventitious roots and lateral roots: similarities and differences. Annu Rev Plant Biol. 2014;65:639–66.
Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602.
Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA. Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science. 1996;273:948–50.
Beyer EM, Morgan PW. Abscission: the role of ethylene modification of auxin transport. Plant Physiol. 1971;48:208–12.
Bhalerao RP, Eklöf J, Ljung K, Marchant A, Bennett M, Sandberg G. Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J. 2002;29:325–32.
Binder BM, O’Malley RC, Wang WY, Zutz TC, Bleecker AB. Ethylene stimulates nutations that are dependent on the ETR1 receptor. Plant Physiol. 2006;142:1690–700.
Bleecker AB, Estelle MA, Somerville C, Kende H. Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science. 1988;241:1086–9.
Boerjan W, Cervera MT, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Van Onckelen H, Van Montagu M, Inze D. Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell. 1995;7:1405–19.
Britz SJ, Galston AW. Physiology of movements in stems of seedling pisum-sativum-L Cv alaska. 2. The role of the apical hook and of auxin in nutation. Plant Physiol. 1982;70:1401–4.
Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, Taiz L, Muday GK. Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol. 2001;126:524–35.
Buer CS, Muday GK. The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell. 2004;16:1191–205.
Buer CS, Wasteneys GO, Masle J. Ethylene modulates root-wave responses in Arabidopsis. Plant Physiol. 2003;132:1085–96.
Buer CS, Sukumar P, Muday GK. Ethylene modulates flavonoid accumulation and gravitropic responses in roots of Arabidopsis. Plant Physiol. 2006;140:1384–96.
Burg SP, Burg EA. The interaction between auxin and ethylene and its role in plant growth. Proc Natl Acad Sci USA. 1966;55:262–9.
Burg SP, Burg EA. Inhibition of polar auxin transport by ethylene. Plant Physiol. 1967;42:1224–8.
Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ, Bennett M. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell. 2001;13:843–52.
Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science. 1993;262:539–44.
Chang SC, Kim YS, Lee JY, Kaufman PB, Kirakosyan A, Yun HS, Kim TW, Kim SY, Cho MH, Lee JS, Kim SK. Brassinolide interacts with auxin and ethylene in the root gravitropic response of maize (Zea mays). Physiol Plant. 2004;121:666–73.
Chapman EJ, Estelle M. Mechanism of auxin-regulated gene expression in plants. Annu Rev Genet. 2009;43:265–85.
Chen RJ, Hilson P, Sedbrook J, Rosen E, Caspar T, Masson PH. The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc Natl Acad Sci USA. 1998;95:15112–7.
Cho M, Lee SH, Cho HT. P-glycoprotein4 displays auxin efflux transporter-like action in Arabidopsis root hair cells and tobacco cells. Plant Cell. 2007;19:3930–43.
Clark DG, Gubrium EK, Barrett JE, Nell TA, Klee HJ. Root formation in ethylene-insensitive plants. Plant Physiol. 1999;121:53–60.
Cluis CP, Mouchel CF, Hardtke CS. The Arabidopsis transcription factor HY5 integrates light and hormone signaling pathways. Plant J. 2004;38:332–47.
Coleman WK, Huxter TJ, Reid DM, Thorpe TA. Ethylene as an endogenous inhibitor of root regeneration in tomato leaf-disks cultured in vitro. Physiol Plantarum 1980;48:519–25.
Collett CE, Harberd NP, Leyser O. Hormonal interactions in the control of Arabidopsis hypocotyl elongation. Plant Physiol. 2000;124:553–61.
De-Klerk G, Krieken W, Wada T, DeJong J. The formation of adventitious roots: new concepts, new possibilities. In Vitro Cell Dev Biol-Plant. 1999;35:481–90.
de Grauwe L, Vandenbussche F, Tietz O, Palme K, Van Der Straeten D. Auxin, ethylene and brassinosteroids: tripartite control of growth in the Arabidopsis hypocotyl. Plant Cell Physiol. 2005;46:827–36.
Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–5.
Dolan L. The role of ethylene in root hair growth in Arabidopsis. J Plant Nutr Soil Sci. 2001;164:141–5.
Estelle MA, Somerville C. Auxin-resistant mutants of Arabidopsis thaliana with an altered morphology. Mol Gen Genet. 1987;206:200–6.
Friml J, Palme K. Polar auxin transport–old questions and new concepts? Plant Mol Biol. 2002;49:273–84.
Fukaki H, Tameda S, Masuda H, Tasaka M. Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J. 2002;29:153–68.
Ganguly A, Lee SH, Cho M, Lee OR, Yoo H, Cho HT. Differential auxin-transporting activities of PIN-FORMED proteins in Arabidopsis root hair cells. Plant Physiol. 2010;153:1046–61.
Garbers C, DeLong A, Deruère J, Bernasconi P, Soll D. A mutation in protein phosphatase 2A regulatory subunit A affects auxin transport in Arabidopsis. EMBO J. 1996;15:2115–24.
Geisler M, Wang B, Zhu J. Auxin transport during root gravitropism: transporters and techniques. Plant Biol (Stuttg). 2014;16(Suppl 1):50–7.
Geisler M, Blakeslee JJ, Bouchard R, Lee OR, Vincenzetti V, Bandyopadhyay A, Titapiwatanakun B, Peer WA, Bailly A, Richards EL, Ejendal KF, Smith AP, Baroux C, Grossniklaus U, Muller A, Hrycyna CA, Dudler R, Murphy AS, Martinoia E. Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J. 2005;44:179–94.
Giovannoni JJ. Fruit ripening mutants yield insights into ripening control. Curr Opin Plant Biol. 2007;10:283–9.
Gupta A, Singh M, Jones AM, Laxmi A. Hypocotyl directional growth in Arabidopsis: a complex trait. Plant Physiol. 2012;159:1463–76.
Guzman P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 1990;2:513–23.
Hall JL, Brummell DA, Gillespie J. Does auxin stimulate the elongation of intact plant stems? New Phyt. 1985;100:341–5.
Hashiguchi Y, Tasaka M, Morita MT. Mechanism of higher plant gravity sensing. Am J Bot. 2013;100:91–100.
Harper RM, Stowe-Evans EL, Luesse DR, Muto H, Tatematsu K, Watahiki MK, Yamamoto K, Liscum E. The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. Plant Cell. 2000;12:757–70.
He W, Brumos J, Li H, Ji Y, Ke M, Gong X, Zeng Q, Li W, Zhang X, An F, Wen X, Li P, Chu J, Sun X, Yan C, Yan N, Xie DY, Raikhel N, Yang Z, Stepanova AN, Alonso JM, Guo H. A small-molecule screen identifies L-kynurenine as a competitive inhibitor of TAA1/TAR activity in ethylene-directed auxin biosynthesis and root growth in Arabidopsis. Plant Cell. 2011;23:3944–60.
Huang Y, Li H, Hutchison CE, Laskey J, Kieber JJ. Biochemical and functional analysis of CTR1, a protein kinase that negatively regulates ethylene signaling in Arabidopsis. Plant J. 2003;33:221–33.
Ikeda Y, Men S, Fischer U, Stepanova AN, Alonso JM, Ljung K, Grebe M. Local auxin biosynthesis modulates gradient-directed planar polarity in Arabidopsis. Nat Cell Biol. 2009;11:731–8.
Ivanchenko MG, Muday GK, Dubrovsky JG. Ethylene-auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J. 2008;55:335–47.
Jacobs M, Rubery PH. Naturally-occurring auxin transport regulators. Science. 1988;241:346–9.
Jensen PJ, Hangarter RP, Estelle M. Auxin transport is required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis. Plant Physiol. 1998;116:455–62.
Jones AR, Kramer EM, Knox K, Swarup R, Bennett MJ, Lazarus CM, Leyser HM, Grierson CS. Auxin transport through non-hair cells sustains root-hair development. Nat Cell Biol. 2009;11:78–84.
Kendrick MD, Chang C. Ethylene signaling: new levels of complexity and regulation. Curr Opin Plant Biol. 2008;11:479–85.
Kepinski S, Leyser O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature. 2005;435:446–51.
Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell. 1993;72:427–41.
Kim H, Helmbrecht EE, Stalans MB, Schmitt C, Patel N, Wen CK, Wang WY, Binder BM. Ethylene receptor ethylene receptor1 domain requirements for ethylene responses in Arabidopsis seedlings. Plant Physiol. 2011;156:417–29.
Kim HJ, Lynch JP, Brown KM. Ethylene insensitivity impedes a subset of responses to phosphorus deficiency in tomato and petunia. Plant, Cell Environ. 2008;31:1744–55.
King JJ, Stimart DP, Fisher RH, Bleecker AB. A mutation altering auxin homeostasis and plant morphology in Arabidopsis. Plant Cell. 1995;7:2023–37.
Laskowski M, Grieneisen VA, Hofhuis H, Hove CA, Hogeweg P, Maree AF, Scheres B. Root system architecture from coupling cell shape to auxin transport. PLoS Biol. 2008;6:14.
Lavenus J, Goh T, Roberts I, Guyomarc’h S, Lucas M, De Smet I, Fukaki H, Beeckman T, Bennett M, Laplaze L. Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci. 2013;18:450–8.
Le J, Vandenbussche F, Van Der Straeten D, Verbelen JP. In the early response of Arabidopsis roots to ethylene, cell elongation is up- and down-regulated and uncoupled from differentiation. Plant Physiol. 2001;125:519–22.
Lee DJ, Park JW, Lee HW, Kim J. Genome-wide analysis of the auxin-responsive transcriptome downstream of iaa1 and its expression analysis reveal the diversity and complexity of auxin-regulated gene expression. J Exp Bot. 2009;60:3935–57.
Lee JS, Chang WK, Evans ML. Effects of ethylene on the kinetics of curvature and auxin redistribution in gravistimulated toots of Zea mays. Plant Physiol. 1990;94:1770–5.
Lehman A, Black R, Ecker JR. HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell. 1996;85:183–94.
Lewis D, Muday G. Ethylene regulates root growth and development. In: Eschel A, Beeckman T, editors. Plant roots: the hidden half. Boca Raton: CRC Press; 2013. p. 1–27.
Lewis D, Olex A, Lundy S, Turkett W, Fetrow J, Muday G. A kinetic analysis of the auxin transcriptome reveals cell wall remodeling proteins that modulate lateral root development in Arabidopsis. Plant Cell. 2013;25:3329–46.
Lewis DR, Negi S, Sukumar P, Muday GK. Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers. Development. 2011a;138:3485–95.
Lewis DR, Ramirez MV, Miller ND, Vallabhaneni P, Ray WK, Helm RF, Winkel BS, Muday GK. Auxin and ethylene induce flavonol accumulation through distinct transcriptional networks. Plant Physiol. 2011b;156:144–64.
Lewis DR, Miller ND, Splitt BL, Wu G, Spalding EP. Separating the roles of acropetal and basipetal auxin transport on gravitropism with mutations in two Arabidopsis multidrug resistance-like ABC transporter genes. Plant Cell. 2007;19:1838–50.
Leyser HM, Pickett FB, Dharmasiri S, Estelle M. Mutations in the AXR3 gene of Arabidopsis result in altered auxin response including ectopic expression from the SAUR-AC1 promoter. Plant J. 1996;10:403–13.
Li H, Johnson P, Stepanova A, Alonso JM, Ecker JR. Convergence of signaling of differential cell growth pathways in the control in Arabidopsis. Dev Cell. 2004;7:193–204.
Li J, Dai X, Zhao Y. A role for auxin response factor 19 in auxin and ethylene signaling in Arabidopsis. Plant Physiol. 2006;140:899–908.
Liu X, Cohen JD, Gardner G. Low-fluence red light increases the transport and biosynthesis of auxin. Plant Physiol. 2011;157:891–904.
Luschnig C, Gaxiola RA, Grisafi P, Fink GR. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 1998;12:2175–87.
Maher EP, Martindale SJ. Mutants of Arabidopsis thaliana with altered responses to auxins and gravity. Biochem Genet. 1980;18:1041–53.
Marchant A, Kargul J, May ST, Muller P, Delbarre A, Perrot-Rechenmann C, Bennett MJ. AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J. 1999;18:2066–73.
Marchant A, Bhalerao R, Casimiro I, Eklöf J, Casero PJ, Bennett M, Sandberg G. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell. 2002;14:589–97.
Markakis MN, De Cnodder T, Lewandowski M, Simon D, Boron A, Balcerowicz D, Doubbo T, Taconnat L, Renou JP, Hofte H, Verbelen JP, Vissenberg K. Identification of genes involved in the ACC-mediated control of root cell elongation in Arabidopsis thaliana. BMC Plant Biol. 2012;12:208.
Masucci JD, Schiefelbein JW. The rhd6 mutation of Arabidopsis thaliana alters root-hair initiation through an auxin- and ethylene-associated process. Plant Physiol. 1994;106:1335–46.
Mockaitis K, Estelle M. Auxin receptors and plant development: a new signaling paradigm. Annu Rev Cell Dev Biol. 2008;24:55–80.
Morgan P, Hall W. Effect of 2,4-Dichlorophenoxyacetic acid on the production of ethylene by cotton and grain sorghum. Physiol Plant. 1962;15:420–7.
Muday GK, Rahman A. Auxin transport and the integration of gravitropic growth. In: Gilroy S, Masson P, editors. Plant tropisms. Oxford: Blackwell Publishing; 2008. p. 47–8.
Muday GK, Lomax TL, Rayle DL. Characterization of the growth and auxin physiology of roots of the tomato mutant, diageotropica. Planta. 1995;195:548–53.
Muday GK, Rahman A, Binder BM. Auxin and ethylene: collaborators or competitors? Trends Plant Sci. 2012;17:181–95.
Muday GK, Brady SR, Argueso C, Deruere J, Kieber JJ, DeLong A. RCN1-regulated phosphatase activity and EIN2 modulate hypocotyl gravitropism by a mechanism that does not require ethylene signaling. Plant Physiol. 2006;141:1617–29.
Müller A, Guan C, Galweiler L, Tanzler P, Huijser P, Marchant A, Parry G, Bennett M, Wisman E, Palme K. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 1998;17:6903–11.
Negi S, Ivanchenko MG, Muday GK. Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J. 2008;55:175–87.
Negi S, Sukumar P, Liu X, Cohen JD, Muday GK. Genetic dissection of the role of ethylene in regulating auxin-dependent lateral and adventitious root formation in tomato. Plant J. 2010;61:3–15.
Nordstrom A, Eliasson L. Regulation of root formation by auxin-ethylene interaction in pea stem cuttings. Physiol Plant. 1984;61:298–302.
Okada K, Shimura Y. Modulation of root growth by physical stimuli. In Arabidopsis. Cold Spring Harbor Press; 1994. pp. 665–84.
Okamoto T, Tsurumi S, Shibasaki K, Obana Y, Takaji H, Oono Y, Rahman A. Genetic dissection of hormonal responses in the roots of Arabidopsis grown under continuous mechanical impedance. Plant Physiol. 2008;146:1651–62.
Overvoorde P, Fukaki H, Beeckman T. Auxin control of root development. Cold Spring Harb Perspect Biol. 2010;2:a001537.
Pacheco-Villalobos D, Sankar M, Ljung K, Hardtke CS. Disturbed local auxin homeostasis enhances cellular anisotropy and reveals alternative wiring of auxin-ethylene crosstalk in Brachypodium distachyon seminal roots. PLoS Genet. 2013;9:e1003564.
Pacurar DI, Pacurar ML, Bussell JD, Schwambach J, Pop TI, Kowalczyk M, Gutierrez L, Cavel E, Chaabouni S, Ljung K, Fett-Neto AG, Pamfil D, Bellini C. Identification of new adventitious rooting mutants amongst suppressors of the Arabidopsis thaliana superroot2 mutation. J Exp Bot. 2014;65:1605–18.
Paponov IA, Paponov M, Teale W, Menges M, Chakrabortee S, Murray JA, Palme K. Comprehensive transcriptome analysis of auxin responses in Arabidopsis. Mol Plant. 2008;1:321–37.
Peer WA, Bandyopadhyay A, Blakeslee JJ, Makam SN, Chen RJ, Masson PH, Murphy AS. Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell. 2004;16:1898–911.
Pickett FB, Wilson AK, Estelle M. The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol. 1990;94:1462–6.
Pitts RJ, Cernac A, Estelle M. Auxin and ethylene promote root hair elongation in Arabidopsis. Plant J. 1998;16:553–60.
Poupart J, Rashotte AM, Muday GK, Waddell CS. The rib1 mutant of Arabidopsis has alterations in indole-3-butyric acid transport, hypocotyl elongation, and root architecture. Plant Physiol. 2005;139:1460–71.
Radwanski ER, Barczak AJ, Last RL. Characterization of tryptophan synthase alpha subunit mutants of Arabidopsis thaliana. Mol Gen Genet. 1996;253:353–61.
Rahman A, Amakawa T, Goto N, Tsurumi S. Auxin is a positive regulator for ethylene-mediated response in the growth of Arabidopsis roots. Plant Cell Physiol. 2001;42:301–7.
Rahman A, Hosokawa S, Oono Y, Amakawa T, Goto N, Tsurumi S. Auxin and ethylene response interactions during Arabidopsis root hair development dissected by auxin influx modulators. Plant Physiol. 2002;130:1908–17.
Rahman A, Bannigan A, Sulaman W, Pechter P, Blancaflor EB, Baskin TI. Auxin, actin and growth of the Arabidopsis thaliana primary root. Plant J. 2007;50:514–28.
Rashotte AM, DeLong A, Muday GK. Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response, and lateral root growth. Plant Cell. 2001;13:1683–97.
Rashotte AM, Poupart J, Waddell CS, Muday GK. Transport of the two natural auxins, indole-3-butyric acid and indole-3-acetic acid, in Arabidopsis. Plant Physiol. 2003;133:761–72.
Rashotte AM, Brady SR, Reed RC, Ante SJ, Muday GK. Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol. 2000;122:481–90.
Raz V, Ecker JR. Regulation of differential growth in the apical hook of Arabidopsis. Development. 1999;126:3661–8.
Reed RC, Brady SR, Muday GK. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol. 1998;118:1369–78.
Robles LM, Deslauriers SD, Alvarez AA, Larsen PB. A loss-of-function mutation in the nucleoporin AtNUP160 indicates that normal auxin signalling is required for a proper ethylene response in Arabidopsis. J Exp Bot. 2012;63:2231–41.
Rogg LE, Lasswell J, Bartel B. A gain-of-function mutation in iaa28 suppresses lateral root development. Plant Cell. 2001;13:465–80.
Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR. Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics. 1995;139:1393–409.
Ruzicka K, Ljung K, Vanneste S, Podhorska R, Beeckman T, Friml J, Benkova E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell. 2007;19:2197–212.
Santisree P, Nongmaithem S, Vasuki H, Sreelakshmi Y, Ivanchenko MG, Sharma R. Tomato root penetration in soil requires a coaction between ethylene and auxin signaling. Plant Physiol. 2011;156:1424–38.
Sargent JA, Atack AV, Osborne DJ. Auxin and ethylene control of growth in epidermal cells of Pisum sativum—biphasic response to auxin. Planta. 1974;115:213–25.
Schiefelbein JW. Constructing a plant cell. The genetic control of root hair development. Plant Physiol. 2000;124:1525–31.
Schlicht M, Samajova O, Schachtschabel D, Mancuso S, Menzel D, Boland W, Baluska F. D’orenone blocks polarized tip growth of root hairs by interfering with the PIN2-mediated auxin transport network in the root apex. Plant J. 2008;55:709–17.
Shibasaki K, Uemura M, Tsurumi S, Rahman A. Auxin response in Arabidopsis under cold stress: underlying molecular mechanisms. Plant Cell. 2009;21:3823–38.
Shin K, Lee RA, Lee I, Lee S, Park SK, Soh MS. Genetic identification of a second site modifier of ctr1-1 that controls ethylene-responsive and gravitropic root growth in Arabidopsis thaliana. Mol Cells. 2013;36:88–96.
Sibout R, Sukumar P, Hettiarachchi C, Holm M, Muday GK, Hardtke CS. Opposite root growth phenotypes of hy5 versus hy5 hyh mutants correlate with increased constitutive auxin signaling. PLoS Genet. 2006;2:e202.
Skottke KR, Yoon GM, Kieber JJ, DeLong A. Protein phosphatase 2A controls ethylene biosynthesis by differentially regulating the turnover of ACC synthase isoforms. PLoS Genet. 2011;7:e1001370.
Smalle J, Haegman M, Kurepa J, Van Montagu M, Straeten DV. Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc Natl Acad Sci U S A. 1997;94:2756–61.
Solano R, Stepanova A, Chao Q, Ecker JR. Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev. 1998;12:3703–14.
Stepanova AN, Alonso JM. Ethylene signaling and response: where different regulatory modules meet. Curr Opin Plant Biol. 2009;12:548–55.
Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM. A Link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell. 2005;17:2230–42.
Stepanova AN, Yun J, Likhacheva AV, Alonso JM. Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell. 2007;19:2169–85.
Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, Schlereth A, Jurgens G, Alonso JM. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell. 2008;133:177–91.
Stowe-Evans EL, Harper RM, Motchoulski AV, Liscum E. NPH4, a conditional modulator of auxin-dependent differential growth responses in Arabidopsis. Plant Physiol. 1998;118:1265–75.
Strader LC, Chen GL, Bartel B. Ethylene directs auxin to control root cell expansion. Plant J. 2010;64:874–84.
Sukumar P. Role of auxin and ethylene in adventitious root formation in Arabidopsis and tomato. In Biology. Winston Salem: Wake Forest University; 2010.
Sukumar P, Edwards KS, Rahman A, Delong A, Muday GK. PINOID kinase regulates root gravitropism through modulation of PIN2-dependent basipetal auxin transport in Arabidopsis. Plant Physiol. 2009;150:722–35.
Suttle JC. Effect of ethylene treatment on polar IAA transport, net IAA uptake and specific binding of N-1-Naphthylphthalamic acid in tissues and microsomes isolated from etiolated pea epicotyls. Plant Physiol. 1988;88:795–9.
Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GT, Sandberg G, Bhalerao R, Ljung K, Bennett MJ. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell. 2007;19:2186–96.
Tian Q, Reed JW. Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development. 1999;126:711–21.
Ulmasov T, Hagen G, Guilfoyle TJ. Dimerization and DNA binding of auxin response factors. Plant J. 1999;19:309–19.
Utsuno K, Shikanai T, Yamada Y, Hashimoto T. AGR, an Agravitropic locus of Arabidopsis thaliana, encodes a novel membrane-protein family member. Plant Cell Physiol. 1998;39:1111–8.
van Norman JM, Xuan W, Beeckman T, Benfey PN. To branch or not to branch: the role of pre-patterning in lateral root formation. Development. 2013;140:4301–10.
Vandenbussche F, Vriezen WH, Smalle J, Laarhoven LJ, Harren FJ, Van Der Straeten D. Ethylene and auxin control the Arabidopsis response to decreased light intensity. Plant Physiol. 2003;133:517–27.
Vandenbussche F, Petrasek J, Zadnikova P, Hoyerova K, Pesek B, Raz V, Swarup R, Bennett M, Zazimalova E, Benkova E, Van Der Straeten D. The auxin influx carriers AUX1 and LAX3 are involved in auxin-ethylene interactions during apical hook development in Arabidopsis thaliana seedlings. Development. 2010;137:597–606.
Vanneste S, De Rybel B, Beemster GT, Ljung K, De Smet I, Van Isterdael G, Naudts M, Iida R, Gruissem W, Tasaka M, Inze D, Fukaki H, Beeckman T. Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14-mediated lateral root initiation in Arabidopsis thaliana. Plant Cell. 2005;17:3035–50.
Wilkinson JQ, Lanahan MB, Yen HC, Giovannoni JJ, Klee HJ. An ethylene-inducible component of signal transduction encoded by never- ripe. Science. 1995;270:1807–9.
Wilson AK, Pickett FB, Turner JC, Estelle M. A dominant mutation in Arabidopsis confers resistance to auxin, ethylene and abscisic acid. Mol Gen Genet. 1990;222:377–83.
Woodward AW, Bartel B. Auxin: regulation, action, and interaction. Ann Bot (Lond). 2005;95:707–35.
Wu G, Lewis DR, Spalding EP. Mutations in Arabidopsis multidrug resistance-like ABC transporters separate the roles of acropetal and basipetal auxin transport in lateral root development. Plant Cell. 2007;19:1826–37.
Yamamoto M, Yamamoto KT. Differential effects of 1-naphthaleneacetic acid, indole-3-acetic acid and 2,4-dichlorophenoxyacetic acid on the gravitropic response of roots in an auxin-resistant mutant of Arabidopsis, aux1. Plant Cell Physiol. 1998;39:660–4.
Yang S, Hoffman N. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol. 1984;35:155–89.
Zadnikova P, Petrasek J, Marhavy P, Raz V, Vandenbussche F, Ding Z, Schwarzerova K, Morita MT, Tasaka M, Hejatko J, Van Der Straeten D, Friml J, Benkova E. Role of PIN-mediated auxin efflux in apical hook development of Arabidopsis thaliana. Development. 2010;137:607–17.
Zazimalova E, Murphy AS, Yang HB, Hoyerova K, Hosek P. Auxin transporters—why so many? Cold Spring Harb Perspect Biol. 2010;2:a00152.
Zhang YJ, Lynch JP, Brown KM. Ethylene and phosphorus availability have interacting yet distinct effects on root hair development. J Exp Bot. 2003;54:2351–61.
Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science. 2001;291:306–9.
Zheng Z, Guo Y, Novak O, Dai X, Zhao Y, Ljung K, Noel JP, Chory J. Coordination of auxin and ethylene biosynthesis by the aminotransferase VAS1. Nat Chem Biol. 2013;9:244–46.
Zobel RW. Some physiological characteristics of the ethylene-requiring tomato mutant diageotropica. Plant Physiol. 1973;52:385–9.
Acknowledgments
This work was supported by grants to GKM from the United States Department of Agriculture National Institute of Food and Agriculture program (2009-65116-20436) and from the National Science Foundation (NSF) Arabidopsis 2010 Program (IOB-0820717). The NSF Major Research Instrumentation Program supported the purchase of the LSCM used to generate the confocal images used in the figures in this article (Grant number MRI-0722926).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Muday, G.K., Maloney, G.S., Lewis, D.R. (2015). Integration of Ethylene and Auxin Signaling and the Developmental Consequences of Their Crosstalk. In: Wen, CK. (eds) Ethylene in Plants. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9484-8_10
Download citation
DOI: https://doi.org/10.1007/978-94-017-9484-8_10
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9483-1
Online ISBN: 978-94-017-9484-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)